Abstract
Proteins of the Hedgehog, Wnt and Epidermal Growth Factor Receptor (EGFR) ligand families are secreted signals that induce concentration-dependent responses in surrounding cells. Although these proteins must diffuse through the aqueous extracellular environment, recent work has shown that hydrophobic lipid modifications are essential for their functions. All three classes of ligands are palmitoylated in the secretory pathway by related enzymes, and Hedgehog also carries a C-terminal cholesterol modification as a result of its autocatalytic cleavage. Palmitoylation is required for Wingless secretion and contributes to the signaling activity of Hedgehog and Wnt3a, but is not required for secretion or receptor activation by the EGFR ligand Spitz. While lipid modifications enhance the long-range activity of Sonic hedgehog, they restrict the range and increase the local concentration of Spitz. We discuss the diverse functions and the possible extent of palmitoylation of secreted ligands.
Keywords: palmitoylation, acyltransferase, lipid, cholesterol, Hedgehog, Wnt, Spitz, Epidermal growth factor receptor, morphogen
Secreted ligands of the Hedgehog (Hh) and Wnt (Wg) families are important regulators of many developmental processes and can act over long distances1. Hh proteins have been shown to undergo autoproteolytic cleavage accompanied by covalent attachment of a cholesterol molecule to the C-terminal residue of the secreted signaling domain2. This finding was followed by studies showing that Hh and Wnt proteins from both Drosophila and mouse are palmitoylated by the related acyltransferases Rasp (also known as Sightless, Skinny Hedgehog and Central Missing) and Porcupine (Porc) respectively, members of the membrane-bound O-acyltransferase (MBOAT) family3. Addition of palmitate, a 16-carbon saturated fatty acid, is required for Hh and Wnt function in vivo4–10. Most recently, we reported that Rasp also palmitoylates the Epidermal Growth Factor Receptor (EGFR) ligand Spitz (Spi) and is essential for Spi function11. The palmitoylation site in Hh and Spi is the N-terminal cysteine residue following the signal peptide11, 12; however, Wg is palmitoylated on an internal cysteine residue8. Lipid modifications of intracellular proteins often promote their attachment to the plasma membrane13, a function not easily reconciled with diffusion to produce a long-range morphogen gradient. In this review, we will compare and contrast the effects of lipid modification on the Hedgehog, Wnt and EGFR ligands (Fig. 1).
Figure 1. Different effects of lipid modifications on Spi, Hh and Wg.
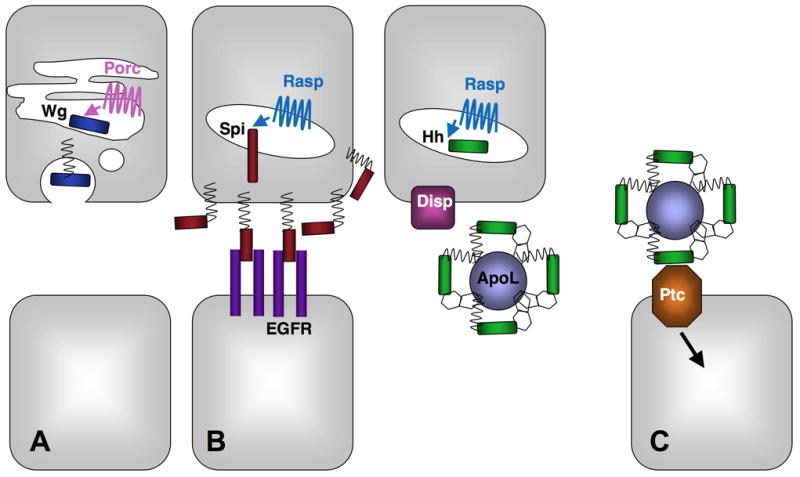
Ligand-producing cells are shown in the upper row and ligand-receiving cells in the lower row. Porc in the ER palmitoylates Wg on an internal cysteine (A), while Rasp in the Golgi palmitoylates Spi and Hh on their N-terminal residues (B, C). Palmitoylation of Wg enhances its glycosylation, lipid raft targeting and secretion (A). Palmitoylation of Spi increases its association with the plasma membrane of producing cells and raises its local concentration to the threshold necessary to activate the EGFR (B). Palmitate and cholesterol modifications on Hh promote its incorporation into multimers that may contain Apolipophorin (ApoL) and enhance its long-range activity. Palmitoylation is also required for Hh to signal productively when bound to Ptc (C).
Palmitoylation is required for Wg secretion
Palmitoylation appears to be required for the secretion of the Drosophila Wnt protein Wingless (Wg). In the absence of either porc or the palmitoylation site, Wg protein accumulates in the cells that synthesize it14–16. Treatment of S2 cells with 2-bromopalmitate, an inhibitor of palmitoylation, also blocks Wg secretion9. The effect of palmitoylation on secretion may be due to its ability to target Wg to lipid raft membrane microdomains that form in the Golgi and are preferentially transported to the plasma membrane9. Palmitoylation of Ras on the cytoplasmic face of the Golgi likewise promotes its transport to the plasma membrane17. Alternatively, decreased secretion of unpalmitoylated Wg may result from protein misfolding and retention in the endoplasmic reticulum (ER), where Porc is localized16. The large number of cysteines in Wg makes misfolding a common response to missense mutations, and lack of palmitoylation would result in a free cysteine14. Wg N-glycosylation is also dependent on Porc; since Wg is glycosylated post-translationally rather than co-translationally, palmitoylation may be required to anchor it close to the oligosaccharyl transferase complex at the membrane18.
Other palmitoylated proteins do not require this modification for their secretion. Mutation of the palmitoylation site of mouse Wnt3a does not prevent its secretion but does reduce its activity, suggesting a second function for Wnt palmitoylation8. Palmitoylation seems to reduce the secretion of Hh and Spi; both proteins are more efficiently recovered from S2 cell culture medium when unpalmitoylated, with this effect being especially dramatic for Spi4, 11. Consistent with the different effects of palmitoylation on Rasp and Porc substrates, Rasp appears to be localized to the Golgi (Fig. 2) rather than the ER. Unpalmitoylated Spi can reach the Golgi and undergo cleavage there by the protease Rhomboid, suggesting that it is correctly folded11, 19.
Figure 2. Rasp is present in the Golgi in Drosophila S2R+ cells.
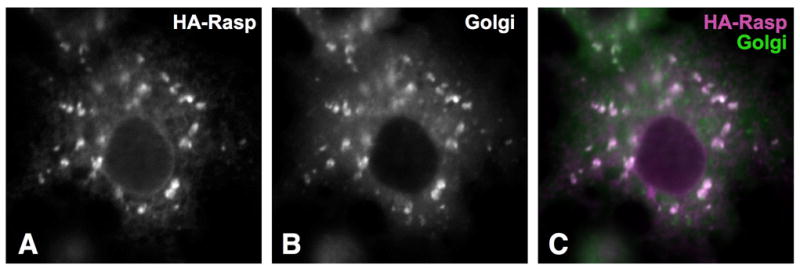
(A–C) show a cell transfected with actin-GAL4, UAS-rasp-HA11 and the Golgi marker UAS-dGRASP65-GFP (kindly provided by Henry Chang). Anti-HA staining (A, magenta in C) colocalizes with GFP (B, green in C).
The different effects of palmitoylation on Wg, Hh and Spi could also be due to the nature of the palmitate linkage. Wg modification occurs through a thioester linkage that is sensitive to cleavage by hydroxylamine or acyl-protein thioesterase-18, 9. Thioester bonds are labile, and proteins modified in this way can undergo cycles of palmitoylation and depalmitoylation20. Reversible palmitoylation targets Ras to the cytoplasmic face of the Golgi apparatus, since depalmitoylation releases it from the plasma membrane17; a similar mechanism might control the localization of Wg within the secretory pathway. In contrast, Hh is palmitoylated on its N-terminal amino acid, and the initial thioester linkage appears to rearrange to form a stable amide bond12. Spi is also modified on its N-terminal cysteine, and like Hh, shows limited incorporation of radioactive palmitate in cell culture, suggesting that only newly synthesized protein can be labeled11. The irreversible modification of Hh and probably Spi may suggest a function in the extracellular environment.
Palmitoylation is required for receptor activation by Hh and Wnt proteins
Palmitoylation has been shown to enhance the signaling activity of some ligands. In assays in which equal amounts of protein are added to cultured cells, unpalmitoylated Wnt3a, Shh and Hh are less active in promoting pathway activation than the palmitoylated forms4, 8, 10, 12. However, Hh binding to its receptor Patched (Ptc) is not significantly affected by palmitoylation12. It has not been determined whether palmitoylation affects the binding of Wnt proteins to Frizzled receptors. An effect on Hh signaling activity can also be seen in vivo, as a transmembrane Hh-CD2 fusion protein fails to activate target genes in neighboring cells when expressed in rasp mutant wing discs4. It is possible that the reduction in activity reflects a loss of access to the membrane domains in which Ptc is located; cholesterol and palmitate modifications have been reported to affect the partitioning of Hh between the apical and basolateral membranes, although the direction of the effect is disputed21, 22. However, a constitutively active chimeric protein in which Hh is directly fused to Ptc23 also shows reduced activity in the absence of rasp (Fig. 3), suggesting that even when bound to Ptc, unpalmitoylated Hh cannot signal productively. Interestingly, other hydrophobic modifications of the N-terminus of Shh can substitute for this effect of palmitoylation24. Finally, mutation of the palmitoylated cysteine to serine confers dominant negative activity on Hh, at least in some contexts22, 25, 26, suggesting that it may competitively inhibit Ptc binding by wildtype Hh. In contrast, palmitoylation does not affect the ability of Spi to bind and activate the EGFR in vitro, and mutation of its palmitoylation site does not result in dominant negative function in vivo11.
Figure 3. A Hh-Ptc fusion protein shows reduced activity in rasp mutant wing discs.
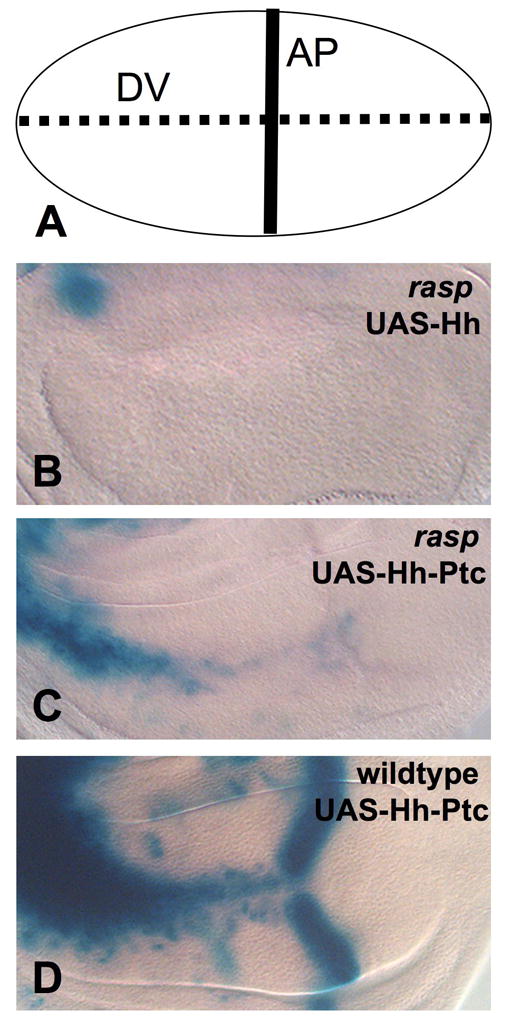
(A) shows a schematic of the wing pouch. The solid line indicates the anterior-posterior (AP) compartment boundary where endogenous dpp is expressed, and the dotted line indicates vestigial (vg)-GAL4 expression at the dorsal-ventral (DV) boundary. (B–D) show third instar wing discs carrying vg-GAL4 and dpp-lacZ and stained with X-Gal. (B–C) raspT392/raspT802; (D) wildtype. (B) expresses UAS-hh and (C–D) express UAS-hh-ptc23. All discs were stained in parallel for the same length of time. The Hh-Ptc fusion activates dpp expression to a slightly greater extent than Hh in the absence of rasp, but its activity is lower than in wildtype discs. Note that endogenous dpp expression at the AP boundary is lost in rasp mutant discs due to the lack of palmitoylation of endogenous Hh4–7. The expression pattern of vg-GAL4 is not altered in rasp mutant wing discs (11 and see Fig. 4).
Palmitoylation has divergent effects on transport
Lipid modification can also regulate ligand transport, but it seems to have different effects on different molecules. Paradoxically, hydrophobic modifications appear to increase Hh solubility; loss of either the palmitate or cholesterol modification on mouse Shh reduces its range of action in vivo10, 27. Interestingly, Shh has been found to form a multimeric complex that is distributed over a long range and signals more efficiently than monomeric Shh10, 28. Both lipid modifications are required for Shh incorporation into this complex10, 29 and probably also for Hh incorporation into a similar complex10, 21. Recent results in Drosophila suggest the possibility that this complex is a lipoprotein particle. Apolipophorins copurify with Hh as well as with Wg, and are required for long-range activity of Hh, although their effect on Wg activity appears weak30. Incorporation of Hh into such a complex may be mediated by Dispatched, a transmembrane protein required for the secretion of cholesterol-modified Hh31, 32. Transport of the complex in vivo requires heparan sulphate proteoglycans (HSPG)33, which may be linked to Hh by Shifted, a secreted Wnt Inhibitory Factor homologue34, 35.
Palmitoylation appears to have the opposite effect on the transport of Spi. Spi normally acts as a short-range ligand, for example in the pairwise recruitment of photoreceptors to an ommatidium36. Misexpression of a truncated form of Spi that does not require cleavage by Rhomboid usually activates target gene expression only in immediately adjacent cells11, 37. However, misexpressed unpalmitoylated Spi can activate weak target gene expression over a long range11. Visualization of tagged Spi molecules suggests that palmitoylation tethers Spi to the plasma membrane of producing cells, preventing its release into the extracellular space11. This would result in a high local concentration of Spi, allowing it to reach the threshold necessary for target gene activation. In contrast, unpalmitoylated Spi would diffuse away and become diluted out, forming a shallower gradient sufficient for target gene activation only under conditions in which Spi is overexpressed11. It is unclear whether Spi can directly bind its receptor while anchored to the membrane by palmitoylation, since Spi is synthesized as a transmembrane protein precursor that is inactive until it is cleaved by the protease Rhomboid19. Intriguingly, Spi isolated from S2 cell culture medium has been shown to initiate at methionine 4511, suggesting that local proteolytic cleavage might generate a soluble, active form of Spi.
Can these apparently different effects of lipid modification be reconciled? One possibility is suggested by a recent paper that uses mathematical modeling to simulate Shh signaling in the neural tube38. The authors obtain the counter-intuitive result that reducing the diffusivity of Shh, an effect that could be produced by lipid modification and/or HSPG binding, extends its effective range of signaling38. The basis for this effect is that untethered Shh diffuses away rapidly and becomes diluted out, failing to reach the threshold required for target gene expression at a distance from the source. Lipid modifications have been shown to reduce the secretion of Hh from cultured cells, supporting the idea that they promote membrane tethering22, 39. This model could explain why loss of cholesterol or palmitate modification of Shh specifically prevents long-range signaling in the mouse limb bud10, 27. Initial reports suggested that removal of cholesterol from Drosophila Hh increased its range of action in the wing disc32. However, this may have been due to its expression in the squamous peripodial cell layer overlying the disc proper, as a reduced range has been seen when Hh lacking cholesterol is restricted to columnar disc epithelial cells22. Interestingly, the range of distribution of GFP-tagged Hh proteins in the wing disc and the range at which they activate low-threshold target genes is increased when they lack the cholesterol or palmitate modifications, although the range at which they can activate high-threshold target genes is decreased21, 22, 40. However, the loss of palmitate causes a much greater functional impairment than loss of cholesterol, suggesting an additional effect on Hh activity as discussed above. It remains to be determined whether incorporation into lipoprotein particles indeed enhances Hh transport, or increases the functional range of Hh by restricting its diffusion.
Lipid modification is only one among a number of mechanisms that have evolved to restrict the range of diffusible ligands. Hh is sequestered and endocytosed by its receptor Ptc; induction of high-level ptc expression by Hh signaling provides a negative feedback mechanism to limit Hh diffusion41, 42. The Wg receptor Dfrizzled2 seems to have the opposite effect, stabilizing Wg to expand its range of function43; however, a receptor-independent endocytic pathway restricts Wg spreading44. Endocytosis and recycling by producing cells also contributes to high Wg levels near the source45. Some ligands are sequestered by secreted proteins rather than receptors; for example, Spi signaling induces expression of the feedback inhibitor Argos, which binds to Spi and prevents it from binding its receptor46, 47. In vertebrates, Shh induces the antagonistic Hedgehog-interacting protein (Hip)48 and a variety of secreted antagonists modulate Wnt function49. In contrast to these mechanisms, restricting the range of a ligand by lipid modification increases the local concentration of the active form, enhancing signaling. Loss of Argos is insufficient to compensate for the loss of palmitoylation of Spi11 and reducing Ptc dosage does not rescue loss of Shh palmitoylation10, suggesting that palmitoylation is necessary to prevent ligand dilution even in the absence of feedback inhibitors.
Are other ligands palmitoylated?
The discovery that Hh, Wnt and Spi proteins are palmitoylated was recent and unexpected, and this modification appears to have a diversity of functions. It is thus tempting to speculate that other secreted ligands may be palmitoylated as well. The basis for acyltransferase specificity is not clear, as the two known Rasp substrates, Hh and Spi, share little sequence homology beyond some basic residues in the vicinity of the palmitoylated cysteine. It is therefore possible that Rasp and Porc have additional substrates. Based on phenotypic analysis, Rasp appears likely to modify the related EGFR ligands Gurken and Keren, but not the long-range Neuregulin-related ligand Vein11. rasp maternally and zygotically mutant embryos show normal mesoderm spreading and tracheal branching, suggesting that rasp is not required for the function of the Fibroblast Growth Factor (FGF) homologues Pyramus, Thisbe and Branchless 50, 51(J. Steinhauer and J.E.T., unpublished data). We have also found that rasp is not essential for the function of the BMP family ligand Decapentaplegic (Dpp), as Dpp is able to activate its target genes when misexpressed in rasp mutant wing discs (Fig. 4). However, other ligands remain to be tested. Rasp and Porcupine belong to the MBOAT family of proteins, which also includes enzymes that acylate lipid substrates3. Several uncharacterized acyltransferases of the MBOAT family are present in the Drosophila and vertebrate genomes. Future investigations will reveal whether they also modify ligands important for development.
Figure 4. Dpp is active in the absence of rasp.
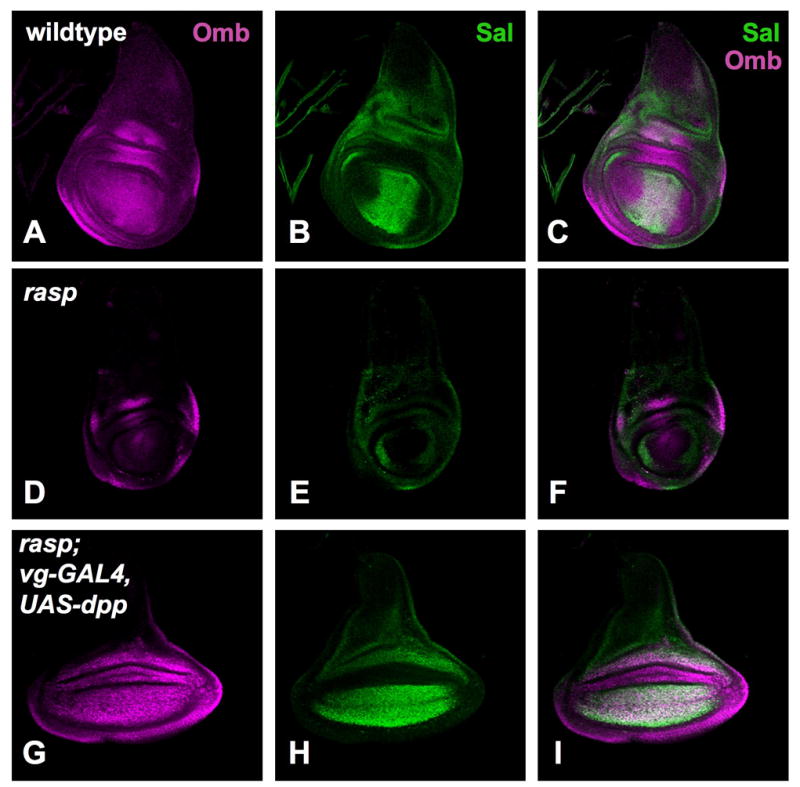
All panels show third instar wing discs. (A–C) wildtype; (D–F) raspT392/raspT802; (G–I) raspT392/raspT802; vg-GAL4/UAS-dpp. The discs were stained to show expression of the Dpp target genes optomotor-blind (Omb; magenta in A, C, D, F, G, I) and spalt (Sal; green in B, C, E, F, H, I). dpp is not expressed in rasp mutant discs due to defective Hh signaling (6 and see Fig. 3); however, when misexpressed at the dorsal-ventral boundary of the wing disc it is able to activate sal and omb expression. Transfections and X-gal and antibody stainings were carried out as described11.
Acknowledgments
We thank Henry Chang, Bertrand Mollereau, Gert Pflugfelder and Gary Struhl for materials. The manuscript was improved by the critical comments of Ines Carrera, Reza Farajian, Kerstin Hofmeyer, Kevin Legent, Jean-Yves Roignant, and Josie Steinhauer. This work was supported by the National Institutes of Health (grant EY13777 to J.E.T.).
References
- 1.Kalderon D. Similarities between the Hedgehog and Wnt signaling pathways. Trends Cell Biol. 2002;12:523–31. doi: 10.1016/s0962-8924(02)02388-7. [DOI] [PubMed] [Google Scholar]
- 2.Porter JA, Ekker SC, Park WJ, von Kessler DP, Young KE, Chen CH, Ma Y, Woods AS, Cotter RJ, Koonin EV, Beachy PA. Hedgehog patterning activity: role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell. 1996;86:21–34. doi: 10.1016/s0092-8674(00)80074-4. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem Sci. 2000;25:111–2. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- 4.Chamoun Z, Mann RK, Nellen D, von Kessler DP, Bellotto M, Beachy PA, Basler K. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–4. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- 5.Amanai K, Jiang J. Distinct roles of Central missing and Dispatched in sending the Hedgehog signal. Development. 2001;128:5119–27. doi: 10.1242/dev.128.24.5119. [DOI] [PubMed] [Google Scholar]
- 6.Lee JD, Treisman JE. Sightless has homology to transmembrane acyltransferases and is required to generate active Hedgehog protein. Curr Biol. 2001;11:1147–52. doi: 10.1016/s0960-9822(01)00323-2. [DOI] [PubMed] [Google Scholar]
- 7.Micchelli CA, The I, Selva E, Mogila V, Perrimon N. Rasp, a putative transmembrane acyltransferase, is required for Hedgehog signaling. Development. 2002;129:843–51. doi: 10.1242/dev.129.4.843. [DOI] [PubMed] [Google Scholar]
- 8.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–52. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 9.Zhai L, Chaturvedi D, Cumberledge S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J Biol Chem. 2004;279:33220–7. doi: 10.1074/jbc.M403407200. [DOI] [PubMed] [Google Scholar]
- 10.Chen MH, Li YJ, Kawakami T, Xu SM, Chuang PT. Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes Dev. 2004;18:641–59. doi: 10.1101/gad.1185804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miura GI, Buglino J, Alvarado D, Lemmon MA, Resh MD, Treisman JE. Palmitoylation of the EGFR ligand Spitz by Rasp increases Spitz activity by restricting its diffusion. Dev Cell. 2006;10:167–76. doi: 10.1016/j.devcel.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Pepinsky RB, Zeng C, Wen D, Rayhorn P, Baker DP, Williams KP, Bixler SA, Ambrose CM, Garber EA, Miatkowski K, Taylor FR, Wang EA, Galdes A. Identification of a palmitic acid-modified form of human Sonic hedgehog. J Biol Chem. 1998;273:14037–45. doi: 10.1074/jbc.273.22.14037. [DOI] [PubMed] [Google Scholar]
- 13.Linder ME, Deschenes RJ. Model organisms lead the way to protein palmitoyltransferases. J Cell Sci. 2004;117:521–6. doi: 10.1242/jcs.00989. [DOI] [PubMed] [Google Scholar]
- 14.Nusse R. Wnts and Hedgehogs: lipid-modified proteins and similarities in signaling mechanisms at the cell surface. Development. 2003;130:5297–305. doi: 10.1242/dev.00821. [DOI] [PubMed] [Google Scholar]
- 15.van den Heuvel M, Harryman-Samos C, Klingensmith J, Perrimon N, Nusse R. Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein. EMBO J. 1993;12:5293–302. doi: 10.1002/j.1460-2075.1993.tb06225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10:3116–28. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- 17.Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PI. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–52. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka K, Kitagawa Y, Kadowaki T. Drosophila segment polarity gene product porcupine stimulates the posttranslational N-glycosylation of wingless in the endoplasmic reticulum. J Biol Chem. 2002;277:12816–23. doi: 10.1074/jbc.M200187200. [DOI] [PubMed] [Google Scholar]
- 19.Shilo BZ. Signaling by the Drosophila epidermal growth factor receptor pathway during development. Exp Cell Res. 2003;284:140–9. doi: 10.1016/s0014-4827(02)00094-0. [DOI] [PubMed] [Google Scholar]
- 20.Bijlmakers MJ, Marsh M. The on-off story of protein palmitoylation. Trends Cell Biol. 2003;13:32–42. doi: 10.1016/s0962-8924(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 21.Callejo A, Torroja C, Quijada L, Guerrero I. Hedgehog lipid modifications are required for Hedgehog stabilization in the extracellular matrix. Development. 2006;133:471–83. doi: 10.1242/dev.02217. [DOI] [PubMed] [Google Scholar]
- 22.Gallet A, Ruel L, Staccini-Lavenant L, Therond PP. Cholesterol modification is necessary for controlled planar long-range activity of Hedgehog in Drosophila epithelia. Development. 2006;133:407–18. doi: 10.1242/dev.02212. [DOI] [PubMed] [Google Scholar]
- 23.Casali A, Struhl G. Reading the Hedgehog morphogen gradient by measuring the ratio of bound to unbound Patched protein. Nature. 2004;431:76–80. doi: 10.1038/nature02835. [DOI] [PubMed] [Google Scholar]
- 24.Taylor FR, Wen D, Garber EA, Carmillo AN, Baker DP, Arduini RM, Williams KP, Weinreb PH, Rayhorn P, Hronowski X, Whitty A, Day ES, Boriack-Sjodin A, Shapiro RI, Galdes A, Pepinsky RB. Enhanced potency of human Sonic hedgehog by hydrophobic modification. Biochemistry. 2001;40:4359–71. doi: 10.1021/bi002487u. [DOI] [PubMed] [Google Scholar]
- 25.Lee JD, Kraus P, Gaiano N, Nery S, Kohtz J, Fishell G, Loomis CA, Treisman JE. An acylatable residue of Hedgehog is differentially required in Drosophila and mouse limb development. Dev Biol. 2001;233:122–36. doi: 10.1006/dbio.2001.0218. [DOI] [PubMed] [Google Scholar]
- 26.Williams KP, Rayhorn P, Chi-Rosso G, Garber EA, Strauch KL, Horan GS, Reilly JO, Baker DP, Taylor FR, Koteliansky V, Pepinsky RB. Functional antagonists of sonic hedgehog reveal the importance of the N terminus for activity. J Cell Sci. 1999;112:4405–14. doi: 10.1242/jcs.112.23.4405. [DOI] [PubMed] [Google Scholar]
- 27.Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, McMahon AP. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105:599–612. doi: 10.1016/s0092-8674(01)00369-5. [DOI] [PubMed] [Google Scholar]
- 28.Zeng X, Goetz JA, Suber LM, Scott WJ, Jr, Schreiner CM, Robbins DJ. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature. 2001;411:716–20. doi: 10.1038/35079648. [DOI] [PubMed] [Google Scholar]
- 29.Goetz JA, Singh S, Suber LM, Kull FJ, Robbins DJ. A highly conserved amino-terminal region of sonic hedgehog is required for the formation of its freely diffusible multimeric form. J Biol Chem. 2006;281:4087–93. doi: 10.1074/jbc.M511427200. [DOI] [PubMed] [Google Scholar]
- 30.Panakova D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y, Erkner A, Gong R, Yao S, Taipale J, Basler K, Beachy PA. Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of dispatched. Cell. 2002;111:63–75. doi: 10.1016/s0092-8674(02)00977-7. [DOI] [PubMed] [Google Scholar]
- 32.Burke R, Nellen D, Bellotto M, Hafen E, Senti KA, Dickson BJ, Basler K. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell. 1999;99:803–15. doi: 10.1016/s0092-8674(00)81677-3. [DOI] [PubMed] [Google Scholar]
- 33.Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–21. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- 34.Glise B, Miller CA, Crozatier M, Halbisen MA, Wise S, Olson DJ, Vincent A, Blair SS. Shifted, the Drosophila ortholog of Wnt inhibitory factor-1, controls the distribution and movement of Hedgehog. Dev Cell. 2005;8:255–66. doi: 10.1016/j.devcel.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Gorfinkiel N, Sierra J, Callejo A, Ibanez C, Guerrero I. The Drosophila ortholog of the human Wnt inhibitor factor Shifted controls the diffusion of lipid-modified Hedgehog. Dev Cell. 2005;8:241–53. doi: 10.1016/j.devcel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–60. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- 37.Schlesinger A, Kiger A, Perrimon N, Shilo BZ. Small wing PLCgamma is required for ER retention of cleaved Spitz during eye development in Drosophila. Dev Cell. 2004;7:535–45. doi: 10.1016/j.devcel.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Saha K, Schaffer DV. Signal dynamics in Sonic hedgehog tissue patterning. Development. 2006;133:889–900. doi: 10.1242/dev.02254. [DOI] [PubMed] [Google Scholar]
- 39.Porter JA, von Kessler DP, Ekker SC, Young KE, Lee JJ, Moses K, Beachy PA. The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature. 1995;374:363–6. doi: 10.1038/374363a0. [DOI] [PubMed] [Google Scholar]
- 40.Dawber RJ, Hebbes S, Herpers B, Docquier F, van den Heuvel M. Differential range and activity of various forms of the Hedgehog protein. BMC Dev Biol. 2005;5:21. doi: 10.1186/1471-213X-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torroja C, Gorfinkiel N, Guerrero I. Patched controls the Hedgehog gradient by endocytosis in a dynamin-dependent manner, but this internalization does not play a major role in signal transduction. Development. 2004;131:2395–408. doi: 10.1242/dev.01102. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Struhl G. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 1996;87:553–63. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- 43.Cadigan KM, Fish MP, Rulifson EJ, Nusse R. Wingless repression of Drosophila frizzled 2 expression shapes the Wingless morphogen gradient in the wing. Cell. 1998;93:767–77. doi: 10.1016/s0092-8674(00)81438-5. [DOI] [PubMed] [Google Scholar]
- 44.Marois E, Mahmoud A, Eaton S. The endocytic pathway and formation of the Wingless morphogen gradient. Development. 2006;133:307–17. doi: 10.1242/dev.02197. [DOI] [PubMed] [Google Scholar]
- 45.Pfeiffer S, Ricardo S, Manneville JB, Alexandre C, Vincent JP. Producing cells retain and recycle Wingless in Drosophila embryos. Curr Biol. 2002;12:957–62. doi: 10.1016/s0960-9822(02)00867-9. [DOI] [PubMed] [Google Scholar]
- 46.Golembo M, Schweitzer R, Freeman M, Shilo BZ. Argos transcription is induced by the Drosophila EGF receptor pathway to form an inhibitory feedback loop. Development. 1996;122:223–30. doi: 10.1242/dev.122.1.223. [DOI] [PubMed] [Google Scholar]
- 47.Klein DE, Nappi VM, Reeves GT, Shvartsman SY, Lemmon MA. Argos inhibits epidermal growth factor receptor signalling by ligand sequestration. Nature. 2004;430:1040–4. doi: 10.1038/nature02840. [DOI] [PubMed] [Google Scholar]
- 48.Chuang PT, McMahon AP. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 1999;397:617–21. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 49.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–34. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 50.Stathopoulos A, Tam B, Ronshaugen M, Frasch M, Levine M. pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev. 2004;18:687–99. doi: 10.1101/gad.1166404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutherland D, Samakovlis C, Krasnow MA. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]


