Abstract
In silico substrate docking of both stereoisomers of the pesticide chlorfenvinphos (CVP) in the phosphotriesterase from Agrobacterium radiobacter identified two residues (F131 and W132) that prevent productive substrate binding and cause stereospecificity. A variant (W131H/F132A) was designed that exhibited ca. 480-fold and 8-fold increases in the rate of Z-CVP and E-CVP hydrolysis, respectively, eliminating stereospecificity.
Synthetic organophosphate pesticides (OPs) can cause acute neurotoxicity in insects and humans as a result of their inhibition of acetylcholinesterase at the nerve synapse (15). The >90% identical bacterial phosphotriesterases (PTEs) from Pseudomonas diminuta (oph; PTEPd) (5) and Agrobacterium radiobacter (opdA; PTEAr) (9) efficiently catalyze the hydrolysis of a broad range of OPs, effectively detoxifying them. This has led to the commercialization of PTEAr as a free-enzyme bioremediant (14) and its use in treating OP poisoning in animal studies (1). However, not all OPs are efficiently turned over by the PTEs. For instance, despite having a reasonably reactive leaving group (Fig. 1), the turnover of chlorfenvinphos (CVP) by PTEAr was not detected in a previous study (9).
FIG. 1.
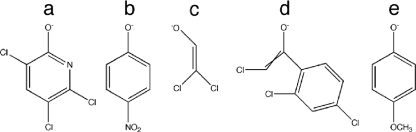
Structures of the leaving groups of all the substrates discussed in this work. (a) 3,5,6-Trichloro-2-pyridinol for methyl chlorpyrifos oxon; (b) 4-nitrophenol for methyl paraoxon and methyl parathion; (c) 2,2-dichloroethenol for dichlorvos; (d) Z/E-2-chloro-1-(2,4-dichlorophenyl)ethanol for E/Z-CVP; (e) 4-methoxyphenol for EPO.
Kinetic analysis.
For a detailed analysis of CVP turnover by PTEAr (expressed and purified as previously described [10]), the E and Z isomers were separated by liquid chromatography, and hydrolysis was measured by liquid chromatography/mass spectrometry (see the supplemental material). These data demonstrate that both isomers of CVP are substrates of PTEAr, although the catalytic efficiencies, particularly for the Z isomer, are very low (Table 1). Additionally, PTEAr exhibits clear isomer specificity, with the kcat/Km of the E isomer exceeding that of the Z isomer by 87-fold. This finding has particular relevance to the application of PTEAr in bioremediation, since the E/Z isomer ratio is approximately 1:9 in commercially available CVP.
TABLE 1.
kcat/Km (s−1 M−1) values for PTEAr and variants with different OPsa
| Substrate |
kcat/Km (s−1 M−1) for PTEAr:
|
|||
|---|---|---|---|---|
| WT | F132A | W131H | W131H/F132A | |
| MPS | 2.7 × 107 | 1.8 × 106 | 1.3 × 106 | 8.7 × 105 |
| DCV | 8.1 × 105 | NA | NA | NA |
| Z-CVP | 9.6 × 10° | 3.9 × 103 | 2.4 × 103 | 4.6 × 103 |
| E-CVP | 8.4 × 102 | 4.3 × 103 | 3.1 × 103 | 6.0 × 103 |
MPS, methyl parathion (ChemService); DCV, dichlorvos (ChemService); CVP, chlorfenvinphos (Bayer Crop Science, Australia); WT, wild type; NA, not available. Error is within 10%.
Substrate docking.
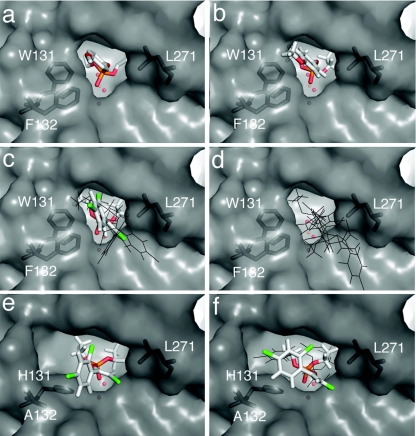
Structures of E- and Z-CVP (obtained from Spartan06; Wavefunction, Inc.) were manually docked at the active site of PTEAr using COOT (6). These poses were then used as starting points for a substrate docking experiment using CDOCKER (16) as previously described (11). As a control, diethyl-4-methoxyphenylphosphate (EPO) was docked, and the resulting pose was found to be effectively identical to the conformation obtained experimentally (Fig. 2b) (11). Of the three unique poses obtained from docking E-CVP (Fig. 2c), only one was productive in the sense that it was aligned such that nucleophilic attack was possible. The hydrogen and chloride atoms of the vinyl moiety straddle the active-site cleft in this pose, with the larger chloride group on the outside of the cleft. No productive poses were obtained with Z-CVP (Fig. 2d), possibly because the analogous pose to the productive E-CVP pose would result in the chloride group (which is positioned on the opposite side of the vinyl bond in the Z-isomer) clashing with the protein. These docking results suggest that the low catalytic efficiency of PTEAr with both isomers is primarily a result of steric hindrance and that the stereospecificity is a result of more severe steric hindrance to Z-CVP binding.
FIG. 2.
Substrate docking using CDOCKER. Unique productive docking poses are shown as colored sticks, whereas unique unproductive docking poses are shown as black lines. The crystal structure of EPO-PTEAr is shown in panel a. Hydrogen atoms are not included since they are not observed in the crystal structure. In silico “docked” poses are also shown as follows: EPO-PTEAr (b), E-CVP-PTEAr (c), Z-CVP-PTEAr (d), E-CVP-PTEAr W131H/F132A (e), and Z-CVP-PTEAr W131H/F132A (f).
Rational design.
Based on the substrate-docking results, the W131A, W131H, F132A, F132L, and F132R mutants were constructed (see the supplemental material) and screened for activity with CVP using a previously described plate-clearing assay (3) at a concentration of 10 mM racemic CVP. The most active variants (W131H and F132A) were identified on the basis of their clearing rates and were combined to produce a W131H/F132A double mutant. Kinetic analysis of these variants was performed (Table 1). The F132A mutation had the largest effect on the rate of CVP hydrolysis, improving kcat/Km by 5.1-fold for E-CVP and 400-fold for Z-CVP, while the W131H mutation improved the rates by 3.7-fold and 250-fold, respectively. Combining the two mutations improved the kcat/Km for E-CVP by 7.1-fold and Z-CVP by 480-fold. The observation that the improvements were nonadditive suggests that the two mutations have similar or overlapping effects. These residues also appear to be responsible for the stereoselectivity of PTEAr, which was relaxed 67-fold from 87:1 in the wild-type enzyme to 1.3:1 in the W131H/F132A variant. Interestingly, the activities of the mutants with the “ideal” substrate methyl parathion were reduced ca. 30-fold, most probably through a loss of stabilizing π-π interactions between W131/F132 and the leaving group in the transition state (reduced kcat; see Table S1 in the supplemental material) and less hydrophobic interaction with the substrate (increased Km; see Table S1 in the supplemental material). The structural effects of the W131H and F132A mutations are shown in Fig. 2. It is clear that both mutations widen the active-site cleft, allowing productive docking of both isomers, and that this improvement is most significant for Z-CVP.
Previous work has shown that a rational redesign of W131 could affect the stereospecificity of PTEPd (2). In that work, W131 was considered part of the small side chain pocket and changes at this position had large effects on the stereospecificity of enantiomers with different-sized alkyl side chains. Here, it is characterized as forming part of the active-site cleft of the enzyme and clearly affects the stereospecificity of the enzyme for substrates with enantiomeric leaving groups. Thus, it appears W131 forms parts of both the small side-chain pocket and the active-site cleft.
Steric and chemical factors in catalysis.
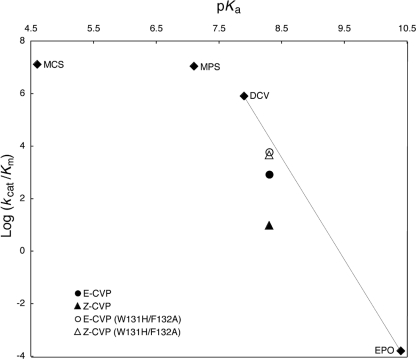
The PTEs exhibit a linear relationship between the pKa value of the leaving group and kcat/Km below a pKa value of ca. 8.0; above this value, other factors, such as diffusion or conformational change, become rate limiting (8). Despite the 480-fold increase in the catalytic efficiency of PTEAr for Z-CVP in the double mutant, its kcat/Km is still ∼103-fold lower than that for methyl paraoxon hydrolysis catalyzed by wild-type PTEAr, and it does not exceed the value that would be expected on the basis of the pKa of the leaving group (Fig. 3). Large catalytic improvements are frequently reported when the substrate specificities of enzymes change as a result of changes in the substrate-binding pocket (3, 4, 17). However, as shown here, the catalytic efficiencies of enzymes are ultimately limited by the underlying reaction chemistry. Improving this facet of catalysis is a looming and markedly more difficult challenge for protein engineers and will most likely require more sophisticated approaches than the relatively simple randomization-based strategies described here and elsewhere.
FIG. 3.
Bronsted plots of log(kcat/Km) versus pKa of the leaving group. The pKa values of the dichlorvos (7.9) and CVP (8.3) leaving groups were calculated using the SPARC online pKa calculator (http://ibmlc2.chem.uga.edu/sparc/) (7), while values for MPO (7.1), MCO (4.6), and EPO were taken from published sources (8, 11, 13). Values of kcat/Km for MCO, MPO, and EPO were taken from previous work (11, 12). The biphasic dependence of the enzyme on pKa is shown, with the curve flattening below a pKa of ∼8.0. A linear dependence on pKa is observed at pKa values below ca. 8.0. The catalysis of Z- and E-CVP hydrolysis by wild-type PTEAr is shown to deviate from the predicted values, while for PTEAr (W131H/F132A), the catalytic rates are close to the expected values.
Supplementary Material
Acknowledgments
This work was supported by Horticulture Australia Ltd. and Orica Australia Ltd.
Footnotes
Published ahead of print on 5 June 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bird, S. B., T. D. Sutherland, C. Gresham, J. Oakeshott, C. Scott, and M. Eddleston. 2008. OpdA, a bacterial organophosphorus hydrolase, prevents lethality in rats after poisoning with highly toxic organophosphorus pesticides. Toxicology 247:88-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen-Goodspeed, M., M. A. Sogorb, F. Wu, S. B. Hong, and F. M. Raushel. 2001. Structural determinants of the substrate and stereochemical specificity of phosphotriesterase. Biochemistry 40:1325-1331. [DOI] [PubMed] [Google Scholar]
- 3.Cho, C. M., A. Mulchandani, and W. Chen. 2004. Altering the substrate specificity of organophosphorus hydrolase for enhanced hydrolysis of chlorpyrifos. Appl. Environ. Microbiol. 70:4681-4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho, C. M., A. Mulchandani, and W. Chen. 2002. Bacterial cell surface display of organophosphorus hydrolase for selective screening of improved hydrolysis of organophosphate nerve agents. Appl. Environ. Microbiol. 68:2026-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumas, D. P., S. R. Caldwell, J. R. Wild, and F. M. Raushel. 1989. Purification and properties of the phosphotriesterase from Pseudomonas diminuta. J. Biol. Chem. 264:19659-19665. [PubMed] [Google Scholar]
- 6.Emsley, P., and K. Cowtan. 2004. COOT: model-building tools for molecular graphics. Acta Crystallogr. D 60:2126-2132. [DOI] [PubMed] [Google Scholar]
- 7.Hilal, S., S. W. Karickhoff, and L. A. Carreira. 1995. A rigorous test for SPARC's chemical reactivity models: estimation of more than 4300 ionization pKa's. Quant. Struct. Act. Rel. 14:348-355. [Google Scholar]
- 8.Hong, S. B., and F. M. Raushel. 1996. Metal-substrate interactions facilitate the catalytic activity of the bacterial phosphotriesterase. Biochemistry 35:10904-10912. [DOI] [PubMed] [Google Scholar]
- 9.Horne, I., T. D. Sutherland, R. L. Harcourt, R. J. Russell, and J. G. Oakeshott. 2002. Identification of an opd (organophosphate degradation) gene in an Agrobacterium isolate. Appl. Environ. Microbiol. 68:3371-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson, C. J., P. D. Carr, H. K. Kim, J. W. Liu, P. Herrald, N. Mitic, G. Schenk, C. A. Smith, and D. L. Ollis. 2006. Anomalous scattering analysis of Agrobacterium radiobacter phosphotriesterase: the prominent role of iron in the heterobinuclear active site. Biochem. J. 397:501-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson, C. J., J. L. Foo, H. K. Kim, P. D. Carr, J. W. Liu, G. Salem, and D. L. Ollis. 2008. In crystallo capture of a Michaelis complex and product-binding modes of a bacterial phosphotriesterase. J. Mol. Biol. 375:1189-1196. [DOI] [PubMed] [Google Scholar]
- 12.Jackson, C. J., J. W. Liu, M. L. Coote, and D. L. Ollis. 2005. The effects of substrate orientation on the mechanism of a phosphotriesterase. Org. Biomol. Chem. 3:4343-4350. [DOI] [PubMed] [Google Scholar]
- 13.Meikle, R. W., and J. W. Hamaker. 1981. The physical properties of 3,5,6-trichloro-2-pyridinol (Dowco 463X) and some environmental consequences. Dow Chemical Company report no. GS-1706. Dow Chemical Company, Midland, MI.
- 14.Scott, C., G. Pandey, C. J. Hartley, C. J. Jackson, M. J. Cheesman, M. C. Taylor, R. Pandey, J. L. Khurana, M. Teese, C. W. Coppin, K. M. Weir, R. K. Jain, R. Lal, R. J. Russell, and J. G. Oakeshott. 2008. The enzymatic basis for pesticide bioremediation. Indian J. Microbiol. 48:65-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomlin, C. D. S. (ed.) 2006. The pesticide manual, 14th ed. British Crop Protection Council, Alton, Hampshire, United Kingdom.
- 16.Wu, G., D. H. Robertson, C. L. Brooks III, and M. Vieth. 2003. Detailed analysis of grid-based molecular docking: a case study of CDOCKER-A CHARMm-based MD docking algorithm. J. Comput. Chem. 24:1549-1562. [DOI] [PubMed] [Google Scholar]
- 17.Yang, H., P. D. Carr, S. Y. McLoughlin, J. W. Liu, I. Horne, X. Qiu, C. M. Jeffries, R. J. Russell, J. G. Oakeshott, and D. L. Ollis. 2003. Evolution of an organophosphate-degrading enzyme: a comparison of natural and directed evolution. Protein Eng. 16:135-145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.