Abstract
Nitrate acts as an electron acceptor in the denitrification process. The effect of nitrate in the range of 0 to 1,000 mg/liter on Pseudomonas mandelii nirS, cnorB, and nosZ gene expression was studied, using quantitative reverse transcription-quantitative PCR. Denitrification activity was measured by using the acetylene blockage method and gas chromatography. The effect of acetylene on gene expression was assessed by comparing denitrification gene expression in P. mandelii culture grown in the presence or absence of acetylene. The higher the amount of NO3− present, the greater the induction and the longer the denitrification genes remained expressed. nirS gene expression reached a maximum at 2, 4, 4, and 6 h in cultures grown in the presence of 0, 10, 100, and 1,000 mg of KNO3/liter, respectively, while induction of nirS gene ranged from 12- to 225-fold compared to time zero. cnorB gene expression also followed a similar trend. nosZ gene expression did not respond to NO3− treatment under the conditions tested. Acetylene decreased nosZ gene expression but did not affect nirS or cnorB gene expression. These results showed that nirS and cnorB responded to nitrate concentrations; however, significant denitrification activity was only observed in culture with 1,000 mg of KNO3/liter, indicating that there was no relationship between gene expression and denitrification activity under the conditions tested.
Denitrification is a critical biological reaction in the global nitrogen cycle that involves the reduction of nitrate (NO3−) or nitrite (NO2−) to gaseous nitric oxide (NO), nitrous oxide (N2O), or molecular nitrogen (N2) under oxygen-limited conditions (26). The main environmental factors that control denitrification include the availability of substrates (i.e., NO3− or NO2−), oxygen limitation, carbon availability, pH, temperature, and the presence of denitrifying microorganisms (21). The presence of N-oxides, as electron acceptors, is essential in maintaining denitrification activity (9, 21, 24). Nitrate is an excellent choice as a parameter to study the effects on denitrification gene expression and activity, as opposed to NO2−, due to its prevalence in nature and lack of toxicity at high concentrations.
The effect of NO3− on denitrification in a pure culture has been studied through biochemical measurements of changes in NO3−, NO2−, and denitrification products (N2O) (4, 5, 19, 22). Thomas et al. (22) measured denitrification in nonproliferating cell suspensions of Pseudomonas aeruginosa and Pseudomonas fluorescens and concluded that the optimum NO3 concentration for denitrification activity in both cultures was 20 mM. A separate study (5) investigated the rate of denitrification in Pseudomonas stutzeri, P. aeruginosa, and Paracoccus denitrificans and concluded that all three organisms had different denitrification rates and accumulated different amounts of NO2−, N2O, and N2. Most research on denitrification gene expression in Pseudomonas spp. have focused on gene regulation (1, 9, 10); however, there are some studies that have investigated the effect of NO3− on denitrification gene expression. Denitrification in pure culture has been studied to understand the effect of NO3− (10, 14, 19) on denitrification gene expression. Körner and Zumft (14) examined the effect of various N-oxides on the production of denitrification enzymes in pure cultures of P. stutzeri and concluded that NO3− resulted in maximum synthesis of all denitrification reductases, compared to NO2− and N2O. An investigation on the effect of NO3− on denitrification gene expression and activity in P. mandelii, a dominant culturable denitrifier isolated from an agricultural field in Canada (7), was limited to measuring cnorB gene expression in the presence (1,000 mg/liter) or absence of KNO3 addition (19). Nitrate-amended cells expressed cnorB at a high level (average of 2.06 × 108 transcripts/μg RNA) for 6 h and had significant N2O accumulations in the presence of acetylene (101 μmol). When NO3− was not present, transient cnorB gene expression was observed for 3 h, beyond which transcript numbers declined to an average of 3.63 × 106 transcripts/μg RNA, from 4 to 6 h. Moreover, insignificant denitrification was measured in cells grown in the absence of nitrate. Although the present study was useful in understanding the effect of NO3− on cnorB gene expression in P. mandelii culture with or without NO3− addition, the effect of different NO3− concentrations, including intermediate NO3− concentrations on cnorB, nirS, and nosZ denitrification genes, was not addressed. Many denitrification studies use the acetylene inhibition method to measure short-term denitrification activity in soil (6, 11, 15, 17, 18, 20, 23) and pure cultures (3, 19, 25). Acetylene blocks the action of nitrous oxide reductase enzyme, thereby inhibiting the reduction of N2O to N2 (3, 12, 25). As a result, N2O becomes the terminal product of denitrification and can be quantified by gas chromatography. Although the mechanism of action of acetylene has been widely investigated and established, the effect of acetylene on denitrification gene expression has not yet been determined.
The objective of this research was to determine the effect of various NO3− concentrations (0, 10, 100, and 1,000 mg/liter) on growth, denitrification gene expression (nirS, cnorB, and nosZ), and denitrification activity in a pure culture of P. mandelii. The effect of acetylene on denitrification gene expression (nirS, cnorB, and nosZ) in P. mandelii cultures was also assessed. It was hypothesized that (i) increasing concentrations of NO3− would result in improved growth, in a longer period of time during which denitrification genes are highly expressed and, subsequently, in higher denitrification activity as measured by N2O product in the presence of acetylene and (ii) the presence of acetylene would not influence denitrification gene expression.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
P. mandelii strain PD30 was cultured in tryptic soy broth (TSB) medium (Difco, Bacto, Dickinson and Company, Sparks, MA) at 30°C and maintained on tryptic soy agar plates as previously described (19).
Denitrification under various NO3− concentrations.
A randomized complete block experimental design was used, with four treatments and three replicates, and with repeated sampling over time. Treatments were four NO3− concentrations (0, 10, 100, and 1,000 mg of potassium nitrate [KNO3]/liter), and sampling was done at six time points: 0, 2, 4, 6, 8, and 24 h. The experiment was conducted twice. The two experiments were treated as blocks for the analysis of variance.
P. mandelii PD30 was precultured aerobically in TSB medium and shaken in phosphate-buffered saline for 1 h, and the cell biomass was used to inoculate liquid cultures containing 0, 10, 100, or 1,000 mg of KNO3/liter. Denitrification conditions were established as previously described by flushing the flask and replacing the headspace atmosphere with helium (19). At each time point, 3-ml samples were collected and assayed for the optical density (OD), RNA isolation, and NO3− and NO2− analyses. In addition, 12-ml headspace gas samples were obtained to allow for N2O analysis.
Denitrification in the presence or absence of acetylene.
A randomized complete block design was used. The experiment had two treatments and three replicates, and used repeated sampling over time. Treatments consisted of two levels (acetylene+, where 10% of the headspace was composed of acetylene, and acetylene−, where 100% of the headspace was composed of helium), and sampling was done at 2 and 6 h to ensure that the NO3− concentration was not limiting. The experiment was conducted once. P. mandelii PD30 cultures were established as described above in TSB medium supplemented with 0.1% KNO3. Upon induction of denitrification conditions, the flasks were made anaerobic, by making the atmosphere either 100% helium (acetylene−) or 10% (wt/vol) acetylene-90% helium (acetylene+). At each time point, 3-ml samples were collected and then assayed for total RNA isolation and subsequent denitrification gene expression. Headspace gas samples were also collected and analyzed for N2O.
Design of P. mandelii nosZ quantitative PCR primers.
P. mandelii nosZ sequences (DQ377802, DQ377780, and DQ377774) were aligned to find unique, conserved regions using the MegAlign (Lasergene 7) software (Applied Biosystems, Foster City, CA). Primers were selected based on standard conditions for real-time quantitative PCR using PrimerSelect (Lasergene 7). The specificity of the primers was tested and verified with P. mandelii genomic DNA in a PCR. The product was sequenced and submitted to BLAST (NCBI) to ensure specificity (S. Henderson, unpublished data).
Gene expression quantification.
The RNeasy minikit (Qiagen, Inc., Mississauga, Ontario, Canada) was used for total RNA isolation and modified as described by Saleh-Lakha et al. (19). RNA was quantified by using Ribogreen RNA quantitation reagent (Molecular Probes, Eugene, OR). Gene expression quantification was performed on the Bio-Rad iCycler iQ detection system (Bio-Rad Laboratories, Mississauga, Ontario, Canada). Previously designed primers from our research group targeting the cnorB region and nirS region were used (8). The conditions for one-step quantitative PCR targeting of P. mandelii cnorB gene and nirS gene were the same as previously described (8).
P. mandelii nirS gene expression was quantified by using one-step quantitative PCR with 50 ng of total RNA template, 12.5 μl of 2× master mix from the Qiagen QuantiTect SYBR green reverse transcription-PCR kit, 300 nM forward primer (5′-ACCGCGGCCAACAACTCCAACA-3′), 500 nM reverse primer (5′-CCGCCCTGGCCCTTGAGC-3′), and 0.25 μl of reverse transcriptase in a final volume of 25 μl. The thermal cycling conditions were as follows: 30 min at 50°C, 15 min at 95°C, followed by 40 cycles of 15 s at 95°C, 30 s at 68.4°C, 30 s at 72°C, and 15 s at 83°C. The data collection was performed during the last step of each cycle at a temperature of 83°C. In a 40-cycle PCR, a no-template control was undetected.
P. mandelii nosZ gene expression was quantified by using one-step quantitative PCR with 50 ng of total RNA template, 12.5 μl of 2× Qiagen QuantiTect SYBR green reverse transcription-PCR kit, 300 nM forward primer (5′-GGACTAAAAAGATCTGGGAC-3′), 300 nM reverse primer (5′-GTGTCACGTCTTCCACCTTATC-3′), and 0.25 μl of reverse transcriptase in a final volume of 25 μl. The thermal cycling conditions were as follows: 30 min at 50°C, 15 min at 95°C, followed by 40 cycles of 15 s at 95°C, 30 s at 56.4°C, 30 s at 72°C, and 15 s at 80°C. The data collection was performed during the last step of each cycle at a temperature of 80°C. In a 40-cycle PCR, a no-template control was undetected.
During each quantitative reverse transcription-PCR run, standard dilutions of digested plasmid carrying a cloned copy of nosZ gene were included to allow for gene quantification. P. mandelii nosZ primers were used to produce a nosZ PCR product that was then cloned in pGEM-TEasy vector according to the manufacturer's instructions (Promega). Plasmid DNA was extracted by using a plasmid minikit (Qiagen, Inc., Mississauga, Ontario, Canada). The plasmid was linearized by digesting with PstI (Roche, Laval, Quebec, Canada) and heat shocking to deactivate the enzymes. The linearized plasmid was quantified by using Picogreen (Molecular Probes), and the size of the nosZ insert was used to calculate the copy number. The curve was linear over a dilution range of 10−3 to 10−9 and sensitive to at least 10 copies of nosZ per reaction. Positive control consisting of P. mandelii genomic DNA isolated as outlined in the DNeasy tissue kit (Qiagen) and quantified by Quant-iT PicoGreen dsDNA assay kit (Molecular Probes) was used in each quantitative reverse transcription-PCR run.
NO3−, NO2−, and N2O analyses.
Frozen culture supernatant samples were analyzed for NO3−-N and NO2−-N concentrations at the Soil and Nutrient Laboratory (Laboratory Services Division, University of Guelph, Guelph, Ontario, Canada). N2O analysis of headspace gas was performed by gas chromatography as previously described (8). Calculated N2O values were corrected for dissolved N2O in the flask by using the Bunsen absorption coefficient (16) and for changes in pressure and gas concentration attributed to sampling.
Statistical methods.
All parameters were tested for normality using the UNIVARIATE function in the SAS System for Windows (version 8; SAS Institute, Inc., Cary, NC), and a log transformation was performed when required. Analysis of variance was performed by using the MIXED procedure of SAS. For the first experiment, the statistical model treated duplicate experiments as blocks in order to pool data from the two experiments, and the REPEATED function was used to account for repeated sampling of flasks over time. Where there was a significant nitrate treatment by time interaction, treatment means were compared by LSMEANS with a Tukey's adjustment. Statistical significance was accepted at P < 0.05. Treatment means and standard errors presented in the figures are calculated from nontransformed data.
RESULTS
P. mandelii growth and denitrification gene expression at 0, 10, 100, and 1,000 mg of KNO3/liter.
Cell density increased over time in all NO3− treatments (Fig. 1). At 8 h, the OD at 600 nm for the 1,000-kg N/liter treatment of 0.36 was higher than for the other three NO3− treatments (average OD of 0.23). Similarly, at 24 h, P. mandelii cell cultures grown at 1,000 mg of N/liter had a higher OD at 600 nm, 1.01, than with all other NO3− treatments (average OD of 0.45).
FIG. 1.
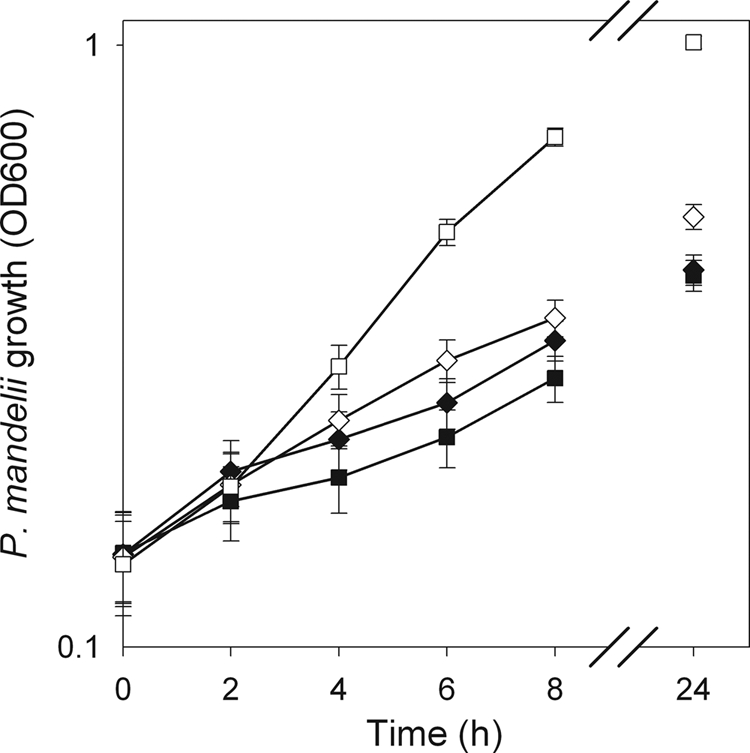
P. mandelii growth in TSB medium supplemented with 0 mg (♦), 10 mg (▪), 100 mg (⋄), or 1,000 mg (□) of KNO3/liter was measured by determining the OD measurements at 600 nm. Error bars indicate ± 1 standard error of the mean (SEM) (n = 6).
NO3− treatment had a significant effect on nirS gene expression in P. mandelii cultures, and the response differed over time. For the 0-mg/liter KNO3 treatment, nirS gene expression increased 12-fold from 0 to 2 h, with 2.7 × 108 transcripts/μg RNA, followed by a significant decline in nirS gene expression at 4 h, and then remained constant thereafter at an average of 3.6 × 107 transcripts/μg RNA between 4 and 24 h (Fig. 2A). In contrast, nirS gene expression increased 143-fold in P. mandelii cultures grown at 10 and 100 mg of KNO3/liter (average of 6.4 × 108 transcripts/μg RNA) and 225-fold in P. mandelii cultures grown at 1,000 mg of KNO3/liter (2.2 × 109 transcripts/μg RNA) to reach a maximum point at 4 and 6 h, respectively, compared to the start of the incubation. Subsequently, a significant decline of in nirS gene expression was measured at 8 and 24 h in P. mandelii cultures grown at 100 and 1,000 mg KNO3/liter, respectively. There was no significant difference in nirS gene expression between 4 and 24 h in P. mandelii cultures grown at 10 mg of KNO3/liter (average of 1.3 × 108 transcripts/μg RNA). nirS gene expression was not significantly different among treatments at 24 h, with an average of 1.3 × 107 transcripts/μg RNA.
FIG. 2.
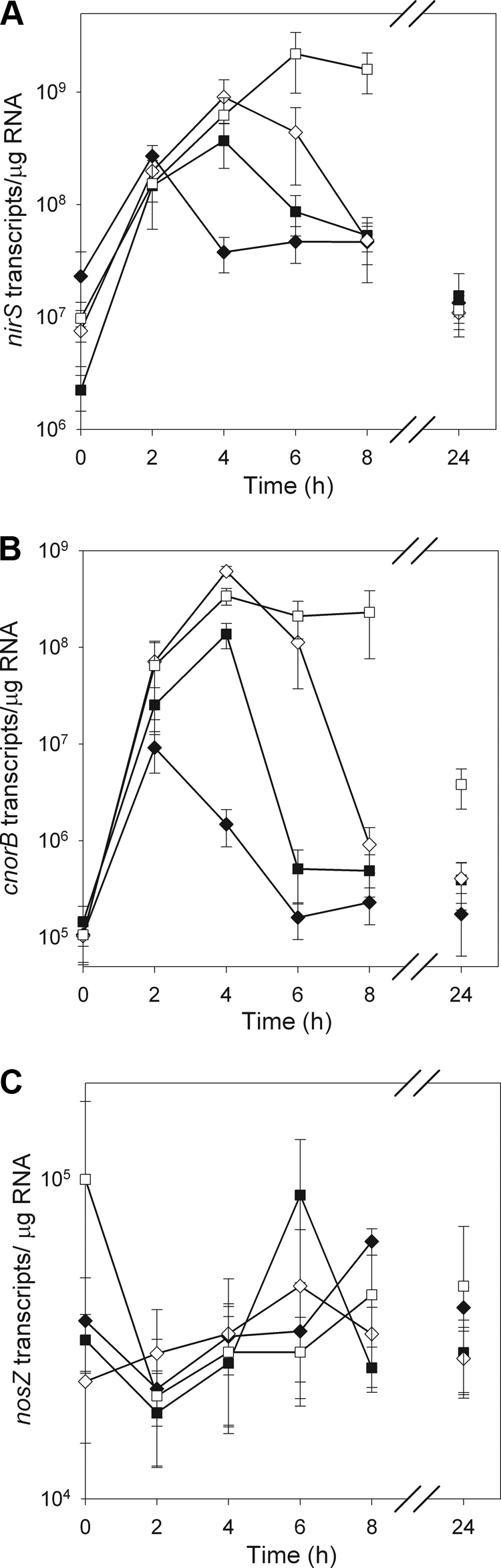
nirS (A), cnorB (B), and nosZ (C) gene expression in P. mandelii grown in TSB medium supplemented with 0 mg (♦), 10 mg (▪),100 mg (⋄), or 1,000 mg (□) of KNO3/liter. Error bars are ± 1 SEM (n = 6 for cnorB and nirS and n = 3 for nosZ). Values were calculated from the line of best fit described by the linear equation for cnorB: y = −4.112x + 46.661 (r2 = 0.99) for the first experiment and y = −4.800x + 53.412 (r2 = 0.99) for the second experiment. For nirS, y = −2.883x + 45.326 (r2 = 0.99) for the first experiment and y = −2.377x + 42.393 (r2 = 0.86) for the second experiment. For nosZ, y = −4.414x + 46.328 (r2 = 0.99).
NO3− treatment had a significant effect on cnorB gene expression in P. mandelii cultures and the response differed over time. Cultures grown at 0 mg of KNO3/liter, demonstrated an 86-fold induction in cnorB gene expression, where cnorB gene transcripts increased from an average of 1.1 × 105 transcripts/μg RNA at 0 h to 9.2 × 106 transcripts/μg RNA at 2 h (Fig. 2B). The number of cnorB transcripts subsequently declined to 1.6 × 105 transcripts/μg RNA at 6 h and then did not change until the end of the incubation at 24 h. Maximum cnorB gene expression in P. mandelii cultures grown at 10, 100, and 1,000 mg of KNO3/liter was measured at 4 h, where P. mandelii cultures exhibited a 949-fold increase for the 10-mg/liter KNO3 treatment (1.4 × 108 transcripts/μg RNA at 4 h) and a 4,550-fold increase for the 100- and 1,000-mg/liter KNO3 treatments (average of 4.8 × 108 transcripts/μg RNA at 4 h), compared to the start of the incubation. For P. mandelii cultures grown at 10 and 100 mg of KNO3/liter, cnorB gene transcripts started to decrease after 4 h and reached time zero transcript levels by 6 h with the 10-mg/liter KNO3 treatment (5.1 × 105 transcripts/μg RNA) and by 8 h with the 100-mg/liter KNO3 treatment (9.2 × 105 transcripts/μg RNA). Both treatments maintained decreased cnorB gene expression at 24 h (average of 4.0 × 105 transcripts/μg RNA). For P. mandelii culture grown at 1,000 of mg KNO3/liter, cnorB gene transcript numbers remained unchanged between 4 and 8 h (average of 2.6 × 108 transcripts/μg RNA) subsequently decreased to 3.8 × 106 transcripts/μg RNA at 24 h.
There was no significant effect of treatment or time and no significant treatment-time interaction on nosZ gene expression in P. mandelii cultures (Fig. 2C). nosZ gene expression averaged 3.8 × 104 transcripts/μg RNA for all treatments over 24 h.
NO3− and NO2− concentration and denitrification in P. mandelii culture grown at 0, 10, 100, and 1,000 mg of KNO3/liter.
NO3− treatment had a significant effect on NO3− and NO2− concentrations in P. mandelii cultures, and the response differed over time.NO3− and NO2− concentrations were not significantly different among P. mandelii cultures grown at 0, 10, and 100 mg KNO3/liter (averages of 1.5 mg of NO3−-N/liter and 1.3 mg of NO2−-N/liter, respectively, from 2 to 8 h (Fig. 3A and B). However, in P. mandelii cultures grown at 1,000 mg of KNO3/liter, NO3− concentrations remained high for 4 h (average of 138 NO3−-N/liter), subsequently declined, and were undetectable by 24 h. NO2− concentrations, in P. mandelii cultures grown at 1,000 mg/liter KNO3 reached a maximum of 60 mg of NO2−-N/liter at 6 h, subsequently declined, and were undetectable by 24 h. The magnitude of NO3− loss (77 ppm) was proportional to the increase in NO2− (54 ppm) at 6 h, which was the time at which significant differences in both NO3− and NO2− concentrations in P. mandelii cultures grown at 1,000 mg of KNO3/liter was observed.
FIG. 3.
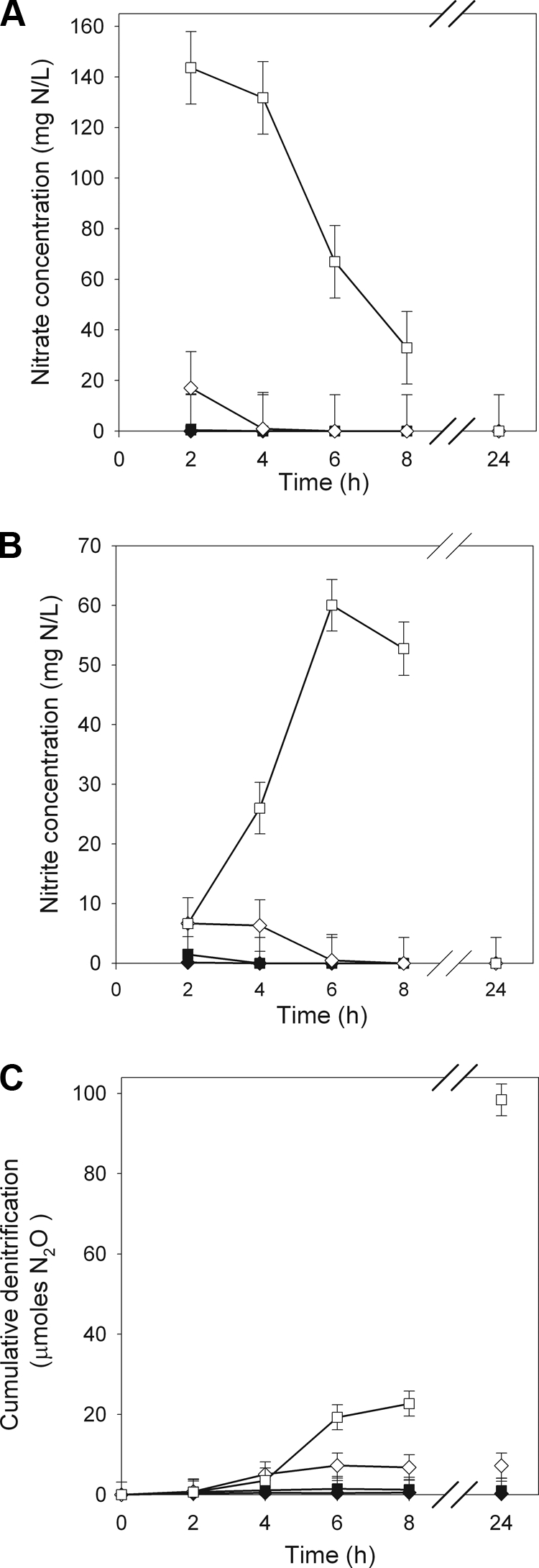
NO3− (A) and NO2− (B) concentrations and denitrification (C) in TSB medium supplemented with 0 mg (♦), 10 mg (▪), 100 mg (⋄), or 1,000 mg (□) of KNO3/liter. Error bars are ± 1 SEM (n = 6).
NO3− treatment had a significant effect on the cumulative denitrification in P. mandelii cultures, and the response differed over time. Denitrification was undetectable in P. mandelii cultures grown at 0 mg of KNO3/liter and, although detectable with the 10- and 100-mg/liter KNO3 treatments, was low, with averages of 1.0 and 7.2 μmol at 24 h, respectively (Fig. 3C). However, in P. mandelii cultures grown at 1,000 mg of KNO3/liter, denitrification was first detected at 4 h and rapidly increased to 98 μmol by 24 h.
Effect of acetylene on P. mandelii denitrification gene expression and denitrification activity.
There was no significant effect of acetylene treatment or time, or acetylene treatment by time interaction, on nirS and cnorB gene expression in P. mandelii cultures (data not shown). In contrast, acetylene treatment had a significant effect on nosZ gene expression in P. mandelii cultures, and the response differed over time. The presence of acetylene in the headspace of denitrifying P. mandelii cultures inhibited nosZ gene expression, with an average of 6.4 × 105 transcripts/μg RNA in the presence of acetylene and 4.2 × 106 transcripts/μg RNA in the absence of acetylene between 2 h and 6 h (Fig. 4).
FIG. 4.
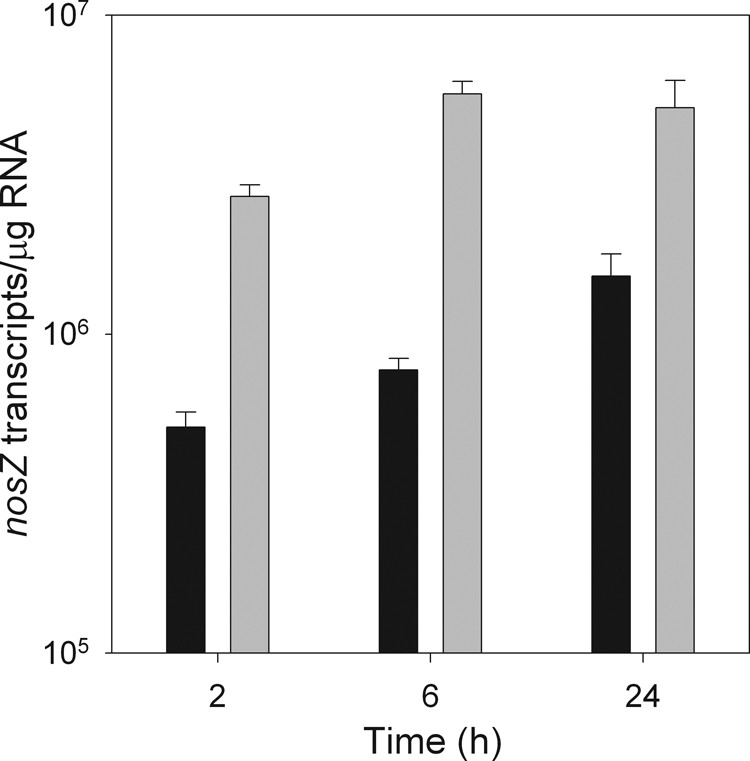
nosZ gene expression in P. mandelii grown in TSB medium supplemented with 1,000 mg of KNO3/liter, grown in the presence (▪) or absence (░⃞) of acetylene. Error bars indicate ± 1 SEM (n = 6 for cnorB and nirS and n = 3 for nosZ). Values were calculated from the line of best fit described by the linear equation for cnorB: y = −3.275x + 35.640 (r2 = 0.98) for the first experiment and y = −2.772x + 34.347 (r2 = 0.99) for the second experiment. For nirS, y = −2.648x + 46.202 (r2 = 0.99) for the first experiment and y = −3.014x + 47.619 (r2 = 0.99) for the second experiment. For nosZ, y = −3.799x + 44.825 (r2 = 0.99).
DISCUSSION
This study demonstrated that the higher the concentration of NO3− present, the greater the induction and the longer the denitrification genes remained expressed. Maximum nirS gene expression was 12-fold at 2 h for cultures grown at 0 mg of KNO3/liter, 43-fold at 4 h in cultures grown at 10 and 100 mg of KNO3/liter and 225-fold at 6 h in cultures grown at 1,000 mg of KNO3/liter. Similarly, cnorB gene expression reached a maximum with an 86-fold increase at 2 h in cultures grown at 0 mg of KNO3/liter, a 949-fold increase at 4 h for the 10-mg/liter KNO3 treatment, and a 4,550-fold increase at 4 h for the 100 and 1,000 mg/liter KNO3 treatments. Increased cnorB gene expression was also measured between 1 and 6 h in a study by Saleh-Lakha et al. (19) in P. mandelii cultures grown in the presence of 1,000 mg of KNO3/liter under anaerobic conditions. Hartig and Zumft (10) also measured nirS and norCB gene expression via Northern blot analysis and reported constant gene expression for a 3-h observation period in denitrifying cultures of Pseudomonas stutzeri grown in the presence of 1,000 mg of KNO3/liter. The present study demonstrated that continued transcription of nirS and cnorB genes in P. mandelii required the presence of NO3−.
In our study, nosZ gene expression was not affected by various NO3− concentrations, nor was the typical pattern of gene induction and gene expression observed, as was the case for nirS and cnorB genes. There was no nosZ gene induction, and the number of nosZ transcripts measured was about 104- and 103-fold lower than both nirS and cnorB gene expression, respectively. This observation is contrary to previous studies where similar induction patterns and coordinated expression of nirS, cnorB, and nosZ genes was observed in P. stutzeri (10, 13). The difference in nosZ gene expression between the present study and previous studies could be attributed to the use of acetylene. Acetylene blockage of the nitrous oxide reductase results in N2O accumulation, which in turn, could affect nosZ regulation. It is also possible that acetylene affects other metabolic processes, which indirectly affects nosZ gene regulation or expression. Alternatively, nosZ gene expression could also have been affected by differences in the experimental conditions used to establish precultures and denitrification conditions, or alternatively, a different mode of nosZ gene regulation in P. mandelii.
We also investigated the effect of variable NO3− concentrations on denitrification intermediates including, specifically, NO2−. Although insignificant NO2− concentrations were observed under limiting (0-, 10-, and 100-mg/liter KNO3) NO3− treatments, 60 mg of NO2−-N/liter accumulated at 6 h in P. mandelii cultures grown with 1,000 mg of KNO3/liter. There are very few studies that have measured NO2− accumulation in the medium of denitrifying cultures. Carlson and Ingraham (5) compared the accumulation of denitrification intermediates in P. stutzeri, P. aeruginosa, and P. denitrificans and concluded that all three species accumulated NO2− at different rates, with P. stutzeri having the highest NO2− accumulation, with an average of 220 μmol, in the presence of 23 mM sodium nitrate. Our previous study also measured insignificant NO2− in P. mandelii cultures grown at 0 mg of KNO3/liter; however, only 8.5 mg of NO2−-N/liter accumulated at 6 h in P. mandelii culture grown with 1,000 mg of KNO3/liter (19). The difference in NO2− accumulation in our previous study and the current one can be explained by slightly different growth conditions (larger flask size and culture volume), suggesting that culture conditions can alter metabolism in P. mandelii cells and thus affect NO2− accumulation.
The effect of NO3− concentrations on denitrification activity, as measured by N2O emissions in the presence of C2H2, demonstrated significant denitrification activity (98 μmol at 24 h) in P. mandelii culture grown with 1,000 mg of KNO3/liter. A recent study also measured 101 μmol of N2O at 24 h in a P. mandelii culture grown with 1,000 mg of KNO3/liter; however, the effects of lower NO3− concentrations on denitrification activity were not investigated (19). Carlson and Ingraham (5) measured different accumulation rates of denitrification intermediates (NO2−) and products (N2O and N2) under unlimited nitrate conditions in pure cultures of P. stutzeri, P. aeruginosa, and P. denitrificans and also concluded that denitrification activity was high under conditions where nitrate was in excess. Our results from the present study demonstrated insignificant denitrification activity under limiting (0-, 10-, and 100-mg/liter) KNO3− treatments, suggesting that KNO3− concentrations of up to 100 mg/liter were not sufficient to allow for significant denitrification activity. There are no other pure culture studies, to our knowledge, that investigated the effect of limited NO3− on denitrification activity. The results presented here demonstrate that nirS and cnorB responded to nitrate concentrations; however, significant denitrification activity was only observed in culture with 1,000 mg of KNO3/liter, indicating that there was no relationship between gene expression and denitrification activity under the conditions tested.
Numerous studies have used acetylene, both in pure culture (3, 19, 25) and soil microcosms (6, 15, 17, 18, 23), as a measure of denitrification. In the present study, denitrification gene expression (nirS, cnorB, and nosZ) was measured in the presence or absence of acetylene in order to determine whether acetylene influences gene expression. Acetylene did not affect nirS or cnorB gene expression in P. mandelii; however, it significantly reduced nosZ gene expression. It has been well established that acetylene inhibits the nitrous oxide reductase enzyme resulting in N2O accumulation (3, 25); however, the effect of acetylene on nosZ gene expression had not yet been determined. The present study provides direct evidence that acetylene decreases nosZ gene expression. This could be due to an effect of acetylene on general metabolism, which in turn, indirectly affects nosZ gene expression. It is also possible that the N2O atmosphere in the flask might interfere with nosZ gene regulation in P. mandelii, although a recent study investigating transcriptional regulation of the nos genes for nitrous oxide reductase in P. aeruginosa concluded that N2O did not affect induction of the promoter for nosZ (2).
In conclusion, we investigated the effect of various NO3− concentrations on growth, denitrification gene expression, and cumulative denitrification. The higher the amount of NO3− present, the greater the induction and the longer the denitrification genes remained expressed. nosZ gene expression did not respond to NO3− treatment under the conditions used. Acetylene negatively influenced nosZ gene expression but did not affect nirS or cnorB gene expression. This observation has important implications in experimental design, especially when investigating nosZ gene expression. This research provided evidence for the effect of varying the NO3− concentration on nirS and cnorB gene expression; however, significant denitrification activity was only observed in culture with 1,000 mg of KNO3/liter, indicating that there was no relationship between gene expression and denitrification activity under the conditions tested.
Acknowledgments
We are grateful to Drucie Janes and Jan Zeng for providing technical support. We are also grateful to Stephen Bowley for providing guidance in the statistical analysis of data.
Funding for this project was supplied by the GAPS program of Agriculture and Agri-Food Canada and an NSERC Strategic Team Grant. S.S.-L. was the recipient of an NSERC doctoral scholarship. S.S.-L. and K.E.S. were also recipients of Ontario Graduate Scholarships. Infrastructure and equipment grants from the Canadian Foundation Innovation and the Ontario Innovation Trust are sincerely acknowledged by J.T.T.
Footnotes
Published ahead of print on 12 June 2009.
REFERENCES
- 1.Arai, H., T. Kodama, and Y. Igarashi. 1999. Effect of nitrogen oxides on expression of the nir and nor genes for denitrification in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 170:19-24. [DOI] [PubMed] [Google Scholar]
- 2.Arai, H., M. Masayuki, and Y. Igarashi. 2003. Transcriptional regulation of the nos genes or nitrous oxide reductase in Pseudomonas aeruginosa. Microbiology 149:29-36. [DOI] [PubMed] [Google Scholar]
- 3.Balderstone, W. L., B. Sherr, and W. J. Payne. 1976. Blockage by acetylene of nitrous oxide reduction in Pseudomonas perfectomarinus. Appl. Environ. Microbiol. 31:504-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergaust, L., J. Shapleigh, Å. Frostegård, and L. Bakken. 2008. Transcription and activities of NOx reductases in Agrobacterium tumefaciens: the influence of nitrate, nitrite, and oxygen availability. Environ. Microbiol. 10:3070-3081. [DOI] [PubMed] [Google Scholar]
- 5.Carlson, C. A., and J. L. Ingraham. 1983. Comparison of denitrification by Pseudomonas stutzeri, Pseudomonas aeruginosa, and Paracoccus denitrificans. Appl. Environ. Microbiol. 45:1247-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dandie, C. E., D. L. Burton, B. J. Zebarth, S. L. Henderson, J. T. Trevors, and C. Goyer. 2008. Changes in bacterial denitrifier community abundance over time in an agricultural field and their relationship with denitrification activity. Appl. Environ. Microbiol. 74:5997-6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dandie, C. E., D. L. Burton, B. J. Zebarth, J. T. Trevors, and C. Goyer. 2007. Analysis of denitrification genes and comparison of nosZ, cnorB, and 16S rDNA from culturable denitrifying bacteria in potato cropping systems. Syst. Appl. Microbiol. 30:128-138. [DOI] [PubMed] [Google Scholar]
- 8.Dandie, C. E., M. N. Miller, D. L. Burton, B. J. Zebarth, J. T. Trevors, and C. Goyer. 2007. Nitric oxide reductase-targeted real-time PCR quantification of denitrifier populations in soil. Appl. Environ. Microbiol. 73:4250-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartig, E., U. Schiek, K. U. Vollack, and W. G. Zumft. 1999. Nitrate and nitrite control of respiratory nitrate reduction in denitrifying Pseudomonas stutzeri by a two-component regulatory system homologous to NarXL of Escherichia coli. J. Bacteriol. 181:3658-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartig, E., and W. G. Zumft. 1999. Kinetics of nirS expression (cytochrome cd1 nitrite reductase) in Pseudomonas stutzeri during the transition from aerobic respiration to denitrification: evidence for a denitrification-specific nitrate- and nitrite-responsive regulatory system. J. Bacteriol. 181:161-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarvis, S. C., D. J. Hatch, and R. D. Lovell. 2001. An improved soil core incubation methods for the field measurement of denitrification and net mineralization using acetylene inhibition. Nutr. Cycl. Agroecosys. 59:219-225. [Google Scholar]
- 12.Knowles, R. 1982. Denitrification. Microbiol. Rev. 46:43-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Körner, H. 1993. Anaerobic expression of nitric oxide reductase from denitrifying Pseudomonas stutzeri. Arch. Microbiol. 159:410-416. [Google Scholar]
- 14.Körner, H., and W. G. Zumft. 1989. Expression of denitrification enzymes in response to the dissolved oxygen level and respiratory substrate in continuous culture of Pseudomonas stutzeri. Appl. Environ. Microbiol. 55:1670-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller, M. N., B. J. Zebarth, C. E. Dandie, D. L. Burton, C. Goyer, and J. T. Trevors. 2008. Crop residue influence on denitrification, N2O emissions and denitrifier community abundance in soil. Soil Biol. Biochem. 40:2553-2562. [Google Scholar]
- 16.Moraghan, J. T., and R. Buresh. 1977. Correction for dissolved nitrous oxide in nitrogen studies. Soil Sci. Soc. Am. J. 41:1201-1202. [Google Scholar]
- 17.Müller, M. M., V. Sundman, and J. Skujins. 1980. Denitrification in low pH spodosols and peats determined with the acetylene inhibition method. Appl. Environ. Microbiol. 40:235-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parkin, T. B., A. J. Sexstone, and J. M. Tiedje. 1985. Adaptation of denitrifying population to low soil pH. Appl. Environ. Microbiol. 49:1053-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saleh-Lakha, S., K. E. Shannon, C. Goyer, J. T. Trevors, B. J. Zebarth, and D. L. Burton. 2008. Nitric oxide reductase gene expression and nitrous oxide production in nitrate-grown Pseudomonas mandelii. Appl. Environ. Microbiol. 74:6876-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith, M. S., M. K. Firestone, and J. M. Tiedje. 1978. The acetylene inhibition method for short-term measurement of soil denitrification and is evaluation using nitrogen-13. Soil Sci. Soc. Am. J. 42:611-615. [Google Scholar]
- 21.Tate, R. L., III. 2000. Denitrification, p. 404-432. In Soil microbiology, 2nd ed. John Wiley, Inc., New York, NY.
- 22.Thomas, K. L., D. Lloyd, and L. Boddy. 1994. Effects of oxygen, pH and nitrate concentration on denitrification by Pseudomonas species. FEMS Microbiol. Lett. 118:181-186. [DOI] [PubMed] [Google Scholar]
- 23.Van Raalte, C. D., and D. G. Patriquin. 1979. Use of the acetylene blockage technique for assaying denitrification in a salt marsh. Mar. Biol. 52:315-320. [Google Scholar]
- 24.Vollack, K. U., and W. G. Zumft. 2001. Nitric oxide signaling and transcriptional control of denitrification genes in Pseudomonas stutzeri. J. Bacteriol. 183:2516-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshinari, T., and R. Knowles. 1976. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem. Biophys. Res. Commun. 69:705-710. [DOI] [PubMed] [Google Scholar]
- 26.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]


