Abstract
Background & Aims
An association between female hormones and symptomatic gastroesophageal reflux disease (GERD) and may be modified by obesity is suggested but not proven. Factors affecting GERD progression, however, are largely unknown.
Methods
At 40 US clinical centers, postmenopausal women with hysterectomy (n = 10,739) were randomly assigned to receive 0.625 mg/d of conjugated equine estrogens or placebo. Women without hysterectomy (n = 16,608) were randomly assigned to receive estrogen plus progestin, given as 0.625 mg conjugated equine estrogens/d plus 2.5 mg medroxyprogesterone acetate/d, or placebo. We performed secondary analyses using data from these trials.
Results
After 1 year, there was a trend toward a higher incidence of symptomatic GER among women randomly assigned to the estrogen treatment (4.2%) than with placebo (3.1%). The estrogen plus progestin treatment did not affect this risk. Neither treatment affected the progression of existing GER symptom. There was a dose-response association between baseline obesity, particularly as measured by waist circumference, with more than double the risk of incident symptomatic GER at 1 year among women with the largest waist circumference (≥114 cm) compared with a normal waist circumference (70–80 cm). Weight gain at 1 year was associated with elevated risk of incident symptomatic GER. Weight loss at 1 year alleviated existing GER symptoms. No interaction between hormone therapy and obesity on symptomatic GER was observed.
Conclusions
Estrogen treatment alone, but not with progestin, may cause GER symptoms in postmenopausal women. Increasing weight and girth increases the risk of developing GER symptoms, whereas weight loss alleviates existing GER symptoms. This trial was registered at www.clinicaltrials.gov as NCT00000611.
Gastroesophageal reflux (GER) is common in industrialized countries.1–3 Eighteen percent of the adult population in the United States report having had heartburn at least once a week and almost half of these people have had their symptoms ≥10 years.1 GER significantly affects the quality of patients’ life and is a strong and independent risk factor for esophageal adenocarcinoma,4,5 a malignancy that has increased rapidly in recent decades.6 More than $10 billion is spent annually for the care of GER, of which $6 billion is for prescription medications.7 Patients were willing to pay nearly $200 additional per month to resolve symptoms and have no side effects.8
The progressive rise in plasma estrogen and progesterone during pregnancy has long been thought to be responsible for symptomatic heartburn in pregnant women,9–12 by way of a mechanism of decreased lower esophageal sphincter pressure. Recently, exogenous estrogen therapy was linked to symptomatic GER,13 but no association was found in other studies.14,15 An interaction between estrogen use and obesity on the risk of symptomatic GER was also noted in one study.13 Obesity is a well-known risk factor for GER in women,13,15–17 even controlling for genetic predisposition among twins.17 However, most of the previous risk factor studies were on prevalent symptomatic GER, and few have examined the incidence and progression of symptomatic GER. In addition, little is known about abdominal obesity and GER risk.18 The effects of estrogen and progesterone on GER and the interactions of hormone therapy with obesity have not been reported from randomized controlled trials.
The Women’s Health Initiative (WHI) Hormone Trials consisted of 2 large, multicenter, randomized controlled and double-blind clinical trials in which women without a uterus received estrogen or a placebo and women with a uterus received estrogen plus progesterone or a placebo. Previous results from these trials showed reduction in waist circumference in women assigned to active treatment with hormone therapy.19–21 In this study, we analyze the relations among hormone therapy, obesity, and the incidence and progression of symptomatic GER at 1 year after randomization.
Materials and Methods
Study Population and Randomization
Detailed eligibility, recruitment methods, study population characteristics, hormone regimens, randomization, blinding, follow-up, and the main outcomes of the WHI hormones trials were published previously.22–24 Briefly, 27,347 postmenopausal women were recruited from 1993 to 1998 at 40 US clinical centers, primarily by mass mailings and other media announcements. Participants were aged 50–79 years at the initial screening, and were likely to reside in the study area for at least 3 years. Women were excluded if their last menstrual period occurred <6 months before they were enrolled in the study (<12 months for women 50–54 years of age) or if they had medical conditions suggesting a <3-year survival, a history of breast cancer, any cancer within the previous 10 years except nonmelanoma skin cancer, venous thromboembolism, hypertriglyceridemia, or were deemed to be at risk of poor medication adherence or retention.
Women who had undergone a hysterectomy were randomly assigned to receive a daily tablet containing either a placebo (n = 5429) or 0.625 mg of conjugated equine estrogens (Premarin; Wyeth, Philadelphia, PA) (n = 5310) (Figure 1). Women with an intact uterus were randomly assigned to receive a single daily tablet containing either a placebo (n = 8102) or 0.625 mg of conjugated equine estrogens plus 2.5 mg of medroxyprogesterone acetate (Prempro; Wyeth) (n = 8506). Participants were randomly assigned to the active treatment or placebo groups using a randomized permuted block algorithm. All participants who were currently using hormone therapy were required to undergo a 3-month “washout” period before enrollment. Participants, clinic staff, investigators, and outcome adjudicators were blinded to treatment assignments.
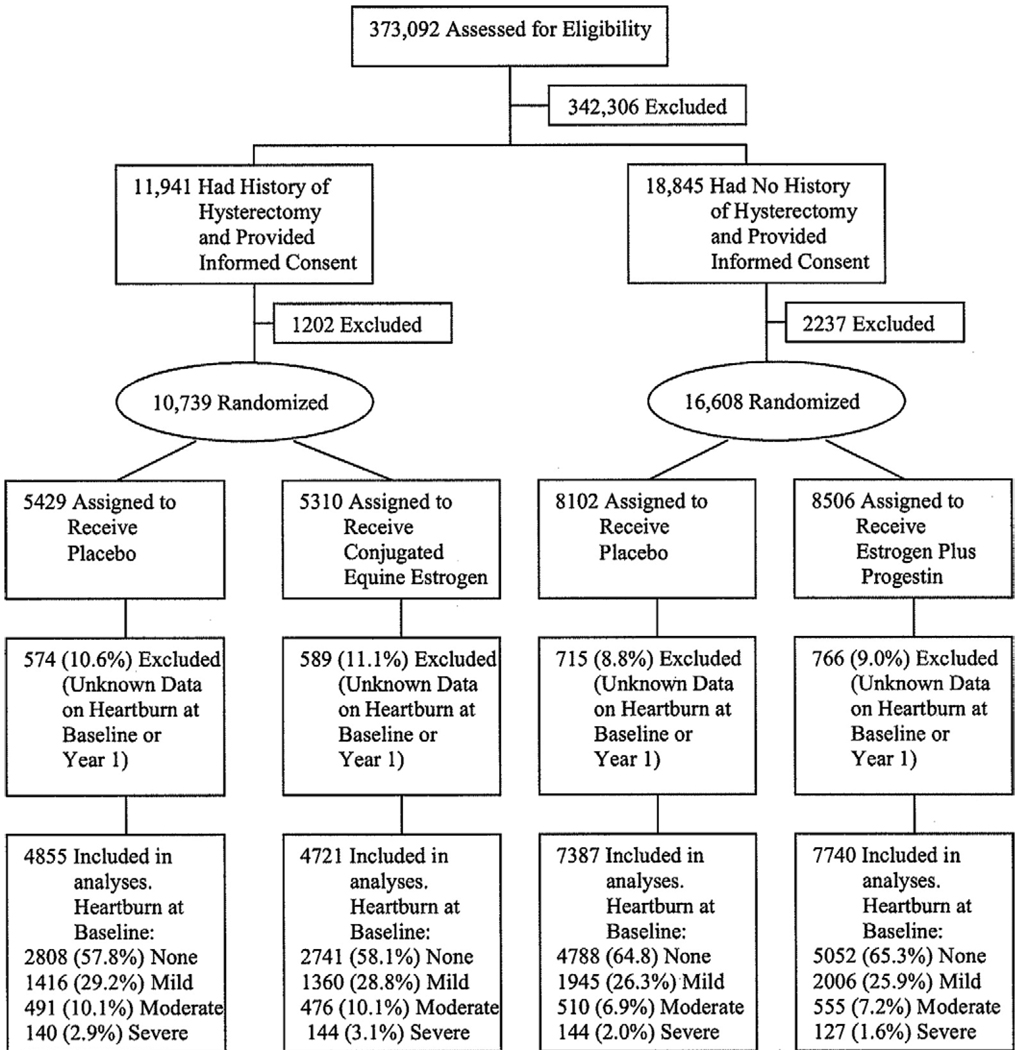
Figure 1.
Flow diagram of women included in the analyses.
Reductions in dosage or frequency were allowed to manage symptoms such as breast tenderness and vaginal bleeding. Permanent discontinuation of the study medication was required for women who developed breast cancer, endometrial atypical hyperplasia or cancer, venous thromboembolism, meningioma, malignant melanoma, triglyceride levels > 11.3 mmol/L, or were prescribed estrogen, testosterone, or selective estrogen receptor modifiers by their personal physicians.
Data Collection, Follow-Up, and Outcome Definition
At baseline, comprehensive information on participants was collected in a standardized fashion using self-administered forms, interviews, and clinical examinations. Height (in cm) was measured using a wall-mounted stadiometer. Weight (in kg) was measured using a balance beam scale with participants dressed in indoor clothing without shoes. The natural waist (in cm) at the end of a participant’s normal expiration and hip circumferences (in cm) were obtained using a standardized measuring tape. Medication history was assessed by clinic staff by asking participants to bring all current prescription and nonprescription medications to their first screening visit. The product or generic names of the medications on the label were matched to the corresponding item in a pharmacy database, the Master Drug Data Base (Medi-Span, Indianapolis, IN). Other baseline variables, including demographic, social, behavioral, and clinical characteristics, were obtained from self-administered, structured questionnaires. All the above data collection was repeated at 1 year.
Study participants were contacted by telephone 6 weeks after random assignment to assess symptoms and to reinforce adherence. Follow-up contact by telephone or a clinic visit occurred at 6 months, with clinic visits required at 1 year. At each clinic contact, adherence to study medication was assessed by pill counts. Nonadherence, which was defined as taking <80% of study medication or taking nonstudy or open-label hormones at 1 year, was ≈5% among both the active and the placebo arms of the estrogen trial, and ≈15% among the active arm, and ≈10% among the placebo arm of the estrogen plus progesterone trial in this study. The higher nonadherence from the estrogen plus progestin active treatment arm compared with placebo was primarily due to persistent vaginal bleeding.23
At baseline and at 1 year, all participants were instructed to indicate how bothersome the GER symptom, “heartburn,” was during the past 4 weeks, and the 4 response choices were symptom did not occur, symptom occurred and was mild (symptom did not interfere with usual activities), moderate (symptom interfered somewhat with usual activities), or severe (symptom was so bothersome that usual activities could not be performed). Because the group with mild symptoms was more likely to be misclassified, we defined incident symptomatic GER cases as those who developed moderate or severe heartburn at 1 year among women who were asymptomatic at baseline. We performed secondary analyses using data from the trials.
Statistical Analyses
Body mass index (BMI; in kg/m2) was calculated and was categorized as underweight (<21, equivalent to below the 5th percentile of our study participants) and, according to the World Health Organization classification, normal (21 to <25), overweight (25–30), class 1 obesity (>30–35), class 2 obesity (>35–40), and class 3 obesity (>40, equivalent to above the 95th percentile of our study participants). Waist circumference and waist-to-hip ratio were categorized into 6 categories, based on their distribution percentiles among our study participants with equivalent to <5th (referred to as lowest), 5th–25th (referred to as normal and used as reference), 26th–50th, 51st–75th, 76th–95th, and >95th (referred to as highest) percentiles. For waist circumference, these categories corresponded to <70 cm, 70 to <80 cm, 80 to <88 cm, 88 to <98 cm, 98 to <114 cm, and ≥114 cm; for waist-to-hip ratio, the categories corresponded to <0.71, 0.71 to <0.77, 0.77 to <0.82, 0.82 to <0.87, 0.87 to <0.95, and ≥0.95. Changes of these measures at 1 year were categorized into 5 categories based on their distribution percentiles with equivalent to <5th (referred to as profound loss), 5th–25th, 26th–75th (referred to as stable and used as reference), 76th–95th, and >95th (referred to as profound gain) percentiles. For BMI these categories corresponded to <−2.5, −2.5 to <−0.8, −0.8 to <0.8, 0.8 to <2.5 and ≥2.5; for waist circumference they corresponded to < −9, −9 to < −3, −3 to <3, 3 to <9, and ≥9; for waist-to-hip ratio change they corresponded to < −0.08, −0.08 to < −0.03, −0.03 to <0.03, 0.03 to <0.08, and ≥0.08.
In the incident symptomatic GER analysis, we compared women with incident symptomatic GER with those who remained asymptomatic at 1 year by logistic regression. Because the outcome was rare (≈3%), the odds ratio (OR) closely approximates the relative risk. Because hormone therapy decreases waist circumference,19–21 which is associated with GER,13,15,16,25 to examine the effect of hormone therapy on symptomatic GER through pathways other than the waist circumference pathway, we adjusted for waist circumference change in separate analyses. To study the progress of symptomatic GER, we calculated a symptom change score, which was the difference of score (none = 0, mild = 1, moderate = 2, severe = 3) at 1 year to that at baseline. Thus, an ordinal dependent variable, at a scale from −3 to 2, with no change at 0, was derived among those women with heart-burn symptoms at baseline. To avoid potential misclassification, the change scores −1 and 1 were not used in the analyses, −2 and −3 were further treated equally as an improvement (recoding to −1), 2 was considered as a worsening (recoding to 1); and 0 was unchanged. We then used cumulative logistic regression to study factors influencing GER symptom change. The primary analyses were based on the intention-to-treat principle, unless otherwise specified, but sensitivity analyses were conducted to examine the effect of nonadherence by excluding those participants who became nonadherent at 1 year. ORs and 95% confidence intervals (95% CIs) derived from regression models were used to estimate relative risks for symptomatic GER incidence and change. Trend tests were based on ordinal categorical values. To evaluate the association between obesity and symptomatic GER, we fit 2 regression models, one adjusted for age only and another one adjusted further for other potential confounders. To examine the potential effect modification by BMI, waist circumference, and waist-to-hip ratio, we fit a series of regression models, including the variables hormone therapy, the potential effect modifier, and their cross-product term.
Results
Baseline Characteristics
Among 27,347 randomly assigned women, ≈90% responded to the questions on heartburn symptoms both at baseline and at 1 year and were nearly equal in the active and placebo assignments in both trials (Figure 1). For the estrogen trial, 5549 (58.0%), 2776 (29.0%), 967 (10.1%), and 284 (3.0%) women reported no, mild, moderate, and severe heartburn at baseline, respectively. For the estrogen plus progestin trial, the corresponding figures were 9840 (65.1%), 3951 (26.1%), 1065 (7.0%), and 271 (1.8%). Baseline characteristics were balanced between active and placebo assignments, including age, race or ethnicity, income, education, occupation, BMI, waist circumference, smoking, alcohol drinking, coffee intake, physical activity, prior hormone use, history of gastric ulcer, gallbladder disease or gallstones, antacid use, and H-2 antagonist use and proton pump inhibitor use (Table 1, some not shown). Women in the estrogen trial were more likely to be black; had higher BMI, waist circumference, and waist-to-hip ratio; and were more likely to report gallbladder disease, H2-blocker use, and heartburn (Table 1; Figure 1).
Table 1.
Baseline Characteristics of Participants Without Gastroesophageal Reflux Symptoms in Different Treatment Arms of Both Trials
| Estrogen trial | Estrogen plus progestin trial | |||||
|---|---|---|---|---|---|---|
| Placebo (n = 2808) |
Estrogen (n = 2741) |
P value* | Placebo (n = 4788) |
Estrogen plus progestin (n = 5052) |
P value* | |
| Age at screening (y) | ||||||
| 50–59 | 824 ± 29.3 | 844 ± 30.8 | 1573 ± 32.9 | 1678 ± 33.2 | ||
| 60–69 | 1289 ± 45.9 | 1216 ± 44.4 | 2145 ± 44.8 | 2279 ± 45.1 | ||
| 70–79 | 695 ± 24.8 | 681 ± 24.8 | .43 | 1070 ± 22.3 | 1095 ± 21.7 | .72 |
| Race or ethnicity, n (%) | ||||||
| American Indian | 15(0.5) | 20(0.7) | 17(0.4) | 12 (0.2) | ||
| Asian or Pacific Islander | 45(1.6) | 53(1.9) | 122(2.5) | 137(2.7) | ||
| Black or African American | 429(15.3) | 389(14.2) | 307 (6.4) | 308 (6.1) | ||
| Hispanic or Latino | 113(4.0) | 129(4.7) | 171(3.6) | 198 (3.9) | ||
| White | 2164(77.1) | 2115(77.2) | 4113 (85.9) | 4324 (85.6) | ||
| Other | 42(1.5) | 35(1.3) | .44 | 58(1.2) | 73(1.4) | .60 |
| Education, n (%) | ||||||
| 0–8 y | 61(2.2) | 69(2.5) | 75(1.6) | 77(1.5) | ||
| Some high school (9–11 y) | 139(5.0) | 158(5.8) | 162 (3.4) | 184(3.7) | ||
| High school diploma or GED | 587(21.0) | 578(21.3) | 892 (18.8) | 870(17.3) | ||
| School after high school | 1217(43.6) | 1173(43.2) | 1755 (36.9) | 1975 (39.3) | ||
| College degree or higher | 789(28.2) | 736(27.1) | .51 | 1871 (39.3) | 1925 (38.3) | .10 |
| Income, n (%) | ||||||
| <$10,000 | 176(6.6) | 172(6.7) | 207 (4.6) | 229 (4.8) | ||
| $10,000–$19,999 | 501 (18.9) | 490 (19.0) | 582 (12.9) | 643 (13.4) | ||
| $20,000–$34,999 | 757(28.5) | 748 (28.9) | 1205 (26.6) | 1333(27.7) | ||
| $35,000–$49,999 | 546 (20.6) | 493(19.1) | 982 (21.7) | 1044(21.7) | ||
| $50,000–$74,999 | 413(15.5) | 415(16.1) | 865 (19.1) | 873(18.2) | ||
| ≥$75,000 | 263 (9.9) | 267(10.3) | .85 | 685(15.1) | 682 (14.2) | .51 |
| Smoking, n (%) | ||||||
| Never smoked | 1434(51.6) | 1429(52.6) | 2392 (50.5) | 2578(51.4) | ||
| Past smoker | 1051 (37.8) | 1032 (38.0) | 1852 (39.1) | 1923 (38.4) | ||
| Current smoker | 294 (10.6) | 257(9.5) | .37 | 488 (10.3) | 511 (10.2) | .68 |
| Physical activity, n(%) | ||||||
| None | 483 (19.0) | 493(19.5) | 693(15.4) | 756 (16.6) | ||
| Some | 1164 (45.8) | 1117(44.2) | 1900 (42.2) | 1903 (41.8) | ||
| 2 to <4 episodes/wk | 360(14.2) | 403 (16.0) | 709(15.7) | 752 (16.5) | ||
| 4 episodes/wk | 533(21.0) | 512(20.3) | .28 | 1201 (26.7) | 1146(25.1) | .17 |
| BMI (kg/m2), n (%) | ||||||
| Underweight (<21) | 118(4.2) | 107 (3.9) | 369 (7.8) | 380 (7.6) | ||
| Normal (21 to <25) | 581 (20.9) | 621(22.8) | 1362 (28.6) | 1424 (28.3) | ||
| Overweight (25–30) | 987 (35.5) | 952 (34.9) | 1648 (34.6) | 1729 (34.4) | ||
| Class 1 obesity (>30–35) | 648(23.3) | 581(21.3) | 847 (17.8) | 956 (19.0) | ||
| Class 2 obesity (>35–40) | 294 (10.6) | 293(10.8) | 354 (7.4) | 381 (7.6) | ||
| Class 3 obesity (>40) | 155(5.6) | 171(6.3) | .28 | 179(3.8) | 153 (3.0) | .31 |
| Waist circumference (cm), n (%) | ||||||
| <70 | 119(4.3) | 143(5.2) | 402 (8.4) | 399 (7.9) | ||
| 70 to <80 | 587(21.0) | 588(21.5) | 1319(27.6) | 1431 (28.4) | ||
| 80 to <88 | 596(21.3) | 602 (22.0) | 1141 (23.9) | 1176(23.4) | ||
| 88 to <98 | 738(26.4) | 647(23.7) | 971 (20.4) | 1033 (20.5) | ||
| 98 to <114 | 601(21.5) | 611(22.3) | 757 (15.9) | 810(16.1) | ||
| ≥114 | 156(5.6) | 143(5.2) | .15 | 181 (3.8) | 185(3.7) | .88 |
| Waist-to-hip ratio, n (%) | ||||||
| <0.71 | 136 (4.9) | 154(5.6) | 328 (6.9) | 300 (6.0) | ||
| 0.71 to <0.77 | 503 (18.0) | 511(18.7) | 1160 (24.4) | 1201 (23.9) | ||
| 0.77 to <0.82 | 726(26.0) | 755(27.6) | 1281 (26.9) | 1457 (29.0) | ||
| 0.82 to <0.87 | 673(24.1) | 603(22.1) | 1037 (21.8) | 1095 (21.8) | ||
| 0.87 to <0.95 | 608(21.8) | 577(21.1) | 766 (16.1) | 766(15.2) | ||
| ≥0.95 | 149(5.3) | 131 (4.8) | .24 | 188 (3.9) | 207 (4.1) | .13 |
| Prior menopausal hormones use, n (%) | ||||||
| Never used | 1400 (49.9) | 1436(52.4) | 3609 (75.4) | 3737 (74.0) | ||
| Past user | 1018(36.3) | 930 (33.9) | 866 (18.1) | 975(19.3) | ||
| Current user | 388 (13.8) | 375(13.7) | .14 | 311 (6.5) | 337 (6.7) | .26 |
| Gallbladder disease or gallstones, n (%) | ||||||
| No | 2282 (81.9) | 2233 (81.9) | 4171(87.5) | 4453 (88.5) | ||
| Yes | 504 (18.1) | 493 (18.1) | 1.00 | 594 (12.5) | 577 (11.5) | .13 |
| Antacid use, n (%) | ||||||
| No | 2747 (97.8) | 2681 (97.8) | 4663 (97.4) | 4903(97.1) | ||
| Yes | 61(2.2) | 60(2.2) | .97 | 125(2.6) | 149(2.9) | .31 |
| H-2 antagonist use, n (%) | ||||||
| No | 2724(97.0) | 2677(97.7) | 4703(98.2) | 4955 (98.1) | ||
| Yes | 84 (3.0) | 64(2.3) | .13 | 85(1.8) | 97(1.9) | .59 |
| Proton pump inhibitor use, n (%) | ||||||
| No | 2781 (99.0) | 2701 (98.5) | 4750(99.2) | 5018 (99.3) | ||
| Yes | 27(1.0) | 40(1.5) | .09 | 38 (0.8) | 34(0.7) | .48 |
BMI, body mass index; GED, General Educational Development.
P value of chi-square test for trend was based on ordinal categorical values.
Effect of Conjugated Equine Estrogens on GER Symptoms
A trend was observed toward a slightly higher incidence of new, moderate, or severe symptomatic GER at 1 year in the estrogen group (4.2%) than in the placebo group (3.1%) (OR, 1.35; 95% CI, 0.99–1.85; Table 2). The number needed to harm was 96 women, indicating that for every 96 women treated with estrogen for 1 year, one additional woman would experience new and moderate to severe symptomatic GER (1 ÷ [0.042–0.031] = 96). When adjusted for waist circumference change at 1 year, the OR was 1.41 (95% CI: 1.02–1.95). Including mild heartburn along with moderate-to-severe heartburn at 1 year in previously asymptomatic women did not result in differences between active treatments and placebo (approximately 21%; OR, 0.98). Among women reporting symptomatic GER at baseline, the estrogen treatment did not affect the severity of existing symptoms compared with placebo treatment (Table 2). Analysis restricted to women who were adherent at 1 year showed similar results (data not shown).
Table 2.
Effect of Menopausal Hormone Therapy on Symptomatic Gastroesophageal Reflux (GER) Incidence and Change
| Estrogen trial | Estrogen plus progestin trial | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo n (%) |
Estrogen n (%) |
Odds ratio (95% CI) |
Odds ratio* (95% CI) |
Placebo n(%) |
Estrogen plus progestin n (%) |
Odds ratio (95% CI) |
Odds ratio* (95% CI) |
|
| Incident symptomatic GER (moderate or severe) | ||||||||
| No | 2205 (96.9) | 2164 (95.8) | 1.0 (reference) | 1.0 (reference) | 3957 (97.6) | 4181 (97.6) | 1.0 (reference) | 1.0 (reference) |
| Yes† | 71 (3.1) | 94 (4.2) | 1.35 (0.99–1.85) | 1.41 (1.02–1.95) | 96 (2.4) | 101 (2.4) | 1.00 (0.75–1.32) | 0.97 (0.73–1.30) |
| Existing symptomatic GER change (score at 1 y – score at baseline)‡ | ||||||||
| −3 | 11 (0.8) | 12 (0.9) | 15(0.9) | 6 (0.4) | ||||
| −2 | 102(7.1) | 108 (8.2) | 97(5.7) | 106 (6.2) | ||||
| −1 | 613 (30.0) | 666 (33.6) | 893 (34.4) | 974 (36.2) | ||||
| 0 | 1030 (71.8) | 927 (70.6) | 1267 (74.3) | 1320(77.0) | ||||
| 1 | 252 (17.6) | 230 (17.5) | 281 (16.5) | 247 (14.4) | ||||
| 2 | 39 (2.7) | 37 (2.8) | 0.89 (0.70–1.13) | 0.92 (0.72–1.17) | 46 (2.7) | 35 (2.0) | 0.94 (0.74–1.19) | 0.96 (0.75–1.23) |
Adjusted for waist circumference change at 1 year to examine the effect of hormone therapy on symptomatic GER through pathways other than the waist circumference pathway.
Among asymptomatic women at baseline, those who developed mild GER symptom at 1 year were excluded from the analysis to avoid potential misclassification.
Only women reporting heartburn symptom at baseline were Included. The ORs were derived from cumulative logistic regression models. For example, the odds of women randomly assigned to CEE is 0.89 times the odds of women randomly assigned to placebo for having worsening GER. (To avoid potential misclassification, the scores −1 and 1 were excluded from the analyses; −2 and −3 were treated equally [recoding to −1] as an improvement; and 2 [recoding to 1] as a worsening.)
Compared with placebo treatment, the estrogen treatment showed no effect on BMI (0.004 reduction; P = .94), a small effect on waist circumference (0.5-cm reduction; P = .001), and a small effect on waist-to-hip ratio (0.005 reduction; P = .001) at 1 year. However, these effects were not observed in regression models to modify the effect of the estrogen on incidence or change of symptomatic GER. Regression models also showed no effect modification by baseline BMI, waist circumference, waist-to-hip ratio, or a history of gallbladder disease or gallstones (data not shown).
Effect of Conjugated Equine Estrogens Plus Medroxyprogesterone on GER Symptoms
No difference was observed in the incidence of symptomatic GER in women randomly assigned to estrogen plus progestin or to placebo, with both at 2.4% (Table 2) and at approximately 17% when including mild heartburn. The estrogen plus progestin treatment did not affect the severity of existing symptoms compared with the placebo treatment. Women randomly assigned to the estrogen plus progestin treatment had a 0.2 reduction in BMI, a 0.8-cm reduction in waist circumference (both P < .001) but no effect on waist-to-hip ratio (0.002 reduction, P = .13) at 1 year compared with women randomly assigned to placebo. Again, none of these factors modified the effect of the estrogen plus progestin treatment on GER incidence or progression. There was also no effect modification by a history of gallbladder disease or change in the results when the analysis was restricted to women who were adherent at 1 year (data not shown).
Obesity and GER
Because there was little difference before and after adjustment for obesity on the effect of hormone therapy on symptomatic GER, we combined the trials for analyses of obesity and symptomatic GER. We observed a dose–response association between baseline obesity (BMI, waist circumference, and waist-to-hip ratio) and incident moderate or severe symptomatic GER (Table 3). Compared with women at normal BMI (21 to <25), class 3 obese women (BMI > 40) had nearly double the risk of incident symptomatic GER at 1 year. The association was somewhat attenuated after further adjustment for education, income, and physical activity (higher education, higher income, and more physical activity showed modest inverse associations with risk of incident symptomatic GER [data not shown]). Compared with women at normal waist circumference (70 to <80 cm), women with highest waist circumference (≥114 cm) had >2-fold the risk of incident symptomatic GER at 1 year. The similar comparison that used waist-to-hip ratio showed nearly twice the risk. Mutual adjustment between BMI, waist circumference, and waist-to-hip ratio, by introducing 2 of them into one regression model at a time when modeling likelihood of incident GER symptoms, showed that waist circumference resulted in better goodness-of-fit than did BMI and that BMI was better than waist-to-hip ratio, irrespective of whether continuous or categorical variables were used (data not shown). However, being under-weight was not associated with incident symptomatic GER, irrespective of the type of measurement scale.
Table 3.
Associations Between Obesity and Incident Symptomatic Gastroesophageal Reflux (GER) in the Combined Estrogen and Estrogen Plus Progestin Trials
| No GER symptoms at baseline n | Incident GER n (%) | Odds ratio* (95% CI) | Odds ratio† (95% CI) | |
|---|---|---|---|---|
| BMI (kg/m2) | ||||
| Underweight (<21) | 863 | 11 (1.3) | 0.72 (0.38–1.38) | 0.63 (0.31–1.29) |
| Normal (21 to <25) | 3389 | 60 (1.7) | 1.0 (reference) | 1.0 (reference) |
| Overweight (25–30) | 4300 | 120 (2.7) | 1.57 (1.15–2.15) | 1.40 (1.00–1.96) |
| Class 1 obesity (>30–35) | 2389 | 102 (4.1) | 2.38 (1.72–3.28) | 1.87 (1.31–2.67) |
| Class 2 obesity (>35–40) | 1001 | 42 (4.0) | 2.29 (1.53–3.42) | 1.69 (1.08–2.64) |
| Class 3 obesity (>40) | 486 | 25 (4.9) | 2.71 (1.68–4.37) | 1.85 (1.06–3.21) |
| P value for trend | <.0001 | .0001 | ||
| Waist circumference (cm) | ||||
| <70 | 941 | 15 (1.6) | 0.92 (0.52–1.63) | 0.92 (0.49–1.70) |
| 70 to <80 | 3361 | 58 (1.7) | 1.0 (reference) | 1.0 (reference) |
| 80 to <88 | 2831 | 75 (2.6) | 1.54(1.09–2.18) | 1.43 (0.98–2.09) |
| 88 to <98 | 2697 | 97 (3.5) | 2.10 (1.51–2.92) | 1.93 (1.34–2.76) |
| 98 to <114 | 2151 | 89 (4.0) | 2.38 (1.70–3.32) | 1.79 (1.22–2.62) |
| ≥114 | 480 | 27 (5.3) | 3.15 (1.97–5.02) | 2.11 (1.23–3.62) |
| P value for trend | <.0001 | .0001 | ||
| Waist-to-hip ratio | ||||
| <0.71 | 803 | 13 (1.6) | 0.75 (0.41–1.37) | 1.00 (0.53–1.91) |
| 0.71 to <0.77 | 2884 | 60 (2.0) | 1.0 (reference) | 1.0 (reference) |
| 0.77 to <0.82 | 3452 | 87 (2.5) | 1.25 (0.89–1.74) | 1.25 (0.86–1.83) |
| 0.82 to <0.87 | 2654 | 93 (3.4) | 1.75 (1.26–2.43) | 1.67 (1.15–2.42) |
| 0.87 to <0.95 | 2143 | 85 (3.8) | 2.00 (1.43–2.81) | 1.70 (1.16–2.50) |
| ≥0.95 | 510 | 22 (4.1) | 2.20 (1.34–3.62) | 1.86 (1.07–3.23) |
| P value for trend | <.0001 | .0007 |
BMI, body mass index.
Adjusted for age only.
Adjusted for age, trial assignment, race or ethnicity, education, income, smoking status, physical activity, history of gastric ulcer, gallbladder disease or gallstone.
Likewise, weight gain (in all of the 3 different scales) at 1 year was dose dependently associated with increased risk of incident symptomatic GER (Table 4). No association between weight loss at 1 year and incident symptomatic GER was observed, irrespective of the type of measurement scale.
Table 4.
Association Between Obesity Change at 1 Year and Incidence and Change of Symptomatic Gastroesophageal Reflux (GER) in the Combined Estrogen and Estrogen Plus Progestin Trials
| Symptomatic GER incidence | Existing symptomatic GER change‡ |
|||||
|---|---|---|---|---|---|---|
| No GER symptoms at baseline n |
Incident GER n (%) |
Odds ratio* (95% CI) |
Odds ratio† (95% CI) |
n | Odds ratio† (95% CI) |
|
| Change in BMI at 1 y | ||||||
| <−2.5 | 771 | 23 (2.9) | 1.16 (0.74–1.81) | 1.02 (0.62–1.66) | 290 | 0.38 (0.26–0.55) |
| −2.5 to <−0.8 | 2379 | 48 (2.0) | 0.80 (0.58–1.11) | 0.77 (0.54–1.11) | 913 | 0.73 (0.56–0.95) |
| −0.8 to <0.8 | 6263 | 156 (2.4) | 1.0 (reference) | 1.0 (reference) | 2566 | 1.0 (reference) |
| 0.8 to <2.5 | 2215 | 83 (3.6) | 1.45 (1.11–1.91) | 1.47 (1.09–1.98) | 1010 | 1.20 (0.92–1.57) |
| ≥2.5 | 482 | 33 (6.4) | 2.59 (1.75–3.81) | 2.35 (1.53–3.63) | 219 | 0.98 (0.59–1.62) |
| P value for trend | <.0001 | <.0001 | <.0001 | |||
| Change in waist circumference at 1 y (cm) |
||||||
| <−9 | 590 | 17 (2.8) | 1.19 (0.71–1.98) | 0.72 (0.39–1.34) | 250 | 0.64 (0.42–0.98) |
| −9 to <−3 | 2526 | 69 (2.7) | 1.15 (0.86–1.54) | 1.11 (0.80–1.52) | 1039 | 0.79 (0.61–1.02) |
| −3 to <3 | 5887 | 140 (2.3) | 1.0 (reference) | 1.0 (reference) | 2321 | 1.0 (reference) |
| 3 to <9 | 2531 | 85 (3.2) | 1.40 (1.07–1.84) | 1.47 (1.09–1.98) | 1081 | 1.15 (0.88–1.49) |
| ≥9 | 496 | 32 (6.1) | 2.66 (1.79–3.95) | 2.31 (1.46–3.64) | 260 | 0.96 (0.61–1.51) |
| P value for trend | .0025 | .0004 | .0078 | |||
| Change in waist-to-hip ratio at 1 y |
||||||
| <−0.08 | 510 | 21 (4.0) | 1.67 (1.05–2.66) | 1.20 (0.69–2.07) | 247 | 1.12 (0.69–1.80) |
| −0.08 to <−0.03 | 2148 | 56(2.5) | 1.06 (0.78–1.44) | 0.86 (0.61–1.22) | 895 | 0.82 (0.63–1.07) |
| −0.03 to <0.03 | 6725 | 167 (2.4) | 1.0 (reference) | 1.0 (reference) | 2594 | 1.0 (reference) |
| 0.03 to <0.08 | 2117 | 78 (3.6) | 1.49 (1.14–1.96) | 1.44 (1.06–1.95) | 967 | 1.22 (0.93–1.60) |
| ≥0.08 | 499 | 20 (3.9) | 1.62 (1.01–2.61) | 1.37 (0.79–2.37) | 237 | 0.70 (0.44–1.10) |
| P value for trend | .17 | .04 | .53 | |||
BMI, body mass index.
Adjusted for age only.
Adjusted for age, trial assignment, corresponding baseline measure, race or ethnicity, education, income, smoking status, physical activity, and history of gastric ulcer, gallbladder disease, or gallstone.
Only women reporting heartburn symptom at baseline were included. The odds ratio, derived from cumulative logistic regression model, indicates, for example, that the odds of women whose BMI increased by >2.5 had 1.25 the odds of those women with stable BMI (−0.8 to <0.8) for having worsening symptomatic GER. (To avoid potential misclassification, the scores −1 and 1 were excluded from the analyses; −2 and −3 were treated equally [recoding to −1] as an improvement; and 2 [recoding to 1] as a worsening.)
Among women who already had symptomatic GER at baseline, profound decrease in BMI had nearly two-thirds and profound decrease in waist circumference had about one-third higher likelihood of improved symptoms than did stable weight, whereas profound decrease in waist-to-hip ratio had no such association (Table 4). Weight gain was not observed to worsen existing GER symptoms. Mutual adjustment of these 3 scales when modeling the likelihood of GER symptom change showed that BMI resulted in best goodness-of-fit, followed by waist circumference, and waist-to-hip ratio the last.
Discussion
WHI is the first large randomized controlled trial to examine the effect of menopausal hormone therapy on the incidence and progression of symptomatic GER and also the first large prospective study on various measures of obesity to determine the most important physiologic factor for GER. Our data show that estrogen may modestly increase the incidence of symptomatic GER but does not affect its progression, and estrogen plus progestin affects neither the incidence nor the progression of symptomatic GER in this population of postmenopausal women. Overweight and obesity increase the risk of developing symptomatic GER, and weight loss alleviates existing symptoms. Hormone therapy leads to a small decrease in weight and waist circumference, which does not affect symptomatic GER at 1 year.
To our knowledge, only a few epidemiologic studies have evaluated the association between estrogen or progestin or both and symptomatic GER. One was a large population-based case–control study conducted in Norway reporting that ever using estrogen therapy was associated with a 70% increased risk of symptomatic GER among all participants and a more than doubled risk among a subset of posthysterectomy women, compared with never receiving estrogen therapy.13 In contrast, a study conducted in a UK twin register reported that use of menopausal hormone therapy or oral contraceptives was associated with a 24% decreased risk of GER. Unfortunately, the latter study did not provide separate data by type of hormone therapy. In the Nurses’ Health Study, use of estrogen only, but not estrogen plus progesterone, was more common among those women with at least weekly GER symptoms than those without symptoms in the past year.15 Our results showed that the use of estrogen but not estrogen plus progesterone caused symptomatic GER and were consistent with the Norwegian and the American studies but were of a lower magnitude. This may be due to a shorter exposure period, ie, 1 year in our study rather than much longer in the former observational studies. No previous studies have evaluated the effect of hormone therapy on the progression of symptomatic GER. We observed no effect. The biologic reasons for the observation that estrogen may play a role only in the initiation but not in the progression of symptomatic GER are unknown. In addition, the mechanisms for the lack of an effect of estrogen when combined with progestin in the initiation of symptomatic GER are unknown.
Obesity is an established risk factor for GER among women.13,15–17 The Nurse’s Health Study in the United States showed that BMI was more important than waist-to-hip ratio in terms of obesity-associated GER.15 This finding was consistent with our results. However, our results showed that waist circumference, which tends to reflect abdominal obesity, was more important in the initiation of symptomatic GER than was BMI, which tends to reflect whole body fat. This result was supported by a recent finding that both BMI and waist circumference increased intragastric pressure and gastroesophageal pressure gradient, but, when examined simultaneously, only waist circumference revealed significant relations with these measures.26 Recent studies also showed that waist circumference was associated with esophageal acid exposure18 and Barrett’s esophagus.27 Our data also showed that waist circumference was less important than BMI in the progression of symptomatic GER. Few data have been available in evaluating the initiation and progression of symptomatic GER separately. It is tempting to reason that the underlying mechanism is an anatomic disruption of lower esophageal sphincter, induced by the pressure imposed by abdominal obesity.26 This may act as a threshold for the development of symptomatic GER, and, once this antireflux barrier is disrupted, other obesity-related pathophysiologic mechanisms such as prolonged transient lower esophageal sphincter relaxations, impaired acid clearance, and delayed gastric emptying may affect the progression of symptomatic GER.28 This is further supported by our data that being underweight or losing weight did not prevent symptomatic GER initiation, but that weight loss alleviated existing symptoms. Weight gain, particularly an increase in abdominal obesity, facilitated symptomatic GER initiation. Taken together, these results suggested an effective threshold for initiating symptomatic GER. The beneficial effect of weight loss and hazardous effect of weight gain were consistent with previous epidemiologic findings.13,15
Our data showed that postmenopausal hormone therapy led to a small decrease in waist circumference, but the effect was negligible as far as symptomatic GER was concerned, at least at 1 year. Although the Norwegian study showed a significant synergic interaction between BMI and estrogen therapy in the association with symptomatic GER, one study from the Swedish Twin Register did not.29 A possible explanation for these discrepancies could be due to the different estrogen formulations and that the Norwegian study studied estrogen use before 1986.
Although a large-scale randomized clinical trial provides compelling epidemiologic evidence, some limitations must be acknowledged. First, WHI studied only one route, one dosage, and one type of postmenopausal hormone, which might make the results not generalizeable to other types of hormone therapy or to premenopausal women. Second, because studying GER was not the primary aim of the WHI study, only one questionnaire item was asked about how bothersome heartburn was in terms of interference with usual activities. This may raise a concern about the validity of GER ascertainment. However, heartburn is the hallmark symptom of GER, and severity of heartburn was shown to have high specificity and good reliability for true GER30,31 The Montreal definition concluded that GER disease can be diagnosed based on symptoms alone, and symptoms that are not troublesome, which is up to the patient to define, should not be diagnosed as GER disease.32 Furthermore, baseline prevalence of heartburn, 42% in women in the estrogen trial and 35% in the estrogen plus progestin trial, is consistent with the prevalence of monthly heartburn reported from previous epidemiologic studies that varied from 34% to 42%, with no sex difference, in the US population.1,2 In addition, our definition of incident symptomatic GER was based on moderate-to-severe heartburn, the prevalence of which at the baseline was approximately 13% in the estrogen trial and 9% in the estrogen plus progestin trial, coincided with the 10%–20% weekly GER symptoms in other studies.15–17 Finally, the incidence of symptomatic GER at 1 year, 3.1% in the estrogen and 2.4% in the estrogen plus progestin placebo arms, again coincided with the yearly incidence of weekly heartburn ≈1.5–3% in previous studies.28 We compared GER symptoms at the group level, and modest misclassification, if any, at the individual level should not alter our findings from double-blind, randomized, placebo-controlled trials.
In conclusion, estrogen therapy may modestly increase the incidence of GER. Obesity causes GER symptoms, and weight loss alleviates the symptoms. Weight control is an effective method (or treatment) for preventing GER and for improving existing symptoms.
Acknowledgments
Supported by Wyeth and by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
The authors thank the dedicated efforts of WHI investigators and staff and the National Heart, Lung, and Blood Institute program office. A short list of WHI investigators is in an appendix. We gratefully acknowledge the WHI participants for their extraordinary commitment to the WHI program.
The National Institutes of Health governed the overall design and conduct of the WHI study and approved the manuscript. Wyeth supplied study drugs but did not participate in any aspect of the aforementioned.
Abbreviations used in this paper
- BMI
body mass index
- GER
gastroesophageal reflux
- GERD
gastroesophageal reflux disease
- OR
odds ratio
- WHI
Women’s Health Initiative
- 95% CI
95% confidence interval.
Appendix 1. WHI Investigators
Program Office (National Heart, Lung, and Blood Institute, Bethesda, MD): Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Linda Pottern, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan (Fred Hutchinson Cancer Research Center, Seattle, WA); Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC); Evan Stein (Medical Research Labs, Highland Heights, KY); Steven Cummings (University of California at San Francisco, San Francisco, CA).
Clinical Centers: Sylvia Wassertheil-Smoller (Albert Einstein College of Medicine, Bronx, NY); Aleksandar Rajkovic (Baylor College of Medicine, Houston, TX); JoAnn Manson (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA); Annlouise R. Assaf (Brown University, Providence, RI); Lawrence Phillips (Emory University, Atlanta, GA); Shirley Beresford (Fred Hutchinson Cancer Research Center, Seattle, WA); Judith Hsia (George Washington University Medical Center, Washington, DC); Rowan Chlebowski (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA); Evelyn Whitlock (Kaiser Permanente Center for Health Research, Portland, OR); Bette Caan (Kaiser Permanente Division of Research, Oakland, CA); Jane Morley Kotchen (Medical College of Wisconsin, Milwaukee, WI); Barbara V. Howard (MedStar Research Institute/Howard University, Washington, DC); Linda Van Horn (Northwestern University, Chicago/Evanston, IL); Henry Black (Rush Medical Center, Chicago, IL); Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, CA); Dorothy Lane (State University of New York at Stony Brook, Stony Brook, NY); Rebecca Jackson (The Ohio State University, Columbus, OH); Cora E. Lewis (University of Alabama at Birmingham, Birmingham, AL); Tamsen Bassford (University of Arizona, Tucson/Phoenix, AZ); Jean Wactawski-Wende (University at Buffalo, Buffalo, NY); John Robbins (University of California at Davis, Sacramento, CA); F. Allan Hubbell (University of California at Irvine, CA); Lauren Nathan (University of California at Los Angeles, Los Angeles, CA); Robert D. Langer (University of California at San Diego, LaJolla/Chula Vista, CA); Margery Gass (University of Cincinnati, Cincinnati, OH); Marian Limacher (University of Florida, Gainesville/Jacksonville, FL); David Curb (University of Hawaii, Honolulu, HI); Robert Wallace (University of Iowa, Iowa City/Davenport, IA); Judith Ockene (University of Massachusetts/Fallon Clinic, Worcester, MA); Norman Lasser (University of Medicine and Dentistry of New Jersey, Newark, NJ); Mary Jo O’Sullivan (University of Miami, Miami, FL); Karen Margolis (University of Minnesota, Minneapolis, MN); Robert Brunner (University of Nevada, Reno, NV); Gerardo Heiss (University of North Carolina, Chapel Hill, NC); Lewis Kuller (University of Pittsburgh, Pittsburgh, PA); Karen C. Johnson (University of Tennessee, Memphis, TN); Robert Brzyski (University of Texas Health Science Center, San Antonio, TX); Gloria E. Sarto (University of Wisconsin, Madison, WI); Denise Bonds (Wake Forest University School of Medicine, Winston-Salem, NC); Susan Hendrix (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI).
Footnotes
All authors declare that they have no conflict of interest to disclose.
References
- 1.Locke GR, III, Talley NJ, Fett SL, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Petersen NJ, Carter J, et al. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology. 2004;126:1692–1699. doi: 10.1053/j.gastro.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson M, Johnsen R, Ye W, et al. Prevalence of gastro-oesophageal reflux symptoms and the influence of age and sex. Scand J Gastroenterol. 2004;39:1040–1045. doi: 10.1080/00365520410003498. [DOI] [PubMed] [Google Scholar]
- 4.Revicki DA, Wood M, Maton PN, et al. The impact of gastroesophageal reflux disease on health-related quality of life. Am J Med. 1998;104:252–258. doi: 10.1016/s0002-9343(97)00354-9. [DOI] [PubMed] [Google Scholar]
- 5.Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 6.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 7.Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 8.Kleinman L, McIntosh E, Ryan M, et al. Willingness to pay for complete symptom relief of gastroesophageal reflux disease. Arch Intern Med. 2002;162:1361–1366. doi: 10.1001/archinte.162.12.1361. [DOI] [PubMed] [Google Scholar]
- 9.Van Thiel DH, Gavaler JS, Joshi SN, et al. Heartburn of pregnancy. Gastroenterology. 1977;72:666–668. [PubMed] [Google Scholar]
- 10.Schulze K, Christensen J. Lower sphincter of the opossum esophagus in pseudopregnancy. Gastroenterology. 1977;73:1082–1085. [PubMed] [Google Scholar]
- 11.Fisher RS, Roberts GS, Grabowski CJ, et al. Inhibition of lower esophageal sphincter circular muscle by female sex hormones. Am J Physiol. 1978;234:E243–E247. doi: 10.1152/ajpendo.1978.234.3.E243. [DOI] [PubMed] [Google Scholar]
- 12.Marrero JM, Goggin PM, de Caestecker JS, et al. Determinants of pregnancy heartburn. Br J Obstet Gynaecol. 1992;99:731–734. doi: 10.1111/j.1471-0528.1992.tb13873.x. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson M, Johnsen R, Ye W, et al. Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA. 2003;290:66–72. doi: 10.1001/jama.290.1.66. [DOI] [PubMed] [Google Scholar]
- 14.Mohammed I, Cherkas LF, Riley SA, et al. Genetic influences in gastro-oesophageal reflux disease: a twin study. Gut. 2003;52:1085–1089. doi: 10.1136/gut.52.8.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson BC, Somers SC, Fuchs CS, et al. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340–2348. doi: 10.1056/NEJMoa054391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Z, Nordenstedt H, Pedersen NL, et al. Lifestyle factors and risk for symptomatic gastroesophageal reflux in monozygotic twins. Gastroenterology. 2007;132:87–95. doi: 10.1053/j.gastro.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Serag HB, Ergun GA, Pandolfino J, et al. Obesity increases oesophageal acid exposure. Gut. 2007;56:749–755. doi: 10.1136/gut.2006.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margolis KL, Bonds DE, Rodabough RJ, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47:1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Bassford T, Green SB, et al. Postmenopausal hormone therapy and body composition–a substudy of the estrogen plus progestin trial of the Women’s Health Initiative. Am J Clin Nutr. 2005;82:651–656. doi: 10.1093/ajcn.82.3.651. [DOI] [PubMed] [Google Scholar]
- 21.Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 22.Stefanick ML, Cochrane BB, Hsia J, et al. The Women’s Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13 Suppl:S78–S86. doi: 10.1016/s1047-2797(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 23.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 24.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 25.Locke GR, III, Talley NJ, Fett SL, et al. Risk factors associated with symptoms of gastroesophageal reflux. Am J Med. 1999;106:642–649. doi: 10.1016/s0002-9343(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 26.Pandolfino JE, El-Serag HB, Zhang Q, et al. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology. 2006;130:639–649. doi: 10.1053/j.gastro.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett’s esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. quiz 311. [DOI] [PubMed] [Google Scholar]
- 28.Moayyedi P, Talley NJ. Gastro-oesophageal reflux disease. Lancet. 2006;367:2086–2100. doi: 10.1016/S0140-6736(06)68932-0. [DOI] [PubMed] [Google Scholar]
- 29.Nordenstedt H, Zheng Z, Cameron AJ, et al. Postmenopausal hormone therapy as a risk factor for gastroesophageal reflux symptoms among female twins. Gastroenterology. 2008;134:921–928. doi: 10.1053/j.gastro.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Locke GR, Talley NJ, Weaver AL, et al. A new questionnaire for gastroesophageal reflux disease. Mayo Clin Proc. 1994;69:539–547. doi: 10.1016/s0025-6196(12)62245-9. [DOI] [PubMed] [Google Scholar]
- 31.Revicki DA, Wood M, Wiklund I, et al. Reliability and validity of the Gastrointestinal Symptom Rating Scale in patients with gastroesophageal reflux disease. Qual Life Res. 1998;7:75–83. doi: 10.1023/a:1008841022998. [DOI] [PubMed] [Google Scholar]
- 32.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. quiz 1943. [DOI] [PubMed] [Google Scholar]