Abstract
Previous studies in our laboratory have shown that the subcutaneous pretreatment of rats with heat-killed cells (HKC) of Cryptococcus neoformans emulsified in complete Freund adjuvant (CFA) promotes protective immunity against an intraperitoneal challenge with C. neoformans. In contrast, subcutaneous treatment with the capsular polysaccharide (PSC) emulsified in CFA exacerbates the cryptococcal infection. The purpose of this study was to analyze the mechanisms involved in these phenomena. Adherent peritoneal cells from rats treated with HKC-CFA showed upregulated ED2, CD80, and CD86 expression; an increase in the level of production of anticryptococcal metabolites; and the enhanced production of interleukin-12 (IL-12) in comparison with the findings for cells from rats treated with CFA-phosphate-buffered saline (PBS). Adherent peritoneal cells from rats treated with PSC-CFA, however, also presented upregulated ED2, CD80, and CD86 expression compared to the level of expression for peritoneal cells from controls, but these cells showed an increase in arginase activity and decreased levels of production of IL-12 and tumor necrosis factor (TNF) compared with the activity and levels of production by peritoneal cells from CFA-PBS-treated rats. In addition, treatment with HKC-CFA resulted in a rise in the phagocytic and anticryptococcal activities of adherent peritoneal cells compared to those for control rats. However, adherent peritoneal cells from rats treated with PSC-CFA presented a reduction in anticryptococcal activity in comparison with that for cells from animals treated with CFA-PBS. These results show the differential activation between adherent peritoneal cells from HKC-CFA- and PSC-CFA-treated rats, with this differential activation at the primary site of infection possibly being responsible, at least in part, for the phenomena of protection and exacerbation observed in our model.
Infection by the encapsulated yeast Cryptococcus neoformans is one of the most common opportunistic infections in patients with impaired cell-mediated immunity, such as those with AIDS (19). The capsule represents the major virulence factor of this fungus, with the main component of this capsule, glucuronoxylomannan (GXM), being continuously released by C. neoformans during its replication (16, 17). GXM has been implicated in multiple deleterious effects to immune function (14, 66, 67). Furthermore, previous studies in our laboratory have provided strong cytometric, molecular, and morphological evidence demonstrating that purified GXM promotes cell death by apoptosis in spleen mononuclear cells (18).
Full protection against C. neoformans is dependent on multiple factors, which include innate and acquired immunity (37, 50). The innate immune system has multiple components to provide resistance to C. neoformans infection, such as cytokines, various cell populations, chemokines, and adhesion molecules. In previous publications, different cytokines have been shown to be important in the control of infections caused by C. neoformans (2, 13, 39). For example, interleukin-12 (IL-12) plays a pivotal role in the induction of a Th1 immune response against this infection, which is essential for protection (24). In relation to this, treatment with IL-12 was found to reduce the fungal load and prolong the survival of mice infected with C. neoformans (20, 38). In addition, another proinflammatory cytokine, tumor necrosis factor alpha (TNF-α), is necessary during the early stage of infection with C. neoformans, since it promotes the maturation and migration of dendritic cells to the draining lymph node (9). Furthermore, TNF-α can also activate macrophages, leading to an increase in the capacity of these cells to phagocytose and kill C. neoformans yeast, with the neutralization of this cytokine resulting in the exacerbation of infection (21, 36). In contrast, Th2-related cytokines, such as IL-4 and IL-10, contribute to the progression of cryptococcal infection (41). IL-10 suppresses T-cell proliferation and downregulates the production of Th1 cytokines, which includes gamma interferon (IFN-γ), IL-12, and TNF-α (23, 47, 52). The role of transforming growth factor beta (TGF-β) in cryptococcosis is still debated. Studies by Shao et al. (61) demonstrated that the early, but not the late, administration of this cytokine during cryptococcal infection results in an increase in the lung fungal burden. In conclusion, the Th1-Th2 cytokine balance is critical for host immunity against infection with C. neoformans.
Neutrophils, macrophages, dendritic cells, and natural killer T cells constitute the cellular effectors of innate immunity against C. neoformans (15, 40, 46, 68). Macrophages have been demonstrated histopathologically to be the predominant cell types after a transient influx of neutrophils into the lungs of rats infected with C. neoformans, which has suggested an important role of macrophages in host defenses against cryptococcal infection (30). These cells are capable of secreting an array of cytotoxic products, including reactive oxygen intermediates (ROI), such as hydrogen peroxide, and reactive nitrogen intermediates, such as nitric oxide (51). In addition, previous studies in our laboratory have shown that macrophages from infected rats appear to be able to kill C. neoformans principally by generating NO (58). Furthermore, other functions have been attributed to macrophages, including phagocytosis, the production of cytokines and chemokines, and antigen presentation (35, 43, 44, 65). On the other hand, alternatively activated macrophages (aaMac) can also appear. In this case, the macrophages have high levels of arginase activity and produce l-ornithine and urea. Other characteristics include increased fungal phagocytosis, decreased intracellular killing, and the promotion of fibrosis. Furthermore, alternative activation is also associated with a high level of expression of macrophage activation markers, such as major histocompatibility complex class II (MHC II), CD80 (B7-1), CD86 (B7-2), and CD163 (ED2 antigen), and low levels of TNF-α production (7, 28, 29, 32, 63).
In previous studies, we have shown that subcutaneous treatment of rats with heat-killed cells (HKC) of C. neoformans emulsified in complete Freund adjuvant (CFA) provides protection against an intraperitoneal challenge with viable C. neoformans. In contrast, cryptococcosis is exacerbated in rats pretreated with capsular polysaccharide (CPS) emulsified in CFA (8). In the study described here, our objective was to evaluate the immunological bases of the protective and nonprotective responses induced by these treatments. For this purpose, given the importance of innate immunity against cryptococcosis and the central role of peritoneal cells in our model, due to the fact that it is in peritoneum where the first contact between the yeast and the host immune system is produced, we studied the activation state of adherent peritoneal cells induced by different treatments, before fungal challenge, which could be responsible for the protection or exacerbation of cryptococcal infection reported previously (8).
MATERIALS AND METHODS
Microorganism.
A clinical isolate, Cryptococcus neoformans var. grubii serotype A, strain 102/85, from the National University of Córdoba stock culture collection was used in all experiments. For each experiment, a fresh culture of C. neoformans was started from −80°C stocks. Cultures were grown on Sabouraud glucose agar slants at 37°C; maintained by not more than four passages on the same medium; and periodically checked for assimilation pattern, urease production, and virulence (59).
Cryptococcal antigens.
Two different sources of C. neoformans antigens were used in this study. One was HKC and the other was CPS. HKC were prepared by incubating the C. neoformans isolate for 1 h at 60°C (10) and were then washed three times in phosphate-buffered saline (PBS) before they were used to treat rats. These washed HKC were assessed for viability by plating them on Sabouraud agar.
As reported by Cherniak et al. (16), CPS was prepared by precipitation with ethanol. The detection of neutral carbohydrates was performed by the phenol-sulfuric acid method of Dubois et al. (25). In order to avoid endotoxin contamination, which may affect the experimental results, the whole process of CPS preparation was performed with sterile water, PBS, and plastic or glassware. The preparations were tested for lipopolysaccharide (LPS) contamination by the Limulus amebocyte lysate assay (E-Toxate; Sigma-Aldrich Co., St. Louis, MO), and the endotoxin content did not exceed 0.1 endotoxin unit per ml of sample.
Animals and treatment.
Female inbred Wistar rats (ages, 8 to 12 weeks) were used in this study. The animals were housed and cared for in the animal resource facilities of the Department of Clinical Biochemistry, Faculty of Chemical Sciences, National University of Córdoba, according to institutional guidelines. Rats were treated subcutaneously at four sites on the lower abdomen with 107 HKC or 0.1 mg of CPS emulsified in CFA (Sigma-Aldrich Co.), and controls for these groups received an equal volume of PBS-CFA by the same route. Three animals per group were used (8).
Peritoneal cells.
At 4 days posttreatment, the rats were killed and peritoneal cells were obtained by washing of the peritoneal cavity with sterile Krebs Ringer phosphate dextrose buffer (KRPG; pH 7.0; Sigma-Aldrich Co.) containing gentamicin (50 mg/liter) and heparin (20 U/ml). Adherent peritoneal cells were obtained by allowing them to adhere for 2 h at 37°C in a 5% CO2 atmosphere, with nonadherent cells being removed by two washes with KRPG. Adherent cells were then removed with cold PBS and a scraper, washed twice, and resuspended in culture medium. The viability of the cells was consistently more than 90%, as determined by a trypan blue exclusion method.
Flow cytometric analysis.
Surface antigen expression on adherent peritoneal cells was determined by flow cytometry. Adherent peritoneal cells were obtained at 4 days posttreatment, washed, and blocked by the addition of rat Fc block (catalog no. 550270; BD Biosciences, San Jose, CA). After blocking of the Fc receptor, 1 × 106 cells were stained with fluorescein isothiocyanate-conjugated monoclonal antibody (MAb) specific for ED2; phycoerythrin-conjugated MAbs specific for MHC II, CD80, and CD86; or appropriate isotype-matched antibodies (all antibodies obtained from BD Biosciences) for 30 min at 4°C. The cells were washed twice with PBS containing 3% fetal calf serum (FCS) and 0.09% (wt/vol) sodium azide, and the cell pellet was resuspended in the residual volume. Stained samples were fixed in 2% formaldehyde and were stored at 4°C in the dark until they were analyzed by flow cytometry (Cytoron Absolute; Ortho Diagnostic Systems).
Measurement of nitrite production.
Nitric oxide production by adherent peritoneal cells was determined by measuring the nitrite content of the culture supernatant by use of the Griess reagent (5). Peritoneal cells (2 × 106/ml) were incubated for 48 h in RPMI medium supplemented with 10% FCS at 37°C in a 5% CO2 atmosphere in the presence of LPS (10 μg/ml). Briefly, the Griess reagent was prepared by mixing equal volumes of sulfanylamide (1.5% in 1 N HCl) and N-(1-naphthyl)ethylenediamide dihydrochloride (0.13% in H2O). A volume of 200 μl of the Griess reagent was then mixed with 100 μl of sample, and the mixture was incubated for 15 min in the dark. The absorbance at 540 nm was measured with an automated microplate reader (Bio-Rad), and the nitrite concentration was calculated by use of a calibration curve created with between 2 and 100 μM of nitrite. No nitrite was detectable in the cell-free medium.
Hydrogen peroxide production.
The level of hydrogen peroxide production was determined as described by Pick and Keisari (53). Briefly, 1 × 106 adherent peritoneal cells were incubated in the presence of phorbol 12-myristate 13-acetate (100 ng/ml; Sigma-Aldrich Co.) with red phenol (0.01 mg/ml) and peroxidase (20 U/ml). After 45 min at 37°C, the reaction was stopped with 10 μl of 1 N NaOH. The optical density at 610 nm was recorded. The hydrogen peroxide concentrations were calculated by comparing the concentration in the supernatant to the concentrations on a standard curve created with known amounts of H2O2.
Superoxide anion production.
Superoxide anion production was qualitatively determined by nitroblue tetrazolium (NBT) reduction. Adherent peritoneal cells (1 × 106) were incubated in the dark for 30 min with 5% CO2 in the presence of NBT (0.1%) and phorbol 12-myristate 13-acetate (100 ng/ml; Sigma-Aldrich Co.). The reaction was stopped with 0.4 ml of 0.1 N HCl. The cells were centrifuged, and insoluble formazan was extracted twice with 1 ml of 1,4-dioxane. The optical densities at 560 nm of the supernatants were determined.
Arginase activity.
Arginase activity was measured as described by Corraliza et al. (22). Adherent peritoneal cells were lysed in 50 μl 0.1% Triton X-100 containing 5 μg pepstatin, 5 μg aprotinin, and 5 μg antipain at room temperature for 30 min. To 50 μl of the lysed cells, 50 μl 10 mM MnCl2 and 50 mM Tris-HCl were added to activate the enzyme, and the mixture was heated at 56°C for 10 min. To 50 μl of this activated lysate, 50 μl of 0.5 M l-arginine, pH 9.7, was added, and the mixture was incubated for 1 h at 37°C. The reaction was stopped with 400 μl of a solution that contained 96% H2SO4, 85% H3PO4, and H2O (at a 1:3:7 ratio, vol/vol/vol). The urea formed was measured colorimetrically at 540 nm after the addition of 25 μl of 9% α-isonitrosopropiophenone (in 100% ethanol); the mixture was then heated at 100°C for 45 min. The results are expressed as μg of urea.
Cytokine measurement.
The supernatants were collected for determination of the levels of cytokines from cultures of adherent peritoneal cells in the presence of LPS (10 μg/ml). The supernatants were collected after 24 h of culture for IL-12 and TNF-α, 48 h of culture for IL-10, and 72 h of culture for TGF-β. These were frozen at −70°C until they were analyzed. Cytokine levels were measured by a sandwich enzyme-linked immunosorbent assay, according to the manufacturer's protocol, by using for each cytokine two cytokine-specific MAbs, one of which was biotinylated. Antibodies purchased from Biosource were used for all cytokines. Dilutions of recombinant rat IL-12, TNF-α, IL-10, and TGF-β were used as standards. After the plates were appropriately washed, they were reacted with horseradish peroxidase-streptavidin, followed by the addition of o-phenylenediamine (5 to 20 min). The reaction was stopped with 25 μl sulfuric acid. The reaction was measured by use of a microplate reader (Bio-Rad).
In vitro phagocytosis.
Rats were treated as indicated above with CFA-PBS, HKC-CFA, or CPS-CFA; and 4 days later adherent peritoneal cells were obtained. These cells were cultured with C. neoformans at a ratio of 5:1 adherent peritoneal cells to fungi in RPMI medium supplemented with 10% FCS at 37°C for 2 h in the presence of 10% rat serum. The cells were washed several times with PBS and cytocentrifuged on a slide. The cells were then fixed and stained with May-Grunwald-Giemsa. The phagocytic index was defined as the number of attached and ingested cryptococci divided by the number of cells per microscope field. The experiments were done in triplicate, and five different fields were counted (26).
Killing assay.
Adherent peritoneal cells from each group of animals and C. neoformans were coincubated for 24 h at 37°C. The cells were lysed by the addition of 0.1 ml of sterile distilled water, and the lysates were incubated for 30 min at room temperature. The mixture was then carefully resuspended, and 100 μl was placed in plates with Sabouraud medium to determine the numbers of CFU (58). The results were expressed as the percentage of C. neoformans yeast cells killed, with the percentage of dead cells being calculated with reference to the level of development of the controls (yeast cells cultured in culture medium only), which was taken to be 100%.
Statistical analysis.
Three animals per group were used in all studies, and experiments were performed in triplicate samples from individual animals from each group. Data are expressed as means ± standard errors of the means (SEMs). The data were analyzed by a one-way analysis of variance with Tukey's post hoc test to determine the statistical significance for all pairwise multiple-comparison procedures. A P value of <0.05 was considered significant. All experiments were repeated, and equivalent results were obtained in each experiment.
RESULTS
Expression of surface molecules on adherent peritoneal cells.
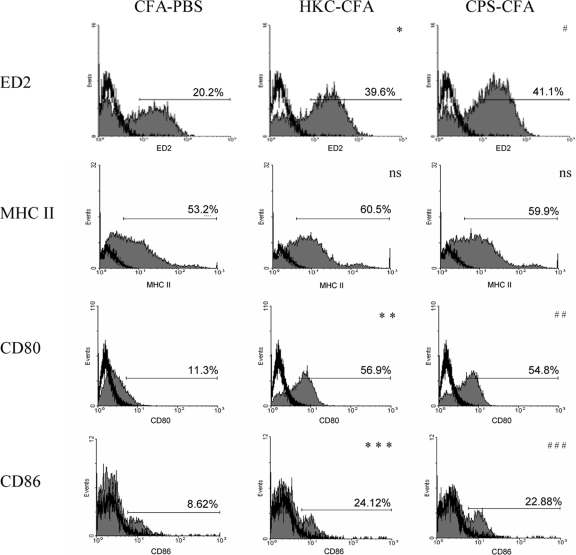
For the induction of immunity against pathogens, the expression of important surface molecules is essential. For this reason, we determined the expression of ED2, CD80, CD86, and MHC II on the surfaces of adherent peritoneal cells obtained at 4 days posttreatment. Figure 1 shows that adherent peritoneal cells from rats treated subcutaneously with HKC-CFA, as well as those treated with CPS-CFA, presented upregulated ED2, CD80, and CD86 expression compared to the level of expression on adherent peritoneal cells from control animals treated with CFA-PBS. On the other hand, no changes were observed in MHC II expression on adherent peritoneal cells obtained from the different experimental groups (Fig. 1).
FIG. 1.
Effect of treatment with cryptococcal antigens on the phenotype of adherent peritoneal cells. Wistar rats were treated with CFA-PBS, HKC-CFA, or CPS-CFA. Four days after treatment, adherent peritoneal cells were obtained; stained with antibodies specific for ED2, MHC II, CD80, and CD86; and analyzed by flow cytometry, as described in Materials and Methods. Filled histograms, levels of expression of the different surface molecules; open histograms, reactivity obtained with isotype-matched control MAbs. *, P < 0.006 for HKC-CFA-treated rats versus the results for CFA-PBS-treated rats; **, P < 0.0003 for HKC-CFA-treated rats versus the results for CFA-PBS-treated rats; ***, P < 0.025 for HKC-CFA-treated rats versus the results for CFA-PBS-treated rats; #, P < 0.005 for CPS-CFA-treated rats versus the results for CFA-PBS-treated rats; ##, P < 0.0005 for CPS-CFA-treated rats versus the results for CFA-PBS-treated rats; ###, P < 0.025 for CPS-CFA-treated rats versus the results for CFA-PBS-treated rats; ns, no significant difference for the results for HKC-CFA- and CPS-CFA-treated rats versus the results for CFA-PBS-treated rats. Data are representative of those from three experiments, with three rats per group per experiment being tested.
In vitro phagocytosis and killing assay.
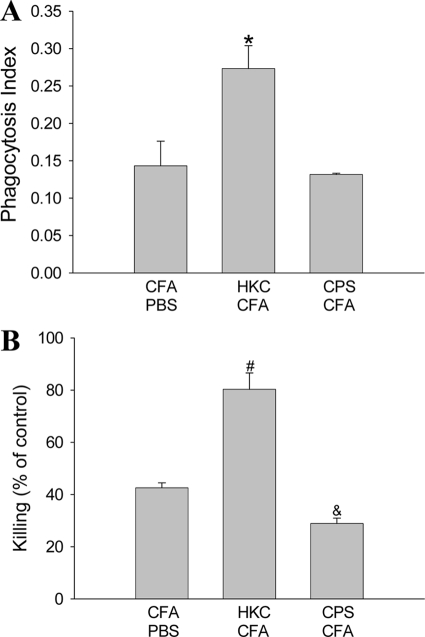
In order to determine the functionality of adherent peritoneal cells in response to antigenic treatment, we studied the ability of these cells to phagocytose and kill C. neoformans yeast. The phagocytic index for adherent peritoneal cells from rats treated with HKC-CFA was significantly higher than that for cells from control rats (Fig. 2A). In contrast, there were no differences in the phagocytosis of C. neoformans between adherent peritoneal cells from animals treated with CPS-CFA and cells from control rats (Fig. 2A). With respect to the anticryptococcal activity, adherent peritoneal cells from rats treated with HKC-CFA were more effective in inhibiting C. neoformans replication than those from CFA-PBS-treated rats (Fig. 2B). On the other hand, the fungicidal activity observed in cultures of adherent peritoneal cells from rats treated with CPS-CFA was lower than that observed in cultures of cells obtained from control animals (Fig. 2B).
FIG. 2.
Phagocytosis and killing of C. neoformans by adherent peritoneal cells. Adherent peritoneal cells obtained from rats treated with CFA-PBS, HKC-CFA, or CPS-CFA were cocultivated with C. neoformans yeast for 2 h to assess the phagocytic activity of experimental cells (A) or 24 h for the killing assay (B), as described in Materials and Methods. Each column shows the mean ± SEM of three independent experiments. *, P < 0.05 for HKC-CFA-treated rats versus the results for CFA-PBS-treated rats; #, P < 0.02 for HKC-CFA-treated rats versus the results for CFA-PBS-treated rats; &, P < 0.04 for CPS-CFA-treated rats versus the results for CFA-PBS-treated rats).
Nitric oxide production by adherent peritoneal cells.
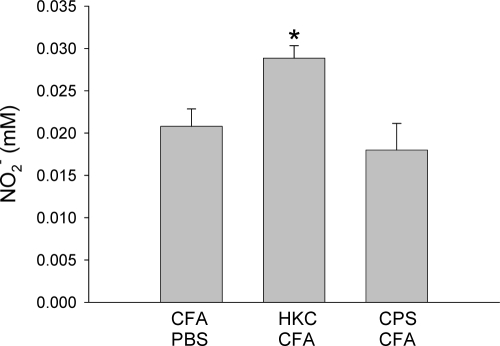
Nitric oxide production has been demonstrated to be involved in reducing the level of C. neoformans replication in different experimental models of infection (3, 45, 58). Therefore, the level of NO production by adherent peritoneal cells from each group of animals was evaluated. The nitrite levels found in culture supernatants of LPS-stimulated cells from HKC-CFA-treated rats were higher than those found in culture supernatants of cells from rats treated with CFA-PBS (Fig. 3). On the other hand, the levels of nitrite produced by LPS-stimulated cells from CPS-CFA-treated rats were similar to those produced by cells from control rats (Fig. 3).
FIG. 3.
Production of nitric oxide by adherent peritoneal cells. Rats were treated subcutaneously with CFA-PBS, HKC-CFA, or CPS-CFA. Four days after treatment, adherent peritoneal cells were obtained and stimulated with LPS for 48 h, and 100 μl of the culture supernatant was used for determination of the nitrite concentration by the Griess reaction. Each column shows the mean ± SEM for three animals in each group, and the results are representative of those from three independent experiments. *, P < 0.001 for HKC-CFA-treated rats versus the results for CFA-PBS-treated rats.
Hydrogen peroxide and superoxide anion release by adherent peritoneal cells.
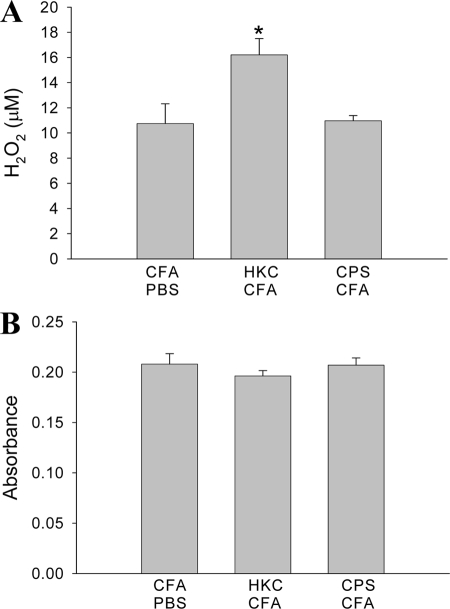
The levels of hydrogen peroxide and superoxide anion (O2−) produced by adherent peritoneal cells obtained at 4 days posttreatment were quantified. The levels of H2O2 produced by adherent peritoneal cells from HKC-CFA-treated rats were higher than those produced by cells from rats treated subcutaneously with CFA-PBS (Fig. 4A). In contrast, the H2O2 concentration produced by adherent peritoneal cells from CPS-CFA-treated rats did not differ significantly from that produced by cells from the control rats (Fig. 4A). On the other hand, the levels of O2− produced by adherent peritoneal cells from rats treated with HKC-CFA or CPS-CFA were similar to those produced by cells from the control rats (Fig. 4B).
FIG. 4.
Production of ROI by adherent peritoneal cells. Rats were treated with the different cryptococcal antigens, and 4 days later, the adherent peritoneal cells were obtained. Afterwards, the levels of H2O2 (A) and O2− (B) generation by these cells were determined. Each column shows the mean ± SEM of three independent experiments. *, P < 0.007 for HKC-CFA-treated rats versus the results for CFA-PBS-treated rats.
Arginase activity in adherent peritoneal cells from experimental animals.
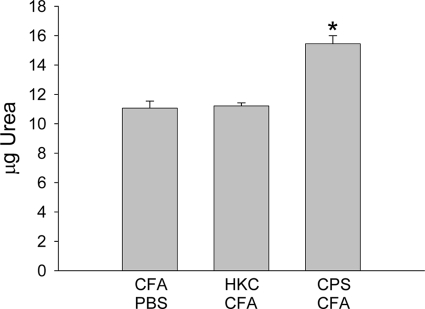
Even though activated phenotypes were presented by adherent peritoneal cells from rats treated with CPS-CFA, these cells were not capable of producing an increase in the level of generation of anticryptococcal molecules, such as NO and ROS, compared with the level generated by cells from the control animals. Furthermore, these cells also presented impaired anticryptococcal activity compared to the activity of the cells from the control animals. Therefore, we hypothesized that treatment with CPS-CFA could have been associated with the induction of alternatively activated macrophages. To confirm this hypothesis, the arginase activity of adherent peritoneal cells from the different experimental groups, obtained at 4 days posttreatment, was evaluated. As shown in Fig. 5, significantly greater levels of urea were detected in cultures of adherent peritoneal cells from CPS-CFA-treated rats than in cultures of adherent peritoneal cells from animals treated with CFA-PBS. In contrast, no difference in arginase activity between adherent peritoneal cells from HKC-CFA-treated rats and those from animals treated with CFA-PBS was observed (Fig. 5).
FIG. 5.
Arginase activity in adherent peritoneal cells. Four days posttreatment, the arginase activity in adherent peritoneal cell lysates was determined. The results are expressed as μg of urea. Each column shows the mean ± SEM for three animals in each group, and the results are representative of those from three independent experiments. *, P < 0.005 for CPS-CFA-treated rats versus the results for CFA-PBS-treated rats.
Cytokine production by adherent peritoneal cells.
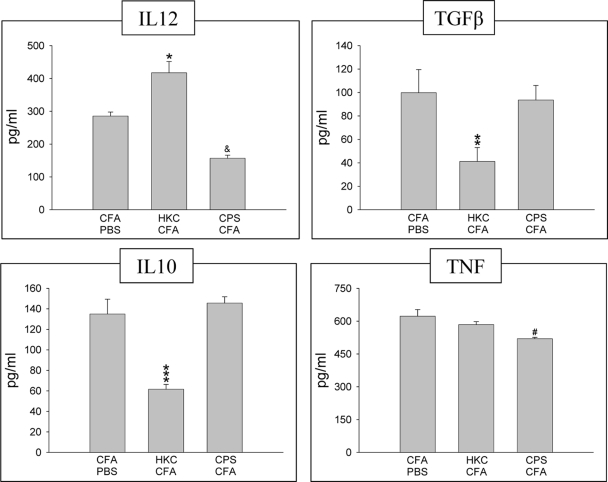
To determine whether treatment with the different antigens could modify the cytokine production by adherent peritoneal cells, the levels of IL-12, TGF-β, IL-10, and TNF-α in cultures of LPS-stimulated cells were measured (Fig. 6). The levels of IL-12 produced by adherent peritoneal cells from rats treated with HKC-CFA were higher that those produced by cells from rats treated with CFA-PBS. In contrast, the levels of production of TGF-β and IL-10 by these cells were lower than those by cells from the control animals. In addition, no significant difference in the level of TNF-α production between adherent peritoneal cells from control rats and cells from HKC-CFA-treated rats was observed. On the other hand, at 4 days after treatment, the levels of IL-12 and TNF-α produced by adherent peritoneal cells from CPS-CFA-treated rats were lower than those produced by cells from the control rats. In contrast, the levels of production of TGF-β and IL-10 by these cells were similar to those found in the supernatants of the control cells (Fig. 6).
FIG. 6.
Cytokine production by adherent peritoneal cells from rats treated with CFA-PBS, HKC-CFA, or CPS-CFA at day 4 posttreatment. Adherent peritoneal cells from different groups were cultured in 5% CO2 at 37°C in the presence of LPS. The supernatants used for the measurement of cytokine levels were collected at 24 h for IL-12 and TNF-α, 48 h for IL-10, and 72 h for TGF-β. A capture enzyme-linked immunosorbent assay was used to determine the cytokine levels. Each column shows the mean ± SEM of three independent experiments. *, P < 0.01 for HKC-CFA-treated rats versus the results for CFA-PBS-treated rats; **, P < 0.003 for HKC-CFA-treated rats versus the results for CFA-PBS-treated rats; ***, P < 0.0002 for HKC-CFA-treated rats versus the results for CFA-PBS-treated rats; &, P < 0.01 for CPS-CFA-treated rats versus the results for CFA-PBS-treated rats; #, P < 0.008 for CPS-CFA-treated rats versus the results for CFA-PBS-treated rats.
DISCUSSION
Previous work carried out in our laboratory has demonstrated that rats treated subcutaneously with HKC-CFA 4 days before intraperitoneal infection with C. neoformans were better able to control this infection than animals treated with PBS. In contrast, those that were pretreated subcutaneously with CPS-CFA presented with an exacerbation of fungal infection (8). In the present study, the immunological basis that could explain this differential response was evaluated.
The role of the innate immune system as a first-line defense against a pathogenic challenge has been well documented. The induction of the appropriate activation of the innate immune system is related to better control of the infection. In this way, the treatment of mice with a Toll-like receptor ligand, such as LPS, before the infection was efficient in reducing the number of C. neoformans yeast cells in tissues (56). In contrast, inadequate innate immunity may facilitate the development of disease. For example, the inoculation of rats with Trypanosoma lewisi induced immunosuppression that was strong and rapid (at 4 to 5 days postinoculation) and that enhanced the susceptibility of these animals to Toxoplasma gondii infection (34). Furthermore, this treatment also affected the capacity of rat alveolar macrophages to kill C. neoformans yeast (33). The results of our studies clearly show that the subcutaneous treatment of animals with different cryptococcal antigens results in the differential activation of the innate immune system and, particularly, adherent peritoneal cells. Those are the first immune cells that come into contact with the C. neoformans yeast after intraperitoneal infection and either restrict or enhance their growth and dissemination.
We observed that treatment with HKC-CFA resulted in the phenotypic activation of adherent peritoneal cells and the upregulation of important surface molecules, such as CD80 and CD86, involved in the initiation of the adaptive immune response. In addition, this treatment also resulted in the induction of expression of the ED2 antigen, which is a member of the scavenger receptor cysteine-rich group B. In this sense, studies by Polfliet et al. (55) have demonstrated that the ligation of this receptor on rat peritoneal macrophages triggers the production of proinflammatory mediators such as NO, TNF-α, and IL-6. On the other hand, it has also been indicated that the ED2 antigen is a marker for aaMac (7).
Surprisingly, the treatment of rats with CPS-CFA, which resulted in an exacerbation of the infection, also increased the levels of expression of these molecules on adherent peritoneal cells compared with the levels of expression on cells from the control rats (Fig. 1). On the other hand, the levels of expression of MHC II on adherent peritoneal cells were similar for the different antigenic treatments.
Phagocytosis is an important host defense mechanism by which different cells are able to ingest microbes and kill them. In cryptococcal infection, the importance of this process has been well established. In this way, cells such as macrophages are capable of ingesting and killing C. neoformans yeast (11, 12). In addition, this process of phagocytosis has also been reported to stimulate the production of chemokines and cytokines by phagocytic cells (31). However, C. neoformans can also replicate intracellularly and lyse or escape from the host cell (4, 27, 64). In this case, macrophages could serve as permissive sites for the growth of fungi. Our findings demonstrate that adherent peritoneal cells from animals treated with HKC-CFA were able to ingest and kill the C. neoformans yeast more efficiently than adherent peritoneal cells from control rats (Fig. 2). In contrast, the phagocytic capacity of adherent peritoneal cells from CPS-CFA-treated rats was similar to that observed for adherent peritoneal cells from animals treated with CFA-PBS (Fig. 2A). However, despite the similarity in their phagocytic activities, the cells from these two groups of animals differed in their abilities to kill C. neoformans cells. Consequently, the fungal growth observed in cocultures of yeast and adherent peritoneal cells from rats treated with CPS-CFA was higher than that found in control cultures (Fig. 2B). Therefore, these peritoneal cells were less efficient in inhibiting C. neoformans replication than those from rats treated with CFA-PBS.
Previous studies in our laboratory and other laboratories have demonstrated that the production of reactive nitrogen intermediates and ROI by different cell types plays an important role in the host defense against cryptococcal infection. Chemically generated NO inhibits C. neoformans growth in vitro (3). Moreover, in a tracheal infection model with this yeast, it has been demonstrated that the production of IFN-γ and NO is necessary for the efficient resolution of the infection (45). In addition, previous studies in our laboratory have shown that macrophages from infected rats appear to be able to kill C. neoformans, principally by generating NO (58). On the other hand, the NADPH oxidase system was found to be important in the mechanism of C. neoformans killing by rat peritoneal cells, with superoxide anion, hydrogen peroxide, and the hydroxyl radical being involved in this process (60). Our results showed an increase in the amount of NO produced by adherent peritoneal cells from rats treated with HKC-CFA compared with the amount produced by cells from rats treated with CFA-PBS. In contrast, the level of NO production by adherent peritoneal cells from rats treated with CPS-CFA was not significantly different from the level of NO production by adherent peritoneal cells from rats treated with CFA-PBS (Fig. 3). With respect to the production of ROI induced by the different treatments, the level of release of H2O2 by adherent peritoneal cells from HKC-CFA-treated animals was significantly higher than that found in culture supernatants of control cells (Fig. 4A). Moreover, unlike H2O2, the levels of O2− observed in cultures of adherent peritoneal cells from rats treated with HKC-CFA and CFA-PBS were not significantly different (Fig. 4B). On the other hand, the synthesis of H2O2 and O2− by adherent peritoneal cells from rats traded with CPS-CFA was similar to that by cells from control animals (Fig. 4).
During pulmonary infection by C. neoformans in IFN-γ-knockout C57BL/6 mice, the number of aaMac increases. Moreover, the presence of this cellular population is accompanied by a high fungal lung burden and progressive infection (6). Therefore, the development of aaMac could be considered a marker of susceptibility to cryptococcosis (49). In addition, in other infections, such as leishmaniasis and schistosomiasis, the induction of aaMac has been related to the persistence of pathogenic microorganisms and the pathogenesis of the disease (1, 42). aaMac produce arginase and have low levels of TNF-α and decreased levels of intracellular killing. Furthermore, these cells have also been implicated in the differentiation of naïve T cells to the Th2 phenotype (57). In our model, the treatment with CPS-CFA caused an increase in the arginase activity of adherent peritoneal cells compared with that observed for cells from the control animals (Fig. 5). In contrast, this activity of adherent peritoneal cells from HKC-CFA-treated rats was not modified compared with the arginase activity of cells from animals treated with CFA-PBS.
Cytokines play an important role in the immunological response to invading microorganisms. In cryptococcosis, the balance between Th1 and Th2 cytokines is critical to the outcome of the disease. It is well known that the development of a Th1-type immune response is associated with protection against C. neoformans, whereas the Th2 response favors the infection (41). Proinflammatory cytokines such as IL-12 and TNF-α are necessary for the induction of Th1 immunity and an effective host defense. Therefore, the early production of IL-12, as induced by the treatment of mice with mannoprotein from C. neoformans, is essential for the development of the anticryptococcal response seen with this treatment (54). On the other hand, the production of other cytokines, such as IL-10 and TGF-β, is largely associated with more severe cryptococcal infections (41). In our work, the cytokine studies demonstrated that the treatment of rats with HKC-CFA induced a Th1-Th2 disequilibrium, thus favoring the Th1-type pathway (Fig. 6). In contrast, the treatment with CPS-CFA resulted in the production of low levels of proinflammatory cytokines, such as IL-12 and TNF-α, which could lead to the development of a Th2-type response (Fig. 6).
In summary, our results have shown different states of activation of adherent peritoneal cells from rats treated with HKC-CFA or CPS-CFA, with adherent peritoneal cells from animals treated with HKC-CFA being more effective than control cells in ingesting and killing C. neoformans yeast. In a consistent way, these cells were also capable of secreting high levels of anticryptococcal metabolites and the proinflammatory cytokine IL-12. These findings are in agreement with those of our previous studies, in which animals treated with HKC-CFA were more efficient than control rats at resolving the intraperitoneal infection with C. neoformans yeast (8). In addition, at day 21 postinfection, these rats also presented a Th1 response, with IFN-γ being produced by CD4+ T cells. Adherent peritoneal cells from animals treated with CPS-CFA, however, showed an increase in arginase activity and a low level of production of IL-12 and TNF-α and had reduced anticryptococcal activity compared with the findings for CFA-PBS-treated rats. These results are also consistent with the exacerbation of the infection observed in those animals treated with CPS-CFA after intraperitoneal challenge with C. neoformans (8). This macrophage polarization toward an alternative means of activation induced by treatment with CPS-CFA could be related to the findings of the in vitro studies reported by Shoham et al. (62), in which the interaction of GXM with Toll-like receptor 4 was found to result in an incomplete activation of CHO cells, without mitogen-activated protein kinase activation or TNF-α production, suggesting a mechanism of immune dysregulation induced by this polysaccharide. On the other hand, the exacerbation of cryptococcal infection observed in rats treated with CPS-CFA could also be promoted in part by a polysaccharide-mediated apoptosis of immune cells. In this sense, GXM induces the upregulation of the Fas ligand in macrophages, which results in the apoptosis of T cells that express Fas (48).
The activation in the peritoneum is only a part of the immune response mounted by the experimental animals, since we have also observed that HKC-CFA and CPS-CFA treatments promote the differential activation of mononuclear spleen and lymphatic node cells (unpublished results), suggesting systemic involvement in the protection or exacerbation of cryptococcal infection after antigenic treatment. However, in the present study we used a model of intraperitoneal infection reported previously (8). In this sense, we consider that further studies are necessary in order to elucidate if these mechanisms are relevant in the activation of alveolar macrophages during an intranasal or intratracheal challenge, which better represent the natural route of cryptococcal infection.
Therefore, subcutaneous treatment with HKC or CPS emulsified in CFA differentially stimulates the immune system in naïve rats and either favors or inhibits an anti-infection response, thus conditioning the further effector response and the course of the cryptococcal infection.
Acknowledgments
This work was supported by grants from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and the Secretaría de Ciencia y Tecnología de la Universidad Nacional de Córdoba (SECyT-UNC). J. L. Baronetti and A. P. Garro are recipients of fellowships from CONICET. D. T. Masih and L. S. Chiapello are members of the Research Career from CONICET, Argentina.
We thank native English speaker Paul Hobson for revision of the manuscript.
Footnotes
Published ahead of print on 3 June 2009.
REFERENCES
- 1.Abdallahi, O. M., H. Bensalem, R. Augier, M. Diagana, M. De Reggi, and B. Gharib. 2001. Arginase expression in peritoneal macrophages and increase in circulating polyamine levels in mice infected with Schistosoma mansoni. Cell. Mol. Life Sci. 581350-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre, K., E. A. Havell, G. W. Gibson, and L. L. Johnson. 1995. Role of tumor necrosis factor and gamma interferon in acquired resistance to Cryptococcus neoformans in the central nervous system of mice. Infect. Immun. 631725-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alspaugh, J. A., and D. L. Granger. 1991. Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect. Immun. 592291-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez, M., and A. Casadevall. 2006. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 162161-2165. [DOI] [PubMed] [Google Scholar]
- 5.Archer, S. 1993. Measurement of nitric oxide in biological models. FASEB J. 7349-360. [DOI] [PubMed] [Google Scholar]
- 6.Arora, S., Y. Hernandez, J. R. Erb-Downward, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J. Immunol. 1746346-6356. [DOI] [PubMed] [Google Scholar]
- 7.Badylak, S. F., J. E. Valentin, A. K. Ravindra, G. P. McCabe, and A. M. Stewart-Akers. 2008. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng. Part A 141835-1842. [DOI] [PubMed] [Google Scholar]
- 8.Baronetti, J. L., L. S. Chiapello, M. P. Aoki, S. Gea, and D. T. Masih. 2006. Heat killed cells of Cryptococcus neoformans var. grubii induces protective immunity in rats: immunological and histopathological parameters. Med. Mycol. 44493-504. [DOI] [PubMed] [Google Scholar]
- 9.Bauman, S. K., G. B. Huffnagle, and J. W. Murphy. 2003. Effects of tumor necrosis factor alpha on dendritic cell accumulation in lymph nodes draining the immunization site and the impact on the anticryptococcal cell-mediated immune response. Infect. Immun. 7168-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauman, S. K., K. L. Nichols, and J. W. Murphy. 2000. Dendritic cells in the induction of protective and nonprotective anticryptococcal cell-mediated immune responses. J. Immunol. 165158-167. [DOI] [PubMed] [Google Scholar]
- 11.Bolaños, B., and T. G. Mitchell. 1989. Killing of Cryptococcus neoformans by rat alveolar macrophages. J. Med. Vet. Mycol. 27219-228. [PubMed] [Google Scholar]
- 12.Bolaños, B., and T. G. Mitchell. 1989. Phagocytosis of Cryptococcus neoformans by rat alveolar macrophages. J. Med. Vet. Mycol. 27203-217. [PubMed] [Google Scholar]
- 13.Buchanan, K. L., and J. W. Murphy. 1994. Regulation of cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect. Immun. 622930-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchanan, K. L., and J. W. Murphy. 1998. What makes Cryptococcus neoformans a pathogen? Emerg. Infect. Dis. 471-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulmer, G. S., and J. R. Tacker. 1975. Phagocytosis of Cryptococcus neoformans by alveolar macrophages. Infect. Immun. 1173-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherniak, R., L. C. Morris, B. C. Anderson, and S. A. Meyer. 1991. Facilitated isolation, purification, and analysis of glucuronoxylomannan of Cryptococcus neoformans. Infect. Immun. 5959-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherniak, R., E. Reiss, and S. H. Turner. 1982. A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr. Res. 103239-250. [Google Scholar]
- 18.Chiapello, L. S., J. L. Baronetti, M. P. Aoki, S. Gea, H. Rubinstein, and D. T. Masih. 2004. Immunosuppression, interleukin-10 synthesis and apoptosis are induced in rats inoculated with Cryptococcus neoformans glucuronoxylomannan. Immunology 113392-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuck, S. L., and M. A. Sande. 1989. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N. Engl. J. Med. 321794-799. [DOI] [PubMed] [Google Scholar]
- 20.Clemons, K. V., E. Brummer, and D. A. Stevens. 1994. Cytokine treatment of central nervous system infection: efficacy of interleukin-12 alone and synergy with conventional antifungal therapy in experimental cryptococcosis. Antimicrob. Agents Chemother. 38460-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins, H. L., and G. J. Bancroft. 1992. Cytokine enhancement of complement-dependent phagocytosis by macrophages: synergy of tumor necrosis factor-alpha and granulocyte-macrophage colony-stimulating factor for phagocytosis of Cryptococcus neoformans. Eur. J. Immunol. 221447-1454. [DOI] [PubMed] [Google Scholar]
- 22.Corraliza, I. M., M. L. Campo, G. Soler, and M. Modolell. 1994. Determination of arginase activity in macrophages: a micromethod. J. Immunol. Methods 174231-235. [DOI] [PubMed] [Google Scholar]
- 23.D'Andrea, A., M. Aste-Amezaga, N. M. Valiante, X. Ma, M. Kubin, and G. Trinchieri. 1993. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1781041-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Decken, K., G. Köhler, K. Palmer-Lehmann, A. Wunderlin, F. Mattner, J. Magram, M. K. Gately, and G. Alber. 1998. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 664994-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28350-355. [Google Scholar]
- 26.Feldmesser, M., and A. Casadevall. 1997. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J. Immunol. 158790-799. [PubMed] [Google Scholar]
- 27.Feldmesser, M., Y. Kress, P. Novikoff, and A. Casadevall. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 684225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goerdt, S., and C. E. Orfanos. 1999. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity 10137-142. [DOI] [PubMed] [Google Scholar]
- 29.Goerdt, S., O. Politz, K. Schledzewski, R. Birk, A. Gratchev, P. Guillot, N. Hakiy, C. D. Klemke, E. Dippel, V. Kodelja, and C. E. Orfanos. 1999. Alternative versus classical activation of macrophages. Pathobiology 67222-226. [DOI] [PubMed] [Google Scholar]
- 30.Goldman, D., S. C. Lee, and A. Casadevall. 1994. Pathogenesis of pulmonary Cryptococcus neoformans infection in the rat. Infect. Immun. 624755-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldman, D., X. Song, R. Kitai, A. Casadevall, M. L. Zhao, and S. C. Lee. 2001. Cryptococcus neoformans induces macrophage inflammatory protein 1alpha (MIP-1alpha) and MIP-1beta in human microglia: role of specific antibody and soluble capsular polysaccharide. Infect. Immun. 691808-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon, S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 323-35. [DOI] [PubMed] [Google Scholar]
- 33.Gross, N. T., O. M. Guerrero, M. Chinchilla, and C. Jarstrand-Hall. 2006. Trypanosoma lewisi-induced immunosuppression: the effects on alveolar macrophage activities against Cryptococcus neoformans. Exp. Parasitol. 113262-266. [DOI] [PubMed] [Google Scholar]
- 34.Guerrero, O. M., M. Chinchilla, and E. Abrahams. 1997. Increasing of Toxoplasma gondii (Coccidia, Sarcocystidae) infections by Trypanosoma lewisi (Kinetoplastida, Trypanosomatidae) in white rats. Rev. Biol. Trop. 45877-882. [PubMed] [Google Scholar]
- 35.He, W., A. Casadevall, S. C. Lee, and D. L. Goldman. 2003. Phagocytic activity and monocyte chemotactic protein expression by pulmonary macrophages in persistent pulmonary cryptococcosis. Infect. Immun. 71930-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawakami, K., M. H. Qureshi, Y. Koguchi, T. Zhang, H. Okamura, M. Kurimoto, and A. Saito. 1999. Role of TNF-alpha in the induction of fungicidal activity of mouse peritoneal exudate cells against Cryptococcus neoformans by IL-12 and IL-18. Cell. Immunol. 1939-16. [DOI] [PubMed] [Google Scholar]
- 37.Kawakami, K. 2004. Regulation by innate immune T lymphocytes in the host defense against pulmonary infection with Cryptococcus neoformans. Jpn. J. Infect. Dis. 57137-145. [PubMed] [Google Scholar]
- 38.Kawakami, K., M. Tohyama, X. Qifeng, and A. Saito. 1997. Expression of cytokines and inducible nitric oxide synthase mRNA in the lungs of mice infected with Cryptococcus neoformans: effects of interleukin-12. Infect. Immun. 651307-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawakami, K., M. Tohyama, Q. Xie, and A. Saito. 1996. IL-12 protects mice against pulmonary and disseminated infection caused by Cryptococcus neoformans. Clin. Exp. Immunol. 104208-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinjo, T., J. Fujita, and K. Kawakami. 2006. Cooperative regulation of the host defense to cryptococcal infection by innate immune lymphocytes. Nippon Ishinkin Gakkai Zasshi 47201-207. [DOI] [PubMed] [Google Scholar]
- 41.Koguchi, Y., and K. Kawakami. 2002. Cryptococcal infection and Th1-Th2 cytokine balance. Int. Rev. Immunol. 21423-438. [DOI] [PubMed] [Google Scholar]
- 42.Kropf, P., J. M. Fuentes, E. Fähnrich, L. Arpa, S. Herath, V. Weber, G. Soler, A. Celada, M. Modolell, and I. Müller. 2005. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J. 191000-1002. [DOI] [PubMed] [Google Scholar]
- 43.Levitz, S. M. 1994. Macrophage-Cryptococcus interactions. Immunol. Ser. 60533-543. [PubMed] [Google Scholar]
- 44.Li, R. K., and T. G. Mitchell. 1997. Induction of interleukin-6 mRNA in rat alveolar macrophages by in vitro exposure to both Cryptococcus neoformans and anti-C. neoformans antiserum. J. Med. Vet. Mycol. 35327-334. [PubMed] [Google Scholar]
- 45.Lovchik, J., M. Lipscomb, and C. R. Lyons. 1997. Expression of lung inducible nitric oxide synthase protein does not correlate with nitric oxide production in vivo in a pulmonary immune response against Cryptococcus neoformans. J. Immunol. 1581772-1778. [PubMed] [Google Scholar]
- 46.Lovchik, J. A., and M. F. Lipscomb. 1993. Role for C5 and neutrophils in the pulmonary intravascular clearance of circulating Cryptococcus neoformans. Am. J. Respir. Cell Mol. Biol. 9617-627. [DOI] [PubMed] [Google Scholar]
- 47.Mariano Andrade, R., G. Monteiro Almeida, G. Alexandre DosReis, and C. Alves Melo Bento. 2003. Glucuronoxylomannan of Cryptococcus neoformans exacerbates in vitro yeast cell growth by interleukin 10-dependent inhibition of CD4+ T lymphocyte responses. Cell. Immunol. 222116-125. [DOI] [PubMed] [Google Scholar]
- 48.Monari, C., E. Pericolini, G. Bistoni, A. Casadevall, T. R. Kozel, and A. Vecchiarelli. 2005. Cryptococcus neoformans capsular glucuronoxylomannan induces expression of Fas ligand in macrophages. J. Immunol. 1743461-3468. [DOI] [PubMed] [Google Scholar]
- 49.Müller, U., W. Stenzel, G. Köhler, C. Werner, T. Polte, G. Hansen, N. Schütze, R. K. Straubinger, M. Blessing, A. N. McKenzie, F. Brombacher, and G. Alber. 2007. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J. Immunol. 1795367-5377. [DOI] [PubMed] [Google Scholar]
- 50.Murphy, J. W. 1998. Protective cell-mediated immunity against Cryptococcus neoformans. Res. Immunol. 149373-386. [DOI] [PubMed] [Google Scholar]
- 51.Nathan, C. F. 1987. Secretory products of macrophages. J. Clin. Investig. 79319-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parry, S. L., M. Sebbag, M. Feldmann, and F. M. Brennan. 1997. Contact with T cells modulates monocyte IL-10 production: role of T cell membrane TNF-alpha. J. Immunol. 1583673-3681. [PubMed] [Google Scholar]
- 53.Pick, E., and Y. Keisari. 1981. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages—induction by multiple nonphagocytic stimuli. Cell. Immunol. 59301-318. [DOI] [PubMed] [Google Scholar]
- 54.Pietrella, D., R. Cherniak, C. Strappini, S. Perito, P. Mosci, F. Bistoni, and A. Vecchiarelli. 2001. Role of mannoprotein in induction and regulation of immunity to Cryptococcus neoformans. Infect. Immun. 692808-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polfliet, M. M., B. O. Fabriek, W. P. Daniëls, C. D. Dijkstra, and T. K. van den Berg. 2006. The rat macrophage scavenger receptor CD163: expression, regulation and role in inflammatory mediator production. Immunobiology 211419-425. [DOI] [PubMed] [Google Scholar]
- 56.Rayhane, N., C. Fitting, O. Lortholary, F. Dromer, and J. M. Cavaillon. 2000. Administration of endotoxin associated with lipopolysaccharide tolerance protects mice against fungal infection. Infect. Immun. 683748-3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodríguez-Sosa, M., A. R. Satoskar, R. Calderón, L. Gomez-Garcia, R. Saavedra, R. Bojalil, and L. I. Terrazas. 2002. Chronic helminth infection induces alternatively activated macrophages expressing high levels of CCR5 with low interleukin-12 production and Th2-biasing ability. Infect. Immun. 703656-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rossi, G. R., L. A. Cervi, M. M. García, L. S. Chiapello, D. A. Sastre, and D. T. Masih. 1999. Involvement of nitric oxide in protecting mechanism during experimental cryptococcosis. Clin. Immunol. 90256-265. [DOI] [PubMed] [Google Scholar]
- 59.Rossi, G. R., L. A. Cervi, D. A. Sastre, and D. T. Masih. 1998. Lack of involvement of nitric oxide in the macrophage-mediated inhibition of spleen cell proliferation during experimental cryptococcosis. Clin. Immunol. Immunopathol. 8616-26. [DOI] [PubMed] [Google Scholar]
- 60.Rossi, G. R., D. A. Sastre, H. R. Rubinstein, and D. T. Masih. 1994. Biochemical basis for the killing of Cryptococcus neoformans by rat peritoneal cells. J. Med. Vet. Mycol. 32405-414. [PubMed] [Google Scholar]
- 61.Shao, X., J. Rivera, R. Niang, A. Casadevall, and D. L. Goldman. 2005. A dual role for TGF-beta1 in the control and persistence of fungal pneumonia. J. Immunol. 1756757-6763. [DOI] [PubMed] [Google Scholar]
- 62.Shoham, S., C. Huang, J. M. Chen, D. T. Golenbock, and S. M. Levitz. 2001. Toll-like receptor 4 mediates intracellular signaling without TNF-alpha release in response to Cryptococcus neoformans polysaccharide capsule. J. Immunol. 1664620-4626. [DOI] [PubMed] [Google Scholar]
- 63.Stein, M., S. Keshav, N. Harris, and S. Gordon. 1992. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tucker, S. C., and A. Casadevall. 2002. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc. Natl. Acad. Sci. USA 993165-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vecchiarelli, A., M. Dottorini, D. Pietrella, C. Monari, C. Retini, T. Todisco, and F. Bistoni. 1994. Role of human alveolar macrophages as antigen-presenting cells in Cryptococcus neoformans infection. Am. J. Respir. Cell Mol. Biol. 11130-137. [DOI] [PubMed] [Google Scholar]
- 66.Vecchiarelli, A., D. Pietrella, M. Dottorini, C. Monari, C. Retini, T. Todisco, and F. Bistoni. 1994. Encapsulation of Cryptococcus neoformans regulates fungicidal activity and the antigen presentation process in human alveolar macrophages. Clin. Exp. Immunol. 98217-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vecchiarelli, A., C. Retini, D. Pietrella, C. Monari, C. Tascini, T. Beccari, and T. R. Kozel. 1995. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1 beta secretion from human monocytes. Infect. Immun. 632919-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wozniak, K. L., J. M. Vyas, and S. M. Levitz. 2006. In vivo role of dendritic cells in a murine model of pulmonary cryptococcosis. Infect. Immun. 743817-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]