Abstract
Background and purpose
The ECASS-3 study demonstrated a benefit of treatment with intravenous tPA for acute stroke in the 3-4.5 hour time-window. Prior studies, however, have failed to demonstrate a significant benefit of tPA for patients treated beyond 3 hours. The purpose of this study was to produce reliable and precise estimates of the treatment effect of tPA by pooling data from all relevant studies.
Methods
A meta-analysis was undertaken to determine the efficacy of tPA in the 3-4.5 hour time-window. The effect of tPA on favorable outcome and mortality was assessed.
Results
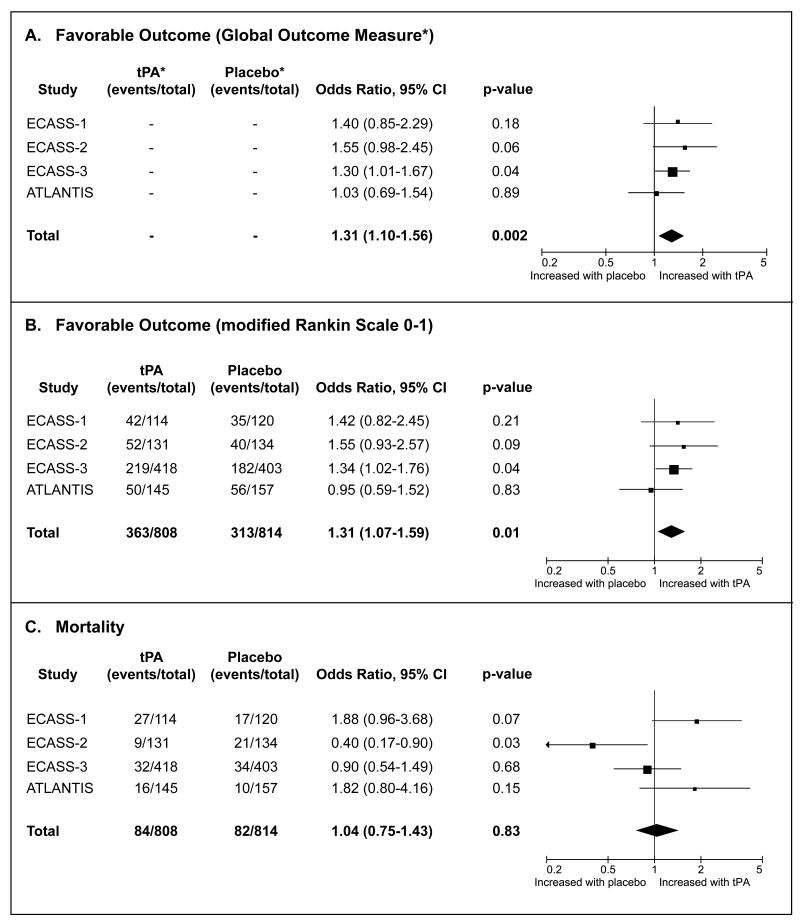
The meta-analysis included data from patients treated in the 3-4.5 hour time-window in ECASS-1 (n=234), ECASS-2 (n=265), ECASS-3 (n=821) and ATLANTIS (n=302). tPA treatment was associated with an increased chance of favorable outcome (OR 1.31, 95% CI 1.10-1.56; p=0.002) and no significant difference in mortality (OR 1.04; 95% CI 0.75-1.43; p=0.83) compared to placebo treated patients.
Conclusions
Treatment with tPA in the 3-4.5 hour time-window is beneficial. It results in an increased rate of favorable outcome without adversely affecting mortality.
Keywords: Acute Stroke, thrombolysis, meta-analysis, Acute Care, Acute Rx, Therapy, Thrombolytic RX, TPA
Background
In 1996, based on the results of the two-part National Institutes of Neurological Disorders and Stroke (NINDS) acute stroke trial, the FDA approved intravenous tissue plasminogen activator (tPA) for treatment of acute ischemic stroke up to 3-hours after symptom onset.1 The recently published ECASS-3 study results are the first data from a randomized placebo-controlled trial that demonstrate efficacy of intravenous tPA beyond the established 3-hour time-window.2 In ECASS-3, 821 stroke patients were randomized between treatment with placebo and tPA in the 3 to 4.5 hour time-window after acute ischemic stroke. Compared to placebo treated patients, tPA treated patients experienced a 7.2% absolute increase in the rate of excellent recovery at 90-day follow-up (p=0.04). And although tPA therapy was associated with an increased rate of symptomatic intracerebral hemorrhage (7.9% for tPA vs 3.5% for placebo, p<0.001), it was not associated with an increased rate of death (7.7% for tPA vs 8.4% for placebo, p=0.68). These results differ from those of previous studies that have assessed the effect of tPA beyond the 3-hour time-window.3-5 In ATLANTIS part B3, which resembles ECASS-3 most closely, tPA was associated with only a 2% increased rate of excellent outcome (not significant), a 5.4% higher rate of symptomatic intracerebral hemorrhage (p<0.001), and a 4% increased rate of death (p=0.08). The inferiority of the ATLANTIS results compared to ECASS-3 may be due to the longer treatment time-window in ATLANTIS part B (3-5 hour window with a median time-to-treatment of 4hr 36min in ATLANTIS part B versus a 3-4.5 hour time window with a median time to treatment of 3hr 59min in ECASS-3). The marginal significance with which superiority of tPA over placebo was demonstrated in ECASS-3 and the lack of a confirmatory randomized controlled trial of tPA in the 3-4.5 hour time-window may cast doubt on the true efficacy of tPA in this time-window. In order to arrive at a more robust estimate of the treatment effect we conducted a meta-analysis of patients treated in the 3-4.5 hour time-window from all major tPA stroke trials to date.2, 6
Materials and methods
Randomized controlled trials (n>100) of intravenous tPA for treatment of acute ischemic stroke with outcome data on patients who were treated between 3 and 4.5 hours after stroke were selected for the meta-analysis. Studies were identified based on a search of the Pubmed database and based on the authors' knowledge of the stroke literature. All analyses were based on the intention-to-treat populations of the identified studies. Outcomes analyzed included 1) good functional outcome on a global outcome measure (a global odds ratio test based on three individual outcome scales at day 90: mRS 0-1, NIHSS 0-1, and Barthel Index>=95); 2) good functional outcome defined as a score of 0-1 on the mRS at day 90; and 3) mortality. The global odds ratio test for this meta-analysis was slightly different from the global odds ratio test used for the individual analyses of the NINDS and ECASS-3 stroke trials in that it did not include the Glasgow Outcome Scale (GOS) as a fourth variable.1,2 The GOS was excluded in the meta-analysis because it was not assessed in all studies. Minor differences between the previously published ECASS-3 results2 and the results reported in this meta-analysis stem from exclusion of the Glascow Outcome Scale. If relevant outcome data were not published, the sponsor of the study was contacted and additional data were requested. Pooled odds ratios describing the treatment effect of tPA were computed with commercially available software (SAS version 9.2, Cary, NC).
Results
The ECASS-1, ECASS-2, ECASS-3 and ATLANTIS studies were included in the analysis. 2-5 Baseline characteristics of the 3-4.5 hour treatment cohort for each study are listed in table 1. Treatment with tPA in the 3-4.5 hour time-window is associated with an increased chance of favorable outcome based on the global outcome measure (ORGlobal Outcome Measure = 1.31, p=0.002) and the modified rankin scale (ORmRS 0-1 = 1.31, p=0.008), without adversely affecting 90-day mortality (ORmortality = 1.04, p=0.83). (Figure) Because a relatively high dose of tPA was administered to patients enrolled in ECASS-1 (1.1 mg/kg) a separate meta-analysis was conducted including only the three studies that used an iv tPA dose of 0.9 mg/kg. In this subset the association between tPA and favorable outcome remained essentially unchanged (ORGlobal Outcome Measure = 1.27, p=0.01; ORmRS 0-1 = 1.28, p=0.03). Exclusion of ECASS-1 from the analysis reduced the odds ratio for 90-day mortality associated with tPA from 1.04 to 0.87, but the association remained non-significant (95% CI 0.60-1.26; p=0.46).
Comparison of tPA versus placebo in the 3-4.5 hour time-window after acute ischemic stroke. Three 90-day endpoints were assessed: favorable outcome using a global outcome measure (panel A), favorable outcome defined as a modified Rankin Scale Score of 0-1 (panel B), and mortality (panel C).
*The global outcome measure is a global odds ratio derived from a statistical model that takes into account the outcome on three individual outcome scales at day 90: mRS 0-1, NIHSS 0-1, and Barthel Index>=95. Number of event ratios are not listed for this outcome measure because these cannot be derived from the model.
Discussion
This meta-analysis provides strong evidence for the efficacy of tPA treatment in the 3-4.5 hour time-window. Pooling of ECASS-3 data with data from prior studies of tPA in the 3-4.5 hour time-window approximately doubled the available sample size (from 821 to 1622). In this large sample the benefit of tPA in the 3-4.5 hour time-window was demonstrated with great statistical confidence as illustrated by a p-value of 0.002. This is in contrast with the ECASS-3 study results and the results of the previously published pooled analysis of NINDS, ECASS-1, ECASS-2 and ATLANTIS, which each showed a benefit, but only with marginal statistical significance. 2,6 Based on this meta-analysis the odds of a favorable outcome are increased by 31% for stroke patients treated with tPA in the 3-4.5 hour time-window.
Given the increased risk of intracerebral hemorrhage with tPA treatment, close attention has been paid to the safety profile of tPA. The ATLANTIS study results raised some safety concern for patients treated with tPA between 3 and 5 hours after symptom onset.3 In this cohort, 90-day mortality was higher in patients treated with tPA (11.0%) compared to placebo (6.9%, p=0.09). Our analysis demonstrates that no consistent association exists between tPA treatment in the 3-4.5 hour time-window and 90-day mortality; whereas tPA was associated with non-significant increases in 90-day mortality in ATLANTIS and ECASS-1, a significant reduction in 90-day mortality was observed in ECASS-2, and a non-significant reduction in ECASS-3. These discrepancies are likely due to chance effects, which are reduced by pooling of the data. Consequently, our meta-analysis provides the most reliable and precise estimate available to date of the effect of tPA treatment in the 3-4.5 hour time-window on mortality. The odds ratio of 1.04 (p=0.83) for 90-day mortality suggests that the effect of tPA on mortality is neutral.
The studies included in this meta-analysis were very similar in design. All four were randomized controlled trials of iv tPA for acute ischemic stroke. Time-to-treatment, a variable that is well known to modify the response to tPA, was well matched between the cohorts of patients from the different studies that were included in this meta-analysis (Table). There were, however, also differences between the studies that may account for some of the variability in the outcomes. For example, the higher dose of iv tPA in ECASS-1 compared to the other studies (1.1 mg/kg versus 0.9 mg/kg) may have led to an increased rate of symptomatic intracranial hemorrhage which, in turn, could partially account for the increased 90-day mortality rate associated with tPA in ECASS-1. Similarly, a relatively high proportion of patients with extended early infarcts signs in ECASS-1, may have contributed to the excess 90-day mortality rate in this study. A separate analysis, excluding ECASS-1 was run to assess the impact of ECASS-1 on the overall meta-analysis results. The association between tPA and favorable outcome remained essentially unchanged in this sub-analysis, whereas the odds ratio for 90-day mortality reduced slightly from 1.05 to 0.87, but remained non-significant. The ECASS-3 study was unique in excluding patients who had a combination of previous stroke and diabetes mellitus. Exclusion of this subgroup, however, does not appear to have improved the efficacy of tPA. In fact, both ECASS-1 and ECASS-2 had slightly higher odds ratios for favorable outcome with tPA than ECASS-3, whereas only ATLANTIS had a lower odds ratio for favorable outcome. The lack of efficacy of tPA therapy in the 3 to 4½ hr cohort of the ATLANTIS study is likely due to chance as there are no obvious differences in study design between ATLANTIS and the ECASS studies to account for this lack of efficacy. Hypothetically, differences in baseline demographics favoring placebo treated patients could have contributed to the lack of observed efficacy of tPA in the 3 to 4½ hr cohort of ATLANTIS. The only data to support this hypothesis is that diabetes was more common in tPA treated patients than controls in the overall 3-5 hour ATLANTIS cohort (n=613).3 Whether a similar imbalance in diabetes or other imbalances were present in the subset of ATLANTIS patients treated between 3 and 4.5 hours (n=302) has not been investigated.
Table.
Characteristics of the cohort of patients treated with tPA between 3-4.5 hours in the ECASS-1, ECASS-2, ECASS-3 and ATLANTIS studies
| Study Characteristic | ECASS-1 | ECASS-2 | ECASS-3 | ATLANTIS |
|---|---|---|---|---|
| n | 234 | 265 | 821 | 302 |
| Mean age (SD) | 64.7 (11.9) | 65.5 (11.0) | 65.2 (11.6) | 65.4 (11.6) |
| Mean NIHSSS (SD) | 15.2 (5.7) | 13.5 (5.7) | 11.2 (5.8) | 13.0 (6.2) |
| Median NIHSSS | 15 | 13 | 10 | 12 |
| Diabetes (%) | 15.8% | 17.7% | 15.7% | 21.2% |
| Median time to treatment | 3hr 50min | 3hr 55min | 3hr 58min | 4hr 1min |
In summary, the results of this meta-analysis strengthen the evidence base that treatment with tPA in the 3-4.5 hour window is beneficial and should therefore be taken into consideration for stroke patients who present during this time-window.
Acknowledgments
The funding for this study was provided by national institutes of health (NIH) grants K23 NS051372, Principal Investigator Maarten G. Lansberg and the Fonds voor Wetenschappelijk Onderzoek, Principal Investigator Vincent N. Thijs.
Footnotes
Conflict of Interest Disclosure: Dr Lansberg received a significant amount of salary support in the form of research grants relevant to this manuscript. Dr Bluhmki is an employee of Boehringer Ingelheim Pharma GmbH & Co, Germany.
Contributor Information
Maarten G. Lansberg, Stanford Stroke Center, Stanford University Medical Center, Palo Alto, CA.
Erich Bluhmki, Boehringer Ingelheim Pharma GmbH & Co, Germany.
Vincent N. Thijs, Department of Neurology, University Hospitals of Leuven, Belgium, Vesalius Research Center, Leuven, Belgium.
References
- 1.Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-pa stroke study group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS study: A randomized controlled trial. Alteplase thrombolysis for acute noninterventional therapy in ischemic stroke. JAMA. 1999;282:2019–2026. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, Boysen G, Bluhmki E, Hoxter G, Mahagne MH, Hennerici M ECASS Study Group. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The european cooperative acute stroke study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 5.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, Schneider D, Diez-Tejedor E, Trouillas P Second European-Australian Acute Stroke Study Investigators. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II) Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 6.Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. The ATLANTIS, ECASS and NINDS rt-PA Study Group Investigators. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]