Abstract
Natural immunity to meningococcal disease in young children is associated epidemiologically with carriage of commensal Neisseria species, including Neisseria lactamica. We have previously demonstrated that outer membrane vesicles (OMVs) from N. lactamica provide protection against lethal challenge in a mouse model of meningococcal septicemia. We evaluated the safety and immunogenicity of an N. lactamica OMV vaccine in a phase I placebo-controlled, double-blinded clinical trial. Ninety-seven healthy young adult male volunteers were randomized to receive three doses of either an OMV vaccine or an Alhydrogel control. Subsequently, some subjects who had received the OMV vaccine also received a fourth dose of OMV vaccine, 6 months after the third dose. Injection site reactions were more frequent in the OMV-receiving group, but all reactions were mild or moderate in intensity. The OMV vaccine was immunogenic, eliciting rises in titers of immunoglobulin G (IgG) against the vaccine OMVs, together with a significant booster response, as determined by an enzyme-linked immunosorbent assay. Additionally, the vaccine induced modest cross-reactive immunity to six diverse strains of serogroup B Neisseria meningitidis, including IgG against meningococcal OMVs, serum bactericidal antibodies, and opsonophagocytic activity. The percentages of subjects showing ≥4-fold rises in bactericidal antibody titer obtained were similar to those previously reported for the Norwegian meningococcal OMV vaccine against the same heterologous meningococcal strain panel. In conclusion, this N. lactamica OMV vaccine is safe and induces a weak but broad humoral immune response to N. meningitidis.
There is great interest in the development of vaccines for protection against serogroup B meningococcal disease, and vaccines based on outer membrane vesicles (OMVs) have been assessed in efficacy trials in Cuba (33), Brazil (8), and Norway (4). Efficacy was demonstrated for teenagers and children aged over 4 years, but in the Brazilian study, protection from disease was not observed in younger children. Protection provided by OMV vaccines is associated with serum bactericidal antibody (SBA) responses (20), but SBA responses to OMV vaccines appear to be dependent on the immunodominant and highly variable PorA surface protein, especially in young children (35). More recently, a meningococcal OMV vaccine has been used in New Zealand to combat an epidemic caused by meningococci with a single PorA serosubtype (P1.7-2,4) (25). An OMV vaccine manufactured from the epidemic strain has been used to immunize infants and children up to 19 years, and an effectiveness rate of 84.8% has been demonstrated after 24 months for children 6 months to 3 years of age (13).
To broaden the protection offered by meningococcal OMV vaccines, a vaccine based on two strains, each engineered to express three PorA proteins, was developed and assessed in several clinical studies (6, 7). Broader protection was observed but restricted primarily to the six PorA subtypes represented in the vaccine, and the rapid change in predominant PorA subtypes, such as those seen in the United Kingdom (19), would probably require periodic reformulation of such a vaccine. Bivalent meningococcal OMV vaccines have also been assessed (5, 31) and heterologous strain SBA responses detected for some individuals. Mining of the meningococcal genome has identified several new antigens capable of generating SBA responses in laboratory animals (28), and a potentially broadly protective vaccine is in development (14). Factor H binding protein has been separately identified as a potential vaccine antigen (12, 21), and this is being evaluated in early-phase clinical studies.
An alternative approach has been the development of an OMV vaccine based on the commensal Neisseria lactamica. N. lactamica colonizes the nasopharynx, particularly in young children, and its presence has been implicated in naturally acquired immunity against the meningococcus (15, 18). N. lactamica does not possess a capsule or PorA, so any protection provided by carriage of this organism would not be serogroup or serosubtype specific. We have demonstrated that vaccines prepared from N. lactamica protect mice from lethal challenge in a mouse model of meningococcal septicemia (17, 26) in the absence of an SBA response (11). This is in contrast to SBA responses observed in children following colonization with this organism (15). To further evaluate this vaccine approach, we have assessed the safety and immunogenicity of an N. lactamica OMV vaccine in a phase I study with adult volunteers.
MATERIALS AND METHODS
Subjects and ethical aspects.
Adult males aged 18 to 55 years with no history of meningococcal disease were enrolled in the study. Male volunteers were recruited, as reproductive toxicology studies were not performed on the vaccine. Individuals were excluded if they had clinically significant acute or chronic illness, had abnormal laboratory hematology or urinalysis results, had received blood products or immunoglobulins in the previous 3 months, had a history of anaphylactic shock or other allergic reaction after previous vaccinations or hypersensitivity to any known vaccine component, or had any known or suspected immune impairment. Subjects were also excluded if they had a skin tattoo at the injection site, had received any vaccine within 60 days of the commencement of the study, were participating in any other clinical trial, or had an oral temperature of ≥37.5°C immediately prior to administration of vaccine. The study was conducted in accordance with the Declaration of Helsinki and the European Clinical Trials Directive and other local legal and regulatory requirements. The trial had independent ethics committee and national regulatory approval from Oxfordshire Research Ethics Committee A and the Medicines and Healthcare Products Regulatory Agency (EudraCT number 2005-002191-15). Participants provided written informed consent before enrollment.
Vaccines.
The N. lactamica OMV vaccine was produced at the Health Protection Agency Centre for Emergency Preparedness and Response. N. lactamica strain Y92-1009 (sequence type 3493/clonal complex 613) was cultured as described previously (11). OMV bulk preparations in 0.2 M glycine, pH 8.0, with 3.0% sucrose were stored at −80°C before being thawed, pooled, and then filtered using two 0.2-μm filters. The filtrate was then diluted to 60 μg ml−1 with the same buffer, and Alhydrogel (Brenntag Biosector, Denmark) was added to give a final concentration of 0.33%, giving a final protein concentration of 25 μg per 0.5 ml dose. Protein concentrations were determined using a Peterson Lowry assay (Sigma, United Kingdom). A placebo vaccine containing 0.2 M glycine, pH 8.0, 3% sucrose, and the same amount of Alhydrogel as the OMV vaccine was also prepared.
Study design.
The study was a double-blinded, randomized, placebo-controlled phase 1 clinical trial. Subjects were randomized to OMV vaccine or placebo groups and immunized on days 0, 42, and 84 by intramuscular injection into the upper arm. Blood samples for assessment of antibody responses were taken before each dose and 3 weeks after the third dose (day 105). Following a satisfactory interim analysis of safety and immunogenicity data, the study was unblinded and subjects who had received the OMV vaccine were invited to receive a fourth dose of OMV vaccine 6 months after the third dose (day 266). The study was designed to enroll 60 subjects, with 30 in each group (vaccine and placebo). This sample size was sufficient for detection of differences between response rates or reaction rates of 12% or more with >95% probability and of 30 to 40% with 80% probability (5% significance level).
Safety assessment and monitoring.
Hematology, blood biochemistry, and urinalysis tests were performed before each immunization and at the end of study follow-up. In addition, blood pressure, pulse, and oral temperature were recorded and a 12-lead electrocardiogram was obtained at each visit. Subjects were observed for at least 2 h following immunization and then at 24 h and local and systemic reactions (pain, tenderness, erythema, and swelling) assessed. Subjects were asked to keep a diary recording body temperature, local reactions, and any unexpected symptoms at the same time each day for 2 weeks after each dose. Subjects received a telephone interview at 3 weeks after the first two doses and a final follow-up 3 weeks after the third and fourth doses.
Enzyme-linked immunosorbent assay (ELISA).
To measure N. lactamica OMV-specific immunoglobulin G (IgG) in human serum, Nunc Maxisorb 96-well plates were coated by overnight incubation at 2 to 8°C with N. lactamica OMVs, prepared in the same way as the OMVs in the vaccine, diluted to 10 μg/ml in 15 mM sodium carbonate, 35 mM sodium bicarbonate buffer, pH 9.6. Plates were then washed five times with 300 μl of phosphate-buffered saline containing 0.1% (vol/vol) Tween 20 (PBST) and blocked with PBST containing 5% (vol/vol) fetal bovine serum for 75 min at 20°C ± 5°C. The remainder of the assay was also performed at this temperature. Test, reference, and quality control (QC) serum serial dilutions were prepared in PBST, 5% (vol/vol) fetal bovine serum in a separate dilution plate, and 100 μl/well of each serial dilution was transferred to the coated plate, incubated for 90 min, and then washed as described above. Bound antibody was detected using a goat anti-human IgG conjugated to alkaline phosphatase, diluted in PBST to 1/2,500, and incubated for 90 min. After a wash as described above, 100 μl/well of AP yellow substrate (p-nitrophenyl phosphate) was added for 55 min, after which the reaction was stopped by the addition of 50 μl/well of 3 M sodium hydroxide for 5 min, and the optical densities were measured with a VERSAmax plate reader (Molecular Devices). A human reference serum sample which contained naturally acquired antibodies that reacted with N. lactamica OMVs was identified. Data analysis was completed using SOFTmax PRO software to fit an unweighted four-parameter logistic curve to the reference serum data. Optical densities from duplicate serial dilutions for each test and QC serum sample were used to interpolate titers from the reference serum dose response curve.
Specific IgG endpoint titers relative to those for reference sera for each strain were also determined for meningococcal OMVs isolated using a deoxycholate extraction method similar to that used for the N. lactamica OMVs, from six target strains which are representative of recent major disease-causing clones in the United Kingdom (Table 1) (10).
TABLE 1.
N. meningitidis strains used in immunologic assaysa
| Designation | Serotype | Serosubtype
|
Sequence type | Clonal cluster | |
|---|---|---|---|---|---|
| VR1 | VR2 | ||||
| 44/76-SL | 15 | 7 | 16 | 32 | 32 |
| M01-240101 | NT | 19-1 | 15-11 | 1049 | 269 |
| M01-240013 | NT | 22 | 9 | 275 | 269 |
| M01-240149 | 4 | 7-2 | 4 | 41 | 41/44 |
| M01-240355 | 1 | 22 | 14 | 213 | 213 |
| M01-240185 | 2a | 5-1 | 10-8 | 11 | 11 |
All strains listed were of serogroup B. VR, variable region; NT, not typeable.
SBA.
SBA assays (10) were performed for the six meningococcal target strains (Table 1) by using a standardized assay with a starting dilution of 1:2. Briefly, human serum, at 25%, was used as an exogenous source of human complement, with titers expressed as the reciprocal of the final dilution giving ≥50% SBA killing at 60 min compared with the level for the control (inactive complement and no test serum). Single determinations were made using each serum sample, the human complement used had previously been screened for lack of intrinsic bactericidal activity, and comparability of complement activity between batches was confirmed using QC sera with known SBA activity. Several sources were used to complete the study.
OPA.
The opsonophagocytosis assay (OPA) was performed as described previously (10), except that immediately before flow cytometric analysis, 50 μl trypan blue solution (Sigma, United Kingdom) was added to each sample to quench the fluorescence of surface-associated but not internalized bacteria, and the fluorescence index of the complement-only control was subtracted from the fluorescence index obtained with each serum sample. Each sample was analyzed in duplicate.
Statistical analysis. (i) Safety.
Proportions for local and systemic reactions outside normal ranges, and biochemical and hematological samples outside normal laboratory ranges, were calculated after each dose and also aggregated across all doses. The results for the vaccine and placebo groups were then compared using Pearson's chi-square or Fisher's exact test.
(ii) Immunogenicity.
Antibody geometric mean titers (GMTs), with 95% confidence intervals, for ELISA, SBA analysis, and OPA results were calculated after each dose. Unpaired t tests for the logarithmically transformed results were used to compare the vaccine and placebo groups, and paired t tests were used to compare results at different time points. The percentages of individuals showing ≥4-fold rises in SBA response between time points and significant rises in OPA levels between time points were calculated and compared between the vaccine and placebo groups by using Fisher's exact test and between time points by using the Wilcoxon signed-rank test. The definition of a significant rise in OPA level for an individual was a rise that exceeded the variability of the data from the duplicates in each assay with a probability of >95%, calculated using a z test.
All analyses were performed using Stata 10.0 software (StataCorp 2008 statistical software, release 10.0; Stata Corporation, College Station, TX).
RESULTS
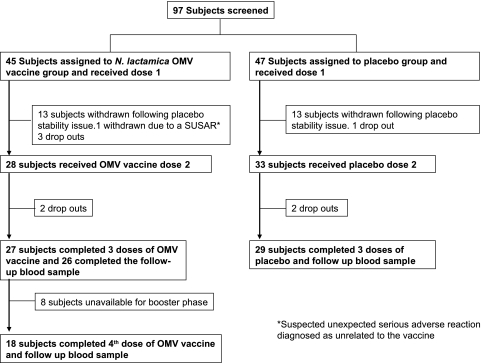
The flow of participants in the study can be seen in Fig. 1. Ninety-seven subjects were screened, enrolled in the study, and randomized to either the OMV vaccine or the placebo group. Five individuals withdrew before the first dose; therefore, 45 subjects received the first dose of the OMV vaccine and 47 the placebo. The median age (interquartile range) for the OMV vaccinees was 22 years (19 to 25 years), and that for the placebo was 22 years (20 to 26 years). Following administration of the first dose to the first 26 participants (13 in each group), the placebo vaccine failed to meet the product specification for the rabbit pyrogenicity test in a stability study. Although quickly attributed to an inappropriate placebo product specification, this resulted in the withdrawal of all these subjects from the study, as the study was blinded and, by the time the product specification was amended, the subjects were beyond the protocol date for dose 2. The study resumed, and the second and third doses of the OMV vaccine were received by 28 and 27 subjects and those of the placebo by 33 and 29 subjects, respectively. Eighteen subjects who received three doses of the OMV vaccine were available to receive the fourth dose and provided a follow-up blood sample 3 weeks later. Subjects withdrew for a variety of reasons, but none because of adverse reactions.
FIG. 1.
Flow of subjects through the trial.
Safety.
Frequencies and proportions for local reactions are shown in Table 2. Following the first dose, tenderness at the injection site was observed more frequently in the OMV vaccine group than in the placebo group (P = 0.01), and a greater proportion of subjects exhibited erythema (P < 0.001). There were also greater proportions of subjects with erythema following the first (P < 0.001) and third (P = 0.05) doses of the OMV vaccine. Tenderness at the injection site was equally frequent in the OMV and placebo groups following the second and third doses of the OMV vaccine. Swelling and pain were observed equally following the three doses of the OMV vaccine or placebo. In addition, the OMV vaccine did not cause fever of >38°C with greater frequency than the placebo. The percentages of subjects with fever following vaccination were low, with 4 of 45 (8.9%) and 2 of 47 (4.3%) showing temperatures of >38°C in the vaccine and placebo groups, respectively. No significant differences in blood pressure, pulse, blood biochemistry measurement, or urine analysis measurement were observed between the OMV vaccine and placebo groups (data not presented).
TABLE 2.
Frequencies and proportions of adverse reactions grouped under “no effect” or “mild or moderate effect”a
| Reaction and dose | Effect | No. (%) of subjects observed within 14 days of dose
|
Pb | |
|---|---|---|---|---|
| Placebo | OMV vaccine | |||
| Pain | ||||
| 1 | None | 34 (72.3) | 27 (60.0) | |
| Mild/moderate | 13 (27.7) | 18 (40.0) | 0.21* | |
| 2 | None | 25 (75.8) | 21 (75.0) | |
| Mild/moderate | 8 (24.2) | 7 (25.0) | 0.94* | |
| 3 | None | 25 (86.2) | 22 (81.5) | |
| Mild/moderate | 4 (13.8) | 5 (18.5) | 0.72** | |
| Booster | None | 13 (72.2) | ||
| Mild/moderate | 5 (27.8) | |||
| Tenderness | ||||
| 1 | None | 19 (40.4) | 7 (15.6) | |
| Mild/moderate | 28 (59.6) | 38 (84.4) | 0.01* | |
| 2 | None | 15 (45.5) | 14 (50.0) | |
| Mild/moderate | 18 (54.6) | 14 (50.0) | 0.72* | |
| 3 | None | 16 (55.2) | 19 (70.4) | |
| Mild/moderate | 13 (44.8) | 8 (29.6) | 0.24* | |
| Booster | None | 8 (44.4) | ||
| Mild/moderate | 10 (55.6) | |||
| Erythema | ||||
| 1 | None | 47 (100.0) | 34 (75.6) | |
| Mild/moderate | 0 (0.0) | 11 (24.4) | <0.001** | |
| 2 | None | 33 (100.0) | 26 (92.9) | |
| Mild/moderate | 0 (0.0) | 2 (7.1) | 0.21** | |
| 3 | None | 29 (100.0) | 23 (85.2) | |
| Mild/moderate | 0 (0.0) | 4 (14.8) | 0.05** | |
| Booster | None | 15 (83.3) | ||
| Mild/moderate | 3 (16.7) | |||
| Swelling | ||||
| 1 | None | 47 (100.0) | 41 (91.1) | |
| Mild/moderate | 0 (0.0) | 4 (8.9) | 0.05** | |
| 2 | None | 33 (100.0) | 26 (92.9) | |
| Mild/moderate | 0 (0.0) | 2 (7.1) | 0.21** | |
| 3 | None | 29 (100.0) | 24 (88.9) | |
| Mild/moderate | 0 (0.0) | 3 (11.1) | 0.11** | |
| Booster | None | 17 (94.4) | ||
| Mild/moderate | 1 (6.6) | |||
Results are shown for all study participants. Local reaction assessments were made in accordance with the following grading system: for pain, mild (does not interfere with daily living activity), moderate (interferes with daily living activity or requires repeated use of pain relievers), and severe (prevents or disrupts daily living activity or requires repeated use of narcotic pain relievers, clinician appointment, or hospital visit); for tenderness, mild (mild pain to touch), moderate (pain with movement), and severe (significant pain at rest); and for erythema and swelling, mild (up to 5 cm), moderate (5.1 to 10 cm), and severe (>10 cm).
*, Pearson's chi-square test; **, Fisher's exact test.
Immunogenicity. (i) Anti-N. lactamica OMV antibody response.
The anti-N. lactamica IgG GMT (Table 3) rose following each OMV vaccine dose and was significantly greater than the level for the placebo in OMV-vaccinated subjects (P < 0.001).
TABLE 3.
Anti-N. lactamica OMV IgG ELISA GMTs and 95% confidence intervals
| Time point (day) | Placebo
|
OMV vaccine
|
Pa | ||
|---|---|---|---|---|---|
| No. of subjects | GMT (95% CI) | No. of subjects | GMT (95% CI) | ||
| Before dose 1 (0) | 47 | 809 (672-974) | 45 | 881 (682-1,137) | 0.59 |
| Before dose 2 (42) | 45 | 801 (653-982) | 40 | 1,710 (1,347-2,172) | <0.001 |
| Before dose 3 (84) | 29 | 773 (571-1,048) | 27 | 2,869 (2,143-3,841) | <0.001 |
| After dose 3 (105) | 29 | 772 (575-1,038) | 26 | 4,645 (3,462-6,232) | <0.001 |
| Before booster dose (266) | 0 | 18 | 2,506 (1,747-3,595) | ||
| After booster dose (287) | 0 | 18 | 10,331 (7,499-14,234) | ||
Two-sample t test.
(ii) Anti-N. meningitidis OMV antibody response.
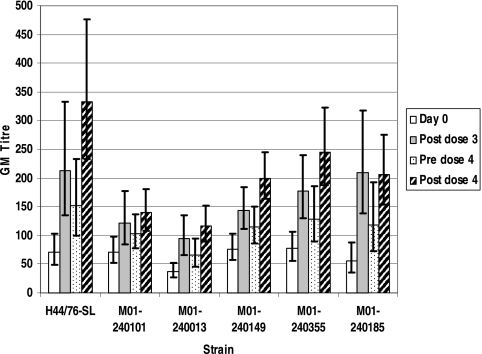
Rises in IgG GMT were seen for OMVs from all six meningococcal strains (Fig. 2), with a significant rise from day 0 to after the third dose (P < 0.05) for all strains. Values declined in the 6 months following the third dose, although titers remained higher than those at day 0 for all strains. Following the fourth dose, greater IgG GMTs than those observed after three doses were achieved for all strains except M01-240185.
FIG. 2.
Meningococcal OMV ELISA IgG GMTs and 95% confidence intervals.
(iii) SBA response.
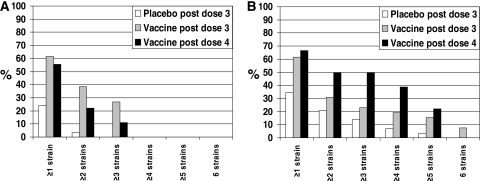
Subjects were not prescreened for low titers of SBA against the target strains. At day 0 in the OMV vaccine group, between 42.3 and 96.1% of the subjects had SBA titers of ≥1:4 (Table 4), depending on the meningococcal strain, with titers up to 1:1,024 determined. The SBA GMTs following three doses of vaccine were not significantly different from the placebo titers (Table 4), except for strain M01-240185 (P = 0.001; Wilcoxon signed-rank test). Unlike the N. lactamica and N. meningitidis ELISA titers, the SBA GMTs did not increase from after the third dose to after the fourth dose. Rises of ≥4-fold in SBA GMT from day 0 to after the third dose varied between 7.7% of subjects for strain M01-240185 to 30.8% for strain M01-240355 (Table 5). Despite the rise in anti-N. lactamica and anti-N. meningitidis OMV ELISA titers following the fourth dose, there was no further rise in the number of subjects showing ≥4-fold rises in SBA, except for strain M01-240185. The percentage of subjects who mounted ≥4-fold rises in SBA against at least one meningococcal strain is shown in Fig. 3A. It can be seen that the OMV vaccine induced a greater cross-reactive SBA response than the placebo vaccine, with 61.5% of subjects mounting ≥4-fold rises in SBA against at least one of the strains assessed (P = 0.006; Wilcoxon signed-rank test). These ≥4-fold rises were observed for different strains in some individuals.
TABLE 4.
SBA GMTs, 95% confidence intervals, and percentages of volunteers with ≥4-fold rises in SBA titer
| Treatment and time point | No. of subjects |
N. meningitidis strain
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 44/76-SL
|
M01-240101
|
M01-240013
|
M01-24149
|
M01-240355
|
M01-240185
|
||||||||
| GMT (95% CI) | % ≥4a | GMT (95% CI) | % ≥4a | GMT (95% CI) | % ≥4a | GMT (95% CI) | % ≥4a | GMT (95% CI) | % ≥4a | GMT (95% CI) | % ≥4a | ||
| Placebo, before dose 1 (day 0) | 32 | 7.7 (4.3-13.7) | 62.1 | 38.4 (23.2-63.6) | 96.6 | 14.4 (8.9-23.4) | 86.2 | 11.5 (6.8-19.5) | 83.8 | 10.9 (6.1-19.6) | 75.9 | 3.5 (2.0-6.1) | 37.9 |
| Placebo, after dose 3 (day 105) | 29 | 8.6 (4.5-16.4) | 65.5 | 25.8 (15.3-43.5) | 93.1 | 13.2 (7.7-22.6) | 79.3 | 9.9 (5.8-17.0) | 79.3 | 10.7 (5.3-21.6) | 69.0 | 2.9 (1.7-4.9) | 37.9 |
| OMV vaccine, before dose 1 (day 0) | 32 | 5.2 (2.9-9.4) | 50 | 36.4 (22.2-59.9) | 96.1 | 12.3 (7.4-20.6) | 88.4 | 10.6 (6.1-18.4) | 76.9 | 15.3 (7.9-29.5) | 80.8 | 3.4 (2.1-5.7) | 42.3 |
| OMV vaccine, after dose 3 (day 105) | 26 | 7.6 (3.5-16.5) | 57.7 | 41.8 (22.8-76.4) | 96.1 | 16.9 (9.2-31.0) | 92.3 | 14.4 (8.2-25.1) | 92.3 | 23.2 (11.4-47.4) | 84.6 | 4.6 (2.5-8.3) | 53.8 |
| OMV vaccine, before booster dose (day 266) | 18 | 5.2 (2.5-10.9) | 61.1 | 24.4 (11.0-54.1) | 88.9 | 14.2 (7.8-26.1) | 88.9 | 12.7 (6.9-23.5) | 94.4 | 12.2 (4.5-33.3) | 66.7 | 4.0 (2.1-7.8) | 55.6 |
| OMV vaccine, after booster dose (day 287) | 18 | 5.9 (2.7-12.6) | 61.1 | 27.4 (13.0-57.7) | 88.9 | 21.8 (12.5-38.0) | 94.4 | 14.2 (7.8-26.1) | 94.4 | 23.5 (10.1-54.8) | 83.3 | 6.6 (3.2-13.8) | 66.7 |
Percentage of volunteers with ≥4-fold rises in SBA titer.
TABLE 5.
Numbers and percentages of subjects having ≥4-fold rises in SBA titer
| Treatment (time point) | No. of subjects | No. (%) of subjects for indicated N. meningitidis strain
|
|||||
|---|---|---|---|---|---|---|---|
| 44/76-SL | M01-240101 | M01-240013 | M01-24149 | M01-240355 | M01-240185 | ||
| Placebo (day 0 after dose 3) | 29 | 2 (7.4) | 1 (3.4) | 1 (3.4) | 2 (6.9) | 3 (10.3) | 1 (3.4) |
| Vaccine (day 0 after dose 3 | 26 | 6 (23.1) | 5 (19.2) | 7 (26.9) | 4 (15.4) | 8 (30.8) | 2 (7.7) |
| Vaccine (day 0 after dose 4) | 18 | 2 (11.1) | 1 (5.6) | 3 (16.7) | 2 (11.1) | 2 (11.1) | 4 (22.2) |
FIG. 3.
Percentages of subjects with ≥4-fold rises in SBA titer for one or more meningococcal strains (A) or significant rises in OPA for one or more meningococcal strains (B). A significant rise in OPA for an individual was considered to have occurred when the observed rise exceeded the variability of the data from the duplicates in each assay with a probability of >95%, calculated using a z test.
(iv) OPA response.
At day 0, there was a large range in OPA values, attributable to preexisting immunity, and OPA FIR geometric means showed little difference from placebo values following three doses of vaccine (data not presented). Between 6.9 and 24.1% of the subjects had significant increases in OPA following three doses of the placebo (Table 6), and between 19.2 and 34.6% had significant increases following three doses of the OMV vaccine, depending on the strain, and this rose to between 27.8 and 55.5% of subjects following the fourth dose. It can be seen that significant rises in OPA occur more for meningococcal strains than for SBA (Fig. 3B), and in contrast to the SBA results, the cross-reactivity of the OPA response is increased following the fourth vaccine dose.
TABLE 6.
Numbers and percentages of subjects having rises in OPAa
| Treatment (time point) | No. of subjects | No. (%) of subjects for indicated N. meningitidis strain
|
|||||
|---|---|---|---|---|---|---|---|
| 44/76-SL | M01-240101 | M01-240013 | M01-24149 | M01-240355 | M01-240185 | ||
| Placebo (day 0 after dose 3) | 29 | 2 (6.9) | 7 (24.1) | 2 (6.9) | 2 (6.9) | 4 (13.8) | 6 (20.7) |
| Vaccine (day 0 after dose 3) | 26 | 8 (30.8) | 5 (19.2) | 7 (26.9) | 9 (34.6) | 7 (26.9) | 5 (19.2) |
| Vaccine (day 0 after dose 4) | 18 | 10 (55.5) | 5 (27.8) | 7 (38.9) | 7 (38.9) | 5 (27.8) | 7 (27.8) |
A significant rise in OPA for an individual was considered to have occurred when the observed rise exceeded the variability of the data from the duplicates in each assay with a probability of >95%, calculated using a z test.
DISCUSSION
This study presents data on the safety and immunogenicity of a candidate meningococcal disease vaccine based on N. lactamica OMVs which we developed first by demonstration of protection of laboratory animals (26) and then by preclinical evaluation (11), manufacture, and clinical testing. The occurrence of local reactions is very common for intramuscularly administered vaccines containing aluminum hydroxide, and similar profiles of reactions were observed with meningococcal OMV vaccines and the N. lactamica OMV vaccine (1, 7, 9, 24, 27, 35). Tenderness and pain at the injection site were the most common reactions with the N. lactamica OMV vaccine, although the majority of reactions were mild and none were severe. A number of meningococcal OMV studies have reported severe local pain, particularly following the first dose (1, 5, 8, 24), but this was not seen with the commensal Neisseria OMV vaccine. None of the withdrawals from the study were due to adverse reactions; all withdrawals were due either to the withdrawal of 26 subjects during the temporary suspension of the study or to an inability to conform to the protocol.
The N. lactamica OMV vaccine was highly immunogenic, inducing large rises in IgG against N. lactamica OMVs after three doses and a large boost response following the fourth dose. The ELISAs using OMVs isolated from six different meningococcal strains demonstrated a rise in the cross-reactive IgG response. As this ELISA has been used to analyze the responses to a meningococcal OMV vaccine (10) given at the same dose and with the same schedule, it can be seen that the IgG concentration for the homologous meningococcal strain (44/76-SL) was 3.7-fold greater than that obtained following the N. lactamica vaccine. However, the concentrations for heterologous strains were very similar to those obtained with the N. lactamica vaccine, indicating that the antibodies elicited by N. lactamica are comparable to those elicited by an N. meningitidis OMV for the same heterologous meningococcal strains (10).
The majority of subjects in this study had preexisting SBA against the meningococcal target strains, and both rises and falls in activity were seen in subjects in both the placebo and the vaccine groups, which may depend on recent or current exposure to meningococci. Carriage of N. meningitidis was not assessed in this study, but most of the subjects were young adults (median age, 22 years) in university education, a group known for elevated carriage rates of up to 35% (3). This is in agreement with both the historical data from the United States showing that the majority of young adults have ≥4-fold rises in SBA titer (16) and prevaccination data presented by Boutriau et al. (5). A difference between this OMV vaccine study and previous studies with meningococcal OMV vaccines is that we do not have a homologous meningococcal strain to include in the analysis, and relatively few of the many published studies included a comprehensive panel of heterologous meningococcal target strains. The standardized SBA-and-strain panel used in this study has been used to study the responses to the Norwegian OMV vaccine (10) and a bivalent OMV vaccine (5). In the study with the Norwegian meningococcal OMV vaccine, the SBA response was clearly greatest against the homologous strain (44/76-SL) (10), with 75% of the adult volunteers having ≥4-fold rises. However, for the heterologous strains, the percentages of subjects achieving ≥4-fold rises (17.4 to 34.8%, depending on the strain) were similar to those showing responses to the N. lactamica vaccine (7.7 to 30.8%) in this study. Greater heterologous-strain responses were elicited by the bivalent meningococcal OMV vaccine (5). Despite the modest increase in percentage of SBA responders in the present study, it is clear that the N. lactamica vaccine induced a greater SBA response than the placebo vaccine, with 61.5% of subjects showing ≥4-fold rises for at least one meningococcal strain and 26.9% of subjects having ≥4-fold rises against three or more meningococcal strains (Fig. 3). The observation that 24.1% of volunteers who received the placebo vaccine showed ≥4-fold rises in SBA against at least one of the meningococcal strains (Fig. 3A) shows the dynamic nature of immunity to N. meningitidis in this population, which most likely reflects recent carriage of the organism. Monitoring of meningococcal carriage in volunteers during the study was considered but not performed, as it may be insensitive (34) and also may miss transient carriage occurring between sampling dates. We also did not prescreen volunteers and exclude those with preexisting bactericidal antibodies, as a meningococcal disease vaccine must be suitable for use without prior antibody assessment. A further observation was that the percentage of individuals with ≥4-fold rises in SBA from day 0 to after the fourth OMV vaccine dose was lower than that observed following three doses. It is unlikely that this is due to hyporesponsiveness to the vaccine, as SBA GMTs did not differ significantly from after the third dose to after the fourth (Table 4).
It has been suggested that OPA is also an important defense mechanism against serogroup B meningococcal disease (30, 36). There are a number of approaches to OPAs for assessment of meningococcal disease vaccines. These include the use of live meningococci and fresh human phagocytic cells followed by quantification of oxidative burst (2); a killing assay using fresh human cells and a complement source depleted in C6 (29); and measurement of uptake of fixed, fluorescent meningococci by HL60 cells. In this study, we utilized an OPA as described previously (10), except that sensitivity was greatly increased by the use of trypan blue to quench the fluorescence of bacteria associated with the surfaces of the HL60 cells. Previously, an increase in OPA was observed with only two of seven meningococcal strains following three doses of Norwegian meningococcal OMV vaccine (10). In the present study, only modest geometric mean rises in OPA activity were detected. However, we observed a greater number of subjects with rises in OPA activity against the panel of meningococcal strains than with the placebo (Table 6 and Fig. 3B). As seen for SBA, some individuals showed a rise in OPA activity following three doses of the placebo vaccine, which is likely to reflect recent exposure to N. meningitidis. In contrast to the SBA response, there was a marked increase in subjects showing significant rises in OPA activity following the fourth dose. Sandbu et al. (31) also report that a higher proportion of subjects responded in the OPA than in the SBA analysis, and they suggest that the protection elicited by the vaccines tested may be higher than indicated by the results of the SBA analysis alone. Additional evidence that the SBA response may underestimate protection arises from the efficacy study of the Cuban OMV vaccine in Brazil. Fourfold or greater rises in SBA were detected in 52% of children older than 4 years, while efficacy was shown to be greater that 70% in this age group (8, 23). Also, protection in the absence of SBA has been demonstrated for the N. lactamica OMV vaccine (11, 26) in an adult mouse disease model and in infant rats by using sera against a number of meningococcal antigens raised in laboratory animals (38, 39) and in human recipients of polysaccharide vaccines (37).
We have assessed the safety and immunogenicity of an N. lactamica OMV vaccine in adult volunteers. The vaccine showed a good safety profile and was immunogenic, eliciting large rises in IgG against the vaccine OMV and also against OMVs isolated from a panel of meningococcal strains. Modest increases in titer of SBA against strains of serogroup B N. meningitidis were observed in some individuals, with a greater number of subjects showing increases in OPA activity against a wider range of strains. The results indicate that this vaccine is unlikely to be a successful meningococcal disease vaccine alone but that it may provide a safe and immunogenic basis for a combination vaccine containing other antigens, both acting as an adjuvant (22, 32) for the additional components and providing protection against some strains.
Acknowledgments
Chris Care, Mary Stringer, and Lynn Smart (Clinical Research Nurses, Sheffield Teaching Hospitals) helped execute the clinical study. Many members of the Manufacturing, Quality Control, and Quality Assurance Departments at the Centre for Emergency Preparedness and Response, including Roger Price, Fenella Walker, and Stuart Smith, were responsible for the vaccines, and we acknowledge the contribution of past members of the vaccine development team. Advice for the study was provided by Keith Cartwright and Ian Feavers.
Funding was provided by the United Kingdom Health Protection Agency.
Footnotes
Published ahead of print on 24 June 2009.
REFERENCES
- 1.Aaberge, I. S., P. Oster, O. S. Helland, A. C. Kristoffersen, E. Ypma, E. A. Høiby, B. Feiring, and H. Nøkleby. 2005. Combined administration of meningococcal serogroup B outer membrane vesicle vaccine and conjugated serogroup C vaccine indicated for prevention of meningococcal disease is safe and immunogenic. Clin. Diagn. Lab. Immunol. 12599-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aase, A., L. M. Naess, R. H. Sandin, T. K. Herstad, F. Oftung, J. Holst, I. L. Haugen, E. A. Høiby, and T. E. Michaelsen. 2003. Comparison of functional immune responses in humans after intranasal and intramuscular immunisations with outer membrane vesicle vaccines against group B meningococcal disease. Vaccine 212042-2051. [DOI] [PubMed] [Google Scholar]
- 3.Ala'Aldeen, D. A., K. R. Neal, K. Ait-Tahar, J. S. Nguyen-Van-Tam, A. English, T. J. Falla, P. M. Hawkey, and R. C. Slack. 2000. Dynamics of meningococcal long-term carriage among university students and their implications for mass vaccination. J. Clin. Microbiol. 382311-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjune, G., E. A. Hoiby, J. K. Gronnesby, O. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A. K. Lindbak, H. Nøkleby, and E. Rosenqvist. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 3381093-1096. [DOI] [PubMed] [Google Scholar]
- 5.Boutriau, D., J. Poolman, R. Borrow, J. Findlow, J. D. Domingo, J. Puig-Barbera, J. M. Baldó, V. Planelles, A. Jubert, J. Colomer, A. Gil, K. Levie, A. D. Kervyn, V. Weynants, F. Dominguez, R. Barberá, and F. Sotolongo. 2007. Immunogenicity and safety of three doses of a bivalent (B:4: 1.19,15 and B:4: 1.7-2,4) meningococcal outer membrane vesicle vaccine in healthy adolescents. Clin. Vaccine Immunol. 1465-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cartwright, K., R. Morris, H. Rümke, A. Fox, R. Borrow, N. Begg, P. Richmond, and J. Poolman. 1999. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (PorA) outer membrane proteins. Vaccine 172612-2619. [DOI] [PubMed] [Google Scholar]
- 7.de Kleijn, E., L. van Eijndhoven, C. Vermont, B. Kuipers, H. van Dijken, H. Rümke, R. de Groot, L. van Alphen, and G. van den Dobbelsteen. 2001. Serum bactericidal activity and isotype distribution of antibodies in toddlers and schoolchildren after vaccination with RIVM hexavalent PorA vesicle vaccine. Vaccine 20352-358. [DOI] [PubMed] [Google Scholar]
- 8.de Moraes, J. C., B. A. Perkins, M. C. Camargo, N. T. Hidalgo, H. A. Barbosa, C. T. Sacchi, I. M. Landgraf, V. L. Gattas, and H. Vasconcelos. 1992. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet 3401074-1078. [DOI] [PubMed] [Google Scholar]
- 9.Feiring, B., J. Fuglesang, P. Oster, L. M. Naess, O. S. Helland, S. Tilman, E. Rosenqvist, M. A. Bergsaker, H. Nøkleby, and I. S. Aaberge. 2006. Persisting immune responses indicating long-term protection after booster dose with meningococcal group B outer membrane vesicle vaccine. Clin. Vaccine Immunol. 13790-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Findlow, J., S. Taylor, A. Aase, R. Horton, R. Heyderman, J. Southern, N. Andrews, R. Barchha, E. Harrison, A. Lowe, E. Boxer, C. Heaton, P. Balmer, E. Kaczmarski, P. Oster, A. Gorringe, R. Borrow, and E. Miller. 2006. Comparison and correlation of Neisseria meningitidis serogroup B immunologic assay results and human antibody responses following three doses of the Norwegian meningococcal outer membrane vesicle vaccine MenBvac. Infect. Immun. 744557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finney, M., T. Vaughan, S. Taylor, M. J. Hudson, C. Pratt, J. X. Wheeler, C. Vipond, I. Feavers, C. Jones, J. Findlow, R. Borrow, and A. Gorringe. 2008. Characterization of the key antigenic components and pre-clinical immune responses to a meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Hum. Vaccin. 423-30. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher, L. D., L. Bernfield, V. Barniak, J. E. Farley, A. Howell, M. Knauf, P. Ooi, R. P. Smith, P. Weise, M. Wetherell, X. Xie, R. Zagursky, Y. Zhang, and G. W. Zlotnick. 2004. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect. Immun. 722088-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galloway, Y., P. Stehr-Green, A. McNicholas, and J. O'Hallahan. 2009. Use of an observational cohort study to estimate the effectiveness of the New Zealand group B meningococcal vaccine in children aged under 5 years. Int. J. Epidemiol. 38413-418. [DOI] [PubMed] [Google Scholar]
- 14.Giuliani, M. M., J. Adu-Bobie, M. Comanducci, B. Aricò, S. Savino, L. Santini, B. Brunelli, S. Bambini, A. Biolchi, B. Capecchi, E. Cartocci, L. Ciucchi, F. Di Marcello, F. Ferlicca, B. Galli, E. Luzzi, V. Masignani, D. Serruto, D. Veggi, M. Contorni, M. Morandi, A. Bartalesi, V. Cinotti, D. Mannucci, F. Titta, E. Ovidi, J. A. Welsch, D. Granoff, R. Rappuoli, and M. Pizza. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. USA 10310834-10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold, R., I. Goldschneider, M. L. Lepow, T. F. Draper, and M. Randolph. 1978. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J. Infect. Dis. 137112-121. [DOI] [PubMed] [Google Scholar]
- 16.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 1291327-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorringe, A., D. Halliwell, M. Matheson, K. Reddin, M. Finney, and M. Hudson. 2005. The development of a meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Vaccine 232210-2213. [DOI] [PubMed] [Google Scholar]
- 18.Gorringe, A. R. 2005. Can Neisseria lactamica antigens provide an effective vaccine to prevent meningococcal disease? Expert Rev. Vaccines 4373-379. [DOI] [PubMed] [Google Scholar]
- 19.Gray, S. J., C. L. Trotter, M. E. Ramsay, M. Guiver, A. J. Fox, R. Borrow, R. H. Mallard, and E. B. Kaczmarski. 2006. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J. Med. Microbiol. 55887-896. [DOI] [PubMed] [Google Scholar]
- 20.Holst, J., B. Feiring, J. E. Fuglesang, E. A. Høiby, H. Nøkleby, I. S. Aaberge, and E. Rosenqvist. 2003. Serum bactericidal activity correlates with the vaccine efficacy of outer membrane vesicle vaccines against Neisseria meningitidis serogroup B disease. Vaccine 21734-737. [DOI] [PubMed] [Google Scholar]
- 21.Koeberling, O., A. Seubert, and D. M. Granoff. 2008. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H-binding protein and genetically attenuated endotoxin. J. Infect. Dis. 198262-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, X., L. M. Wetzler, and P. Massari. 2008. The PorB porin from commensal Neisseria lactamica induces Th1 and Th2 immune responses to ovalbumin in mice and is a potential immune adjuvant. Vaccine 26786-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milagres, L. G., S. R. Ramos, C. T. Sacchi, C. E. Melles, V. S. Vieira, H. Sato, G. S. Brito, J. C. Moraes, and C. E. Frasch. 1994. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect. Immun. 624419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nøkleby, H., P. Aavitsland, J. O'Hallahan, B. Feiring, S. Tilman, and P. Oster. 2007. Safety review: two outer membrane vesicle (OMV) vaccines against systemic Neisseria meningitidis serogroup B disease. Vaccine 253080-3084. [DOI] [PubMed] [Google Scholar]
- 25.O'Hallahan, J., D. Lennon, P. Oster, R. Lane, S. Reid, K. Mulholland, J. Stewart, L. Penney, T. Percival, and D. Martin. 2005. From secondary prevention to primary prevention: a unique strategy that gives hope to a country ravaged by meningococcal disease. Vaccine 232197-2201. [DOI] [PubMed] [Google Scholar]
- 26.Oliver, K. J., K. M. Reddin, P. Bracegirdle, M. J. Hudson, R. Borrow, I. M. Feavers, A. Robinson, K. Cartwright, and A. R. Gorringe. 2002. Neisseria lactamica protects against experimental meningococcal infection. Infect. Immun. 703621-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oster, P., D. Lennon, J. O'Hallahan, K. Mulholland, S. Reid, and D. Martin. 2005. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 232191-2196. [DOI] [PubMed] [Google Scholar]
- 28.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Aricò, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 2871816-1820. [DOI] [PubMed] [Google Scholar]
- 29.Plested, J. S., and D. M. Granoff. 2008. Vaccine-induced opsonophagocytic immunity to Neisseria meningitidis group B. Clin. Vaccine Immunol. 15799-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross, S. C., P. J. Rosenthal, H. M. Berberich, and P. Densen. 1987. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement-deficient individuals. J. Infect. Dis. 1551266-1275. [DOI] [PubMed] [Google Scholar]
- 31.Sandbu, S., B. Feiring, P. Oster, O. S. Helland, H. S. Bakke, L. M. Naess, A. Aase, I. S. Aaberge, A. C. Kristoffersen, K. M. Rydland, S. Tilman, H. Nøkleby, and E. Rosenqvist. 2007. Immunogenicity and safety of a combination of two serogroup B meningococcal outer membrane vesicle vaccines. Clin. Vaccine Immunol. 141062-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sardiñas, G., K. Reddin, R, Pajon, and A. Gorringe. 2006. Outer membrane vesicles of Neisseria lactamica as a potential mucosal adjuvant. Vaccine 24206-214. [DOI] [PubMed] [Google Scholar]
- 33.Sierra, G. V., H. C. Campa, N. M. Varcacel, I. L. Garcia, P. L. Izquierdo, P. F. Sotolongo, G. V. Casanueva, C. O. Rico, C. R. Rodriguez, and M. H. Terry. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14195-207. [PubMed] [Google Scholar]
- 34.Sim, R. J., M. M. Harrison, E. R. Moxon, and C. M. Tang. 2000. Underestimation of meningococci in tonsillar tissue by nasopharyngeal swabbing. Lancet 3561653-1654. [DOI] [PubMed] [Google Scholar]
- 35.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Høiby, J. Holst, H. Nøkleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 2811520-1527. [DOI] [PubMed] [Google Scholar]
- 36.Toropainen, M., L. Saarinen, G. Vidarsson, and H. Käyhty. 2006. Protection by meningococcal outer membrane protein PorA-specific antibodies and a serogroup B capsular polysaccharide-specific antibody in complement-sufficient and C6-deficient infant rats. Infect. Immun. 742803-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welsch, J. A., and D. Granoff. 2004. Naturally acquired passive protective activity against Neisseria meningitidis group C in the absence of serum bactericidal activity. Infect. Immun. 725903-5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welsch, J. A., G. R. Moe, R. Rossi, J. Adu-Bobie, R. Rappuoli, and D. M. Granoff. 2003. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J. Infect. Dis. 1881730-1740. [DOI] [PubMed] [Google Scholar]
- 39.Welsch, J. A., R. Rossi, M. Comanducci, and D. M. Granoff. 2004. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J. Immunol. 1725606-5615. [DOI] [PubMed] [Google Scholar]