Abstract
To identify the Toll-like receptor 2 ligand critically involved in infections with gram-positive bacteria, lipoprotein lipase (LPL) or hydrogen peroxide (H2O2) is often used to selectively inactivate lipoproteins, and hydrofluoric acid (HF) or platelet-activating factor-acetylhydrolase (PAF-AH) is used to selectively inactivate lipoteichoic acid (LTA). However, the specificities of these chemical reactions are unknown. We investigated the reaction specificities by using two synthetic lipoproteins (Pam3CSK4 and FSL-1) and LTAs from pneumococci and staphylococci. Changes in the structures of the two synthetic proteins and the LTAs were monitored by mass spectrometry, and biological activity changes were evaluated by measuring tumor necrosis factor alpha production by mouse macrophage cells (RAW 264.7) following stimulation. PAF-AH inactivated LTA without reducing the biological activities of Pam3CSK4 and FSL-1. Mass spectroscopy confirmed that PAF-AH monodeacylated pneumococcal LTA but did not alter the structure of either Pam3CSK4 or FSL-1. As expected, HF treatment reduced the biological activity of LTA by more than 80% and degraded LTA. HF treatment not only deacylated Pam3CSK4 and FSL-1 but also reduced the activities of the lipoproteins by more than 60%. Treatment with LPL decreased the biological activities by more than 80%. LPL also removed an acyl chain from the LTA and reduced its activity. Our results indicate that treatment with 1% H2O2 for 6 h at 37°C inactivates Pam3CSK4, FSL-1, and LTA by more than 80%. Although HF, LPL, and H2O2 treatments degrade and inactivate both lipopeptides and LTA, PAF-AH selectively inactivated LTA with no effect on the biological and structural properties of the two lipopeptides. Also, the ability of PAF-AH to reduce the inflammatory activities of cell wall extracts from gram-positive bacteria suggests LTA to be essential in inflammatory responses to gram-positive bacteria.
Bacterial sepsis is a leading cause of death within intensive care units (43). Although bacterial sepsis was traditionally associated with gram-negative (Gr−) bacteria, recently, the prevalence of sepsis caused by gram-positive (Gr+) bacteria has rapidly increased (2, 3, 38). In fact, in 2000, Gr+ bacteria accounted for 52% of sepsis cases whereas Gr− bacteria accounted for only 37.6% (7, 31, 38). In bacterial sepsis, the innate immune system provides both the initial immune responses and the early inflammatory responses (1, 8, 12). Early responses to infections with Gr+ and Gr− bacteria have been shown in previous studies to involve different cytokine profiles (9, 16, 25, 51, 54). Other studies have found that infections with Gr− bacteria activate Toll-like receptor 4 (TLR4) primarily with lipopolysaccharide (LPS), a membrane component of Gr− bacteria (26, 27, 44, 53). In contrast, infections with Gr+ bacteria involve TLR2, but the nature of the key TLR2 ligand is still controversial (34, 52, 56).
Two components of the cell walls of Gr+ bacteria have been proposed to be TLR2 ligands. One group of studies suggests that lipoteichoic acid (LTA) is the key ligand (10, 46, 49, 57). LTA is a polyphosphate attached to the cell membrane via a diacyl glycolipid and is an abundant component of the envelopes of Gr+ bacteria (47). Highly purified LTA, as well as its synthetic analogs, has been shown to trigger TLR2-mediated inflammatory responses (10, 15, 20, 35). However, the biological role of the LTA is unclear because it is difficult to purify natural LTA without introducing contaminants or damaging the structure of the LTA (41). Another group proposes bacterial lipoproteins as the critical ligand (22). Lipoproteins are a functionally diverse class of bacterial membrane proteins characterized by an N-terminal lipid moiety (4) and are TLR2 ligands (22-24). Although synthetic analogs of lipoproteins were found to be potent TLR2 ligands (5, 6, 42), natural lipoproteins are difficult to purify, and their properties are poorly understood.
To avoid the technical difficulties involved in purification, a different investigational approach was developed. This approach uses methods to selectively inactivate either LTA or lipoproteins in bacterial culture supernatants or crude bacterial cell wall extracts (22-24, 49). LTA inactivation is usually performed with hydrofluoric acid (HF) or platelet-activating factor-acetylhydrolase (PAF-AH) (23, 48, 49), which, respectively, hydrolyzes the phosphodiester bonds in the LTA or deacylates one of its acyl chains (17, 28, 36, 55). Lipoprotein inactivation is commonly achieved by deacylation with a lipoprotein lipase (LPL) or by oxidation with hydrogen peroxide (H2O2) (22, 24, 62). Despite their wide use, the reaction selectivities of these methods have not been evaluated. Thus, we investigated the reaction specificities of these methods by studying the impacts of these four reactions on the biological properties as well as the chemical structures of LTA and lipoprotein analogs.
MATERIALS AND METHODS
Reagents.
Recombinant human plasma PAF-AH was kindly provided by ICOS Corporation (Bothell, WA). Catalase was obtained from Worthington (Lakewood, NJ). Pefabloc SC (a serine protease inhibitor), 48% HF, 30% hydrogen peroxide (H2O2), and LPL (EC 3.1.1.34) from a Pseudomonas species were purchased from Sigma-Aldrich (St. Louis, MO). LPS of E. coli O55:B5 was purchased from Sigma-Aldrich, and impurities were removed by further purification, as described previously (26). A synthetic diacylated lipopeptide (FSL-1) and a synthetic triacylated lipopeptide (Pam3CSK4) were obtained from InvivoGen (San Diego, CA).
Purification of LTA.
Staphylococcal and pneumococcal LTAs were prepared using an organic solvent extraction and octyl-Sepharose chromatography method, as described previously (13, 33, 40). Staphylococcal LTA was further purified using a DEAE Sepharose chromatography method, as described previously (10, 40).
Bacterial strains and generation of supernatants and crude extracts.
Streptococcus pneumoniae (strain TIGR4) and Staphylococcus aureus (strain ATCC 6538) were obtained from the American Type Culture Collection (ATCC; Manassas, VA). A nonencapsulated pneumococcal strain (TIGR4JS) was kindly provided by Susan K. Hollingshead (University of Alabama at Birmingham). Bacteria were cultured in 60 ml of a chemically defined medium (58) to mid-log phase (optical density at 600 nm, 0.3 to 0.4) and pelleted by centrifugation, and the supernatants were filter sterilized with a 0.22-μm membrane filter and then lyophilized. The pellets were resuspended with 20 ml of 0.05 M sodium acetate buffer (pH 4.7) and sonicated. Twenty milliliters of n-butanol was added to each cell wall extract, and the resulting mixtures were stirred for 30 min at 4°C. The mixtures were then centrifuged to separate the aqueous phase from the butanol and cell debris. The aqueous phase was dialyzed at 4°C and lyophilized.
Inactivation methods.
Lyophilized, purified LTA (250 μg), lipopeptides (Pam3CSK4 and FSL-1 [10 μg each]), LPS (10 μg), the bacterial culture supernatants, and the butanol extracts from bacteria were incubated with 100 μl of 48% HF at 4°C for 24 h, 1% H2O2 in 1× phosphate-buffered saline (PBS) at 37°C for 6 h, 50 μg/ml LPL in 1× PBS at 37°C for 12 h, or 30 μg/ml PAF-AH at 37°C for 3 to 12 h. After incubation, the HF was removed from the reaction mixture by lyophilization and the mixture was resuspended with the original volume of 1× PBS. The H2O2 reaction was stopped by adding 1,000 U of catalase (at 37°C for 1 h), and the mixture was lyophilized. The PAF-AH in the reaction mixture was inactivated by adding 100 μM Pefabloc SC, as reported previously (48).
Cells and culture conditions.
The mouse macrophage cell line RAW 264.7 (ATCC TIB-71) was obtained from the ATCC (Manassas, VA) and was cultured in Dulbecco's modified Eagle's medium (Cellgro Mediatech, Herndon, VA) supplemented with 10% defined fetal bovine serum (HyClone, Logan, UT), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified incubator with 5% CO2.
In vitro stimulation and analysis of TNF-α production.
RAW 264.7 cells (2 × 105 cells/well) were cultured in 96-well plates (Costar, Corning, NY) and incubated with stimulants for 24 h. Supernatants were harvested, and the amount of mouse tumor necrosis factor alpha (TNF-α) in the culture supernatant was determined with a commercially available sandwich-type enzyme-linked immunosorbent assay using the protocol of the manufacturer (eBioscience, San Diego, CA).
Data analysis.
All of the experiments in this study were conducted at least three times. The data shown below are representative results. Experimental values are expressed as means ± standard deviations. The statistical significance of a difference between two means was evaluated with a one-way analysis of variance (ANOVA) test.
RESULTS
Biochemical effects of LPL treatment.
Since LPL can deacylate mono-, diacyl-, or triacylglycerols (45, 60, 63) (Fig. 1), LPL is often used to inactivate bacterial lipoproteins in culture supernatants or crude extracts from Gr+ bacteria (22, 24). Since we found previously that PAF-AH, a phospholipase, can inactivate LTA (48, 49), we considered that LPL may deacylate and inactivate LTA as well. To compare the reaction specificities of LPL and PAF-AH, synthetic lipopeptides (Pam3CSK4 and FSL-1) and pneumococcal LTA were incubated with LPL (50 μg/ml) or PAF-AH (30 μg/ml) at 37°C for 12 h, as reported previously (22, 48). The reaction products were then analyzed by electrospray (ES) mass spectrometry or by matrix-assisted laser desorption ionization-time of flight mass spectrometry.
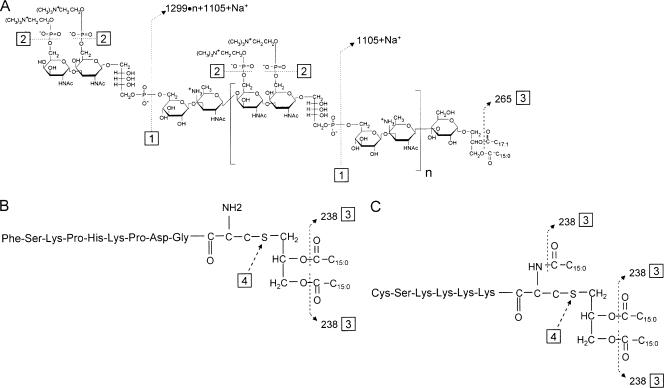
FIG. 1.
Molecular models of pneumococcal LTA (A) and the two synthetic lipopeptides, FSL-1 (B) and Pam3CSK4 (C). Dashed lines (numbered 1 to 4) indicate the sites of chemical reactions, which are described in the text. Numbers next to the arrowheads of the dashed lines indicate the loss of mass (in AMU) associated with the chemical degradation.
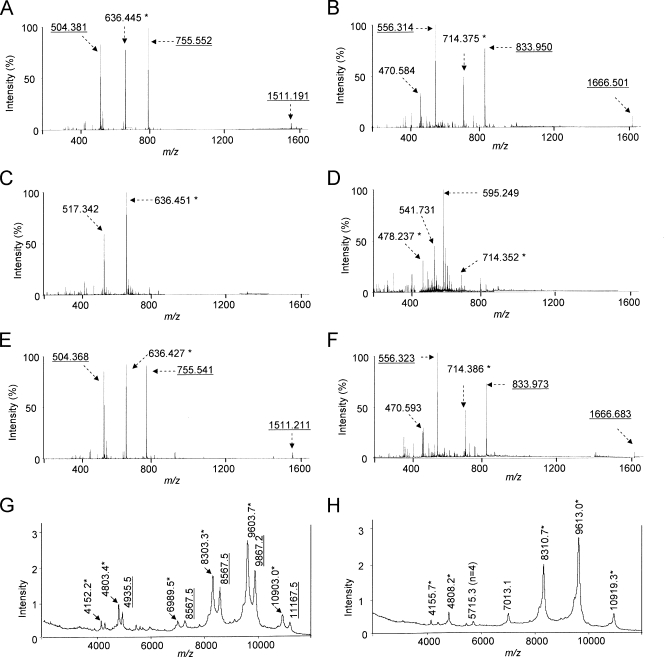
ES mass spectrometry analysis of intact Pam3CSK4 (molecular weight [MW] = 1,509.2) showed peaks at m/z 1,511.191 (M + H+), 755.552 (M + 2H+), and 504.381 (M + 3H+) (Fig. 2A), which corresponded to Pam3CSK4 with one, two, and three positive charges. As most ions have two or three charges, the peak at m/z 1,511.191 was small. Also, this preparation of Pam3CSK4 has a prominent peak at m/z 636.451 (M + 2H+), which corresponds to Pam3CSK4 that has lost one acyl chain. After LPL treatment (Fig. 2C), the three peaks at m/z 1,511.191, 755.552, and 504.381 (those representing intact Pam3CKS4) became very small while the m/z 636.451 peak became dominant and a new peak appeared at m/z 517.342 (M + 2H+) (Fig. 2C). The new peak corresponded to Pam3CSK4 that had lost two acyl chains (site 3 in Fig. 1B). These results clearly demonstrate that LPL treatment degrades Pam3CSK4.
FIG. 2.
Mass spectra of intact Pam3CSK4 (A), intact FSL-1 (B), Pam3CSK4 after LPL treatment (C), FSL-1 after LPL treatment (D), Pam3CSK4 after PAF-AH treatment (E), FSL-1 after PAF-AH treatment (F), LPL-treated pneumococcal LTA (G), and PAF-AH-treated LTA (H). For enzyme treatments, lipoproteins and LTA were incubated with LPL (50 μg/ml) for 12 h at 37°C or with PAF-AH (30 μg/ml) for 12 h at 37°C. The underlined numbers indicate AMU of the intact Pam3CSK4, intact FSL-1, or intact LTA with one, two, or three charges. The numbers with an asterisk indicate AMU of Pam3CSK4, FSL-1, or LTA that has undergone monodeacylation. The number in parentheses in panel H indicates the number of repeating units in the LTA molecule.
When intact FSL-1 (MW = 1,665.0) was examined by mass spectroscopy (Fig. 2B), it showed two prominent peaks at m/z 833.950 (M + 2H+) and 556.314 (M + 3H+), which correspond to FSL-1 with two and three charges. A very small peak at m/z 1,666.501 (M + H+) corresponds to FSL-1 with one charge. A large peak at m/z 714.375 (M + 2H+) corresponds to monoacyl FSL-1. The peak at m/z 470.584 was not identified. After LPL treatment (Fig. 2D), the three peaks representing intact FSL-1 became unrecognizable, but a new peak at m/z 595.249 became prominent. This peak corresponds to fully deacylated FSL-1. Peaks at m/z 714.352 (M + 2H+) and 478.237 (M + 3H+) represent monoacyl FSL-1 with two and three charges. Thus, LPL can degrade FSL-1 by removing acyl chains. In contrast to LPL treatment, PAF-AH treatment had no effect on the structure of Pam3CKS4 (Fig. 2E) or FSL-1 (Fig. 2F), even though the lipopeptides were treated with PAF-AH for more than 12 h. Thus, LPL readily removed one or two acyl chains from both lipopeptides, whereas PAF-AH had no effect on these lipoproteins.
After 24 h of LPL treatment, pneumococcal LTA showed minor peaks at m/z 8,567.5, 9,867.2, and 1,1167.5 and major peaks at m/z 8,303.3, 9,603.7, and 1,0903.0 (Fig. 2G). The minor peaks represent intact pneumococcal LTA (with six, seven, and eight repeating units), and the major peaks represent pneumococcal LTA with only one acyl chain since the loss of one acyl chain reduces the mass of LTA by 264 to 266 atomic mass units (AMU) (47). Minor peaks at m/z 4,935.5, 4,803.4, and 4,152.2 represent LTA ions with double charges (Fig. 2G). In contrast, following 3 h of incubation with PAF-AH, the PAF-AH-treated LTA showed peaks at only m/z 8,310.7, 9,613.0, and 10,919.3 (Fig. 2H). These results demonstrate that LPL treatment converted the majority of pneumococcal LTA to monoacyl LTA (33), although LPL was not as efficient as PAF-AH. Thus, LPL degraded both LTA and lipopeptides, whereas PAF-AH deacylated LTA but not lipopeptides.
Biochemical effects of HF treatment.
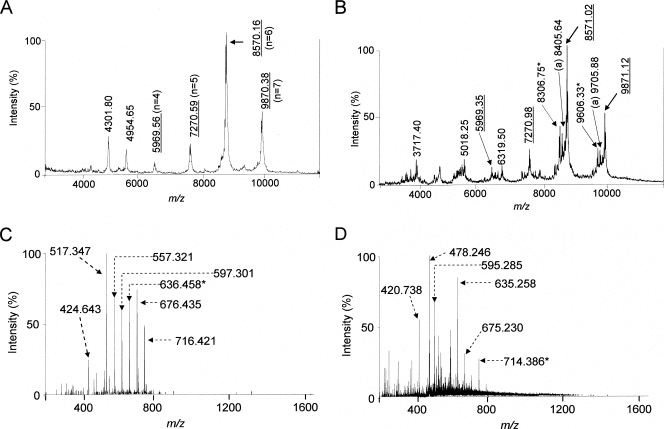
HF is considered to inactivate LTA but not lipoproteins (23, 62) because HF preferentially hydrolyzes phosphodiester bonds (17, 28, 36, 47, 55) (sites 1 and 3 in Fig. 1A). However, we observed previously that HF can also hydrolyze acyl linkages of pneumococcal LTA (47) and have confirmed this in the present study. The matrix-assisted laser desorption ionization-time of flight mass spectrum of intact pneumococcal LTA shows three major peaks at approximately m/z 7,270.6, 8,570.2, and 9,870.4, which correspond to LTA with five, six, and seven repeating units (47) (Fig. 3A). Following a relatively short hydrolysis (3 h) of pneumococcal LTA with 48% HF at 4°C, new peaks appeared (Fig. 3B). The peaks at m/z 3,717.4, 5,018.3, and 6,319.5 (Fig. 3B) correspond to the LTA fragments resulting from the breakage of the phosphodiester bonds between the repeating units (site 1 in Fig. 1A). Peaks at m/z 8,405.64 and 9,705.88 (Fig. 3B), which show reductions equivalent to about 165 AMU compared to the peaks for intact LTA, correspond to LTA that has lost one phosphocholine residue, which has 165 AMU (site 2 in Fig. 1A). The peaks at m/z 8,306.8 and 9,606.3 in Fig. 3B, which show reductions equivalent to 264 AMU compared to the peaks for intact LTA, represent the LTA fragments that lost one acyl chain (site 3 in Fig. 1A).
FIG. 3.
Mass spectra of intact pneumococcal LTA (A), HF-treated LTA (B), HF-treated Pam3CSK4 (C), and HF-treated FSL-1 (D). LTA was treated with 48% HF at 4°C for only 3 h. Lipoproteins were treated with HF for 24 h. The underlined numbers indicate AMU of intact LTA, and the numbers in parentheses in panel A indicate the number of repeating units in the LTA molecule. Numbers labeled “a” indicate AMU of the LTA that lost one phosphocholine group. Numbers labeled “b” or “★” indicate AMU of LTA or lipoproteins that lost one acyl chain.
To determine if HF can deacylate and/or degrade lipoproteins, two synthetic lipopeptides (Pam3CSK4 and FSL-1) were treated with 48% HF for 24 h, with the reaction products being analyzed by ES mass spectrometry. Prior to the HF reaction, Pam3CSK4 (MW = 1,509.6) had peaks at m/z 1,511.139 (M + H+), 755.548 (M + 2H+), 504.039 (M + 3H+), and 636.446 (M + 2H+) (Fig. 2A). The mass spectrum of HF-treated Pam3CSK4 did not show these three peaks. Instead, it showed many new peaks representing ions with lower MWs. Two large peaks at m/z 636.458 (M + 2H+) and 517.347 (M + 2H+) correspond to Pam3CSK4 with two acyl chains and one acyl chain (Fig. 3C). Although we did not fully identify other new peaks, such as those at m/z 716.421 (M + 2H+) and 676.435 (M + 2H+), the mass spectrometry analysis clearly showed that the HF treatment degraded Pam3CKS4 (Fig. 3C).
The mass spectrum of the lipopeptide FSL-1 had peaks at m/z 1,666.501 (M + H+), 833.950 (M + 2H+), and 556.313 (M + 3H+), which correspond to FSL-1 with one, two, and three charges (Fig. 2C). Also, another peak at m/z 714.375 (M + 2H+) corresponds to monoacylated FSL-1 with two charges, which is normally found among FSL-1 preparations like Pam3CSK4. After the HF reaction, the three peaks corresponding to the intact FSL-1 disappeared completely but many new peaks appeared. The peaks at m/z 714.386 (M + 2H+) and 595.285 (M + 2H+) correspond to monoacylated and deacylated FSL-1 (Fig. 3D). Although we did not identify other new peaks (e.g., peaks at m/z 635.258, 478.246, and 420.738), our data clearly show that HF treatment degrades FSL-1 (Fig. 3D).
Biological effects of various treatments.
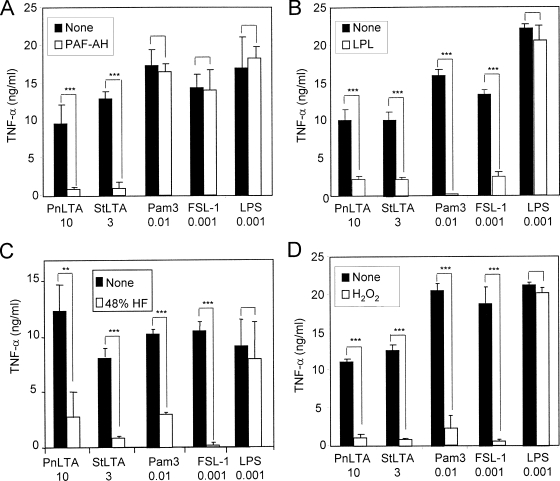
After studying the effects of chemical treatments on the molecular structures of the stimulants, we directly investigated the effects of chemical treatments on the biological properties of the stimulants. We treated the four stimulants (i.e., two LTA preparations, FSL-1, and Pam3CSK4) with one of the four treatment agents (PAF-AH, LPL, HF, and H2O2), stimulated RAW 264.7 cells with either untreated or treated stimulants, and measured the levels of TNF-α production. The PAF-AH treatment reduced the activities of Pam3CSK4, FSL-1, and LPS by less than 10%, but it reduced the inflammatory activities of both LTAs by over 85% (Fig. 4A). Thus, PAF-AH specifically inactivates LTA, but not LPS or lipoproteins, as described previously (48, 49). These findings also support the contention that our LTA preparations are clean and not contaminated with LPS or lipoproteins.
FIG. 4.
TNF-α production by RAW 264.7 cells in response to stimulation by pneumococcal LTA (PnLTA), staphylococcal LTA (StLTA), Pam3CSK4 (Pam3), FSL-1, or LPS before (black bars) and after (white bars) treatment with 30 μg/ml of PAF-AH for 3 h at 37°C, 50 μg/ml of LPL for 12 h at 37°C, 48% HF for 24 h at 4°C, or 1% H2O2 for 6 h at 37°C. Numbers at the bottom indicate the concentrations (in micrograms per milliliter) of stimulants used in the experiments. Bars indicate the means of results for triplicate wells in a representative experiment. Error bars indicate standard deviations. Statistical significance was calculated using one-way ANOVA: **, P < 0.01; ***, P < 0.001; no asterisks, P > 0.05.
In contrast to PAF-AH treatment, LPL treatment reduced the inflammatory potencies of both LTAs by more than 70%, that of Pam3CSK4 almost completely (by over 95%), and that of FSL-1 by about 70% (Fig. 4B), while the treatment did not affect the activity of LPS (Fig. 4B). Similarly, HF treatment reduced the biological potencies of both LTAs and of the two lipopeptides by more than 80% (Fig. 4C). HF-treated LPS retained almost all of its inflammatory activities (Fig. 4C). In addition to these inactivation methods, we also tested a newly developed lipoprotein inactivation method, H2O2 treatment, which oxidates lipoproteins to make them inactive. H2O2 treatment reduced the inflammatory properties of both lipopeptides by over 80% (Fig. 4D), which is in agreement with previous findings (62). It also reduced the bioactivities of both LTA preparations (Fig. 4D). Thus, LPL, HF, and H2O2 treatments, unlike PAF-AH treatment, inactivated both LTAs and lipoproteins.
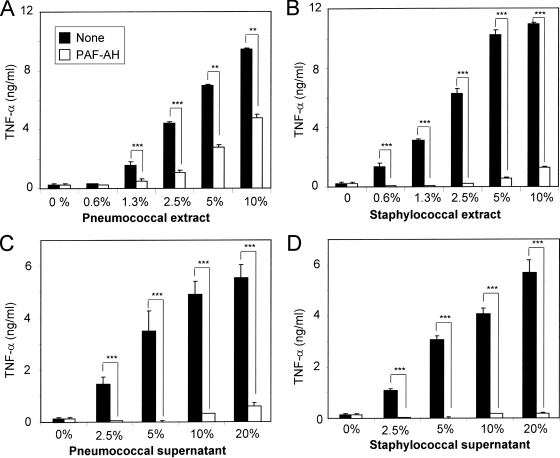
LTA is a critical PAMP in the culture supernatant and crude extract.
Cell wall extracts with prepared butanol are widely used to purify LTA (13, 14, 33, 40, 46). Recent studies have shown that the active pathogen-associated molecular patterns (PAMPs) in crude butanol extract are lipoproteins rather than LTA (22, 23). Since PAF-AH is found to selectively inactivate LTA but not lipoproteins, we used it to investigate the nature of PAMPs in the crude butanol extracts. As expected, untreated supernatants and extracts dramatically increased TNF-α production (Fig. 5) and PAF-AH treatment inactivated the early culture supernatants from S. pneumoniae and S. aureus by more than 95% (Fig. 5A and B) (49). Following the PAF-AH treatment, the crude butanol extract from S. aureus lost more than 90% of its potency, whereas the pneumococcal extract lost only 50% of its potency (Fig. 5D and C). While our findings do not exclude the presence of factors that may synergistically enhance the potency of LTA in the extracts, they do indicate that LTA is a critically necessary PAMP in the extracts of these two Gr+ bacteria. In the case of pneumococci, there may be another critical PAMP in addition to LTA.
FIG. 5.
TNF-α production by RAW 264.7 cells in response to the butanol extracts (A and B) or the culture supernatants (C and D) from S. pneumoniae (A and C) and S. aureus (B and D). The amounts of the extracts or culture supernatants used for stimulation are indicated as percentages along the x axis. The stimulants were treated with nothing (black bars) or with 30 μg/ml of PAF-AH (white bars). White bars with 0% stimulants represent data for cells stimulated with PAF-AH alone. Bars indicate the means of results for triplicate wells in a representative experiment. Error bars indicate standard deviations. Statistical significance was calculated using one-way ANOVA: **, P < 0.01; ***, P < 0.001; no asterisks, P > 0.05.
DISCUSSION
Chemical treatments of bacteria with HF, H2O2, LPL, or PAF-AH are often used to inactivate PAMPs (22-24, 48, 62). HF and PAF-AH methods were introduced to inactivate LTA, and LPL and H2O2 have been used to inactivate lipoproteins. Despite the wide usage of these methods (22-24, 62), the reaction specificities have not previously been systematically evaluated. Therefore, in the present study, we assessed the impacts of the chemical reactions on two bacterial lipoproteins and two LTAs by analyzing the chemical structures and biological activities (TNF-α production) of the reaction products. We provide evidence that PAF-AH selectively and efficiently inactivates LTA without inactivating lipoproteins (Fig. 2E, 2F, and 4A) and that the other three inactivation agents are nonspecific and render both lipoproteins and LTAs inactive (Fig. 2, 3, and 4). Studies of nitric oxide production provided results equivalent to TNF-α results (data not shown).
Our present study is limited since we could not directly investigate lipoproteins isolated from Gr+ bacteria but used model lipoproteins instead. Nevertheless, FSL-1 is a synthetic lipopeptide representing an actual mycoplasma lipoprotein (molecular mass, 44 kDa) (50). Also, Pam3CSK4 has been extensively used for studies of its interaction with TLR, including crystallographic studies (29). Another limitation is that we evaluated the specificities of the reaction conditions that are currently used. One may find in the future that a new reaction condition (e.g., a new buffer, temperature, or pH) provides the desired reaction specificity. Nevertheless, our findings emphasize the need to evaluate the specificities of the inactivation methods, especially by analyzing molecular structures. Although our present study does not show the biochemical effect of H2O2 on LTAs, H2O2 treatment dramatically reduced the inflammatory potencies of both LTAs (Fig. 4D). Thus, the H2O2 method cannot be used to selectively inactivate lipoproteins from Gr+ bacteria either. Furthermore, our findings strongly suggest that previous conclusions based on the presumed specificities of the reactions must be reexamined.
In addition to these chemical inactivation methods, evidence from genetic studies supports the contention that lipoproteins are the dominant TLR2 ligand in infections with Gr+ bacteria (21, 23, 24, 37). The genetic studies are based on the observations made with the bacteria lacking the gene for lipoprotein diacylglycerol transferase (lgt), which is the critical enzyme for producing lipoproteins. The lgt-deficient bacteria express no lipoproteins on their membranes and induce much less inflammatory activity than do wild-type bacteria (18, 24, 37). However, inasmuch as lipoproteins may be involved in LTA biosynthesis, the lgt-deficient bacteria may produce LTA with an abnormal structure and subnormal biological potency. Thus, one should examine the structure of LTA from lgt-deficient Gr+ bacteria to be certain that inactive LTA is not produced by lgt-deficient bacteria.
Although our study does not exclude the presence of unusual inflammatory agents in select bacterial fractions, we provide evidence suggesting that LTA is a critically necessary PAMP in unfractionated extracts from Gr+ bacteria. Nevertheless, the potency of purified LTA is significantly less than that of the purified LPS. We have previously reported that other bacterial factors may enhance the inflammatory potency of LTA. It is well known that LTA can synergize with peptidoglycan (19) and with muramyl dipeptide (32), which are both known to stimulate nucleotide-binding oligomerization domain-containing receptors. Furthermore, it has been shown previously that LTA (unlike LPS) can induce septic shock in animal models only in the presence of peptidoglycan (30). Also, the results of a recent study suggested a synergistic factor independent of nucleotide-binding oligomerization domain-containing receptors (24). Another study showed that lipoproteins and glycosphingolipids have the ability to enhance LTA-mediated inflammation by some unknown mechanism (39). The mechanism may involve altered presentation of LTA molecules since the presentation of LTA molecules has been shown to be important (11). Thus, in actual infections with Gr+ bacteria, several additional bacterial molecules may synergize with LTA to greatly increase its inflammatory potential.
Because of the increasing prevalence of sepsis caused by Gr+ bacteria, there is a great need for a new therapeutic approach for treatment. For instance, antibodies to LTA are currently being investigated as a basis for the treatment of infections with Gr+ bacteria (61). Others have proposed sulfonylamide compounds as therapeutic agents, since sulfonylamide may selectively inactivate LTA without inactivating lipoproteins (59). These new approaches may be less fruitful if LTA is not critically involved in infections with Gr+ bacteria. In the present study, we demonstrate that LTA is critically necessary for the butanol extracts to be inflammatory. This finding is consistent with the results from studies of synthetic LTA analogs (10) and extends our previous results regarding the critical importance of LTA in bacterial culture supernatants (49). The findings from these studies, taken together, provide additional evidence that LTA inactivation may be a valid therapeutic approach.
Acknowledgments
This work was supported by Public Health Service grants AI-69695 and AI-30021 from the National Institutes of Health.
We thank ICOS Corporation of Bothell, WA, for providing us with PAF-AH. We thank Sue Michalek for her careful reading and advice.
Footnotes
Published ahead of print on 24 June 2009.
REFERENCES
- 1.Akira, S. 2009. Innate immunity to pathogens: diversity in receptors for microbial recognition. Immunol. Rev. 2275-8. [DOI] [PubMed] [Google Scholar]
- 2.Angus, D. C., W. T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M. R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 291303-1310. [DOI] [PubMed] [Google Scholar]
- 3.Angus, D. C., and R. S. Wax. 2001. Epidemiology of sepsis: an update. Crit. Care Med. 29S109-S116. [DOI] [PubMed] [Google Scholar]
- 4.Babu, M. M., M. L. Priya, A. T. Selvan, M. Madera, J. Gough, L. Aravind, and K. Sankaran. 2006. A database of bacterial lipoproteins (DOLOP) with functional assignments to predicted lipoproteins. J. Bacteriol. 1882761-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buwitt-Beckmann, U., H. Heine, K. H. Wiesmuller, G. Jung, R. Brock, S. Akira, and A. J. Ulmer. 2006. TLR1- and TLR6-independent recognition of bacterial lipopeptides. J. Biol. Chem. 2819049-9057. [DOI] [PubMed] [Google Scholar]
- 6.Buwitt-Beckmann, U., H. Heine, K. H. Wiesmuller, G. Jung, R. Brock, S. Akira, and A. J. Ulmer. 2005. Toll-like receptor 6-independent signaling by diacylated lipopeptides. Eur. J. Immunol. 35282-289. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, J., and E. Abraham. 1999. Microbiologic findings and correlations with serum tumor necrosis factor-alpha in patients with severe sepsis and septic shock. J. Infect. Dis. 180116-121. [DOI] [PubMed] [Google Scholar]
- 8.Creagh, E. M., and L. A. O'Neill. 2006. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 27352-357. [DOI] [PubMed] [Google Scholar]
- 9.Cui, W., D. C. Morrison, and R. Silverstein. 2000. Differential tumor necrosis factor alpha expression and release from peritoneal mouse macrophages in vitro in response to proliferating gram-positive versus gram-negative bacteria. Infect. Immun. 684422-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deininger, S., A. Stadelmaier, S. von Aulock, S. Morath, R. R. Schmidt, and T. Hartung. 2003. Definition of structural prerequisites for lipoteichoic acid-inducible cytokine induction by synthetic derivatives. J. Immunol. 1704134-4138. [DOI] [PubMed] [Google Scholar]
- 11.Deininger, S., S. Traub, D. Aichele, T. Rupp, T. Baris, H. M. Moller, T. Hartung, and S. von Aulock. 2008. Presentation of lipoteichoic acid potentiates its inflammatory activity. Immunobiology 213519-529. [DOI] [PubMed] [Google Scholar]
- 12.Doherty, T. M., and M. Arditi. 2005. Innate immunity, Toll-like receptors and host response to infection. Pediatr. Infect. Dis. J. 24643-644. [DOI] [PubMed] [Google Scholar]
- 13.Draing, C., M. Pfitzenmaier, S. Zummo, G. Mancuso, A. Geyer, T. Hartung, and S. von Aulock. 2006. Comparison of lipoteichoic acid from different serotypes of Streptococcus pneumoniae. J. Biol. Chem. 28133849-33859. [DOI] [PubMed] [Google Scholar]
- 14.Draing, C., S. Sigel, S. Deininger, S. Traub, R. Munke, C. Mayer, L. Hareng, T. Hartung, S. von Aulock, and C. Hermann. 2008. Cytokine induction by Gram-positive bacteria. Immunobiology 213285-296. [DOI] [PubMed] [Google Scholar]
- 15.Ellingsen, E., S. Morath, T. Flo, A. Schromm, T. Hartung, C. Thiemermann, T. Espevik, D. Golenbock, D. Foster, R. Solberg, A. Aasen, and J. Wang. 2002. Induction of cytokine production in human T cells and monocytes by highly purified lipoteichoic acid: involvement of Toll-like receptors and CD14. Med. Sci. Monit. 8BR149-BR156. [PubMed] [Google Scholar]
- 16.Feezor, R. J., C. Oberholzer, H. V. Baker, D. Novick, M. Rubinstein, L. L. Moldawer, J. Pribble, S. Souza, C. A. Dinarello, W. Ertel, and A. Oberholzer. 2003. Molecular characterization of the acute inflammatory response to infections with gram-negative versus gram-positive bacteria. Infect. Immun. 715803-5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer, W. 1987. ‘Lipoteichoic acid’ of Bifidobacterium bifidum subspecies pennsylvanicum DSM 20239. A lipoglycan with monoglycerophosphate side chains. Eur. J. Biochem. 165639-646. [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto, Y., S. Inamura, A. Kawasaki, Z. Shiokawa, A. Shimoyama, T. Hashimoto, S. Kusumoto, and K. Fukase. 2007. IEIIS Meeting minireview: chemical synthesis of peptidoglycan fragments for elucidation of the immunostimulating mechanism. J. Endotoxin Res. 13189-196. [DOI] [PubMed] [Google Scholar]
- 19.Girardin, S. E., I. G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D. J. Philpott, and P. J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2788869-8872. [DOI] [PubMed] [Google Scholar]
- 20.Han, S. H., J. H. Kim, M. Martin, S. M. Michalek, and M. H. Nahm. 2003. Pneumococcal lipoteichoic acid (LTA) is not as potent as staphylococcal LTA in stimulating Toll-like receptor 2. Infect. Immun. 715541-5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto, M., M. Furuyashiki, R. Kaseya, Y. Fukada, M. Akimaru, K. Aoyama, T. Okuno, T. Tamura, T. Kirikae, F. Kirikae, N. Eiraku, H. Morioka, Y. Fujimoto, K. Fukase, K. Takashige, Y. Moriya, S. Kusumoto, and Y. Suda. 2007. Evidence of immunostimulating lipoprotein existing in the natural lipoteichoic acid fraction. Infect. Immun. 751926-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto, M., K. Tawaratsumida, H. Kariya, K. Aoyama, T. Tamura, and Y. Suda. 2005. Lipoprotein is a predominant Toll-like receptor 2 ligand in Staphylococcus aureus cell wall components. Int. Immunol. 18355-362. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto, M., K. Tawaratsumida, H. Kariya, A. Kiyohara, Y. Suda, F. Krikae, T. Kirikae, and F. Gotz. 2006. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J. Immunol. 1773162-3169. [DOI] [PubMed] [Google Scholar]
- 24.Henneke, P., S. Dramsi, G. Mancuso, K. Chraibi, E. Pellegrini, C. Theilacker, J. Hubner, S. Santos-Sierra, G. Teti, D. T. Golenbock, C. Poyart, and P. Trieu-Cuot. 2008. Lipoproteins are critical TLR2 activating toxins in group B streptococcal sepsis. J. Immunol. 1806149-6158. [DOI] [PubMed] [Google Scholar]
- 25.Hessle, C., B. Andersson, and A. E. Wold. 2000. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infect. Immun. 683581-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J. Immunol. 165618-622. [DOI] [PubMed] [Google Scholar]
- 27.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1623749-3752. [PubMed] [Google Scholar]
- 28.Jennings, H. J., C. Lugowski, and N. M. Young. 1980. Structure of the complex polysaccharide C-substance from Streptococcus pneumoniae type 1. Biochemistry 194712-4719. [DOI] [PubMed] [Google Scholar]
- 29.Jin, M. S., S. E. Kim, J. Y. Heo, M. E. Lee, H. M. Kim, S. G. Paik, H. Lee, and J. O. Lee. 2007. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 1301071-1082. [DOI] [PubMed] [Google Scholar]
- 30.Kengatharan, K. M., S. De Kimpe, C. Robson, S. J. Foster, and C. Thiemermann. 1998. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J. Exp. Med. 188305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kieft, H., A. I. Hoepelman, W. Zhou, M. Rozenberg-Arska, A. Struyvenberg, and J. Verhoef. 1993. The sepsis syndrome in a Dutch university hospital. Clinical observations. Arch. Intern. Med. 1532241-2247. [PubMed] [Google Scholar]
- 32.Kim, H. J., J. S. Yang, S. S. Woo, S. K. Kim, C. H. Yun, K. K. Kim, and S. H. Han. 2007. Lipoteichoic acid and muramyl dipeptide synergistically induce maturation of human dendritic cells and concurrent expression of proinflammatory cytokines. J. Leukoc. Biol. 81983-989. [DOI] [PubMed] [Google Scholar]
- 33.Kim, J. H., H. Seo, S. H. Han, J. Lin, M. K. Park, U. B. Sorensen, and M. H. Nahm. 2005. Monoacyl lipoteichoic acid from pneumococci stimulates human cells but not mouse cells. Infect. Immun. 73834-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirschning, C., and R. Schumann. 2002. TLR2: cellular sensor for microbial and endogenous molecular patterns. Curr. Top. Microbiol. Immunol. 270121-144. [DOI] [PubMed] [Google Scholar]
- 35.Lotz, S., E. Aga, I. Wilde, G. Van Zandbergen, T. Hartung, W. Solbach, and T. Laskay. 2004. Highly purified lipoteichoic acid activates neutrophil granulocytes and delays their spontaneous apoptosis via CD14 and TLR2. J. Leukoc. Biol. 75467-477. [DOI] [PubMed] [Google Scholar]
- 36.Lovett, E. G., and D. Lipkin. 1977. Base-catalyzed reactions of 1,3-disubstituted uracils. J. Org. Chem. 422574-2580. [DOI] [PubMed] [Google Scholar]
- 37.Machata, S., S. Tchatalbachev, W. Mohamed, L. Jansch, T. Hain, and T. Chakraborty. 2008. Lipoproteins of Listeria monocytogenes are critical for virulence and TLR2-mediated immune activation. J. Immunol. 1812028-2035. [DOI] [PubMed] [Google Scholar]
- 38.Martin, G. S., D. M. Mannino, S. Eaton, and M. Moss. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 3481546-1554. [DOI] [PubMed] [Google Scholar]
- 39.Meron-Sudai, S., A. Matityahou, Y. Keisari, K. H. Cox, D. L. Hasty, and I. Ofek. 2008. Lipoteichoic acid synergizes with glycosphingolipids to potently stimulate secretion of interleukin-6 from human blood cells. Clin. Vaccine Immunol. 151309-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morath, S., A. Geyer, and T. Hartung. 2001. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 193393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morath, S., A. Geyer, I. Spreitzer, C. Hermann, and T. Hartung. 2002. Structural decomposition and heterogeneity of commercial lipoteichoic acid preparations. Infect. Immun. 70938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakao, Y., K. Funami, S. Kikkawa, M. Taniguchi, M. Nishiguchi, Y. Fukumori, T. Seya, and M. Matsumoto. 2005. Surface-expressed TLR6 participates in the recognition of diacylated lipopeptide and peptidoglycan in human cells. J. Immunol. 1741566-1573. [DOI] [PubMed] [Google Scholar]
- 43.Parrillo, J. E., M. M. Parker, C. Natanson, A. F. Suffredini, R. L. Danner, R. E. Cunnion, and F. P. Ognibene. 1990. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann. Intern. Med. 113227-242. [DOI] [PubMed] [Google Scholar]
- 44.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 2822085-2088. [DOI] [PubMed] [Google Scholar]
- 45.Preiss-Landl, K., R. Zimmermann, G. Hammerle, and R. Zechner. 2002. Lipoprotein lipase: the regulation of tissue specific expression and its role in lipid and energy metabolism. Curr. Opin. Lipidol. 13471-481. [DOI] [PubMed] [Google Scholar]
- 46.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 27815587-15594. [DOI] [PubMed] [Google Scholar]
- 47.Seo, H. S., R. T. Cartee, D. G. Pritchard, and M. H. Nahm. 2008. A new model of pneumococcal lipoteichoic acid structure resolves biochemical, biosynthetic, and serologic inconsistencies of the current model. J. Bacteriol. 1902379-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seo, H. S., J. H. Kim, and M. H. Nahm. 2006. Platelet-activating factor-acetylhydrolase can monodeacylate and inactivate lipoteichoic acid. Clin. Vaccine Immunol. 13452-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seo, H. S., S. M. Michalek, and M. H. Nahm. 2008. Lipoteichoic acid is important in innate immune responses to gram-positive bacteria. Infect. Immun. 76206-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shibata, K., A. Hasebe, T. Into, M. Yamada, and T. Watanabe. 2000. The N-terminal lipopeptide of a 44-kDa membrane-bound lipoprotein of Mycoplasma salivarium is responsible for the expression of intercellular adhesion molecule-1 on the cell surface of normal human gingival fibroblasts. J. Immunol. 1656538-6544. [DOI] [PubMed] [Google Scholar]
- 51.Sriskandan, S., and J. Cohen. 1999. Gram-positive sepsis. Mechanisms and differences from gram-negative sepsis. Infect. Dis. Clin. N. Am. 13397-412. [DOI] [PubMed] [Google Scholar]
- 52.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11443-451. [DOI] [PubMed] [Google Scholar]
- 53.Tapping, R. I., S. Akashi, K. Miyake, P. J. Godowski, and P. S. Tobias. 2000. Toll-like receptor 4, but not Toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J. Immunol. 1655780-5787. [DOI] [PubMed] [Google Scholar]
- 54.Tavares, E., R. Maldonado, M. L. Ojeda, and F. J. Minano. 2005. Circulating inflammatory mediators during start of fever in differential diagnosis of gram-negative and gram-positive infections in leukopenic rats. Clin. Diagn. Lab. Immunol. 121085-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toon, P., P. E. Brown, and J. Baddiley. 1972. The lipid-teichoic acid complex in the cytoplasmic membrane of Streptococcus faecalis N.C.I.B. 8191. Biochem. J. 127399-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Triantafilou, M., F. G. Gamper, R. M. Haston, M. A. Mouratis, S. Morath, T. Hartung, and K. Triantafilou. 2006. Membrane sorting of Toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J. Biol. Chem. 28131002-31011. [DOI] [PubMed] [Google Scholar]
- 57.Triantafilou, M., M. Manukyan, A. Mackie, S. Morath, T. Hartung, H. Heine, and K. Triantafilou. 2004. Lipoteichoic acid and Toll-like receptor 2 internalization and targeting to the Golgi is lipid raft dependent. J. Biol. Chem. 27940882-40889. [DOI] [PubMed] [Google Scholar]
- 58.van de Rijn, I., and R. E. Kessler. 1980. Growth characteristics of group A streptococci in a new chemically defined medium. Infect. Immun. 27444-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warshakoon, H. J., M. R. Burns, and S. A. David. 2009. Structure-activity relationships of antimicrobial and lipoteichoic acid-sequestering properties in polyamine sulfonamides. Antimicrob. Agents Chemother. 5357-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weinstock, P. H., S. Levak-Frank, L. C. Hudgins, H. Radner, J. M. Friedman, R. Zechner, and J. L. Breslow. 1997. Lipoprotein lipase controls fatty acid entry into adipose tissue, but fat mass is preserved by endogenous synthesis in mice deficient in adipose tissue lipoprotein lipase. Proc. Natl. Acad. Sci. USA 9410261-10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weisman, L. E. 2007. Antibody for the prevention of neonatal nosocomial staphylococcal infection: a review of the literature. Arch. Pediatr. 14(Suppl. 1)S31-S34. [DOI] [PubMed] [Google Scholar]
- 62.Zahringer, U., B. Lindner, S. Inamura, H. Heine, and C. Alexander. 2008. TLR2—promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology 213205-224. [DOI] [PubMed] [Google Scholar]
- 63.Zechner, R. 1997. The tissue-specific expression of lipoprotein lipase: implications for energy and lipoprotein metabolism. Curr. Opin. Lipidol. 877-88. [DOI] [PubMed] [Google Scholar]