Abstract
To determine whether Korean red ginseng (KRG) has beneficial effects on human immunodeficiency virus type 1 (HIV-1)-infected patients administered highly active antiretroviral therapy (HAART), we analyzed the CD4 T-cell count, viral load, and resistance mutations to HAART in 46 individuals. Thirteen patients harbored resistance mutations at baseline. The study population was divided into two groups: specifically, a group treated with a combination of HAART plus KRG (23 patients) and a group treated with HAART alone (23 patients). The annual increase in CD4 T-cell count in the combination group was significantly higher than that in the group treated with HAART alone (P < 0.05). Overall, 21 patients harbored resistance mutations after 3 years of therapy. Following exclusion of 13 patients displaying baseline resistance mutations, 7.1% of patients (1/14) in the combination group and 42.1% (8/19) in the HAART group were identified with resistance mutations. One patient with baseline resistance mutations in the combination group did not display resistance mutations 3 years after HAART therapy. High-level resistance mutations were significantly lower in the combination group than in the group treated with HAART alone. Five patients showed no improvement in viral copy number (26.3% [5/19]) in the combination group and 9 (45.0% [9/20]) showed no improvement in the HAART-only group. Our data support the clinical utility of KRG intake during HAART therapy.
The rates of mortality and morbidity related to human immunodeficiency virus (HIV) disease have decreased significantly following the introduction of antiretroviral therapy for HIV-1 infections (25). The current recommendation for treatment of HIV-1 infections is combination therapy with at least three antiretroviral agents in cases where patients display a CD4 T-cell count of <200 to 350/μl or become symptomatic (1). While highly active antiretroviral therapy (HAART) suppresses replication of HIV-1, its effectiveness is often limited by the emergence of antiretroviral drug-resistant mutants (28). Replication of drug-resistant HIV-1 during HAART is a major cause of treatment failure (32). Consequently, novel approaches to delay treatment failure are urgently required.
In Korea, zidovudine (AZT) monotherapy has been offered free of charge by the Korean Health Authority from 1991 onwards for HIV-1-infected patients displaying a CD4 T-cell count of <500/μl (11), followed by three-drug combination therapy with AZT or didanosine, lamivudine, and indinavir in 1997. Korean red ginseng (KRG) has been used singly or together with AZT for HIV-1-infected patients since late 1991 as an alternative medicine (6). Several beneficial effects have been reported, including delayed development of AZT resistance mutations and maintenance of CD4 T-cell counts with KRG therapy only for 10 years (6-8). Surprisingly, the frequency of genetic defects of HIV-1 appears dependent on the level of KRG intake (5). Thus, we could maintain CD4 T cells in long-term survivors for more than 20 years in the absence of antiviral therapy (13).
In the present study, we investigated whether KRG has a favorable effect on the CD4 T-cell response and development of resistance mutations in Korean HIV-1 patients treated with HAART. While this is essentially a pilot study, the results support the potential benefits of combination therapy of KRG with HAART. We recommend that clinicians advocate the use of KRG to improve the prognosis of HIV-1-infected patients.
MATERIALS AND METHODS
Patients.
Forty-six HIV-1-infected patients (39 men and 7 women) receiving HAART were recruited from eight tertiary care hospitals located in four metropolitan cities and three provinces in Korea (Table 1). At the commencement of HAART, nine patients were classified as stage B and the remaining patients as stage C, according to the 1993 revised classification system for HIV-1 infection and the expanded AIDS surveillance case definition for adolescents and adults by the Centers for Disease Control and Prevention (2). Among the 46 patients, 41 were infected with HIV-1 subtype B, 4 with subtype CRF02_AG, and 1 with subtype J. The duration of HAART was 39.2 ± 4.0 months.
TABLE 1.
Baseline characteristics of patients treated with HAART plus KRG or HAART alone
| Parameter | Result for:
|
|
|---|---|---|
| HAART + KRG (n = 23) | HAART alone (n = 23) | |
| Sex (male/female) | 18/5 | 21/2 |
| Mean age in yr (range) | 36.5 (17-48) | 36.4 (11-60) |
| Subtype | ||
| B | 19 | 22 |
| CRF02_AG | 3 | 1 |
| J | 1 | 0 |
| CD4 T-cell count/μl | 127.0 ± 112.5 | 138.4 ± 96.6 |
| No. (%) of patients with CD4 T-cell count of <200/μl | 17 (73.9) | 17 (73.9) |
| No. (%) of patients with history of previous monotherapy | 13 (56.5) | 12 (52.2) |
| No. (%) of patients with resistance mutations | 9 (39.1) | 4 (17.4) |
KRG therapy.
KRG was administered as capsules prepared commercially by the Korea Ginseng Corporation (Daejeon, Korea). Capsules were made with 100% KRG powder products ground to a grain size of 0.125 mm (120 mesh). The crude ginseng saponin content was 44.84 mg/g, and the water content of KRG capsules was <5% (4). In addition to HAART, 23 (50.0%) of the 46 patients were administered KRG in the third year, with men receiving 5.4 g/day (six 300-mg capsules three times a day) and women receiving 2.7 g/day (three 300-mg capsules three times a day) with no other additives (6, 7). The dose was adjusted to half for women in view of potential adverse effects, such as weight gain and vaginal hemorrhage. The remaining 23 patients were not administered KRG during the study period. In the first and second years, only 17 and 19 patients commenced KRG intake, respectively. The median duration of KRG intake by the 17 patients who received KRG prior to HAART was 62 months (range, 6 to 148 months). The average amount of KRG supplied to the 23 patients during HAART was 2,419 ± 1,713 g (range, 720 to 5,940 g), corresponding to 66.2 ± 47.5 g per month (range, 20.0 to 165.0 g per month). Four out of the 23 patients subjected to therapy with HAART plus KRG purchased additional KRG. The KRG intakes during HAART therapy were <1,000 g in 5 patients, 1,000 to 2,000 g in 8 patients, and >2,000 g in 10 patients. In terms of monthly dose, KRG intakes were <30 g/month in 6 patients, 30 to 60 g/month in 7 patients, and >60 g/month in 10 patients. Informed written consent was obtained from all study participants.
Specimens.
At 6-month intervals, blood samples were collected from all patients for the measurement of HIV-1 RNA levels, CD4 T-cell counts, and HIV resistance genotype. Plasma samples treated with EDTA for HIV-1 RNA estimation were centrifuged, separated within 2 h of collection, and stored in multiple aliquots at −70°C.
CD4 T-cell count and plasma RNA copies.
Peripheral blood mononuclear cells (PBMCs) were incubated with phycoerythrin- and fluorescein isothiocyanate-conjugated antibodies against CD4 and CD8 antigens, respectively (Simultest reagent; Becton Dickinson, San Jose, CA), and the levels of CD4 and CD8 T cells were measured using FACScan (Becton Dickinson) flow cytometry. The plasma concentration of HIV-1 RNA was quantified using the Roche Amplicor HIV-1 monitor (v1.5 or v1.0 test; Roche Diagnostics Systems, Branchburg, NJ) following the manufacturer's recommendations. The lower limits of detection of the standard and ultrasensitive formats are 400 and 50 RNA copies per ml, respectively.
DNA preparation and pol gene amplification.
DNA was prepared from PBMC samples as described previously (8, 9). Denatured DNA samples (10 μl) were amplified by nested PCR. Details are described in earlier reports (29-31). The outer and inner primer pairs employed were HXB2/half RT and PO1/RIT137, The amplified products were purified and sequenced directly using the primers PO1 and RIT137 or 527.
Subtyping.
Subtyping was based on the analysis of pol gene sequences. Initially, each sequence was compared to reference sequences using the Stanford HIV drug resistance website (http://hivdb.stanford.edu), and subsequent phylogenetic analyses were performed using the PHYLIP program DNAdist (http://evolution.genetics.washington.edu/phylip.html) and Neighbor program.
Analysis of genotypic resistance.
Drug resistance mutations were determined on the basis of published guidelines (19). The details are provided in an earlier report (31).
Statistical analysis.
Data are expressed as means ± standard deviations. Statistical significance was estimated using Student's two-tailed t test or Fisher's exact test with α = 0.05 (SPSS package, version 12.0). Descriptive analysis was performed to determine the proportions of patients with various types of drug resistance. The level of significance was set as 0.05 (5%).
RESULTS
Changes in the CD4 T-cell count.
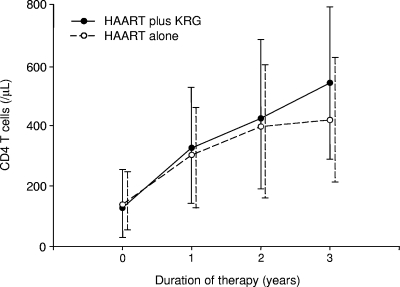
Over the 39.2 ± 4.0 months of HAART therapy, the CD4 T-cell count increased significantly from 133 ± 104/μl to 480 ± 236/μl (P < 0.0001), corresponding to an annual increase of 104 ± 61/μl. The 46 patients were divided into two groups according to KRG intake (HAART plus KRG versus HAART alone, hereafter designated “combination” and “HAART” groups, respectively). The annual increase in the CD4 T-cell count was significantly higher in the combination group than in the HAART group (124 ± 63/μl versus 84 ± 53/μl; P < 0.05) (Table 2). Over the 3-year treatment period, the CD4 T-cell count increased from 127 ± 113/μl (range, 3 to 346 μl) to 541 ± 251/μl (range, 131 to 1,030 μl) in the combination group and from 138 ± 97/μl (range, 2 to 318 μl) to 419 ± 206/μl (range, 143 to 865 μl) in the HAART group (Fig. 1). Additionally, the CD4 T-cell count increase varied, depending on whether the patient was previously subjected to monotherapy. Taking this factor into consideration, the increases in CD4 T-cell counts compared to baseline were 489 ± 198/μl (n = 10), 357 ± 206/μl (n = 13), 333 ± 209/μl (n = 11), and 232 ± 135/μl (n = 12), respectively, in the groups representing combination without previous monotherapy, combination with previous monotherapy, HAART without previous monotherapy, and HAART with previous monotherapy (Fig. 2).
TABLE 2.
Summary of treatment outcomes after 3 years
| Parameter | Result for:
|
P value | |
|---|---|---|---|
| HAART + KRG (n = 23) | HAART alone (n = 23) | ||
| Annual increase in no. of CD4 T cells/μl | 124 ± 63 | 84 ± 53 | <0.05 |
| No. (%) of patients without improvement in viral copy no./totala | 5/19 (26.3) | 9/20 (45.0) | NSb |
| No. (%) of patients with drug resistance mutations | 9 (39.1) | 12 (52.2) | NS |
| No. (%) of patients with additional drug resistance mutations after 3 yr/totalc | 1/14 (7.1) | 8/19 (42.1) | <0.05 |
| No. (%) of patients with additional drug resistance mutations at any time point | 2 (8.7) | 10 (43.5) | <0.05 |
| No. (%) of patients with high-level resistance mutations to ≥2 classes of drugs | 1 (4.3) | 7 (30.4) | <0.05 |
Plasma HIV-1 RNA loads were measured in 19 patients from the group treated with HAART plus KRG and 20 patients from the group treated with HAART alone.
NS, not significant.
With exclusion of 13 patients with baseline resistance mutations.
FIG. 1.
Changes in CD4 T-cell counts during HAART. The increase in the CD4 T-cell count was significantly higher in patients treated with HAART plus KRG (n = 23) than in those treated with HAART alone (n = 23) in the third year (P < 0.05).
FIG. 2.
Comparison of the increase in CD4 T-cell according to the presence or absence of previous monotherapy (PM) and KRG treatment. The groups with combination therapy and combination with previous monotherapy showed mildly significant increases in CD4 T cells compared with those in the groups treated with HAART only and HAART with previous monotherapy, respectively (P = 0.096 and 0.089, respectively). In contrast, absence of previous monotherapy did not show significant increase in CD4 T cells compared with the level in the corresponding group.
Virologic response to treatment.
After 3 years of HAART, the plasma HIV-1 RNA viral loads were measured in 39 patients. Fourteen patients (35.9%) displayed no improvement in viral copy number (i.e., incomplete virological suppression), with a viral load of >400 copies/ml. The annual increases in CD4 T cells were 80.6 ± 49.1/μl in patients displaying no improvement in viral copy number and 107.8 ± 56.7/μl in patients with complete virological suppression (P > 0.05). Five patients showed no improvement in viral copy number (26.3% [5/19]) in the combination group and 9 showed no improvement (45.0% [9/20]) in the HAART-only group (P > 0.05) (Table 2). Excluding 13 patients with baseline resistance mutations, no viral copy number improvement was detected in 1 of 14 patients in the combination group and 7 of 19 patients in the HAART group (P = 0.098).
Overall drug resistance mutations.
We determined the pol sequences encompassing protease and reverse transcriptase (RT) codons for 152 PBMC samples obtained at different time points from the 46 patients during HAART. At baseline, patients previously subjected to monotherapy (44% [11/25]) displayed a significantly higher frequency of resistance mutations than those not exposed to monotherapy (9.5% [2/21]) (P < 0.05).
Among the 13 patients displaying baseline resistance mutations, 1 of 9 patients in the combination group and 2 of 4 patients in the HAART group developed additional drug resistance mutations. In the remaining 33 patients without baseline resistance mutations, 7.1% of patients (1/14) in the combination group and 42.1% of patients (8/19) in the HAART group (P < 0.05) were identified with resistance mutations (Fig. 3 and Table 2). Since therapy-naïve patients in Korea displayed very low rates of resistance mutations (2.3% on the protease inhibitor (PI) gene and 3.4% on the RT inhibitor gene) (29, 30), we assumed that patients with no history of previous monotherapy harbored wild-type genotypes. However, one patient with baseline resistance mutations in the combination group did not display resistance mutations 3 years after HAART therapy. Resistance mutations were recorded in 9 of 23 patients (39.1%) in the combination group and 12 of 23 patients (52.2%) in the HAART-alone group. The data collectively show that 3 years after the commencement of HAART, 21 (45.7%) of the 46 patients harbored drug resistance mutations. Seven of the 44 patients (15.9%) exposed to protease inhibitors and 2 of 12 (16.7%) exposed to nonnucleoside reverse transcriptase inhibitors (NNRTIs) developed resistance mutations to their respective drugs.
FIG. 3.
Comparison of the frequency of additional resistance mutations to the class of drugs used in HAART to the baseline in 33 patients without baseline resistance mutations. The frequency of additional resistance mutations analyzed according to KRG intake was significantly lower in the combination group (7.1% [1/14]) than the HAART-alone group (42.1% [8/19]) (P < 0.05).
Delayed development of resistance mutations to lamivudine and protease inhibitors.
M184V was detected in 11 out of the 46 patients subjected to lamivudine therapy. Four and seven of these patients belonged to the combination and HAART groups, respectively. Resistance mutations appeared after the second year in the combination group but within the second year in three of seven patients in the HAART group. Among the four and seven patients with M184V in both groups, the earliest time points for the development of M184V were 25 months in the combination group and 13 months in the HAART group, suggesting earlier development of M184V in patients administered HAART alone. On the other hand, in Korean patients treated with lamivudine only, the earliest development of M184V was observed at 7 months (3). M184V in two patients (identified at 25 and 29 months) in the combination group disappeared in the subsequent sample but persisted in the HAART group.
One (4.5%) and six (27.3%) out of 22 patients with and without KRG displayed resistance mutations to PIs, respectively (P = 0.09). Following the exclusion of 13 patients with baseline resistance mutations, M184V was not detected in 14 patients subjected to combination therapy but was present in 4 of 19 patients administered HAART alone.
High-level resistance mutations.
After 3 years of HAART, 8 patients contained resistance mutations to two classes and 14 patients contained resistance to one of the three classes of drugs (nucleoside RT inhibitors [NRTIs], NNRTIs, and PIs). Analysis of resistance mutations to more than one drug class revealed a significantly lower rate of mutations in the combination group (1/23) than in the HAART-alone group (7/23) (P < 0.05) (Table 2).
DISCUSSION
To assess the efficacy of combination therapy with HAART plus KRG, we compared the frequencies of resistance mutations, virologic responses, and CD4 T-cell counts between patients subjected to combination and HAART therapy for 3 years. We observed a high frequency of resistance mutations to antiretroviral drugs, along with a significant percentage of patients displaying no improvement in viral copy number. The high frequency and nature of drug resistance mutations are indicative of their significant contribution to virological outcome.
The increase in CD4 T-cell count of the combination group was higher than that of the HAART group over the 3-year period. While patients treated with HAART plus KRG displayed more resistance mutations at baseline than those administered HAART only, the increase in the CD4 T-cell count after 3 years was greater in the former group, suggesting that the effect of KRG intake is underestimated. A report on responses after HAART in patients not subjected to previous monotherapy in Korea shows a 167/μl increase in CD4 T cells (from 207/μl to 374/μl) after 12 months of HAART (21). The increase in the combination group without previous monotherapy in this study was from 127 ± 113/μl to 326 ± 199/μl. Both viral load and immune activation are associated with progression to AIDS (15, 24). However, patients who fail virologically on HAART but have low levels of T-cell activation and proliferation show a continuous increase in CD4 T cells (16, 18). In long-term-asymptomatic HIV-1-infected patients with high viral loads, significant immune activation or proliferation was not evident (14). Thus, low immune activation despite a high viral load preserves HIV-1-specific T-cell responses and results in a long-term-asymptomatic phenotype (14). The long-term effectiveness of KRG intake may be attributed to a combination of mechanisms related to the pathogenesis of HIV-1 infection (9). Patients treated with KRG exhibit a significant and consistent decrease in soluble CD8 antigen (4), indicating that KRG intake suppresses generalized immune activation. Notably, the acid polysaccharide of ginseng induces Th1 cytokines (20), including interleukin-12 (22), thereby modulating the immune responses in vivo. An earlier placebo-controlled study showed that ginseng significantly enhances the activities of neutrophils, CD4 T cells, and natural killer cells (27). Moreover, extracts of North American ginseng (CVT-E002) prevent acute respiratory illness (23, 26). KRG has a mild antiviral effect on serum p24 (10), and an aqueous extract of KRG significantly decreases p24 secretion in an in vivo culture system (unpublished data). In view of these data, we attribute the additional increase in CD4 T cells in the combination group to the immunomodulatory influence of KRG.
In this study, we focus on patient response during the first 3 years of HAART administered in combination with KRG. The modality appears very promising, since several patients treated with HAART plus KRG for more than 8 to 9 years did not display significant resistance mutations to HAART or additional resistance mutations, compared to the baseline (unpublished data). However, our study has several limitations. Patients were enrolled at different time points and received variable doses of KRG, and four individuals additionally purchased KRG. Moreover, patients were not assigned in a blind and random manner. Thus, randomized, blinded, and placebo-controlled studies are required to confirm the dose-dependent effects of KRG on HIV-1-infected patients administered HAART. Since KRG activity was not standardized, we attempted to overcome this problem by using the same KRG product. In terms of optimal dose, patients who ingested more than 60 g of KRG per month did not develop resistance mutations. Additionally, a recent report by our group showed that the frequency of gross deletion in the nef gene depends on the amount of KRG (>60 g per month versus <60 g) (12). Thus, the minimal effective dose for future studies is recommended as 60 g KRG per month.
In total, 15.2% (7/46) of our patients developed major resistance mutations to PIs. V82A and M46I/L mutations were identified in patients treated with indinavir and D30N in patients administered nelfinavir. Some major mutations are not associated with PI cross-resistance, specifically, D30N and I50L atazanavir resistance mutations. In contrast, several other major mutations to PIs, such as those at codons 82, 84, and 90, are associated with significant cross-resistance. Accumulation of minor mutations, which by themselves do not cause resistance, can increase cross-resistance in the presence of major mutations (3, 17).
In conclusion, our findings support the clinical value of KRG in patients treated with HAART. However, further well-designed studies are essential to evaluate whether the potency of HAART plus KRG in HIV-1 infected patients is maintained in the long term.
Acknowledgments
This work was supported by a grant from the Korean Society of Ginseng funded by the Korea Ginseng Corporation (KGC).
Footnotes
Published ahead of print on 17 June 2009.
REFERENCES
- 1.Carpenter, C. C., D. A. Cooper, M. A. Fischl, J. M. Gatell, B. G. Gazzard, S. M. Hammer, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, M. Schechter, R. T. Schooley, M. A. Thompson, S. Vella, P. G. Yeni, and P. A. Volberding. 2000. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society—USA Panel. JAMA 283381-390. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1992. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb. Mortal. Wkly. Rep. 41(RR-17)1-19. [PubMed] [Google Scholar]
- 3.Cho, Y. K., H. S. Sung, H. J. Lee, Y. K. Kim, H. S. Chi, G. J. Cho, and M. W. Kang. 2000. Detection of resistance mutation to lamivudine in HIV-1 infected patients. J. Korean Soc. Microbiol. 35181-190. [Google Scholar]
- 4.Cho, Y. K., and H. Sung. 2007. Effects of Korean red ginseng on serum soluble CD8 in HIV-1-infected patients. J. Ginseng Res. 31175-180. [Google Scholar]
- 5.Cho, Y. K., and Y. S. Jung. 2008. High frequency of gross deletions in the 5′ LTR and gag regions in HIV type 1-infected long-term survivors treated with Korean red ginseng. AIDS Res. Hum. Retrovir. 24181-193. [DOI] [PubMed] [Google Scholar]
- 6.Cho, Y. K., H. Sung, H. J. Lee, C. H. Joo, and G. J. Cho. 2001. Long-term intake of Korean red ginseng in HIV-1-infected patients: development of resistance mutation to zidovudine is delayed. Int. Immunopharmacol. 11295-1305. [DOI] [PubMed] [Google Scholar]
- 7.Cho, Y. K., H. Sung, S. H. Ahn, I. G. Bae, J. H. Woo, Y. H. Won, D. G. Kim, and M. W. Kang. 2002. Frequency of mutations conferring resistance to nucleoside reverse transcriptase inhibitors in human immunodeficiency virus type 1-infected patients in Korea. J. Clin. Microbiol. 401319-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho, Y. K., H. Sung, T. K. Kim, J. Y. Lim, Y. S. Jung, and S. M. Kang. 2004. KRG significantly slows CD4 T cell depletion over 10 years in HIV-1 infected patients: association with HLA. J. Ginseng Res. 28173-182. [Google Scholar]
- 9.Cho, Y. K., J. Y. Lim, Y. S. Jung, S. K. Oh, H. J. Lee, and H. Sung. 2006. High frequency of grossly deleted nef genes in HIV-1 infected long-term slow progressors treated with Korean red ginseng. Curr. HIV Res. 4447-457. [DOI] [PubMed] [Google Scholar]
- 10.Cho, Y. K., Y. K. Kim, I. Lee, M. H. Choi, and Y. O. Shin. 1996. The effect of Korean red ginseng (KRG), zidovudine (ZDV), and the combination of KRG and ZDV on HIV-infected patients. J. Korean Soc. Microbiol. 31353-360. [Google Scholar]
- 11.Cho, Y. K., Y. K. Kim, Y. O. Shin, and Y. J. Cho. 1993. Change of serum β2-microglobulin, p24 antigen and CD4+ T lymphocyte in persons with human immunodeficiency virus infection after azidothymidine treatment. Korean J. Infect. Dis. 25211-219. [Google Scholar]
- 12.Cho, Y. K., Y. S. Jung, and H. Sung. 2009. Frequent gross deletion in the HIV-1 nef gene in hemophiliacs treated with Korean red ginseng: inhibition of detection by highly active antiretroviral therapy. AIDS Res. Hum. Retrovir. 25419-424. [DOI] [PubMed] [Google Scholar]
- 13.Cho, Y. K., Y. S. Jung, H. Sung, M. K. Sim, and Y. K. Kim. 2009. High frequency of gross deletions in 5′ LTR/gag and nef genes in patients infected with CRF02_AG of HIV-1 who survived for over 20 years: an association with Korean red ginseng. AIDS Res. Hum. Retrovir. 25535-541. [DOI] [PubMed] [Google Scholar]
- 14.Choudhary, S. K., N. Vrisekoop, C. A. Jansen, S. A. Otto, H. Schuitemaker, F. Miedema, and D. Camerini. 2007. Low immune activation despite high levels of pathogenic human immunodeficiency virus type 1 results in long-term asymptomatic disease. J. Virol. 818838-8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deeks, S. G., C. M. Kitchen, L. Liu, H. Guo, R. Gascon, A. B. Narváez, P. Hunt, J. N. Martin, J. O. Kahn, J. Levy, M. S. McGrath, and F. M. Hecht. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104942-947. [DOI] [PubMed] [Google Scholar]
- 16.Deeks, S. G., R. Hoh, R. M. Grant, T. Wrin, J. D. Barbour, A. Narvaez, D. Cesar, K. Abe, M. B. Hanley, N. S. Hellmann, C. J. Petropoulos, J. M. McCune, and M. K. Hellerstein. 2002. CD4+ T cell kinetics and activation in human immunodeficiency virus-infected patients who remain viremic despite long-term treatment with protease inhibitor-based therapy. J. Infect. Dis. 185315-323. [DOI] [PubMed] [Google Scholar]
- 17.Gallant, J. E. 2005. Antiretroviral drug resistance and resistance testing. Top. HIV Med. 13138-142. [PubMed] [Google Scholar]
- 18.Hazenberg, M. D., S. A. Otto, F. W. Wit, J. M. Lange, D. Hamann, and F. Miedema. 2002. Discordant responses during antiretroviral therapy: role of immune activation and T cell redistribution rather than true CD4 T cell loss. AIDS 161287-1289. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, V. A., F. Brun-Vezinet, B. Clotet, H. F. Gunthard, D. R. Kuritzkes, D. Pillay, J. M. Schapiro, and D. D. Richman. 2008. Update of the drug resistance mutations in HIV-1. Top. HIV Med. 16138-145. [PubMed] [Google Scholar]
- 20.Kim, K. H., Y. S. Lee, I. S. Jung, S. Y. Park, H. Y. Chung, I. R. Lee, and Y. S. Yun. 1996. Acidic polysaccharide from Panax ginseng, ginsan, induces Th1 cell and macrophage cytokines and generates LAK cells in synergy with rIL-2. Planta Med. 64110-115. [DOI] [PubMed] [Google Scholar]
- 21.Kim, M. S., S. Y. Shin, Y. S. Park, Y. A. Kim, N. S. Ku, J. H. Kim, Y. K. Kim, J. Y. Choi, Y. G. Song, and J. M. Kim. 2007. Therapeutic response of HAART and analysis of related factors in Korean HIV-infected persons. Infect. Chemother. 39142-150. [Google Scholar]
- 22.Larsen, M. W., C. Moser, N. Høiby, Z. Song, and A. Kharazmi. 2004. Ginseng modulates the immune response by induction of interleukin-12 production. APMIS 112369-673. [DOI] [PubMed] [Google Scholar]
- 23.McElhaney, J. E., S. Gravenstein, S. K. Cole, E. Davidson, D. O'Neill, S. Petitjean, B. Rumble, and J. J. Shan. 2004. A placebo-controlled trial of a proprietary extract of North American ginseng (CVT-E002) to prevent acute respiratory illness in institutionalized older adults. J. Am. Geriatr. Soc. 5213-19. [DOI] [PubMed] [Google Scholar]
- 24.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 2721167-1170. [DOI] [PubMed] [Google Scholar]
- 25.Palella, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, et al. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338853-860. [DOI] [PubMed] [Google Scholar]
- 26.Quan, F. S., R. W. Compans, Y. K. Cho, and S. M. Kang. 2007. Ginseng and Salviae herbs play a role as immune activators and modulate immune responses during influenza virus infection. Vaccine 25272-282. [DOI] [PubMed] [Google Scholar]
- 27.Scaglione, F., F. Ferrara, S. Dugnani, M. Falchi, G. Santoro, and F. Fraschini. 1990. Immunomodulatory effects of two extracts of Panax ginseng C. A. Meyer. Drugs Exp. Clin. Res. 16537-542. [PubMed] [Google Scholar]
- 28.Shafer, R. W., M. A. Winters, S. Palmer, and T. C. Merigan. 1998. Multiple concurrent reverse transcriptase and protease mutations and multidrug resistance of HIV-1 isolates from heavily treated patients. Ann. Intern. Med. 128906-911. [DOI] [PubMed] [Google Scholar]
- 29.Sung, H., B. T. Foley, I. G. Bae, H. S. Chi, and Y. K. Cho. 2001. Phylogenetic analysis of reverse transcriptase in antiretroviral drug-naive Korean HIV type 1 patients. AIDS Res. Hum. Retrovir. 171549-1554. [DOI] [PubMed] [Google Scholar]
- 30.Sung, H., B. T. Foley, S. H. Ahn, Y. B. Kim, J. D. Chae, Y. O. Shin, H. I. Kang, and Y. K. Cho. 2003. Natural polymorphisms of protease in protease inhibitor-naive HIV-1 infected patients in Korea: a novel L63M in subtype B. AIDS Res. Hum. Retrovir. 19525-530. [DOI] [PubMed] [Google Scholar]
- 31.Sung, H., Y. S. Jung, M. W. Kang, I. G. Bae, H. H. Chang, J. H. Woo, and Y. K. Cho. 2007. High frequency of drug resistance mutations in human immunodeficiency virus type 1-infected Korean patients treated with HAART. AIDS Res. Hum. Retrovir. 231223-1229. [DOI] [PubMed] [Google Scholar]
- 32.Vergne, L., C. T. Kane, C. Laurent, N. Diakhaté, N. F. Gueye, P. M. Gueye, P. S. Sow, M. A. Faye, F. Liégeois, A. Ndir, I. Lanièce, M. Peeters, I. Ndoye, S. Mboup, and E. Delaporte. 2003. Low rate of genotypic HIV-1 drug-resistant strains in the Senegalese government initiative of access to antiretroviral therapy. AIDS 17(Suppl. 3)S31-S38. [DOI] [PubMed] [Google Scholar]