Abstract
The chimeric protein that relies on the T-cell epitopes of antigen 85B (Ag85B) and the 6-kDa early secreted antigen target (ESAT-6) has been demonstrated to augment the Th1 immune response. In this study, we developed a recombinant Mycobacterium bovis BCG (rBCG) strain that secretes the chimeric protein of Ag85B and ESAT-6 (rBCG-AN-E-AC). Immunization with this rBCG strain induced stronger antigen-specific gamma interferon (IFN-γ) activities, as determined by an enzyme-linked immunospot assay, and higher levels of antigen-specific CD4+ and CD8+ T-cell responses than those in the control groups immunized with either rBCG expressing the Ag85B-ESAT-6 fusion protein (rBCG-A-E) or BCG. Likewise, rBCG-AN-E-AC significantly increased the level of production of the major Th1 cytokines IFN-γ and tumor necrosis factor alpha in splenocyte cultures to levels comparable to those elicited by control BCG. Moreover, the antigen-specific immunoglobulin 2c (IgG2c)/IgG1 ratio for mice immunized with rBCG-AN-E-AC was also much higher than the ratios for the other immunized groups. Together, these results indicate that this rBCG-AN-E-AC strain enhances the Th1 cell-mediated response and may serve as a potential vaccine against M. tuberculosis.
Mycobacterium bovis bacillus Calmette Guérin (BCG) is the only vaccine against tuberculosis (TB) currently available and exhibits various levels of efficacy for the prevention of pulmonary TB (range, 0 to 80%) in different trials (9). BCG has a protective effect in children, particularly against tuberculous meningitis; however, it does not satisfactorily prevent the development of pulmonary TB in adults and fails to protect individuals against reinfection (1). Given the rate of mortality from TB worldwide, with more 8 million new cases and 2 million deaths occurring annually (2), newer strategies need to be implemented to improve BCG or vaccines more effective than BCG urgently need to be developed.
One approach that might be used to increase the efficacy of BCG could be to construct a recombinant BCG (rBCG) which either overexpresses immunogenic antigens or modulates the ensuing immune response (8). rBCG vaccines are attractive because of the widespread experience with their use, the known immunogenicity associated with protection against the worst forms of the disease in children, and the safety profiles of standard BCG strains (13). Two rBCG vaccines have been entered into clinical trials. This includes rBCG30, which expresses the antigen 85B (Ag85B) protein, and ΔureC hly-positive rBCG, which expresses listeriolysin and which is urease deficient (12, 15). It is hoped that these vaccines will provide a strong and perhaps longer-lasting immune response than that achieved with the conventional BCG vaccine.
The most effective defined-antigen TB vaccines will likely require the induction of both cell-mediated and humoral immune responses. Ag85B and the 6-kDa early secreted antigen target (ESAT-6) have been identified as two of the most promising vaccine candidates which are strongly recognized by T lymphocytes (3, 19). In a previous study, we relied on the T-cell epitopes of Ag85B and ESAT-6 to design a chimeric protein by inserting ESAT-6 into Ag85B from amino acids 167 to 182 and demonstrated that this recombination of Ag85B and ESAT-6 could improve the immunogenicity and enhance the T-helper type 1 (Th1) cell-mediated immune response (27). This finding prompted us to explore further the efficacy of rBCG overexpressing this chimeric protein. In this study, we constructed rBCG expressing chimeric protein Ag85BN-ESAT-6-Ag85BC (rBCG-AN-E-AC) and further compared the immune response to that protein with that to rBCG expressing the Ag85B-ESAT-6 fusion protein (rBCG-A-E) and BCG.
MATERIALS AND METHODS
Bacterial strains and cultures.
Mycobacterium bovis BCG (obtained from Shanghai Biological Products Institute) and rBCG were grown on Middlebrook 7H9 medium (Difco Laboratories, Detroit, MI) supplemented with 0.5% glycerol, 0.05% Tween 80, and 10% albumin-dextrose-catalase or on solid Middlebrook 7H11 medium (Difco Laboratories) supplemented with oleic acid-albumin-dextrose-catalase. When it was required, the antibiotic kanamycin was added at a concentration of 25 μg/ml. Escherichia coli DH5α was grown in Luria-Bertani medium and was used for cloning.
rBCG preparation.
The coding sequence for the signal sequence of ag85b was amplified from M. tuberculosis H37Rv genomic DNA by PCR with the following primers: 5′-ATATGGCCAATGACAGACGTGAGCC-3′ (upper primer) and 5′-TTAGGATCCCGCGCCCGCGGTTG-3′ (lower primer). The PCR product was digested with BalI and BamHI and cloned into pMV261. The recombinant plasmid was termed pMV261-SA. The coding region of AN-E-AC was amplified from Escherichia coli DH5α, including the chimeric plasmid ag85b1-501-esat-61-295-ag85b545-855, by PCR with primers 5′-ATAGGATCCTTCTCCCGGCCGGG-3′ (upper primer) and 5′-TTAGAATTCGCGAACATCCCAGTGA-3′ (lower primer). The PCR product was digested with BamHI and EcoRI and cloned into pMV261-AS. The insertion of the genes was confirmed by sequencing. The recombinant plasmid was transformed into BCG by electroporation (24). The transformed BCG cells were plated on 7H11 medium supplemented with 25 μg/ml kanamycin and were grown at 37°C for 3 weeks. Individual colonies were picked and identified. rBCG expressing the chimeric protein AN-E-AC was analyzed by immunoblotting with anti-Ag85B or anti-ESAT-6 rabbit polyclonal antisera. rBCG-A-E was prepared as described previously (28). The stability of the plasmid in rBCG-AN-E-AC was assessed by subculturing the strain (1:10 dilution) three consecutive times for 4 weeks each in the absence of kanamycin and analyzing the filtrate of the subculture.
Mice and immunization.
The female C57BL/6 mice (age, 6 to 8 weeks) used in this study were obtained from the Animal Center of the Second Military Medical University. The mice were maintained under specific-pathogen-free conditions. The mice (five per group) were immunized subcutaneously at the base of the tail vein with 5 × 106 CFU of BCG or rBCG in 100 ml phosphate-buffered saline (PBS). The mice were killed after 6 weeks to prepare sera and splenocytes. All experiments were performed in accordance with the guidelines of the local ethics committee.
Antibody analysis by indirect ELISA.
Sera were collected from the immunized mice to monitor the antibody response by an indirect enzyme-linked immunosorbent assay (ELISA). Briefly, ELISA plates (Maxisorb type 96F; Nunc, Roskilde, Denmark) were coated overnight at 4°C with Ag85B (0.5 μg/well) and ESAT-6 (1 μg/well) separately in carbonate buffer (pH 9.6). The plates were blocked with 200 ml/well PBS containing 1% bovine serum albumin for 30 min at 37°C and washed with PBS containing 0.05% Tween 20 three times. Sera were added at serial twofold dilutions (beginning at a 1/500 dilution) for 2 h at 37°C and washed, followed by addition of 150 ml/well horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G (IgG) (Dingguo Biotechnology, Beijing, People's Republic of China), IgG1, and IgG2c diluted 1/10,000, 1/1,000, and 1/1,000 in PBS, respectively. The plates were incubated for 1 h at 37°C, washed, and developed with 0.1 M citrate-phosphate buffer, pH 5.0, containing 1 mg/ml o-phenylenediamine and 0.03% hydrogen peroxide. Antibody titers are expressed as reciprocal endpoint titers. The reactions were stopped by addition of 50 ml/well of 2 M H2SO4 and were read on an ELISA plate reader at 492 nm.
IFN-γ ELISPOT assay.
The mice were euthanized, and their spleens were removed aseptically. The spleens were gently ground through a 70-μm cell strainer, and then single-cell suspensions were prepared by density-gradient centrifugation with a Lympholyte-M apparatus (Cedar Lane Lab, Burlington, NC). Lymphocytes from spleen cells were diluted in culture medium containing an appropriate stimulus (2 μg/ml Ag85B and 5 μg/ml ESAT-6, respectively) were placed in the wells of the enzyme-linked immunospot (ELISPOT) assay plate at 5 × 105 cells/well in duplicate. A mouse gamma interferon (IFN-γ) ELISPOT assay kit (CT317-PR5; U-Cytech Biosciences, Utrecht, The Netherlands) was used to determine the relative number of IFN-γ-expressing cells in the suspensions of single spleen cells by following the manufacturer's instructions. Finally, the spots were visualized and counted. Wells with fewer than 10 spots were not used for calculations.
Cytokine measurement by sandwich ELISA.
The spleen cells were adjusted to a concentration of 2 × 106 cells/well before they were cultured in 24-well plates (Nunc) in RPMI 1640 medium containing 10% fetal calf serum, 2 mM glutamine, 50 mM β-mercaptoethanol, 100 mg/ml streptomycin, and 100 U/ml penicillin. For each group, the mixture of purified recombinant Ag85B and recombinant ESAT-6 (final protein concentrations, 5 μg/ml) was added to stimulate the lymphocytes. The cells were incubated at 37°C in a humidified CO2 incubator, and the supernatants were harvested after 72 h. Supernatants were screened for IFN-γ, tumor necrosis factor alpha (TNF-α), and interleukin-4 (IL-4) by ELISA with a mouse IFN-γ, TNF-α, and IL-4 ELISA kit (eBioscience, Inc. San Diego, CA). The ELISAs were carried out according to the protocol recommended by the manufacturer. The concentrations of the cytokines were calculated from standard curves generated with the CurveExpert (version 3.0) program.
Lymphocyte subpopulation analysis by flow cytometry.
Lymphocytes from spleen cells were washed and incubated with 2 μg fluorescein isothiocyanate (FITC)-conjugated anti-CD4 and 2 μg phycoerythrin (PE)-conjugated anti-CD8 (eBioscience, Inc.). The cell samples were incubated at 4°C for 30 min, washed, and resuspended in PBS. Isotype controls consisting of FITC- and PE-conjugated anti-rat IgG2b were used. Flow cytometric analysis was carried out with 10,000 events by using a FACSCalibur fluorescence-activated cell sorter (Becton Dickinson, Mountain View, CA).
Statistical methods.
The data were statistically analyzed by one-way analysis of variance, followed by Tukey's test. P values of less than 0.05 were considered statistically significant.
RESULTS
Expression of chimeric protein AN-E-AC on rBCG.
The chimeric plasmid ag85b1-501-esa-t61-295-ag85b545-855 was placed into a site immediately downstream of the Ag85B signal sequence. When BCG was transformed with this construction, the chimeric protein was readily detectable in both rBCG lysates and culture supernatants by immunoblotting, and a control BCG strain showed no specific band on the same blots (Fig. 1). To determine whether rBCG-AN-E-AC expresses Ag85B and ESAT-6 in the absence of selective pressure, like that which would occur in vivo, we cultured three consecutive generations and studied the expression of Ag85B and ESAT-6 in the absence of kanamycin, an antibiotic to which the recombinant plasmid conferred resistance. The cultures showed no decrease in the level of Ag85B and ESAT-6 expression and indicated that the recombinant plasmid is stably maintained in the absence of selective pressure.
FIG. 1.
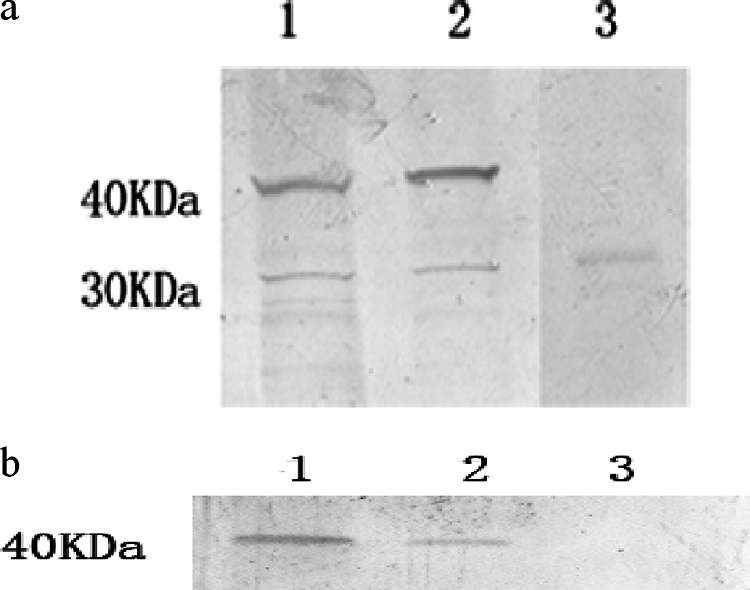
Western blot analysis. rBCG-AN-E-AC lysates and culture supernatants were analyzed for expression of the chimeric protein by immunoblotting with antibodies recognizing Ag85B (a) and ESAT-6 (b). Lanes: 1, cell lysates of rBCG-AN-E-AC; 2, culture supernatants of rBCG-AN-E-AC; 3, culture supernatants of BCG.
Antibody response.
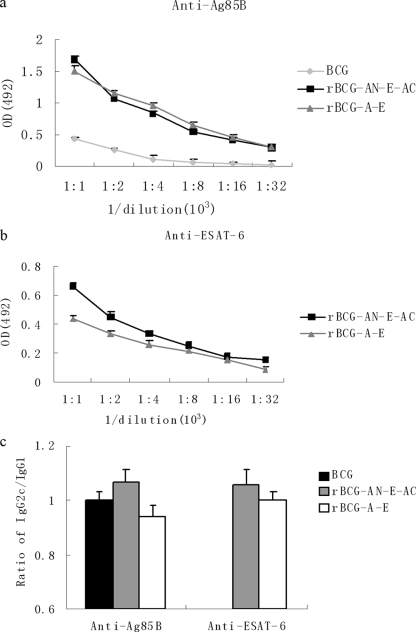
Groups of mice were immunized with rBCG-AN-E-AC, rBCG-A-E, or BCG; and the control group was immunized with PBS. Antisera were collected from the immunized mice; and the Ag85B- or ESAT-6-specific IgG, IgG1, and IgG2c levels were determined. We found that mice immunized with rBCG-AN-E-AC gave a little stronger specific IgG response against the ESAT-6 antigen than the mice in the group immunized with rBCG-A-E (Fig. 2b). The titers of antibodies specific to Ag85B in the two groups were not significantly different (Fig. 2a).
FIG. 2.
Antibody response against Ag85B or ESAT-6 in mice immunized with BCG and rBCG. C57BL/6 mice (five mice per group) were immunized with PBS, BCG, rBCG-A-E, or rBCG-AN-E-AC and killed after 6 weeks to prepare serum for examination of the IgG antibody response (a and b) and the ratio of IgG2c/IgG1 (c). The results are expressed as the means ± standard errors.
The gene coding for IgG2a is deleted in C57BL/6 mice (17). Therefore, in the absence of a functional IgG2a gene, the IgG2c isotype was used as an indicator of a Th1 response. The ratios of IgG2c/IgG1 were calculated to determine the levels of induction of the Th1/Th2 responses in animals. Figure 2c illustrates the levels of the IgG1 and IgG2c isotype antibodies against the Ag85B or the ESAT-6 protein. As a result, the ratio of IgG2c/IgG1 for the mice immunized with rBCG-AN-E-AC was higher than that for the mice immunized with rBCG-A-E or BCG whether the antibodies were against the Ag85B or the ESAT-6 protein. Therefore, the ability to induce Th1 and Th2 responses increased in the group immunized with rBCG-AN-E-AC. The antibody titers in the PBS-treated control group were only 1:50 (data not shown in Fig. 2). Since the esat-6 gene does not exist in the BCG strain, no obvious IgG, IgG1, or IgG2a titers were detected in the mice immunized with the BCG strain. Therefore, we show only the IgG titers and the ratios of IgG2c/IgG1 against the ESAT-6 protein for mice immunized with rBCG.
IFN-γ ELISPOT activities.
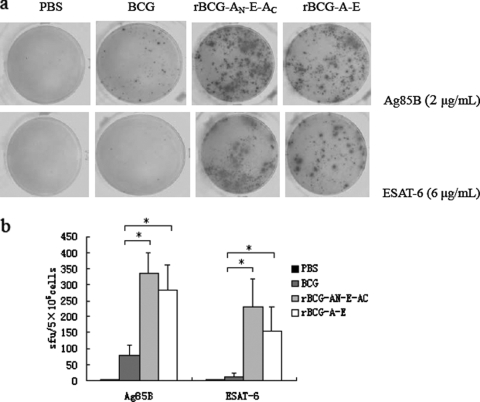
IFN-γ, a key cytokine involved in cellular immune responses, is a direct indicator of an ongoing Th1 type of immune response. We used an ELISPOT assay to detect the antigen-specific IFN-γ activities in suspensions of single spleen cells from immunized mice. Figure 3 illustrates that the IFN-γ responses to the Ag85B or the ESAT-6 antigen by rBCG vaccinees exceeded (P < 0.05) those by the BCG vaccinees, and these responses in the mice immunized with rBCG-AN-E-AC were higher than those in the mice immunized with rBCG-A-E.
FIG. 3.
Analysis of antigen-specific IFN-γ production. The cellular immune response was measured by an ELISPOT assay with splenocytes isolated from C57BL/6 mice immunized with PBS, rBCG-AN-E-AC, rBCG-A-E, or BCG. Splenocytes were stimulated with 2 μg/ml Ag85B or 5 μg/ml ESAT-6. (a) Result from the immunospot image analyzer; (b) number of cells secreting IFN-γ per 5 × 105 cells. Bars represent the mean number of spot-forming units (sfu) ± standard error. *, the endpoint number of spot-forming units was significantly higher than that for the group inoculated with the BCG strain (P < 0.05).
Cytokine production.
The secretion of some cytokines by mouse spleen cells was assayed by a sandwich ELISA after restimulation of the mixture of the Ag85B and the ESAT-6 antigens to examine the effect of rBCG on the Th1 or Th2 immune response. IFN-γ, TNF-α, and IL-4 were detected, as shown in Table 1. Mice immunized with rBCG-AN-E-AC showed an enhanced release of IFN-γ and TNF-α (54.56 ± 6.46 pg/ml and 92.47 ± 18.36 pg/ml, respectively) in response to stimulation with the mixture of the Ag85B and the ESAT-6 antigens compared with the levels of release by the group immunized with rBCG-A-E (48.35 ± 3.05 pg/ml for IFN-γ, 80.25 ± 17.99 pg/ml for TNF-α) and the group immunized with BCG (37.46 ± 2.06 pg/ml for IFN-γ, 47.43 ± 11.26 pg/ml for TNF-α). On the other hand, the production of IL-4 was below the sensitivity limits of our assays and was hardly detected.
TABLE 1.
Cytokine productiona
| Cytokine | Cytokine concn (pg/ml) after immunization with:
|
||
|---|---|---|---|
| BCG | rBCG-AN-E-AC | rBCG-A-E | |
| IFN-γ | 37.46 ± 2.06 | 54.56 ± 6.46 | 48.35 ± 3.05 |
| TNF-α | 47.43 ± 11.26 | 92.47 ± 18.36 | 80.25 ± 17.99 |
| IL-4 | <4b | <4 | <4 |
Cytokine production by mouse spleen cells was assayed by a sandwich ELISA after stimulation of the cells with a mixture of Ag85B and ESAT-6.
The limit sensitivity of the kit was 4 pg/ml, and the level of production of IL-4 was too low to be detected.
Quantitative CD4+ and CD8+ responses.
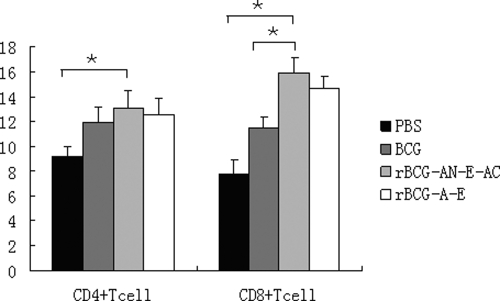
The most effective vaccination strategies in animal models are those that stimulate both CD4+ and CD8+ T cells to produce Th1-associated cytokines (29). After vaccination, the lymphocyte subsets were examined for differences in their percentages by flow cytometry. As shown in Fig. 4, the levels of the CD4+ and CD8+ T-cell populations were higher in the animals immunized with rBCG than in the control group. In the group vaccinated with rBCG-AN-E-AC, the mean percentage of CD4+ T cells was 1.1-fold and the mean percentage of CD8+ T cells was 1.4-fold higher than those observed in mice vaccinated with BCG. Moreover, the CD8+/CD4+ ratio in mice immunized with PBS or BCG was about 0.9, that in mice immunized with rBCG-AN-E-AC was 1.2, and that in mice immunized with rBCG-A-E was 1.1. Therefore, the group vaccinated with rBCG-AN-E-AC had enhanced CD4+ and CD8+ T-cell responses and had an increased CD8+/CD4+ ratio.
FIG. 4.
Analysis of T-cell percentages. Splenocytes were extracted from the immunized mice and incubated with FITC-conjugated anti-CD4 and PE-conjugated anti-CD8. The percentages of CD4+ and CD8+ T cells were determined by flow cytometry. The results are expressed as the mean ± standard error. *, the percentages of CD4+ and CD8+ T cells were significantly greater than those in the group immunized with PBS or BCG (P < 0.05).
DISCUSSION
rBCG expressing protective antigens represents a very promising possibility for use as an efficient vaccine against TB. Furthermore, the fact that the vast majority of individuals exposed to M. tuberculosis do not develop TB suggests that an improvement of the immunogenicity of BCG could reap huge dividends in terms of protection (23). Therefore, the improvement of BCG remains the best choice for the rational design of a vaccine against TB (8). Our previous study suggested that the chimeric protein AN-E-AC, which was designed according to the T-cell epitopes of Ag85B and ESAT-6, could enhance the Th1 response. In the present work, we recombined this chimeric plasmid into BCG to construct rBCG-AN-E-AC and compared its immunogenicity with the immunogenicities of rBCG-A-E and BCG.
M. tuberculosis is a typical intracellular pathogen, and hence, the cellular immune response comprising CD4+ T cells or CD8+ T cells, or both, can be important in controlling infection and preventing or delaying the onset of disease (6). The contribution of CD4+ T cells is thought to be mediated mainly through the production of cytokines (10, 14), and CD8+ T cells contribute to protective responses against mycobacteria (11, 22). In our study, analysis of the T-cell subpopulations indicated that both CD4+ and CD8+ T cells are significantly stimulated in mice vaccinated with rBCG, especially in the group immunized with rBCG-AN-E-AC. Interestingly, the CD8+/CD4+ ratio increased in the mice immunized with rBCG-AN-E-AC compared with the ratio in mice immunized with BCG. Andersen and colleagues have demonstrated that the depletion of CD8+ T cells but not the depletion of CD4+ T cells impairs bacterial control in the Cornell model of latent M. tuberculosis infection (26). Moreover, CD8+ T cells have been shown to contribute to the killing of intracellular mycobacteria in humans (5, 25). Our results suggest that the design of rBCG-AN-E-AC could elicit M. tuberculosis-specific CD8+ T cells, and it is hoped that they would have increased protective efficacy and reduce the bacterial loads in vaccinated mice.
Immunity to M. tuberculosis depends on a robust Th1 cell-mediated response and, in particular, the continued production of IL-12, IFN-γ, and TNF-α (7). Moreover, IFN-γ and TNF-α are key cytokines, in that they activate macrophages to control bacterial proliferation by increasing the level of fusion of the phagosome with the lysosome (16, 18). An effective vaccine must induce a lasting Th1 memory response; over the longer term it might also need to inhibit the development of a Th2 response or downregulate it if it is already present (7). Our data indicate that rBCG stimulated a significantly larger number of IFN-γ-secreting T cells than the BCG vaccines, and mice immunized with rBCG-AN-E-AC had the strongest response. In agreement with these data, the TNF-α recall response was associated with the appearance of IFN-γ secretion. Therefore, rBCG-AN-E-AC could increase the Th1 responses when memory is suppressed by regulatory mechanisms.
The levels of IgG1 and IgG2c reflect the stimulation of Th2 and Th1 cells, respectively (4). In this study, we used the antigen-specific IgG2c/IgG1 ratio as an indicator of whether a predominant Th1 or Th2 response was induced by vaccination. We showed that vaccination with rBCG-AN-E-AC resulted in a biased toward IgG2c production and Th1-type antibody isotypes. The IgG2c/IgG1 ratios were consistent with enhanced Th1 cytokine (IFN-γ and TNF-α) activity.
The study of Palendira et al. showed that the rBCG strain secreting the Ag85B-ESAT-6 fusion protein displayed a satisfactory safety profile and improved the protective efficacy of the existing vaccine against M. tuberculosis challenge within the lung (20). In this study, we relied on the T-cell epitopes of Ag85B and ESAT-6 to design rBCG expressing the chimeric protein AN-E-AC. ESAT-6, one of the secretory proteins, has been shown to be strongly and broadly recognized by Th1 cells in the early phase of infection in patients and experimental animals with active TB (21). When Ag85B is fused with ESAT-6, the fusion site of ESAT-6 is significant, and this influences the Th1 responses. The sequence of the Ag85B and ESAT-6 chimeric recombinant may change the availability of the peptide and enhance the Th1 response (27).
In conclusion, the immunogenicity studies revealed that rBCG-AN-E-AC would be a possible candidate for development as a potential vaccine against M. tuberculosis. It enhanced the Th1 response by inducing higher levels of IFN-γ and TNF-α, increasing the IgG2c/IgG1 ratio, and producing more CD4+ and CD8+ T cells, especially CD8+ T cells. The findings of this study encourage the further evaluation of the protective effect of rBCG-AN-E-AC against M. tuberculosis.
Acknowledgments
This work was supported by the Infectious Disease Project of the 11th Five-Year Plan (grant 2008ZX-10003-013) and the China Postdoctoral Science Foundation (grant 20070420086).
Footnotes
Published ahead of print on 10 June 2009.
REFERENCES
- 1.Andersen, P., and T. M. Doherty. 2005. The success and failure of BCG-implications for a novel tuberculosis vaccine. Nat. Rev. 3656-662. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 2008. Global tuberculosis control 2008: surveillance, planning, financing. WHO report. World Health Organization, Geneva, Switzerland.
- 3.Brandt, L., M. Elhay, I. Rosenkrands, E. B. Lindblad, and P. Andersen. 2000. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 68791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai, H., D. H. Yu, X. Tian, and Y.X. Zhu. 2005. Coadministration of interleukin 2 plasmid DNA with combined DNA vaccines significantly enhances the protective efficacy against Mycobacterium tuberculosis. DNA Cell Biol. 24605-613. [DOI] [PubMed] [Google Scholar]
- 5.Cho, S., V. Mehra, S. Thoma-Uszynski, S. Stenger, N. Serbina, R.J. Mazzaccaro, et al. 2006. Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc. Natl. Acad. Sci. USA 9712210-12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darrah, P. A., D. T. Patel, P. M. De Luca1, R. W. B. Lindsay, D. F. Davey, B. J. Flynn, S. T. Hoff, P. Andersen, S. G. Reed, S. L. Morris, M. Roederer, and R. A. Seder. 2007. Multifunctional TH1 cells define a correlate of vaccine mediated protection against Leishmania major. Nat. Med. 13843-850. [DOI] [PubMed] [Google Scholar]
- 7.Doherty, T. M., and G. Rook. 2006. Progress and hindrances in tuberculosis vaccine development. Lancet 367947-949. [DOI] [PubMed] [Google Scholar]
- 8.Eddine, A. N., and S. H. E. Kaufmann. 2005. Improved protection by recombinant BCG. Microbes Infect. 7939-946. [DOI] [PubMed] [Google Scholar]
- 9.Fine, P. E. M. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 3461339-1345. [DOI] [PubMed] [Google Scholar]
- 10.Flynn, J. L. 2004. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinburgh) 8493-101. [DOI] [PubMed] [Google Scholar]
- 11.Flynn, J. L., M. M. Goldstein, K. J. Triebold, B. Koller, and B. R. Bloom. 1992. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 8912013-12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grode, L., P. Seiler, S. Baumann, J. Hess, V. Brinkmann, A. N. Eddine, et al. 2005. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J. Clin. Investig. 1152472-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoft, D. F. 2008. Tuberculosis vaccine development: goals, immunological design, and evaluation. Lancet 372164-175. [DOI] [PubMed] [Google Scholar]
- 14.Hogg, A. E., A. Worth, P. Beverley, C. J. Howarda, and B. Villarreal-Ramos. 2009. The antigen-specific memory CD8+γσ T-cell response induced by BCG in cattle resides in the CD8+/TCR−CD45RO+ T-cell population. Vaccine 27270-279. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz, M. A., G. Harth, B. J. Dillon, and S. Maslesa-Galic. 2000. Recombinant bacillus Calmette-Guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 9713853-13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacMicking, J. D., G. A. Taylor, and J. D. McKinney. 2003. Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science 302654-659. [DOI] [PubMed] [Google Scholar]
- 17.Martin, R. M., and A. M. Lew. 1998. Is IgG2a a good Th1 marker in mice? Immunol. Today 1949. [DOI] [PubMed] [Google Scholar]
- 18.North, R. J., and Y. J. Jung. 2004. Immunity to tuberculosis. Annu. Rev. Immunol. 22599-623. [DOI] [PubMed] [Google Scholar]
- 19.Olsen, A. W., L. A. H. van Pinxteren, L.M. Okkels, P. B. Rasmussen, and P. Andersen. 2001. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Infect. Immun. 692773-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palendira, U., J. M. Spratt, W. J. Britton, and J. A. Triccas. 2005. Expanding the antigenic repertoire of BCG improves protective efficacy against aerosol Mycobacterium tuberculosis infection. Vaccine 231680-1685. [DOI] [PubMed] [Google Scholar]
- 21.Ravn, P., A. Demissie, T. Eguale, H. Wondwosson, D. Lein, H. A. Amoudy, et al. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179637-645. [DOI] [PubMed] [Google Scholar]
- 22.Sousa, A. O., R. J. Mazzaccaro, R. G. Russell, F. K. Lee, O. C. Turner, S. Hong, et al. 2000. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA 974204-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephen, T. R., and S. H. E. Kaufmann. 2008. Rational design of vaccines against tuberculosis directed by basic immunology. Int. J. Med. Microbiol. 298143-150. [DOI] [PubMed] [Google Scholar]
- 24.Stover, C. K., G. P. Bansal, M. S. Hanson, J. E. Burlein, S. R. Palaszynski, J. F. Young, S. Koenig, D. B. Young, A. Sadziene, and A. G. Barbour. 1993. Protective immunity elicited by recombinant bacille Calmette-Guerin (BCG) expressing outer surface protein A (OspA) lipoprotein: a candidate Lyme disease vaccine. J. Exp. Med. 178197-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner, J., and H. M. Dockrell. 1996. Stimulation of human peripheral blood mononuclear cells with live Mycobacterium bovis BCG activates cytolytic CD8+ T cells in vitro. Immunology 87339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Pinxteren, L. A., J. P. Cassidy, B. H. Smedegaard, E. M. Agger, and P. Andersen. 2000. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur. J. Immunol. 303689-3698. [DOI] [PubMed] [Google Scholar]
- 27.Xu, Y., B. Wang, J. Chen, Q. Wang, B. Zhu, H. Shen, Y. Qie, J. Wang, and H. Wang. 2006. Chimaeric protein improved immunogenicity compared with fusion protein of Ag85B and ESAT-6 antigens of Mycobacterium tuberculosis. Scand. J. Immunol. 64476-481. [DOI] [PubMed] [Google Scholar]
- 28.Xu, Y., B. Zhu, Q. Wang, J. Chen, Y. Qie, J. Wang, H. Wang, B. Wang, and H. Wang. 2007. Recombinant BCG coexpressing Ag85B, ESAT-6 and mouse-IFN-γ confers effective protection against Mycobacterium tuberculosis in C57BL/6 mice. FEMS Immunol. Med. Microbiol. 51480-487. [DOI] [PubMed] [Google Scholar]
- 29.Yu, D. H., M. Li, X. D. Hu, and H. Cai. 2007. A combined DNA vaccine enhances protective immunity against Mycobacterium tuberculosis and Brucella abortus in the presence of an IL-12 expression vector. Vaccine 256744-6754. [DOI] [PubMed] [Google Scholar]