Abstract
Antigens of Mycobacterium bovis elicit a cell-mediated immune response upon intradermal injection in cattle. In vitro, such antigens stimulate the production of gamma interferon (IFN-γ) by bovine T cells in whole-blood culture (IFN-γ assay). We have analyzed various parameters of the in vitro IFN-γ assay, ranging from blood sampling to execution of the IFN-γ test, in view of potential simplifications of the assay. Here, we show that IFN-γ responses may be reduced under certain animal handling/holding conditions and that a delayed time from blood collection to culture may lead to a reduced in vitro IFN-γ response. Delayed initiation of culture in a purified-protein-derivative-based assay (24 h compared to 8 h after blood collection), however, resulted in a significant improvement of specificity (97% compared to 85%), whereas there was only a modest reduction of sensitivity (from 96% to 90%), which was statistically not significant. Furthermore, we show that the stimulation temperature needs to be 33°C or higher; that carbon dioxide is not required for stimulation; and that various plate formats, ranging from 24 to 96 wells per plate, can be utilized. The produced IFN-γ is stable at 4°C for 28 days as well as after repeated freeze-thaw cycles. Thus, stimulation of samples may be initiated in the field without the need for a carbon dioxide source, and bovine IFN-γ is stable under various routine laboratory temperature scenarios. These findings demonstrate opportunities for improvements in the bovine IFN-γ test platform and flexibilities in test application.
Bovine tuberculosis (TB), caused by Mycobacterium bovis, has an important and adverse effect on socioeconomic conditions, public health, and trade of animals and animal products (2). Eradication of bovine TB in cattle is based on detection and slaughter of infected animals or whole herds. The standard antemortem screening test for detection of TB is the intradermal tuberculin skin test (i.e., intradermal injection of tuberculin eliciting a cell-mediated immune response [CMI] at the site, which in turn leads to skin thickening). As an alternative, the CMI can be measured in vitro by stimulating blood cells with tuberculin, which in turn leads to production of gamma interferon (IFN-γ), which can then be quantified by an enzyme-linked immunosorbent assay (ELISA; Bovigam IFN-γ assay) (15).
The Bovigam assay constitutes a laboratory-based TB test and is widely used complementarily to the tuberculin skin test (4, 11), as it offers national TB control programs and industry an additional tool for curtailing the spread of TB in cattle and other Bovidae. The assay critically depends on the sample quality, culture conditions, and quality control of stimulation reagents. The CMI, both in vivo and in vitro, may be negatively affected by stress or corticosteroid application (5). Thus, parallel stimulation of blood leukocytes with mitogen or superantigen in the IFN-γ assay is commonly used as an indicator of sample quality and potential for underlying CMI suppression, thereby reducing the risk of false-negative test results. Conditions such as the anticoagulant used for blood collection, the temperature and time of blood storage, and the culture duration also affect IFN-γ production in whole-blood culture (8). Furthermore, delays in culture setup, initial high sample temperatures (2 h at 37°C, followed by 22 h at 22°C, prior to 24 h of culture at 37°C), or a low sample temperature (4°C) diminishes IFN-γ responses with samples from M. bovis-infected cattle (14). Thus, sample quality, as affected by pre- and postcollection parameters, affects the accuracy of the IFN-γ test. Once the sample reaches the laboratory, additional variables, such as the culture plate format, the culture conditions, the antigens used for TB-specific stimulation, the nonspecific stimulation control reagents, and the cutoff for the final test interpretation, may also influence test performance. While the ability to modify IFN-γ test parameters offers the possibility to adapt the assay more closely to the needs within a TB program, this option also provides challenges to ensure standardization of testing procedures and quality assurance. Indeed, variations in assay protocols, with both the skin test and the IFN-γ test, have resulted in disparate results in test accuracy between studies (reviewed in reference 4).
We therefore analyzed the capacities of animals under various field conditions to produce IFN-γ. Furthermore, we evaluated different parameters of the in vitro assay, including the influence of time to culture initiation, culture vessel geometry, the cell culture temperature, the need for carbon dioxide (generally used in tissue culture to stabilize pH), the animal holding conditions, and the stability of the produced IFN-γ under various standard storage scenarios. All of these parameters were analyzed in view of defining a range of possible conditions and potentially simplifying the assay for use in bovine TB eradication programs.
MATERIALS AND METHODS
Animals.
The groups consisted of cattle experimentally and naturally infected with M. bovis and uninfected animals (Table 1). Five male, TB-free Holstein-Friesian calves were housed according to institutional guidelines at the National Animal Disease Center (NADC), Ames, IA, in a biosafety level 3 facility. All animal care and use procedures were reviewed and approved by the NADC Animal Care and Use Committee. Calves received M. bovis strain 95-1315 by aerosol at 6 months of age as described previously (12). Blood samples for this study were collected 4 months after infection. Cattle naturally infected with M. bovis were obtained from herds with histories of bovine TB, as determined by Animal Health, United Kingdom (previously the State Veterinary Service). Reactors positive by the single intradermal comparative cervical tuberculin test (SICCT) were kept in the animal units at the Veterinary Laboratories Agency (VLA), Weybridge, Surrey, United Kingdom. Animal experiments at the VLA were undertaken under a license granted by the United Kingdom Home Office that was obtained after approval by the local ethical review committee. The animals were predominantly Holstein-Friesians and were all older than 12 months of age. TB was confirmed for all animals by postmortem analysis including culture. Uninfected control animals were obtained from herds free of TB in Great Britain, France, and Switzerland. Additionally, field samples were collected from TB cattle herds and skin test-positive cattle at an abattoir in Northern Ireland. The group of cattle from Mexico comprised dairy cows of the Holstein-Friesian breed originating from two high-prevalence herds (in which approximately 70% of the animals were positive by the caudal fold test).
TABLE 1.
Summary of experiments and cattle groups
| Experimental variable | Animal group(s) | Antigen(s) in IFN-γ assay (concn [μg/ml]) | No. of cattle | Location | Cattle breed(s) | Age |
|---|---|---|---|---|---|---|
| Effect of blood storage duration on: | ||||||
| Stimulation control | Cattle naturally infected with M. bovis | SEB (1) | 34 | VLA | Holstein-Friesian | >12 mo |
| Cattle naturally infected with M. bovis | PWM (5) | 198 | Mexico | Holstein-Friesian | >12 mo | |
| Sensitivity and specificity | Cattle naturally infected with M. bovis | Weybridge PPDs | 107 | VLA | Holstein-Friesian | >12 mo |
| Uninfected cattle | Weybridge PPDs | 146 | VLA | Holstein-Friesian | >12 mo | |
| Sensitivity and specificity with regard to skin test result | Cattle naturally infected with M. bovis | Weybridge PPDs | 107 or 324 | VLA | Holstein-Friesian | >12 mo |
| Uninfected cattle | Weybridge PPDs | 874 | VLA | Holstein-Friesian | >12 mo | |
| Effect of vessel geometry | Cattle experimentally infected with M. bovis | E:C and PWM (5) | 5 | NADC | Holstein-Friesian | 6 mo (at time of infection) |
| Cattle naturally or experimentally infected with M. bovis and uninfected controls | Weybridge PPDs and SEB (1) | 23 | VLA | Holstein-Friesian | >12 mo | |
| Effect of culture temp | Cattle experimentally infected with M. bovis | Prionics PPDs, E:C, and PWM (5) | 5 | NADC | Holstein-Friesian | 6 mo (at time of infection) |
| Effect of carbon dioxide | Uninfected cattle | SEB (1 and 0.1) | 20 | Prionics | Holstein-Friesian and Swiss Brown | 2-9 yr |
| Stability of natural IFN-γ | Uninfected cattle | SEB (1, 0.1, and 0.01) | 5 | Prionics | Holstein-Friesian and Swiss Brown | 2-9 yr |
| Effect of animal holding conditions | TB-positive herd | PWM (4) | 7,988 | Northern Ireland | Holstein-Friesian | >6 mo |
| Cattle at abattoir | PWM (4) | 513 | Northern Ireland | Holstein-Friesian | >6 mo | |
| Uninfected cattle | PWM (5) | 317 | France | Camargue and Brava | >24 mo | |
| Cattle naturally infected with M. bovis | PWM (5) | 100 | Mexico | Holstein-Friesian | >12 mo |
Antigens.
Bovine (purified protein derivative [PPD] B) and avian (PPD A) PPDs were supplied by Prionics AG, Schlieren, Switzerland, and by the Tuberculin Production Unit at the VLA, Weybridge, Surrey, United Kingdom. They were used to stimulate whole blood at 20 μg/ml (Prionics PPDs) and 10 μg/ml (Weybridge PPDs). Recombinant ESAT-6:CFP-10 fusion protein (E:C) was received as a kind gift from F. C. Minion, Iowa State University, and has been produced as described by Waters et al. (13). E:C was used at a concentration of 5 μg/ml in whole-blood culture. Pokeweed mitogen (PWM; Sigma) and staphylococcal enterotoxin B from Staphylococcus aureus (SEB; Sigma) were used as positive controls at the indicated concentrations.
IFN-γ assay.
Whole-blood cultures were performed with 96-well plates by mixing 0.25 ml of heparinized blood with 25 μl of antigen/mitogen-containing solution. For comparison of different culture formats, we used, in addition, 24-well plates with 1.5 ml of whole blood per well and 48-well plates with 1 ml of whole blood per well. In general, the supernatants were harvested after 24 h of culture at 37°C and 5% CO2 in a humidified incubator. For certain experiments, variations in culture temperature and CO2 conditions were evaluated. IFN-γ concentrations were determined using a Bovigam ELISA kit (Prionics AG, Schlieren, Switzerland). Optical density at 450 nm (OD450) was determined. Values of ≥0.1 for PPD B OD450 minus PPD A OD450 and antigen OD450 minus NIL OD450 were used as criteria for determination of positive versus negative responses where appropriate. Only samples with an unstimulated (NIL) OD450 of <0.2 and PWM/SEB-stimulated OD450 of >0. 5 were considered valid for analysis.
Statistical analysis.
Data were analyzed as a completely randomized design by using Statview software (version 5.0; SAS Institute, Inc., Cary, NC). In vitro IFN-γ secretion was analyzed as a split plot with factorial analysis of variance. Sample handling conditions before culture, type of culture plate, and incubation temperature during culture were included in the model as fixed effects, and type of animal was included in the model as the random effect. Fisher's protected-least-significant-difference test was applied when effects (P < 0.05) were detected by the model. Differences between responses for fresh blood and those observed following overnight storage were assessed by Student's t test. The effects of blood storage on test sensitivity and specificity were analyzed using Fisher's exact test. Both kinds of analysis were made using GraphPad Instat software (version 3; GraphPad, San Diego, CA).
RESULTS
Effect of blood storage duration on IFN-γ production.
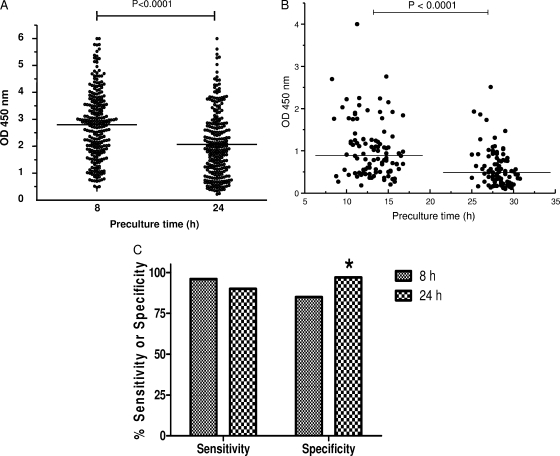
Blood samples from 34 naturally TB infected cattle from the United Kingdom, collected on several occasions, were stimulated either within 8 h of collection or after 24 h at room temperature with SEB (Fig. 1A). IFN-γ responses to SEB were significantly reduced (P < 0.0001) in samples held for 24 h compared to the levels observed for freshly stimulated samples. A similar effect was observed when PWM was used (Fig. 1B). Blood samples from TB-infected animals from Mexico were stimulated with PWM either 8 to 17 h or 25 to 31 h after collection. The two groups showed a significant difference in their levels of IFN-γ production in response to PWM (P < 0.0001), and subsequently, more responses fell below the cutoff of 0.5 OD450 units in the group with the longer holding time (i.e., 24% in the group with the 8- to 17-h holding time versus 52% in the group with the 25- to 31-h holding time).
FIG. 1.
Effects of preculture time on IFN-γ production. (A) IFN-γ responses to SEB were reduced (P < 0.0001) after 24 h versus 8 h of blood storage. Data are presented as OD values for naturally infected animals (n = 34), each tested at multiple sampling points. (B) Blood was obtained from cattle within a TB-positive herd. IFN-γ responses to PWM were reduced (P < 0.0001) after 25 to 31 h (n = 98) compared to the levels observed after 8 to 17 h (n = 100) of blood storage. The horizontal lines indicate the median OD values. (C) Blood samples were obtained from skin test-positive cattle with bovine TB confirmed by culture and/or pathology (n = 107) and from TB-free animals from areas where TB is not endemic (n = 146). Bars indicate percent sensitivity and specificity values for samples obtained within 8 h of blood sampling and after 24 h of storage. IFN-γ responses were determined using the standard criterion for test interpretation (i.e., PPD B OD450 − PPD A OD450 ≥ 0.1). *, increased (P < 0.05) specificity with samples stored for 24 h.
We furthermore compared the impacts on test sensitivity and specificity (Fig. 1C) of initiating the whole-blood culture on the day of sampling and after overnight storage with samples obtained from 107 naturally infected cattle and 146 TB-free cattle. According to standard criteria for test interpretation (PPD B OD450 − PPD A OD450 ≥ 0.1), overnight storage resulted in reduced sensitivity (from 96% to 90%), although this effect was not statistically significant (P = 0.16). Conversely, we observed a significant (P = 0.03) improvement in specificity in blood stored overnight (from 85% to 97%) with this sample set.
We next determined whether the reduced sensitivity resulting from overnight storage was more evident in diseased cattle that were skin test negative, as had been suggested previously (7). For this study, we performed receiver operator comparison (ROC) analyses using samples from tuberculous animals that tested skin test positive or negative as well as disease-free animals (Table 2). All cultures were initiated after overnight storage. The results demonstrate comparable sensitivities in detecting diseased animals whether they tested skin test positive or not (89.7% versus 91.4%, with areas under the curve of 0.941 and 0.947, for skin test-positive and -negative cows, respectively). Please note that a large number of animals with confirmed TB presented as skin test negative.
TABLE 2.
Bovigam test performance in cattle with confirmed bovine TB presenting with positive or negative skin test responses
| Skin test status of diseased animalsa | Data set size for ROC analysisb | AUC (95% CI)c | Cutoff pointc,d | Sensitivity (%)c | Specificity (%)c |
|---|---|---|---|---|---|
| Positive (n = 107) | 981 | 0.941 (0.903-0.977) | 0.1 | 89.7 | 96.5 |
| Negative (n = 324) | 1,198 | 0.947 (0.924-0.965) | 0.1 | 91.4 | 96.5 |
| Positive and negative combined | 0.944 (0.926-0.962) | 0.1 | 90.9 | 96.5 |
Diseased animals included cattle with culture-confirmed disease and/or lesions.
Values shown are numbers of diseased animals plus numbers of disease-free animals used in this study for ROC analysis. There were 874 TB-free animals in both analyses.
Data from ROC analysis performed with culture-positive and/or lesioned animals together with disease-free cattle. AUC, area under the curve. Blood samples from cattle with confirmed TB, presenting as either skin test positive or skin test negative, were stimulated after overnight storage. Sensitivity and specificity for the two groups individually or combined are shown.
The cutoff point was defined as the PPD B OD450 minus the PPD A OD450.
Effects of vessel geometry, culture temperature, and presence of carbon dioxide on IFN-γ production.
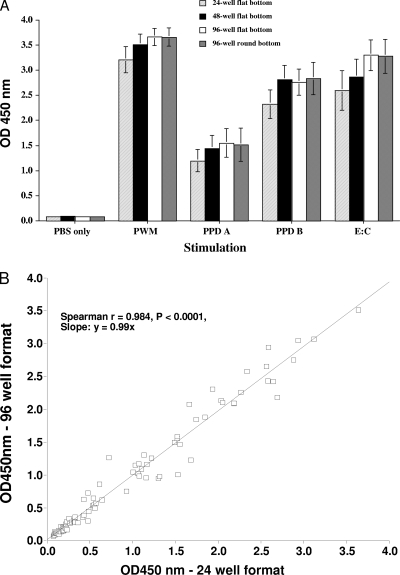
The effects of vessel geometry were evaluated using different stimulating antigens (Fig. 2A and B). Whole-blood samples from M. bovis-infected cattle (n = 5; aerosol infected; United States) were incubated with phosphate-buffered saline (PBS) or RPMI medium (no stimulation) or PPD B, PPD A, PWM, or E:C in 96-well (round- or flat-bottom), 48-well, or 24-well plates for 24 h. The responses were not found to differ (P > 0.05) between vessel geometry types, even when evaluated over a range of stimuli (Fig. 2A). Additionally, IFN-γ responses to PPD B, PPD A, SEB, or PBS from cattle naturally infected with M. bovis (n = 23; United Kingdom) were compared to determine the effects of 24- versus 96-well-plate formats (Fig. 2B). The responses did not differ (P > 0.05) between 24- and 96-well-plate formats, and the responses were positively correlated (Spearman r = 0.9836; P < 0.0001).
FIG. 2.
Effects of vessel geometry on IFN-γ response. (A) Data are presented as the mean OD450 ± standard error of the mean (SEM) for IFN-γ responses to various stimuli (indicated on the x axis) in blood samples from M. bovis-infected cattle (n = 5; aerosol infected; United States) in 24-, 48-, or 96-well flat-bottomed plates as well as 96-well round-bottomed plates. Responses did not differ (P > 0.05) between vessel geometry types. (B) IFN-γ responses to PPD B, PPD A, SEB, or PBS (n = 23; United Kingdom; 16 cattle naturally infected with M. bovis, 5 experimentally infected cattle, and 2 uninfected cattle) were compared to determine the effects of 24- versus 96-well-plate formats. There was a highly significant correlation of responses between 24- and 96-well-plate formats, with a slope close to 1 (Spearman r = 0.9836; P < 0.0001).
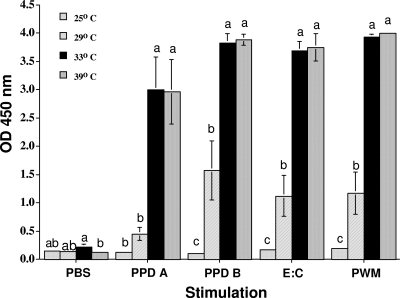
To evaluate the effects of stimulation temperature, whole-blood samples from M. bovis-infected cattle (n = 5; aerosol infected; United States) were incubated at various temperatures (39, 33, 29, 25, and 22°C) in the presence of PPD A, PPD B, E:C, PWM, or PBS (Fig. 3). Responses to antigen or mitogen did not differ when cultured at 39°C versus 33°C (P > 0.05); however, responses were significantly decreased (P < 0.05) at 29°C or less.
FIG. 3.
Effects of culture temperature on IFN-γ response. Data are presented as the mean OD450 ± SEM for IFN-γ responses to various stimuli (indicated on the x axis) in blood samples from M. bovis-infected cattle (n = 5; aerosol infected) incubated at various temperatures (39, 33, 29, 25, and 22°C). The responses did not differ when the samples were cultured at 39°C versus 33°C (P > 0.05); however, responses were significantly decreased (P < 0.05) at 29°C or less.
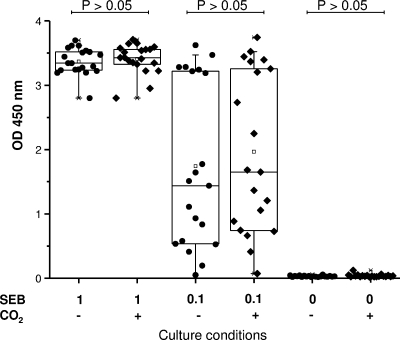
Cell culture is conventionally done in the presence of carbon dioxide (CO2). In view of the practicality of stimulation under field conditions, we investigated the need for CO2. Whole-blood samples from 20 uninfected animals were stimulated with two concentrations of SEB or PBS (Fig. 4). No significant difference (P > 0.05) was found between the groups with and without CO2, indicating that carbon dioxide is not needed for adequate stimulation.
FIG. 4.
Effects of carbon dioxide on IFN-γ response. Whole-blood samples from TB-free cattle (n = 20) were cultured with or without CO2 (indicated in the lower margin) in air at 37°C. Stimulation was performed with SEB at 1, 0.1, and 0 μg/ml as indicated. ODs obtained for the individual animals are shown. Boxes indicate the lower quartile (25%), the median (50%), and the higher quartile (75%). Responses with and without CO2 did not differ (P > 0.05).
Stability of IFN-γ.
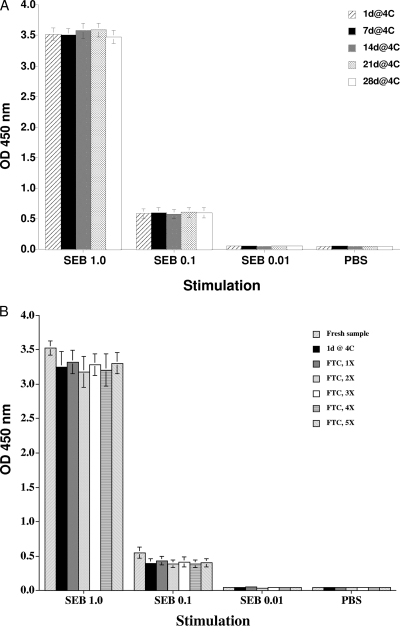
IFN-γ produced in whole-blood culture was analyzed for its stability, which determines the time span in which the ELISA part of the assay has to be carried out. Surprisingly, IFN-γ was stable for at least 4 weeks at 4°C (Fig. 5A). Additionally, responses in plasma stored at 4°C for 1 day or at −80°C with up to five freeze-thaw cycles did not differ (P > 0.05) from responses obtained with freshly harvested plasma (Fig. 5B). These results indicate a high level of stability of bovine IFN-γ in plasma.
FIG. 5.
Effects of plasma storage on IFN-γ response. Results are shown for plasma collected from TB-negative cattle (n = 5) after stimulation with SEB at 1, 0.1, 0.01, and 0 μg/ml. (A) Plasma samples were analyzed directly after collection and after prolonged storage at 4°C for up to 28 days (28d@4C). (B) Additionally, plasma samples were analyzed after storage at −80°C with one to five (5X) freeze-thaw cycles. Data are presented as the mean OD450 ± SEM. The responses observed after the various storage conditions did not differ (P > 0.05).
Effect of environmental conditions on IFN-γ production.
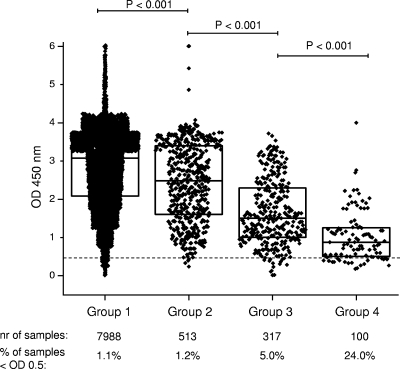
The IFN-γ responses of animals sampled under four different conditions were evaluated (Fig. 6). The conditions of the different groups included bleeding only in the field (group 1; Northern Ireland); bleeding after transport and holding at an abattoir (group 2; Northern Ireland); bleeding of animals at a fighting bull herd facility (group 3; France); and bleeding of malnourished, heavily parasitized animals after transport and holding at an abattoir (group 4; Mexico). The distribution of responses to PWM did not differ between groups 1 and 2, while the other two groups (groups 3 and 4) had lower (P < 0.05) IFN-γ responses to PWM than groups 1 and 2. The percentages of invalid IFN-γ responses to PWM stimulation increased from group 1 to group 4: 1.1% in group 1 (85 of 7,988 samples), 1.2% in group 2 (6 of 513 samples), 5% in group 3 (16 of 317 samples), and 24% in group 4 (24 of 100 samples). These results demonstrate that IFN-γ responses may be reduced under certain animal handling/holding conditions.
FIG. 6.
Comparison of IFN-γ production levels obtained after PWM stimulations performed with different animal groups. Group 1, cattle from TB-positive herds in Northern Ireland (normal on-farm sampling; low stress conditions); group 2, cattle tested at an abattoir in Northern Ireland (moderate stress conditions due to transport and waiting at the slaughterhouse); group 3, cattle from a fighting bull herd in France (severe stress conditions due to handling); group 4, cattle from Mexico (severe, persistent stress conditions due to nutritional shortage, parasitic burden, and transport). The dashed line indicates the cutoff for a valid PWM stimulation (0.5 OD units). Boxes indicate the lower quartile (25%), the median (50%), and the higher quartile (75%).
DISCUSSION
The aim of our study was to analyze various parameters of the in vitro IFN-γ assay for bovine TB (Bovigam) to standardize and improve the flexibility of its use as a tool for eradication of bovine TB. As the assay relies on functional leukocytes, environmental conditions before and during the culture period may influence the test outcome. We investigated various parameters of the IFN-γ assay and their effect on in vitro IFN-γ production and detection in view of defining a range of possible assay conditions and simplifications on the one hand and in view of standardizing the assay on the other hand, which is a requirement for reliable disease control and eradication.
Setting up the blood culture on the day of sampling is logistically and financially unrealistic in many situations. It is therefore important to investigate the impact of blood storage on test sensitivity and specificity. Studies in New Zealand have concluded that similar percentages of animals were diagnosed positive when the Bovigam test was performed on the day of sampling and within 24 h of sampling (9). It had, however, been observed that the OD values obtained after overnight storage of the blood samples were substantially lower than those obtained when the blood culture was started on the day of sampling. These results were obtained with reactor animals with confirmed TB and formed the basis of New Zealand's policy requiring initiation of the assay within 24 h of sampling (9). Despite decreases in signal strength (OD), we observed only an insignificant drop in sensitivity with samples from SICCT field reactors with confirmed TB while we noted a significant increase in specificity when the blood was kept overnight rather than cultured freshly (from 85% to 97%) (Fig. 1C). In a recent study by Coad and coworkers, it was confirmed that testing blood 24 h after collection did not change the diagnostic outcome when PPDs were used but that it did when a defined antigen, such as ESAT-6, was used (3). It may be necessary to apply more-stringent test cutoffs or to test blood on the day of collection to ensure optimal test sensitivity with defined antigens (3). In contrast to these findings, which suggest that overnight storage of blood has a relatively minor impact on sensitivity, a recent paper by Gormley and colleagues (7) suggested that the delay in blood culture significantly reduced test sensitivity. In their study, three groups of IFN-γ test-positive animals were assessed, namely, SICCT reactors with visible lesions (confirmed TB), a group of SICCT-positive animals from surveillance operations, and a group of 60 SICCT-negative animals. When the Bovigam test was applied to the group with confirmed TB, the sensitivity did not change within 8 h or 24 h, while the percentage of IFN-γ-positive animals that had previously been tested only with the skin test decreased dramatically for the 24-h time point. This decrease in positive animals could be due to reduced sensitivity or increased specificity. Gormley and colleagues (7) had suggested decreased sensitivity, even though the true disease statuses of these animals had not been determined. Testing animals with defined disease status by performing ROC analysis, we were able to show that the test performances were identical regardless of whether skin test-positive or -negative diseased animals were tested (Table 2). In the present study, delays in initiation of culture significantly reduced IFN-γ production after both SEB and PWM stimulations. Conversely, overnight blood storage caused only a modest decrease in the sensitivity of the PPD-based IFN-γ assay but a significant increase in the specificity. Further studies are needed to evaluate these effects on alternative antigens, such as ESAT-6 and CFP-10. Starting stimulation in the field may overcome low signal strength by using defined antigens and result in maximum sensitivity and specificity.
Some factors, such as time to stimulation and culture temperature, were expected to have an effect, while other factors, like carbon dioxide level and culture plate geometry, did not influence the assay. Incubation in 96-well culture plates allows for automation of handling. Since there is no need for CO2 during stimulation, this assay might be done in a decentralized way, with the only condition being that the incubation temperature needs to be kept above 33°C. A minor disadvantage of 96- versus 48- or 24-well plates is lower sample volume, which results in fewer opportunities for repeat testing with the ELISA step of the assay. Much to our surprise, IFN-γ produced in cell culture was very stable at 4°C. Obviously, the sample remained sterile, as we would expect degradation if there were bacterial growth. As an alternative to prolonged storage at 4°C, the sample can be frozen and thawed at a later time for analysis. Five such freeze-thaw cycles did not alter the amount of IFN-γ detected in the ELISA. These results suggest that the assay is very robust and allows for centralized test performance after stimulation.
Our data suggest that certain animal holding and handling procedures may result in reduced IFN-γ production, as measured by mitogen stimulation (Fig. 6). Aagaard and colleagues (1) had in fact seen somewhat lower reactivity in normal Mexican cattle than in Northern Irish animals, yet this might have been due to differential exposures to environmental mycobacteria. Even if there is a tendency for Mexican cattle to lower IFN-γ production, we find the massive difference in mitogen stimulation quite surprising. In the absence of technical reasons, we suggest different levels of stress in these populations; however, we have not measured any stress parameters. It is intriguing that under normal field conditions, the percentage of samples with suboptimal stimulation (i.e., invalid samples) is low (1.1% for bleeding in the field and 1.2% for bleeding at the abattoir), while in populations with handling stress (fighting bulls) or difficult holding conditions (nutritional stress and parasitic burden), a significantly higher number of animals were below the validity cutoff for mitogen stimulation (Fig. 6), suggesting that we might find more false-negative diagnoses under such conditions. Unfortunately, our results did not allow us to verify this hypothesis, but it may be worthwhile to measure stress parameters in future studies in order to substantiate our suggestion. Parasitic burden (Fasciola hepatica) has been shown to alter macrophage function and therefore interleukin-4 and IFN-γ expression in animals coinfected with M. bovis BCG (6). Please note that a low stimulation control level does not automatically mean a negative outcome for the TB IFN-γ test. Interestingly, 20 of 75 animals from Mexico with invalid PWM stimulation were positive by the IFN-γ assay using TB-specific antigens (data not shown). This indicates that TB-specific antigens may result in a more potent stimulation of lymphocytes than PWM in some cases, although the mechanisms for this observation are unclear.
In conclusion, these findings demonstrate opportunities for improvements in the bovine IFN-γ test platform and flexibilities in test application. In particular, it may be possible to perform the stimulation under appropriate conditions out in the field, followed by shipping to a diagnostic laboratory.
Acknowledgments
We thank Jessica Pollock, Rachel Huegel, Bart Olthof, and Mike Howard for excellent technical support. We also express our appreciation to the staff of the Animal Service Unit at the VLA, the NADC, and the ETH Forschungsanstalt Chamau.
Footnotes
Published ahead of print on 1 July 2009.
REFERENCES
- 1.Aagaard, C., M. Govaerts, V. Meikle, A. J. Vallecillo, J. A. Gutierrez-Pabello, F. Suarez-Guemes, J. McNair, A. Cataldi, C. Espitia, P. Andersen, and J. M. Pollock. 2006. Optimizing antigen cocktails for detection of Mycobacterium bovis in herds with different prevalences of bovine tuberculosis: ESAT6-CFP10 mixture shows optimal sensitivity and specificity. J. Clin. Microbiol. 444326-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buddle, B. M., A. R. McCarthy, T. J. Ryan, J. M. Pollock, H. M. Vordermeier, R. G. Hewinson, P. Andersen, and G. W. de Lisle. 2003. Use of mycobacterial peptides and recombinant proteins for the diagnosis of bovine tuberculosis in skin test-positive cattle. Vet. Rec. 153615-620. [DOI] [PubMed] [Google Scholar]
- 3.Coad, M., R. G. Hewinson, D. Clifford, H. M. Vordermeier, and A. O. Whelan. 2007. Influence of skin testing and blood storage on interferon-gamma production in cattle affected naturally with Mycobacterium bovis. Vet. Rec. 160660-662. [DOI] [PubMed] [Google Scholar]
- 4.de la Rua-Domenech, R., A. T. Goodchild, H. M. Vordermeier, R. G. Hewinson, K. H. Christiansen, and R. S. Clifton-Hadley. 2006. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, gamma-interferon assay and other ancillary diagnostic techniques. Res. Vet. Sci. 81190-210. [DOI] [PubMed] [Google Scholar]
- 5.Dondo, A., M. Goria, M. C. Abete, M. Giammarino, G. Allasia, and L. Nicolandi. 1996. Effect of dexamethasone on gamma interferon test in cattle infected with M. bovis, p. 25-28. Proceedings Società Italiana delle Scienze Veterinarie, vol. L. Perugia, Italy. [Google Scholar]
- 6.Flynn, R. J., C. Mannion, O. Golden, O. Hacariz, and G. Mulcahy. 2007. Experimental Fasciola hepatica infection alters responses to tests used for diagnosis of bovine tuberculosis. Infect. Immun. 751373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gormley, E., M. B. Doyle, K. McGill, E. Costello, M. Good, and J. D. Collins. 2004. The effect of the tuberculin test and the consequences of a delay in blood culture on the sensitivity of a gamma-interferon assay for the detection of Mycobacterium bovis infection in cattle. Vet. Immunol. Immunopathol. 102413-420. [DOI] [PubMed] [Google Scholar]
- 8.Rothel, J. S., S. L. Jones, L. A. Corner, J. C. Cox, and P. R. Wood. 1990. A sandwich enzyme immunoassay for bovine interferon-gamma and its use for the detection of tuberculosis in cattle. Aust. Vet. J. 67134-137. [DOI] [PubMed] [Google Scholar]
- 9.Ryan, T. J., B. M. Buddle, and G. W. De Lisle. 2000. An evaluation of the gamma interferon test for detecting bovine tuberculosis in cattle 8 to 28 days after tuberculin skin testing. Res. Vet. Sci. 6957-61. [DOI] [PubMed] [Google Scholar]
- 10.Reference deleted.
- 11.Vordermeier, H. M., A. Whelan, K. Ewer, T. Goodchild, R. Clifton-Hadley, J. Williams, and R. G. Hewinson. 2006. The BOVIGAM® assay as ancillary test to the tuberculin skin test. Gov. Vet. J. 1672-80. [Google Scholar]
- 12.Waters, W. R., M. V. Palmer, D. L. Whipple, M. P. Carlson, and B. J. Nonnecke. 2003. Diagnostic implications of antigen-induced gamma interferon, nitric oxide, and tumor necrosis factor alpha production by peripheral blood mononuclear cells from Mycobacterium bovis-infected cattle. Clin. Diagn. Lab. Immunol. 10960-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waters, W. R., B. J. Nonnecke, M. V. Palmer, S. Robbe-Austermann, J. P. Bannantine, J. R. Stabel, D. L. Whipple, J. B. Payeur, D. M. Estes, J. E. Pitzer, and F. C. Minion. 2004. Use of recombinant ESAT-6:CFP-10 fusion protein for differentiation of infections of cattle by Mycobacterium bovis and by M. avium subsp. avium and M. avium subsp. paratuberculosis. Clin. Diagn. Lab. Immunol. 11729-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waters, W. R., B. J. Nonnecke, S. C. Olsen, and M. Palmer. 2007. Effects of pre-culture holding time and temperature on interferon-gamma responses in whole blood cultures from Mycobacterium bovis-infected cattle. Vet. Microbiol. 119277-282. [DOI] [PubMed] [Google Scholar]
- 15.Wood, P. R., L. A. Corner, and P. Plackett. 1990. Development of a simple, rapid in vitro cellular assay for bovine tuberculosis based on the production of gamma interferon. Res. Vet. Sci. 4946-49. [PubMed] [Google Scholar]