Abstract
Emergency vaccination as part of the control strategies against foot-and-mouth disease virus (FMDV) has the potential to limit virus spread and reduce large-scale culling. To reduce the time between vaccination and the onset of immunity, immunostimulatory CpG was tested for its capacity to promote early protection against FMDV challenge in pigs. To this end, CpG 2142, an efficient inducer of alpha interferon, was injected intramuscularly. Increased transcription of Mx1, OAS, and IRF-7 was identified as a sensitive measurement of CpG-induced innate immunity, with increased levels detectable to at least 4 days after injection of CpG formulated with Emulsigen. Despite this, CpG combined with an FMD vaccine did not promote protection. Pigs vaccinated 2 days before challenge had disease development, which was at least as acute as that of unvaccinated controls. All pigs vaccinated 7 days before challenge were protected without a noticeable effect of CpG. In summary, our results demonstrate the caution required when translating findings from mouse models to natural hosts of FMDV.
Foot-and-mouth disease (FMD) represents one of the most economically important diseases of cloven-hoofed livestock. Its causative agent, FMD virus (FMDV), belongs to the Picornaviridae family and is a member of the genus Aphthovirus, together with equine rhinitis A virus. Infection with FMDV occurs commonly via the respiratory tract following contact or inhalation of airborne virus, with the major site of primary replication being the mucosal epithelia of the nasopharynx.
In Europe, the current control strategy of an FMD outbreak relies on a nonvaccination policy. In the case of an epidemic, the primary actions against the spread of the disease have been culling of the affected herds, including preemptive slaughtering in a control zone, together with strict control of animal movement. Such drastic measures resulted in the slaughter of 6.5 million animals during the European outbreak in 2001. As a consequence, a strong desire to reduce reliance on large-scale culling of animals to control future outbreaks of FMD has developed, and one of the control measures being considered is the application of emergency vaccination. However, due to the exceptional rapidity of virus spread during FMD outbreaks, one important element for improving current vaccines is the promotion of an early induction of protection.
FMDV infection elicits a rapid humoral response, followed by clearance of the opsonized virus complexes by phagocytic cells (20). Protection against FMDV correlates with the induction of high levels of neutralizing antibodies in serum, already detectable after 3 to 4 days postinfection (8). However, trials using high-dose conventional vaccines based on inactivated FMDV in cattle (9), pigs (26), and sheep (6) have shown that animals can be protected as early as 4 days postvaccination, in the absence of significant levels of neutralizing antibodies in the serum. This indicates that mechanisms other than specific antibodies were involved. For the current conventional high-potency vaccines, it has been proposed that early protection could be attributed to induction of a combination of innate and early adaptive immune responses. In the peripheral blood compartment, such vaccines induced proinflammatory cytokines, such as interleukin-6 (IL-6), IL-8, and IL-12, and increased the migratory and chemotactic activities of leukocytes, but no direct antiviral activity was found (3, 4, 25). The latter was achieved using an adenovirus-based vaccine, which also included an adenovirus-derived vector for expression of alpha interferon (IFN-α). With this vaccine, pigs were protected against challenge infection as early as 1 day postvaccination (7, 11).
Altogether, the results uphold the principle that targeting innate immune responses represents a promising strategy to develop improved emergency vaccines capable of rapidly establishing a protected status. Based on such ideas, IFN inducers have been tested for their capacity to promote innate protection against FMDV. Such an approach found some success in mouse models using polyinosinic:poly(C) (24) and CpG (16). Stimulation of innate immunity with CpG has also been shown to protect against a number of other bacterial and viral infections, including herpesviruses (13), orthopoxvirus (23), influenza virus (10), and birnavirus (15) in mouse models.
Based on this knowledge, the aim of the present study was to test the suitability of immunostimulatory CpG ODN (CpG) applied together with a conventional FMD vaccine in a challenge model using a natural host of FMDV, the pig. In order to evaluate this strategy, we optimized the delivery of CpG to pigs in terms of rapid induction of IFN type I-responsive genes and tested the capacity of CpG to improve current inactivated FMD vaccines toward inducing early protection against a severe FMDV challenge infection by contact.
MATERIALS AND METHODS
Virus preparation and vaccine formulation.
The FMDV type O UK/2001 isolate was grown in baby hamster kidney 21 (BHK-21) cell monolayers as described previously (19). Briefly, BHK-21 cells in serum-free Glasgow modified Eagle medium (Invitrogen) supplemented with 2 mM l-glutamine and 7.5% (wt/vol) bicarbonate were infected at a multiplicity of infection of 0.001 50% tissue culture infective dose (TCID50) per cell and incubated for 24 h at 37°C with 6% (vol/vol) CO2 until a cytopathic effect (CPE) was observed by light microscopy. Cells were harvested, sonicated twice for 10 s, and clarified at 3,000 × g for 30 min at 4°C. Virus-containing supernatant was stored at −70°C, and the virus titer was calculated from thawed virus stock by titration on BHK-21 cells. The vaccine O1 Manisa was formulated in a double oil emulsion (DOE; Merial Pirbright, United Kingdom) and used at 2 ml per animal. The CpG ODN was formulated in Emulsigen (MVP Laboratories, Inc., Omaha, NE), Montanide ISA 206 (Seppic, France), or DOE (Merial, Pirbright, United Kingdom).
Determination of CpG efficacy in vitro and in vivo.
Swiss White Landrace pigs, kept under specific pathogen-free conditions at the institute, were used as blood donors for the in vitro testing of the CpG ODN. The ODN 2142 with the sequence TCGCGTGCGTTTTGTCGTTTTGACGTT (Merial, France) was compared with the previously characterized ODN 2216 (Invivogen) (12) in terms of its ability to induce IFN-α in porcine CD172a-enriched plasmacytoid dendritic cells (pDC) prepared as previously described (12). In order to determine CpG efficacy in vivo, intramuscular injections of four animals of 3 months of age per group were performed with CpG (0.5 or 2 mg/ml in a total volume of 2 ml) formulated either in phosphate-buffered saline (PBS) (control animals), Montanide ISA 206 (Seppic, France), or Emulsigen (MVP Laboratories, Inc., Omaha, NE). As readouts, either serum cytokine for IFN-α and IL-6 or the levels of IFN-responsive genes detected using real-time PCR were determined. In order to evaluate the immunostimulatory properties of DOE formulations in vivo, CpG formulated in either DOE or Emulsigen was compared by real-time PCR in terms of OAS, IRF7, and Mx1 mRNA upregulation. To do so, three animals per group (DOE alone, CpG formulated in DOE, and CpG formulated in Emulsigen) were injected at a rate of 0.5 mg CpG ODN 2142 formulated in a total volume of 2 ml, and total RNA of PBMCs was harvested 24, 48, and 72 h postinjection.
FMD animal experimentation.
Swiss White Landrace pigs were kept under specific pathogen-free conditions at the institute and used at 3 months of age. In order to test the vaccines, a challenge infection of vaccinated pigs was performed, followed by clinical scoring and virus detection and isolation. A total of six groups with five animals in each group (except group 6, with only four animals) were used. Group 1 was not vaccinated, group 2 was vaccinated with a DOE-FMD vaccine 2 days before challenge, group 3 was vaccinated with a DOE-FMD vaccine and CpG in Emulsigen 2 days before challenge, group 4 was vaccinated with a DOE-FMD vaccine 7 days before challenge, group 5 was vaccinated with a DOE-FMD vaccine and CpG in Emulsigen 7 days before challenge, and group 6 was vaccinated 21 days before challenge with conventional DOE type O1 Manisa vaccine. The vaccination was done by intramuscular injection with 2 ml of DOE type O1 Manisa vaccine and, where indicated, with 2 ml of CpG formulated in Emulsigen 5 cm apart from the vaccine injection. The rationale of this was to avoid any disturbance by the Emulsigen of the DOE FMD antigen formulation optimized for a rapid induction of antibody responses. The animals were then placed into contact for 2 h with three FMDV-infected “disease-generator” pigs. The latter were infected with FMDV O UK/2001 at 105 TCID50/animal 2 days before the contact and showed clinical signs of FMD, such as vesicles on all four feet. All animals were sampled daily for blood and examined for disease symptoms and fever. A clinical score was determined by the addition of points distributed as follows: (i) an elevated body temperature of 40°C (score of 1), >40.5 (score of 2), or >41 (score of 3); (ii) reduced appetite (1 point) or no food intake and food left over from the day before (2 points); (ii) lameness (1 point) or reluctance to stand (2 points); (iv) presence of heat and pain after palpation of the coronary band (1 point) or not standing on the affected foot (2 points); (v) vesicles on the feet, dependent on the number of feet affected and with a maximum of 4 points; and (vi) visible mouth lesions on the tongue (1 point), gums or lips (1 point), or snout (1 point), with a maximum of 3 points. For reasons of animal welfare, pigs with generalized FMD, open lesions on several feet, and severe lameness were sacrificed.
PBMC preparation.
Peripheral blood mononuclear cells (PBMCs) were obtained by density gradient centrifugation over Ficoll-Paque (1.077 g/liter; Amersham Pharmacia Biotech) as described previously (2).
Cytokine ELISAs.
Quantification of IFN-α in serum samples by enzyme-linked immunosorbent assay (ELISA) was as described elsewhere (12), using anti-pig IFN-α monoclonal antibody K9 (R&D Systems, Minneapolis, MN) as capture antibody and biotinylated anti-pig IFN-α monoclonal antibody F17 (kindly donated by Bernard Charley, INRA, Jouy-en-Josas, France) as a detection antibody. IL-6 levels in serum samples were quantified by ELISA using the DuoSet kit from R&D Systems (Minneapolis, MN).
Quantification of IFN-responsive genes.
To determine changes in IFN-responsive gene transcripts in PBMCs, total RNA was prepared using the RNeasy mini kit (Qiagen, Hombrechtikon, Switzerland), followed by treatment with RNase-free DNase I (Ambion, Huntingdon, United Kingdom). The reverse transcription-PCR (RT-PCR) measurements were performed using the ABI Prism 7700 sequence detector system (Applied Biosystems, Foster City, CA). The relative expression of each mRNA was calculated by the threshold cycle (ΔCT) method, and the amount of target mRNA relative to 18S mRNA was expressed as 2−ΔCT (18). Data are presented as the ratio of target mRNA to 18S mRNA. The following primers and probes were employed: OAS forward primer (5′-GCGCCGAGGAGAATTCATC-3′), OAS reverse primer (5′-TGGACCTCAAACGTCACTTTAAAC-3′), OAS probe (5′-CTCTTTGACAGGCTTCCAGCTGTCTCC-3′), IRF7 forward primer (5′-CTGCGATGGCTGGATGAA-3′), IRF7 reverse primer (5′-TAAAGATGCGCGAGTCGGA-3′), IRF7 probe (5′-CCGCGTGCCCTGGAAGCACTT-3′), Mx1 forward primer (5′-CAGCACCTGATTGCCTACCA-3′), Mx1 reverse primer (5′-GGTCCGGAGGATGAAGAACTG-3′), Mx1 probe (5′-AAGCGCATCTCCAGCCACATCCCT-3′), 18S forward primer (5′-CGCCGCTAGAGGTGAAATTC-3′), 18S reverse primer (5′-GGCAAATGCTTTCGCTCTG-3′), and 18S probe (5′-TGGACCGGCGCAAGACGGA-3′).
Quantification of FMDV RNA in serum.
To isolate FMDV RNA from serum samples, the RNeasy mini kit was used (Qiagen) according to the manufacturer's protocol. The RT-PCR measurements were performed using the ABI Prism 7700 sequence detector system (Applied Biosystems, Foster City, CA). The relative expression of each mRNA was calculated by the ΔCT method. Data are relative to a FMDV-positive serum sample provided by our diagnostic department. The following were the primers and probe used: FMDV forward primer (5′-CACTGGTGACAGGCTAAGG-3′), FMDV reverse primer (5′-CCCTTCTCAGATTCCGAGT-3′), and FMDV probe (5′-TGCCCTTCAGGTACCCCGAGGTAACA-3′). The real-time PCR data were confirmed by a cell culture methodology. Briefly, 1:10-diluted serum samples from infected animals were added to monolayers of BHK-21 cells in serum-free Glasgow minimal Eagle medium (Invitrogen) supplemented with 2 mM l-glutamine and 7.5% (wt/vol) bicarbonate. Subsequently, the cells were incubated for 6 days at 37°C and 6% (vol/vol) CO2 and the CPE was analyzed by light microscopy.
Quantification of neutralizing antibodies.
Neutralizing antibodies against FMDV in serum samples were detected by using a serum neutralization test. Briefly, serum samples were heat inactivated for 30 min at 56°C and clarified by centrifugation. Twofold-dilution series were performed in serum-free Glasgow minimal essential medium (Invitrogen) containing 1 U/ml penicillin, 1 mg/ml streptomycin, and 625 ng/ml amphotericin B (all from Invitrogen) and incubated with 200 TCID50/100 μl FMDV O UK/2001 for 1 h at 37°C. In replicates of four, the virus-serum suspension was then inoculated on a monolayer of BHK-21 cells in microtiter plates and scored for CPE 4 days postinoculation. Based on nonspecific virus neutralization with serum from naive animals, the cutoff was defined as a titer of 10 TCID50/ml.
Statistical analysis.
The statistical significance of differences in the clinical scores between two groups was calculated by using the Mann-Whitney rank sum test using the SigmaStat software.
RESULTS
Establishment of vaccine formulation and a readout for CpG efficacy.
For this study, we selected CpG 2142, which induces both strong IFN-α responses from pDC and B-cell activation in terms of B-cell proliferation and CD80/86 upregulation (V. Juillard et al., unpublished results). When CpG 2142 was compared with the previously characterized type A CpG 2216 (12) for its capacity to activate enriched pDC, it induced similar levels of IFN-α. With cell preparations from three different pigs, CpG 2142 induced an average of 13,007 ± 3,335 U/ml and CpG 2216 induced an average of 14,034 ± 3,774 U/ml IFN-α. Based on these characteristics and the higher stability of CpG 2142 due to its phosphorothioate backbone, we selected this ODN for the in vivo studies.
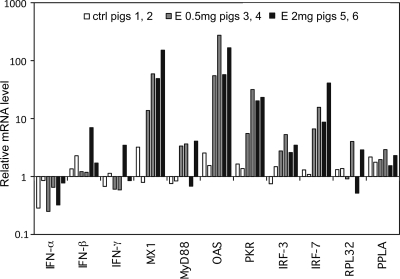
In order to evaluate vaccine formulation and determine CpG efficacy in vivo in pigs, intramuscular injections of CpG (2 or 0.5 mg/ml in a total volume of 2 ml) formulated in PBS (control animals), Montanide ISA 206, or Emulsigen were performed. As a readout, IFN-α and IL-6 serum levels were analyzed based on the known capacity of the CpG to induce these cytokines in porcine pDC (27). Due to the known difficulty of detecting serum cytokines after parenteral injection (17), the levels of IFN-responsive genes were also analyzed using real-time PCR. While we were not able to detect IFN-α or IL-6 proteins or transcripts at 8, 24, 48, or 72 h postinjection (data not shown), it was possible to detect an upregulation of IFN-responsive gene transcripts with a five- to 70-fold increase in mRNA levels compared to that of PBS controls. The data shown in Fig. 1 demonstrate that CpG in Emulsigen efficiently stimulated some of the IFN response genes while formulation in PBS was inefficient. The most sensitive genes were for Mx1, OAS, IRF-7, and PRKRA, which were significantly upregulated at 24 h postinjection (Fig. 1) with both a high (2 mg) and a low (0.5 mg) dose of CpG. Similar mRNA profiles were observed at 8 h postinjection (data not shown). Based on the observation that no significant differences were found between the two doses of CpG, the 0.5-mg dose was selected for the remaining experiments. We have also observed that CpG formulated in Emulsigen was more potent at inducing IFN-responsive genes than a formulation in Montanide (data not shown).
FIG. 1.
Induction of IFNs and IFN type I-responsive genes by CpG formulated in Emulsigen. PBMCs were injected intramuscularly with CpG ODN 2142 formulated in Emulsigen at 0.5 or 2 mg per animal, or not injected into control animals (ctrl), and data were collected at 24 h postinjection. After total RNA was harvested, IFNs (α, β, and γ), and mRNA levels of IFN-responsive genes (Mx1, MyD88, OAS, PKR, IRF-3, IRF-7, RPL32, and PPLA) were quantified by real-time PCR. The mRNA levels were normalized to 18S mRNA levels and are expressed as fold induction over the samples obtained before injection. The bars represent individual pigs (two for each group).
Since the selected FMD vaccine to be used was formulated in a DOE proprietary to Merial, it was decided to also compare the effects of CpG in the DOE FMD vaccine or in Emulsigen (without FMD antigen) at a dose of 0.5 mg per animal using Mx1, OAS, and IRF-7 mRNA levels as the most sensitive readouts for the efficacy of innate antiviral response induced by the different vaccine formulations. Increased expressions of these genes were found 24 h postinjection and were still detectable at 72 h depending on the gene and the animal tested (Fig. 2). A comparison of the DOE vaccine with CpG formulated in DOE or in Emulsigen demonstrated that the latter was more efficient at inducing OAS and Mx1 mRNAs, although DOE CpG was as efficient in the induction of OAS (Fig. 2). Based on these results, it was decided that a separated injection of CpG in Emulsigen was more promising with respect to the induction of a wider range of antiviral genes. This decision is related to the aim of the present study, which was primarily to promote innate immune defenses. Consequently, for the vaccination challenge experiment described below, animals were vaccinated with a separate intramuscular injection of DOE vaccine, and 5 cm apart (reaching the same draining lymph node) a second injection was carried out with CpG (0.5 mg) formulated in Emulsigen.
FIG. 2.
Induction of OAS, IRF7, and Mx1 mRNAs by CpG formulated in the DOE-FMD vaccine or Emulsigen. PBMCs from pigs (n = 2) injected with the DOE-FMD (conventional commercial FMD vaccine), CpG formulated in DOE-FMD, or CpG formulated in Emulsigen (Emul-CpG) were isolated at 24, 48, and 72 h postinjection. Total RNA was harvested and quantified by real-time PCR and normalized to 18S mRNA levels. For CpG injections, 0.5 mg of ODN 2142 was formulated and injected in a total of 2 ml. The fold induction of target genes was calculated based on mRNA levels before injection. The averages from three independent experiments with standard deviation error bars are shown.
Establishing a challenge model for FMDV O Pan Asia UK/2001.
Infection of pigs in the heel pad with 105 TCID50 FMDV O Pan Asia UK/2001 resulted in the first clinical symptoms at 24 h postinfection (mild lameness) and clear FMDV symptoms with vesicles on the coronary bands of all feet at 48 h. When three unvaccinated pigs were placed in contact for 2 h with two pigs, which were infected by a needle with the dose described above, all three animals clearly got disease, with severe lameness and foot lesions within 48 to 72 h after contact (data not shown). Based on these results, this procedure was selected as a challenge model to test vaccine efficacy.
Efficacy of CpG as an immunostimulant for the improvement of early protection.
To test the selected CpG formulations described above, a challenge infection of vaccinated pigs was performed, followed by clinical scoring and virus detection and isolation. While all pigs vaccinated 21 (n = 4) and 7 (n = 5) days before challenge were protected against lesions, unvaccinated animals (n = 5) and pigs vaccinated only 2 days before challenge (n = 10) developed severe lesions, independent of the additional injection of the CpG formulation. As demonstrated in Fig. 3A, the maximum clinical scores found in unvaccinated animals (group 1) were similar to those of animals vaccinated 2 days before contact, independent of CpG application (groups 2 and 3, respectively). There was no statistical difference between these groups. In contrast, the maximum clinical scores of animals vaccinated 7 and 21 days before challenge (groups 4 to 6) differed significantly from groups 1 to 3. As mentioned above, none of the diseased animals developed lesions and all returned to a clinical score of 0 (Fig. 3B).
FIG. 3.
Maximum and end point clinical scores of infected animals. (A) Box plot showing the maximum clinical scores recorded for the individual animals. No statistical difference was shown among groups 1, 2, and 3 and among groups 4, 5, and 6. Statistically significant differences are between groups 1 and 3 and groups 4 and 6. (B) Box plot showing the endpoint clinical score recorded at 6 days postchallenge or when the pigs were slaughtered for animal welfare reasons. There was no statistical difference among groups 1, 2, and 3 and among groups 4, 5, and 6. Statistically significant differences were between groups 1 and 3 and groups 4 and 6. The different populations were compared according to the Mann-Whitney rank sum test.
In Fig. 4A and B, the development of body temperature and clinical scores is shown. In group 1, foot lesions typical of FMD were first observed 2 to 5 days postcontact, with four out of five animals developing severe FMD with lesions on all four feet; for some animals, lesions were also on the tongue, gums, lips, or snout. Such animals were slaughtered for animal welfare reasons. Surprisingly, the animals vaccinated at 2 days before challenge appeared to have a more acute disease progression, with all pigs developing severe lesions as early as 2 to 3 days after contact with challenge. Nevertheless, a statistical analysis comparing the groups 2 days postchallenge did not reveal significant differences between groups 1, 2, and 3.
FIG. 4.
Development of body temperature, clinical scores, and serum neutralizing antibody titers of infected animals. (A) Rectal body temperature was measured 0 to 6 days postchallenge in each group. (B) The clinical score was evaluated according to clinical symptoms (see Materials and Methods for a definition). A total of six groups, with five animals per group (except group 6, with only four animals) were used. Group 1 was not vaccinated, group 2 was vaccinated with a DOE-FMD vaccine 2 days before challenge, group 3 was vaccinated with DOE-FMD and CpG in Emulsigen 2 days before challenge, group 4 was vaccinated with DOE-FMD 7 days before challenge, group 5 was vaccinated with DOE-FMD and CpG in Emulsigen 7 days before challenge, and group 6 was vaccinated 21 days before challenge with a conventional DOE type O1 Manisa vaccine. Each animal among a group is depicted by an open symbol (circle, square, triangle, diamond, or cross). The line represents the median value in each group. (C) Based on nonspecific virus neutralization with sera from naive animals, the cutoff was defined at a titer of 10 TCID50/ml. A total of six groups, with five animals per group (except group 6, with only four animals) were used. Group 1 was not vaccinated, group 2 was vaccinated with a DOE-FMD vaccine 2 days before challenge, group 3 was vaccinated with a DOE-FMD and CpG in Emulsigen 2 days before challenge, group 4 was vaccinated with DOE-FMD 7 days before challenge, group 5 was vaccinated with DOE-FMD and CpG in Emulsigen 7 days before challenge, and group 6 was vaccinated 21 days before challenge with a conventional DOE type O1 Manisa vaccine. Each animal among a group is depicted by an open symbol (circle, square, triangle, diamond, or cross).
Next, we analyzed the induction of FMDV-specific neutralizing antibodies induced by the vaccination/challenge. The results presented in Fig. 4C demonstrated that while 2 unvaccinated animals (group 1) developed weak neutralizing activities at 4 and 6 days postchallenge, none of the animals vaccinated 2 days before challenge developed FMDV-neutralizing antibodies—not unexpected, since they were slaughtered for reasons of animal welfare at 2 days post-challenge infection. In contrast, three out of five animals of group 4 and four out of five animals from group 5 had serum-neutralizing activities above the threshold titer of 10 before challenge. The titers in all animals from these two groups showed a rapid increase after challenge infection with no clear difference between these groups. As expected, animals from group 6 vaccinated 21 days before challenge already possessed high titers at the time of challenge (Fig. 4C).
The presence of viral RNA in serum samples collected at different time points postinfection was quantified using real-time RT-PCR. The results are shown in Fig. 5 and demonstrated that samples from group 3 were positive for viral RNA already at 2 days postinfection, while only two out of five were positive in group 2 and only one out of five in group 1. This was surprising, considering that four out of five animals developed severe vesicular lesions on all four feet in the unvaccinated group. These results were confirmed by cell culture isolation of the challenge virus from serum samples (data not shown). Live virus was found in the sera of groups 1 and 2 in two out of five animals, corresponding to the animals with positive results determined by RT-PCR, while all sera from group 3 contained virus 2 days after challenge.
FIG. 5.
Viral RNA in serum samples following challenge infection. Viral RNA was detected using real-time PCR. A total of six groups with five animals per group (except group 6, with only four animals) were used. Group 1 was not vaccinated, group 2 was vaccinated with a DOE-FMD vaccine 2 days before challenge, group 3 was vaccinated with a DOE-FMD and CpG in Emulsigen 2 days before challenge, group 4 was vaccinated with DOE-FMD 7 days before challenge, group 5 was vaccinated with DOE-FMD and CpG in Emulsigen 7 days before challenge, and group 6 was vaccinated 21 days before challenge with a conventional DOE type O1 Manisa vaccine. The averages from three independent experiments with standard deviation error bars are shown.
DISCUSSION
Based on the promising effect of CpG in mediating early protection during the innate phase of the immune response in mouse models (16, 28), the present study has evaluated the feasibility of CpG to promote early protection against FMDV in a large animal representing a natural host of this virus. While CpGs have been applied to pigs with the aim of stimulating adaptive immune responses, their ability to stimulate innate immune responses has not been described. Consequently, we have established a CpG formulation able to induce innate antiviral responses based on IFN-responsive genes as a readout. Mx1, OAS, and IRF-7 were particularly sensitive, with elevated mRNA levels detectable 8 h to 3 days after injection. The observation that IFN-responsive genes are upregulated but not IFN-α/β mRNA can be explained by the fact that in pigs only pDC are activated to produce IFN-α by CpG, and these cells have a very low frequency in PBMCs (0.1 to 0.5%) (12). Our results indicate that the adjuvant Emulsigen is suitable for CpG formulation, giving superior activation compared to injection of CpG in PBS, a Montanide ISA 206-based formulation, or a DOE-based formulation. Although the CpG ODN 2142 used in this study is a potent inducer of IFN-α in pDC in vitro (Juillard et al., unpublished), the cytokine was not detectable in the serum at 8, 16, 24, and 48 h postinjection. This is probably a consequence of a rapid turnover of IFN-α in vivo.
With respect to the capacity of CpG to enhance early protection against FMDV, our results indicate that this goal is difficult to achieve. Vaccination of animals 2 days before challenge clearly did not have a protective effect, even with the addition of CpG as an immunostimulant. In contrast, our results indicate a negative effect on the capacity of pigs to control FMDV early postinfection, although the differences of unvaccinated animals with regards to clinical scores were not statistically significant. This was surprising considering that CpG injection induced antiviral genes still detectable 4 days postinjection. It is possible that this antiviral activity was not induced at the site of FMDV entry, which is the upper respiratory tract. For the interpretation of the present results, it is important to consider that the short incubation time and the rapid development of severe lesions in unprotected animals demonstrated that the infection model used represented a severe challenge. Also, with respect to adaptive immune responses, we were not able to identify a positive effect of CpG in terms of antibody responses in the animals vaccinated 7 days before challenge. This could be related to the high potency of the FMD vaccine used in terms of inducing rapid seroconversion and/or to the selected separate injection of CpG at a site distant from that of the FMD vaccine.
The higher viral RNA content 2 days after challenge in the sera of animals vaccinated with the CpG formulation compared to the conventional DOE vaccine indicates that CpG when applied early before challenge could even have negative effects. A possible interpretation of this surprising observation would be an inflammatory response induced by the vaccine components with a negative effect on early protection against FMDV. Previous studies have demonstrated the induction of proinflammatory cytokines and chemotactic activity in the serum by a conventional FMD vaccine (4, 25). CpG might further amplify such inflammatory responses. The inflammation at the site of vaccine entry could “distract” the innate immune system and thereby weaken its response at the site of challenge virus entry. This might also be related to the negative effects of CpG on the immune response observed after repeated administration (14).
The observation that adenoviruses expressing IFN-α protected pigs against FMDV challenge only when high levels of IFN-α were found in the serum for several days (5, 7, 21) could explain why CpG did not promote early protection against FMDV. The tested formulations of CpG did not induce systemic IFN-α detectable in the serum. Our difficulty in detecting IFN-α after CpG administration in the serum is consistent with CpG injections of humans (17). IFN-α was found in only a minority of the volunteers injected and required a high dose of CpG (80 μg/kg). Furthermore, the levels were low and IFN-α was found only transiently. A further optimization of delivery, including slow-release technologies, could have the potential to reach this goal.
In conclusion, our results indicate that the use of CpG as an immunostimulant for emergency vaccines against FMDV in pigs is not efficacious. It is important to emphasize that these results are specific to FMD in young pigs and may not be extrapolated to other species and virus diseases. FMDV is one of the most aggressive and rapidly replicating viruses. Furthermore, infected pigs excrete extremely high quantities of FMDV after infection. This poses a severe challenge for animals within the same group, making vaccination challenge experiments difficult for this species (1, 8). The general concept of targeting innate immune responses to improve vaccines has been proven effective in many models and still represents a promising approach for vaccine improvement (22).
Acknowledgments
We are very grateful to Daniel Brechbühl for helping with the animal experimentation.
This work was supported by the State Secretariat for Education and Research (grant no. 03. 0519 linked to EU project FMD-Improcon FP6 503603).
Footnotes
Published ahead of print on 24 June 2009.
REFERENCES
- 1.Alexandersen, S., and N. Mowat. 2005. Foot-and-mouth disease: host range and pathogenesis. Curr. Top. Microbiol. Immunol. 2889-42. [DOI] [PubMed] [Google Scholar]
- 2.Alves, M. P., V. Neuhaus, L. Guzylack-Piriou, N. Ruggli, K. C. McCullough, and A. Summerfield. 2007. Toll-like receptor 7 and MyD88 knockdown by lentivirus-mediated RNA interference to porcine dendritic cell subsets. Gene Ther. 14836-844. [DOI] [PubMed] [Google Scholar]
- 3.Barnard, A. L., A. Arriens, S. Cox, P. Barnett, B. Kristensen, A. Summerfield, and K. C. McCullough. 2005. Immune response characteristics following emergency vaccination of pigs against foot-and-mouth disease. Vaccine 231037-1047. [DOI] [PubMed] [Google Scholar]
- 4.Barnett, P. V., S. J. Cox, N. Aggarwal, H. Gerber, and K. C. McCullough. 2002. Further studies on the early protective responses of pigs following immunisation with high potency foot and mouth disease vaccine. Vaccine 203197-3208. [DOI] [PubMed] [Google Scholar]
- 5.Chinsangaram, J., M. P. Moraes, M. Koster, and M. J. Grubman. 2003. Novel viral disease control strategy: adenovirus expressing alpha interferon rapidly protects swine from foot-and-mouth disease. J. Virol. 771621-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox, S. J., P. V. Barnett, P. Dani, and J. S. Salt. 1999. Emergency vaccination of sheep against foot-and-mouth disease: protection against disease and reduction in contact transmission. Vaccine 171858-1868. [DOI] [PubMed] [Google Scholar]
- 7.de Avila Botton, S., M. C. Brum, E. Bautista, M. Koster, R. Weiblen, W. T. Golde, and M. J. Grubman. 2006. Immunopotentiation of a foot-and-mouth disease virus subunit vaccine by interferon alpha. Vaccine 243446-3456. [DOI] [PubMed] [Google Scholar]
- 8.Doel, T. R. 2005. Natural and vaccine induced immunity to FMD. Curr. Top. Microbiol. Immunol. 288103-131. [DOI] [PubMed] [Google Scholar]
- 9.Doel, T. R., L. Williams, and P. V. Barnett. 1994. Emergency vaccination against foot-and-mouth disease: rate of development of immunity and its implications for the carrier state. Vaccine 12592-600. [DOI] [PubMed] [Google Scholar]
- 10.Dong, L., I. Mori, M. J. Hossain, B. Liu, and Y. Kimura. 2003. An immunostimulatory oligodeoxynucleotide containing a cytidine-guanosine motif protects senescence-accelerated mice from lethal influenza virus by augmenting the T helper type 1 response. J. Gen. Virol. 841623-1628. [DOI] [PubMed] [Google Scholar]
- 11.Grubman, M. J. 2005. Development of novel strategies to control foot-and-mouth disease: marker vaccines and antivirals. Biologicals 33227-234. [DOI] [PubMed] [Google Scholar]
- 12.Guzylack-Piriou, L., C. Balmelli, K. C. McCullough, and A. Summerfield. 2004. Type-A CpG oligonucleotides activate exclusively porcine natural interferon-producing cells to secrete interferon-alpha, tumour necrosis factor-alpha and interleukin-12. Immunology 11228-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harandi, A. M., K. Eriksson, and J. Holmgren. 2003. A protective role of locally administered immunostimulatory CpG oligodeoxynucleotide in a mouse model of genital herpes infection. J. Virol. 77953-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heikenwalder, M., M. Polymenidou, T. Junt, C. Sigurdson, H. Wagner, S. Akira, R. Zinkernagel, and A. Aguzzi. 2004. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat. Med. 10187-192. [DOI] [PubMed] [Google Scholar]
- 15.Jørgensen, J. B., L.-H. Johansen, K. Steiro, and A. Johansen. 2003. CpG DNA induces protective antiviral immune responses in Atlantic salmon (Salmo salar L.). J. Virol. 7711471-11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamstrup, S., T. H. Frimann, and A. M. Barfoed. 2006. Protection of Balb/c mice against infection with FMDV by immunostimulation with CpG oligonucleotides. Antivir. Res. 7242-48. [DOI] [PubMed] [Google Scholar]
- 17.Krieg, A. M., S. M. Efler, M. Wittpoth, M. J. Al Adhami, and H. L. Davis. 2004. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J. Immunother. 27460-471. [DOI] [PubMed] [Google Scholar]
- 18.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 19.McCullough, K. C., and R. Butcher. 1982. Monoclonal antibodies against foot-and-mouth disease virus 146S and 12S particles. Arch. Virol. 741-9. [DOI] [PubMed] [Google Scholar]
- 20.McCullough, K. C., D. Parkinson, and J. R. Crowther. 1988. Opsonization-enhanced phagocytosis of foot-and-mouth disease virus. Immunology 65187-191. [PMC free article] [PubMed] [Google Scholar]
- 21.Moraes, M. P., J. Chinsangaram, M. C. Brum, and M. J. Grubman. 2003. Immediate protection of swine from foot-and-mouth disease: a combination of adenoviruses expressing interferon alpha and a foot-and-mouth disease virus subunit vaccine. Vaccine 22268-279. [DOI] [PubMed] [Google Scholar]
- 22.Pashine, A., N. M. Valiante, and J. B. Ulmer. 2005. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 11S63-S68. [DOI] [PubMed] [Google Scholar]
- 23.Rees, D. G., A. J. Gates, M. Green, L. Eastaugh, R. A. Lukaszewski, K. F. Griffin, A. M. Krieg, and R. W. Titball. 2005. CpG-DNA protects against a lethal orthopoxvirus infection in a murine model. Antivir. Res. 6587-95. [DOI] [PubMed] [Google Scholar]
- 24.Richmond, J. Y., and L. D. Hamilton. 1969. Foot-and-mouth disease virus inhibition induced in mice by synthetic double-stranded RNA (polyriboinosinic and polyribocytidylic acids). Proc. Natl. Acad. Sci. USA 6481-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rigden, R. C., C. P. Carrasco, P. V. Barnett, A. Summerfield, and K. C. McCullough. 2003. Innate immune responses following emergency vaccination against foot-and-mouth disease virus in pigs. Vaccine 211466-1477. [DOI] [PubMed] [Google Scholar]
- 26.Salt, J. S., P. V. Barnett, P. Dani, and L. Williams. 1998. Emergency vaccination of pigs against foot-and-mouth disease: protection against disease and reduction in contact transmission. Vaccine 16746-754. [DOI] [PubMed] [Google Scholar]
- 27.Vincent, I. E., C. Balmelli, B. Meehan, G. Allan, A. Summerfield, and K. C. McCullough. 2007. Silencing of natural interferon producing cell activation by porcine circovirus type 2 DNA. Immunology 12047-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vollmer, J. 2005. Progress in drug development of immunostimulatory CpG oligodeoxynucleotide ligands for TLR9. Expert Opin. Biol. Ther. 5673-682. [DOI] [PubMed] [Google Scholar]