Abstract
Pending removal from the market of a commercial assay (the AUSAB [Abbott Laboratories] enzyme immunoassay [EIA]) for the determination of antibodies to hepatitis B surface antigen (HBsAg), a new in-house quantitative enzyme-linked immunosorbent assay (ELISA) to measure antibodies against HBsAg (anti-HBs) was developed (anti-HBs in-house). Specific anti-HBs antibodies were sandwiched between the precoated HBsAg ad and ay subtypes purified from plasma from hepatitis B virus (HBV) human carriers and the recombinant HBsAg adw2 subtype tagged with horseradish peroxidase. The assay was calibrated against the 1st International Reference Preparation for anti-hepatitis B immunoglobulin (lot 1977-W1042). Analytical sensitivity and the limit of quantitation were estimated at 0.43 mIU/ml and 2.0 mIU/ml, respectively. Overall reproducibility was 11.86%, and accuracy was estimated to be 94.89%. More than 4,000 samples from seven clinical trials were tested with the anti-HBs in-house assay and compared to results generated with AUSAB EIA and AUSAB radioimmunoassay (RIA). During the technical validation, the anti-HBs in-house assay was compared to the AUSAB RIA as a reference (n = 919). Overall assessment of concordance and Deming's regression analysis were performed. The coefficient of correlation between the AUSAB RIA and anti-HBs in-house assay was 0.9815 with a slope of 0.9187. The overall agreement between anti-HBs in-house and AUSAB RIA was 97.61%, considering the clinical cutoffs at 3.3 mIU/ml and 1.0 mIU/ml for the respective assays. From a clinical perspective, seroprotection rates and anti-HBs geometric mean antibody concentrations for individual studies calculated with either the in-house assay or the reference assays were similar. Conclusions of individual studies were confirmed. The performance characteristics of the in-house assay are acceptable. There is no evidence that use of the new assay would lead to different clinical conclusions from the reference method.
Hepatitis B infection is a global health problem but most acutely affects developing countries (16). Currently there is no effective therapy against hepatitis B, whose disease spectrum ranges from asymptomatic disease to chronic liver diseases, including cirrhosis and hepatocellular carcinoma. Prevention of the illness through vaccination remains the method of choice for its control and eradication. Active immunization against hepatitis B infection can be achieved using vaccines containing either inactivated hepatitis B virus (HBV) surface protein (HBsAg) physicochemically purified from plasma from HBV human carriers or recombinant surface antigen produced by transfer of the S gene of HBV coding for HBsAg to an appropriate plasmid that is then inserted into the desired expression vector. The recombinant vaccine manufactured by GlaxoSmithKline Biologicals (GSK) is produced in yeast and is antigenically similar to plasma-derived HBsAg (4). Clinical studies of this recombinant vaccine either formulated as a single-component vaccine (Engerix-B; trademark of GSK) or formulated in combination with other antigens such as hepatitis A vaccine (Twinrix; trademark of GSK) or pediatric diphtheria-tetanus-pertussis-based vaccines (such as Infanrix hexa and Tritanrix HepB; trademarks of GSK) have proven its efficacy and immunogenicity (5).
To date, commercial assays from Abbott Laboratories have been used at GSK to quantify the immune response to HBV vaccines in terms of antibodies against HBsAg (anti-HBs). However, since these assays are no longer commercially available in Europe, GSK has developed an in-house assay with adequate technical and clinical performance to ensure long-term supply of an assay with consistent quality. This paper describes the development, technical validation, and the clinical assessment of the new anti-HBs in-house assay.
MATERIALS AND METHODS
Selection of the immunological ELISA format.
A sandwich enzyme-linked immunosorbent assay (ELISA) format was selected whereby specific anti-HBs antibodies were sandwiched between precoated HBsAg passively adsorbed onto the microplate and HBsAg tagged with horseradish peroxidase (HRP). Because the purpose of HBV vaccination is to protect against possible future contact with the wild-type virus, native HBsAg ad and ay subtypes (11), purified from plasma from HBV human carriers, were used as the solid phase. Quantification of antibodies raised by vaccination used HRP conjugate prepared from the recombinant HBsAg adw2 subtype, similar to that contained in the GSK vaccine (8), tagged with HRP.
Preparation of the HBsAg-precoated plates.
Microplates were passively coated with 100 μl of a mixture (wt/wt) of native human HBsAg ad and ay subtypes (15) from The Binding Site (San Diego, CA) at 1 μg/ml (wt/vol) in carbonate buffer (pH 9.5) for 24 h at room temperature. The plates were then washed four times with distilled water supplemented with NaCl (9 g/liter) and Tween 20 (Merck) and blocked with 200 μl of phosphate-buffered saline (PBS; 0.0095 M [pH 7.4]) (Cambrex) supplemented with enzymatically hydrolyzed 0.1% casein (Sigma) and 0.5 ml/liter Proclin-300 (Supelco) as a preservative for 24 h at room temperature. Plates were washed four times with distilled water with 0.05% Tween 20 and 10g/liter saccharose (Sigma) and then dried overnight in a laminar flux. Each microplate was individually packed in a sealed aluminum bag that included desiccant.
Preparation of HBsAg-HRP conjugate.
The recombinant HBsAg adw2 subtype was conjugated to sodium periodate-oxidized HRP (type VI RZ 3.0; Sigma) by a modification of the original method of Nakane et al. (7). Half a milliliter of a freshly prepared aqueous solution of 0.14 M sodium periodate solution (Aldrich) was added to 1 ml of 50 mg/ml HRP solution in distilled water and gently stirred for 30 min in the dark in order to generate active aldehyde functions on the hydroxyl groups of the several carbohydrate moieties. Then the HRP-aldehyde solution was chromatographically separated from periodate by gel filtration (Sephadex G-25) and quantified by absorbance at 403 nm. (A 1-mg/ml amount of oxidized HRP displays an optical density at 403 nm [OD403] of 1.6). The molar ratio between oxidized HRP after separation and the HBsAg to be coupled was 2.0.
The HBsAg solution previously dialyzed overnight at 4 to 8°C against 0.1 mol/liter sodium carbonate buffer (pH 9.5) was mixed with the HRP-aldehyde solution for 2 h at room temperature in the dark in order to generate Schiff's bases between the primary amine groups on HBsAg and the aldehyde groups present on the oxidized HRP. Remaining reactive aldehyde groups were blocked by adding 2 M Tris solution at a rate of 22 μl per mg of HRP-aldehyde and incubation for 30 min at room temperature. Subsequently the Schiff bases were reduced for 30 min at 4°C by the addition of 0.21 M sodium borohydrure (Aldrich) at a rate of 10 μl/mg of HRP-aldehyde engaged in the conjugation process. The HBsAg-HRP was chromatographically separated by gel filtration on Ultrogel AcA-34 (Amersham Biosciences) equilibrated with 0.05 M Tris buffer (pH 7.5) containing 0.5 ml/liter Tween 20. Finally, the fractions containing the HBsAg-HRP conjugate were pooled, supplemented with 10 g/liter bovine serum albumin (BSA) (Cohn fraction V; Serologicals) and 2 ml/liter Proclin-300 and stored at 4 to 8°C after filtration on a 0.2-μm-pore filtering device.
Preparation and calibration of anti-HBs antibody standard.
A pool of sera from HBV vaccine recipients with high levels of anti-HBs was diluted in fetal bovine serum (FBS; HyClone) containing 0.2 g/liter thymol (Sigma) and 100 μg/ml gentamicin sulfate (Sigma) as preservatives. After adjustment to around 190 mIU/ml, referring to the 1st International Reference Preparation (1st IRP) for anti-hepatitis B immunoglobulin (1), the standard preparation was dispensed in 1-ml aliquots and freeze-dried. To conduct an assay, one freeze-dried vial was reconstituted with 1 ml of distilled water and then serially diluted (six times) with standard and sample diluent (SSD; FBS containing 2 ml/liter Proclin-300 as a preservative). SSD was used as a zero standard. FBS was used instead of recalcified human plasma free of anti-HBs (human plasma) in order to prevent future availability issues. The standard was calibrated against the anti-HBV immunoglobulin 1st IRP, lot 1977-W1042. For calibration purposes, four in-house anti-HBs standard vials were reconstituted with 1 ml of distilled water and then serially diluted (neat plus six steps) with FBS and incubated on four different lots of precoated plates. At the same time, serial dilutions in human recalcified plasma free of anti-HBs antibody ranging between 1.58 mIU/ml and 200.00 mIU/ml of one reference ampoule (1st IRP, lot 1977-W1042), freshly reconstituted according to IRP instructions, were incubated. Consequently, 96 measures based on serial dilutions were obtained. After correction for their respective dilution factor, the average concentration of the 96 individual values was determined. The values obtained before correction for dilution were extrapolated from the standard curve generated by the WHO preparation using a four-parameter logistic fitting algorithm.
Preparation of assay controls.
Four anti-HBs controls were prepared from separated pools of recalcified human plasma. The negative control (CN-1) was nonreactive for anti-HBs antibody. CB1-1 was spiked with serum obtained from a recipient of HBV vaccine and had an antibody level of 6.7 mIU/ml. CM-1 had a level of 18.1 mIU/ml and was spiked with serum of hospital origin. CM1-100 was unspiked and had a level of 6,436 mIU/ml. CN1-1, CB1-1, and CM1-1 were freeze-dried and stored at 4 to 8°C, whereas CH-100 was liquid and stored at −20°C. At the time of use, CN1-1, CB1-1, and CM1-1 were reconstituted with 0.5 ml of distilled water. Control CH1-100 was thawed and prediluted 1 to 100 with SSD before use.
Collection, preparation, and storage of test samples.
Whole blood from healthy recipients of HBV vaccine was collected after informed consent was obtained. Blood collection was approved by the relevant ethics committees. Sera were stored at −20°C until testing. Undiluted samples with responses that were higher than the highest anti-HBs antibody standard were diluted by successive factors of 10 until the response was quantifiable.
ELISA procedure.
Fifty microliters each of standards, controls, and specimen were added in duplicate to the precoated microplate. The plate was covered and incubated overnight at room temperature and then washed four times with 350 μl of distilled water containing Tween 20 (0.05%) and NaCl (0.154 M). One hundred microliters of HBsAg-HRP conjugate at a working dilution of 1:1,000 in the HRP diluent (0.0095 M PBS [pH 7.4] supplemented with 10% BSA and 1 ml/liter Proclin-300 as a preservative) was added across the entire plate. Plates were covered and gently shaken for 2 h at room temperature. Unbound conjugate was removed by washing as previously described. One hundred microliters of the single-component tetramethylbenzidine-peroxidase substrate (Bio-Rad) was added to all wells. After 30 min of incubation in the dark, the color reaction was stopped by the addition of 100 μl of diluted 0.36 N sulfuric acid in distilled water. Plates were read in an ELISA reader at 450 nm against a reference filter at 630 nm within 30 min after stopping.
The calibration curve and its fitting algorithm.
The dose response curve expressed in terms of the response y (ODs on a linear scale) versus the dose x (mIU/ml on a logarithmic scale) commonly display a pronounced sigmoidal shape. The four-parameter logistic function fits these data with a high degree of accuracy (3, 9, 10). An unweighted four-parameter logistic function was used to fit the standard curve for the anti-HBs in-house assay. In order to estimate the accuracy of the standard curves, r2 and the percentage of the relative error (RE) or the relative bias (also called percentage of backfit dose error) were determined for each standard point.
The backfit dose is the recalculated concentration of each standard obtained by extrapolating its response (ODs) through the fitted curve. The RE of each standard point (zero standard is excluded from the analysis) is as follows:
% RE = [(recalculated value − assigned value)/assigned value] × 100%. To evaluate the overall quality of the fitting of the standard curve, 167 standard curves collected during the development phase of the assay were assessed for r2 and RE.
The literature and current guidelines recommend an absolute value of RE of ≤20% and an r2 of >0.99 (2, 13).
Analytical sensitivity.
Sixty-four replicates of the SSD were incubated on one plate along with one set of anti-HBs standards. The average response and its standard deviation (SD) were calculated in terms of ODs. Then the upper limit of the one-sided 95% confidence interval (CI) was determined, injected into the standard curve, and interpolated in terms of mIU/ml from the fitted standard curve.
LOD.
To determine the limit of detection (LOD), 140 serum samples collected from healthy adults and shown to be negative in the AUSAB RIA were tested in the assay. The average and SD were calculated after logarithmic transformation of the OD responses. Then the upper limit of the one-sided 95% CI was determined, injected into the standard curve, and interpolated in terms of mIU/ml from the fitted standard curve.
LOQ.
To determine the limit of quantitation (LOQ), serial twofold dilutions (six consecutive ones) were made for 56 samples (postvaccinees and hospital subjects). If required, samples were prediluted with SSD to around 10 mIU/ml in order to limit the number of dilutions per samples. For each sample, the lowest calculated concentration that did not differ no more than twofold from the expected concentration (with the concentration obtained at the first dilution considered the most accurate) was considered as still reliable. Data were logarithmically transformed before statistical analysis. The average lowest reliable concentration was calculated with its SD. The LOQ was set at the upper limit of the one-sided 95% CI.
Comparison of FBS and human-plasma as standard and sample diluent.
Ninety-eight samples covering a large range of anti-HBs concentrations were assayed either with FBS or with human plasma as SSD, and the results were compared by linear regression analysis after logarithmic transformation of the data. In addition, a paired t test was performed on the geometric mean concentrations (GMCs) obtained with each diluent.
Accuracy of recovery.
In order to assess accuracy, one ampoule of the 1st IRP for anti-HBs immunoglobulin (lot 1977-W1042) was reconstituted according to the 1st IRP instructions and diluted to 10,000 mIU/ml in human plasma. Furthermore, a 25-μl aliquot was added to different volumes of human plasma free of anti-HBs antibodies in order to cover a concentration range from 3.3 to 100,000 mIU/ml. Then each sample was measured in the assay. The accuracy was expressed as the geometric mean recovery percentage calculated for the whole set.
Assessment of repeatability and reproducibility of the anti-HBs in-house assay under routine conditions.
Precision was evaluated by a repeatability and reproducibility experiment made on 12 sera covering a broad range of anti-HBs antibody concentrations observed in routine conditions. To measure repeatability (precision under minimum variable conditions), the same dilution of serum was tested twice by the same certified operator on the same day, in the same run, on the same plate, and with the same lot of reagents. To estimate the reproducibility (precision obtained under maximal variable conditions), all sera were tested twice on the same plate on 4 different days (within a period of 2 weeks) by two certified operators. A random three-way analysis of variance (ANOVA) with day, operator, and sample as random factor was carried out (using proc mixed from statistical SAS software) on log10-transformed concentrations. Computations were done on transformed values, but CVs were expressed from nontransformed concentrations. In conclusion, 192 observations were generated (2 operators × 4 days × 2 repeats × 12 samples).
Linearity and parallelism.
Ten samples displaying concentrations between 100 and 51,895 mIU/ml were prediluted depending on their initial concentration to reach the upper limit of the analytical range. Then six serial twofold dilutions were applied to each sample. Each measured concentration was corrected for its dilution. For the analysis, samples were gathered into four groups depending on the first predilution applied: neat for group 1, 1 to 10 for group 2, 1 to 100 for group 3, and finally 1 to 1,000 for group 4. A statistical analysis was performed on the logarithmically transformed data in order to answer the following questions. Is the model globally linear, and are the slopes equal (parallel lines) from one group to another? Are the slopes equal from one sample to the other within each group? And finally, are the measured concentrations proportional to the applied successive dilutions?
Specificity.
In order to establish specificity of the assay, 3 samples displaying a concentration below the cutoff and 10 samples displaying a very large range of concentrations were preincubated (vol/vol) with an excess of either a mixture of native ad and ay HBsAg subtypes (the combination used for the solid phase) or the adw2 HBsAg subtype (the one used for the preparation of the HRP conjugate) for 2 h at 37°C. The excess of antigen corresponded to 40 times the concentration of antigen used for the coated-plate preparation. The reference condition was similar to the experimental one but with PBS-BSA buffer instead of HBAg moieties. Then samples were tested with the anti-HBs in-house assay but also with the AUSAB EIA as a specificity control. Specificity was expressed as the difference in concentration observed before and after inhibition expressed as a percentage of the corresponding concentration measured without inhibition.
Assessment of high-dose hook effect.
In order to rule out underestimation of the antibody concentration in samples with high levels of the analyte, three sera highly concentrated for anti-HBs were tested undiluted and at five consecutive 10-fold serial dilutions. There is no hook effect if the neat sample reports a concentration higher than the upper limit of the analytical range.
Clinical cutoff and correlation with AUSAB RIA.
The assay cutoff has to be equal to or higher than the LOQ. In order to determine the most appropriate cutoff for seropositivity, different cutoffs from 2 to 3.9 mIU/ml were compared during the technical bridge of the new assay compared to AUSAB RIA. In this respect, the concentrations obtained with the new assay for 919 samples including prevaccination (7%) and postvaccination samples were compared to the historical results observed with the reference method. The cutoff was elected at a level for which the best balance was observed in terms of overall agreement, sensitivity, and specificity. In addition Deming's regression was performed between double-positive samples in order to evaluate the correlation between both assays.
Clinical validation of the assay.
The accepted serological correlate of protection of ≥10 mIU/ml against HBV infection was determined from studies that employed the AUSAB RIA. Therefore, the clinical validation included several studies having used AUSAB RIA. Three additional studies having used AUSAB EIA were also considered. In total, 4,180 samples from seven clinical studies were retested. These studies had been conducted in infants, adolescents, adults, and the elderly (>50 years of age). Subjects had received vaccination with various HBV vaccine-containing products, including vaccine combinations such as combined hepatitis A-HBV vaccine and combined diphtheria-tetanus-acellular pertussis-HBV-inactivated poliovirus vaccine (Table 1).
TABLE 1.
Clinical studies retested with the anti-HBs in-house assay
| Study | Population | Vaccine groupa | Reference assay | No. of patients in group to be tested | Time point(s)b | Rationale |
|---|---|---|---|---|---|---|
| A | 50-70 yr old | Experimental HepB formulation | RIA | 72 (subset) | PI, mo 1 | Verify concordance with AUSAB RIA in an elderly population which reaches lower seroprotection rate and GMT than in adults 18-40 yr old |
| Engerix-B | PI, mo 1 | |||||
| PII, mo 6 | ||||||
| PIII, mo 7 | ||||||
| B | Adult nonrespondersc | Experimental HepB formulation | RIA | 58 (all) | Pre | Verify concordance with AUSAB RIA in a difficult population and check specificity of anti-HBs in-house assay on prevaccination samples |
| Engerix-B | PI, mo 1 | |||||
| PII, mo 2 | ||||||
| PII, mo 6 | ||||||
| PIII, mo 7 | ||||||
| C | 11-18 yr old | Engerix-B | RIA | 72 (subset) | PI, mo 1 | Verify concordance with AUSAB RIA after vaccination with a vaccine from another manufacturer |
| Recombivaxd | PII, mo 2 | |||||
| PII, mo 6 | ||||||
| PIII, mo 7 | ||||||
| D | 18-40 yr old | Engerix old 1 | EIA | 72 (subset) | PII, mo 6 | Verify concordance with AUSAB EIA following different formulations of the same vaccine |
| Engerix old 2 | PIII, mo 7 | |||||
| Engerix | ||||||
| E | 12-15 yr old | Ambirix 720/20 | RIA, EIA | 140 (all) | PIII, mo 7 | Verify concordance with AUSAB RIA and AUSAB EIA |
| Twinrix 360/10 | ||||||
| F | Children <1 yr old | Infanrix hexa | RIA | 50 (subset) | Pre | Verify concordance with AUSAB RIA after vaccination with HBV in combined pediatric vaccines |
| Infanrix penta + Hiberix | PII, mo 6 | |||||
| PII, mo 8 | ||||||
| PIII, mo 9 | ||||||
| G | Children <1 yr old | Infanrix hexa | EIA | 62 (all) | Pre, mo 1.5 | Verify concordance with AUSAB EIA after vaccination with HBV in combined pediatric vaccines |
| Infanrix penta + Hiberix | PIII, mo 7 | |||||
| PIII, mo 15 | ||||||
| PIV, mo 6 |
Engerix old 1, previous formulation, containing thiomersal as a preservative; Engerix old 2, previous formulation, using thiomersal during the production process.
Pre, sample taken prior to vaccination; PI to PIV, postdose months 1 to 4; mo 1, mo 2, etc., study month 1, study month 2, etc.
Nonresponders are healthy adults with an anti-HBs antibody concentration of <10 mIU/ml 2 to 5 months after having received at least four doses of HBV.
Recombivax is a trademark of Merck & Co.
RESULTS
Preparation of the anti-HBs standard and its calibration to anti-HBs IRP lot 1702-1977.
The distribution of the 96 individual concentrations obtained from the serial dilutions was Gaussian, and the CV of the recalculated concentrations was 4%. The final concentration of the freeze-dried in-house standard is expressed in mIU/ml. The calibration curve of the assay consists of one zero standard and seven positive standards ranging from 2.9 to 187.0 mIU/ml, namely 187.0, 93.5, 48.8, 23.4, 11.7, 5.8, and 2.9 mIU/ml. The loss of immunoreactivity after freeze-drying was 1.5% on average for two successive anti-HBs standard batches and therefore was considered negligible.
The calibration curve and its fitting algorithm.
For all of the 167 standard curves, r2 was >0.998. The 0.95th percentiles of the absolute values of RE were determined (Table 2). Accordingly we conclude that the accuracy of the standard curve within the analytical range set from 3.3 mIU/ml to 187 mIU/ml meets current guidelines (2, 13).
TABLE 2.
Calibration curvea
| Antibody concn (mIU/ml) | 0.95th percentile |
|---|---|
| 2.92 | 14.41 |
| 5.84 | 8.59 |
| 11.69 | 6.31 |
| 23.38 | 4.51 |
| 46.75 | 4.11 |
| 93.50 | 3.12 |
| 187.00 | 1.60 |
The calibration curve is based on the distribution of the percentage of the absolute values of the relative bias (RE) for the complete set of standard points (0.95 percentile), where % RE = [(recalculated value − assigned value)/assigned value] × 100%.
Analytical sensitivity.
The individual ODs of the 64 replicates of the zero standard were normally distributed. The average response and the SD of all replicates were calculated. Then the upper limit of a one-sided 95% CI of the mean OD was injected into the standard curve generated at the same time and the corresponding concentration was extrapolated from the curve. Accordingly, the analytical sensitivity was estimated at 0.43 mIU/ml.
LOD.
The OD corresponding to the upper limit of the one-sided 95% CI of the average OD response of the 142 negative samples was interpolated from the standard curve allowing the determination of an equivalent concentration expressed in mIU/ml. Accordingly, the LOD was set at 1.0 mIU/ml.
LOQ.
The average lowest measured concentration was 0.68 mIU/ml with an SD of 0.26. The LOQ of the in-house test was set at 2.0 mIU/ml, equivalent to the one-sided upper limit of the 95% CI.
Comparison of FBS and human plasma as standard and sample diluent.
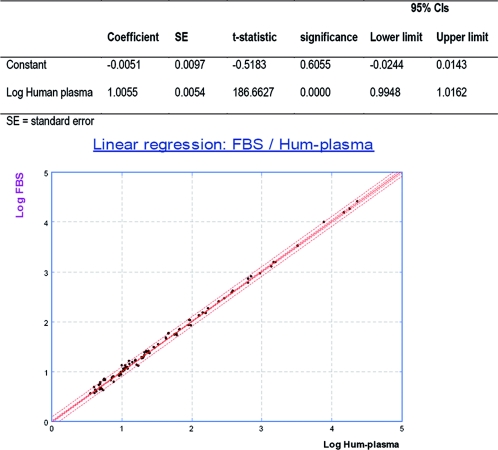
The standard curves generated with either FBS or human plasma were superimposable (data not shown). The concentrations of the 98 samples measured with either FBS or human plasma were statistically compared by linear regression analysis. The interval for the slope was 1.0055 (95% CI, 0.9948 to 1.0162), and the intercept was −0.0051 (95% CI, −0.0244 to 0.0143) comprising 1 and 0, respectively, which indicated no significant difference between the two diluents (Fig. 1). Moreover, a paired t test showed no significant difference between means. (The observed difference in log was 0.0036, for which the 95% CI included 0 [−0.0060 to 0.0132].) Consequently FBS can be used instead of human plasma free of anti-HBs antibody as the sample and standard diluent without affecting sample concentration determination.
FIG. 1.
Linear regression between FBS and human-plasma anti-HBs free as diluent. Hum-plasma, human plasma.
Accuracy of recovery.
The spiking experiments were performed on two different days. Recovery was calculated by expressing the measured concentrations in percentage of the theoretical concentration. Recovery ranged from 79.3% to 101.2%. The geometric mean recovery was 94.89% (95% CI, 92.24% to 97.62%) (Table 3). Accordingly, the anti-HBs in-house assay is considered to be accurate vis-à-vis the 1st IRP for anti-HBV immunoglobulin (1977-W1042).
TABLE 3.
Accuracy of anti-HBs in-house assay in terms of human anti-HBV immunoglobulina
| Theoretical antibody concn (mIU/ml) | Measured antibody concn (mIU/ml) | % Recovery |
|---|---|---|
| 3.3 | 3.4 | 100.4 |
| 6.3 | 5.6 | 90.4 |
| 6.3 | 5.8 | 93.4 |
| 10.4 | 9.8 | 94.2 |
| 10.4 | 9.4 | 90.3 |
| 20.8 | 19.9 | 95.5 |
| 20.8 | 18.9 | 90.8 |
| 41.5 | 42.0 | 101.2 |
| 41.5 | 41.7 | 100.4 |
| 82.6 | 81.1 | 98.2 |
| 82.6 | 80.1 | 96.9 |
| 163.9 | 129.9 | 79.3 |
| 163.9 | 150.0 | 91.5 |
| 476.2 | 446.0 | 93.7 |
| 909.1 | 840.6 | 92.5 |
| 2,000.0 | 1,967.9 | 98.4 |
| 3,333.3 | 3,304.4 | 99.1 |
| 5,000.0 | 5,048.4 | 101.0 |
| 10,000.0 | 9,885.2 | 98.9 |
The geometric mean recovery was 94.9%. The 95% CI lower and upper limits were 92.2% and 97.6%, respectively.
Precision.
A random three-way ANOVA with day, operator, and sample as random factor was carried out (using proc mixed of SAS) on log10-transformed concentrations. Computations were done on transformed values, but CVs were expressed from nontransformed concentrations. The CV for repeatability of the assay was 4.72%. The global CV reproducibility and repeatability under routine conditions were 11.86%. Precision is in agreement with what is currently displayed by the ELISA (6).
Linearity and parallelism.
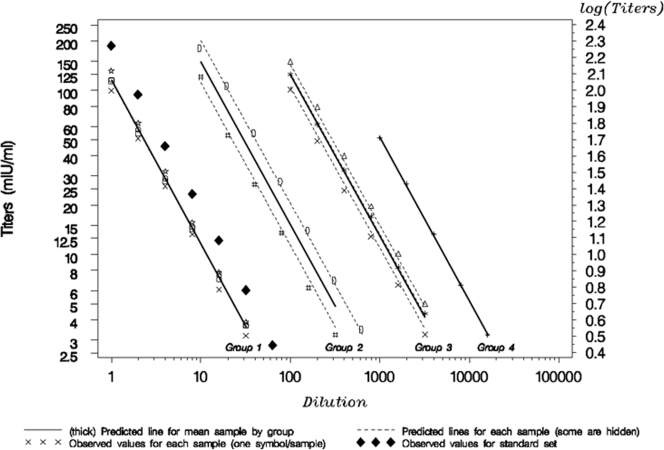
A mixed model was used with the factors log dilution (fixed and continuous), group (fixed, four groups), and samples (random, nested into the groups, 10 samples). The test for curvature of the log dilution effect was not statistically significant (P value of 0.5940). Consequently a linear model was fit to the data (Fig. 2). The test of the interaction of the log dilution and the group factors gave a nonsignificant P value of 0.8903, meaning that the log dilution effect (slope) can be considered equal from one group to another. The P value of the interaction between the log dilution and the sample factors was nonsignificant (0.24526), meaning that the slope of one sample is equal to the slope of another within each group. Finally, the estimated slope with its 95% CI was calculated for each group (Table 4). None of the slopes was significantly different from −1, supporting the proportionality between dilutions. Consequently linearity and parallelism are demonstrated over the analytical range.
FIG. 2.
Observed values and the model predictions for linearity assessment. For groups 1 and 4, the prediction line for each sample is superimposed with the prediction line for the mean sample within each group.
TABLE 4.
Demonstration of assay linearitya
| Group | Slope | 95% CI
|
|
|---|---|---|---|
| Lower limit | Upper limit | ||
| 1 | −0.9990 | −1.0318 | −0.9662 |
| 2 | −0.9910 | −1.0348 | −0.9472 |
| 3 | −0.9802 | −1.0180 | −0.9423 |
| 4 | −0.9976 | −1.0722 | −0.9230 |
Ten samples with concentrations between 100 and 51,895 mIU/ml were grouped into four groups according to the predilution applied to reach the upper limit of the analytical range. Shown are the estimated slope for each predilution group with its 95% CI using a mixed model with log dilution, group, and samples as factors.
Specificity.
As shown in Table 5, the specificity of the anti-HBs in-house assay is around 100%, similar to the specificity displayed by the AUSAB EIA.
TABLE 5.
Specificity of the anti-HBs in-house assay compared to the AUSAB EIA
| AUSAB EIA antibody concn (mIU/ml) before preincubation | Anti-HBs in-house assay antibody concn (mIU/ml) before preincubation | HBsAg preincubation with subtype(s)a:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ad + ay
|
adw2
|
||||||||
| AUSAB EIA
|
Anti-HBs in-house assay
|
AUSAB EIA
|
Anti-HBs in-house assay
|
||||||
| Antibody concn (mIU/ml) | % Specificity | Antibody concn (mIU/ml) | % Specificity | Antibody concn (mIU/ml) | % Specificity | Antibody concn (mIU/ml) | % Specificity | ||
| 20 | 15 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 |
| 94 | 61 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 |
| 119 | 125 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 |
| 346 | 352 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 |
| 382 | 428 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 |
| 1,556 | 1,111 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 |
| 1,957 | 2,410 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 |
| 2,237 | 2,575 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 | <3.3 | 100 |
| 49,680 | 42,660 | <3.3 | 100 | <3.3 | 100 | 86 | 99.8 | 49 | 99.9 |
| 54,490 | 61,065 | <3.3 | 100 | <3.3 | 100 | 651 | 98.8 | 103 | 99.8 |
| <3.3 | <3.3 | <3.3 | NA | <3.3 | NA | <3.3 | <3.3 | ||
| <3.3 | <3.3 | <3.3 | NA | <3.3 | NA | <3.3 | <3.3 | ||
| <3.3 | <3.3 | <3.3 | NA | <3.3 | NA | <3.3 | <3.3 | ||
Preincubation was performed with either an excess of a mix of native ad and ay HBsAg subtypes or adw2 HBsAg subtype for 2 h at 37°C. Specificity represents the difference in concentrations observed before and after inhibition expressed as a percentage of the corresponding concentration measured without inhibition.
Hook effect.
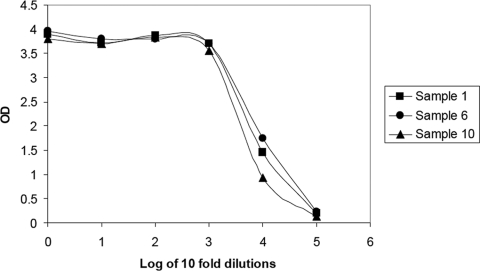
The three undiluted samples gave a higher response than that displayed by the upper standard point, for which the response was an OD of 3.0 in this experiment (upper standard specifications, ODs of 2.5 to 3.3) (Fig. 3). In addition, the CV calculated at dilutions of 10,000 and 100,000 was as good as 2% (Table 6). Concentrations as high as 823,976 mIU/ml can be reliably measured with the new assay without displaying any hook effect.
FIG. 3.
OD response of serial dilutions of three highly concentrated anti-HBs samples.
TABLE 6.
Calculation of the average concentration and CV in three samples with high anti-HBs concentrations
| Sample | Dilution | OD | Anti-HBs antibody concn (mUI/ml) | Avg anti-HBs antibody concn (mUI/ml)a | CV (%) |
|---|---|---|---|---|---|
| 1 | 1 | >3.000 | >187 | ||
| 10,000 | 1.652 | 772,731 | 754,411 | 3 | |
| 100,000 | 0.200 | 736,091 | |||
| 2 | 1 | >3.000 | >187 | ||
| 10,000 | 1.438 | 644,921 | 649,409 | 1 | |
| 100,000 | 0.180 | 654,897 | |||
| 3 | 1 | >3.000 | >187 | ||
| 10,000 | 1.705 | 833,075 | 823,976 | 2 | |
| 100,000 | 0.215 | 814,878 |
Average concentration determined at dilutions of 10,000 and 100,000.
Clinical cutoff and correlation with the AUSAB RIA.
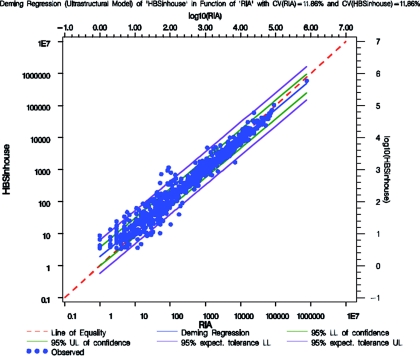
Table 7 displays the overall agreement, specificity, and sensitivity for the comparison of the anti-HBs in-house assay and AUSAB RIA during the technical validation of the assay. The cutoff of the anti-HBs in-house assay was fixed at a value above the LOQ, which gave the best balance with respect to overall agreement, sensitivity, and specificity vis a vis the reference assay while keeping a nonsignificant P value for the McNemar test of dissymmetry in concordance. Accordingly, the cutoff was fixed at 3.3 mIU/ml. At this level, the overall agreement was 97.61% (CI, 96.40 to 98.49%), with a sensitivity and a specificity calculated at 98.04% (CI, 96.84 to 98.88%) and 94.06% (CI, 87.52 to 97.79%), which were considered as good. Deming's regression was performed on the double-positive samples (n = 802) considering a CV at 11.86% as determined during the repeatability and reproducibility experiment (Fig. 4). Finally, regression was considered as good between the anti-HBs in-house assay and the AUSAB RIA. Fuller's coefficient of correlation (simple correction on the AUSAB RIA) was calculated to be 0.9815, which can be considered as high. The estimation of the slope and its 95% CI limit were established at 0.9187 (CI, 0.9060 to 0.9315). However in Table 8, the test of the bioequivalence of the slope was demonstrated because the 95% CI was within the 0.90 to 1.11 equivalence range (12, 14).
TABLE 7.
Contingency table between anti-HBs in-house assay and the AUSAB RIA with different cutoffsa
| Antibody concn cutoff (mIU/ml) | Agreement (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Kappa | McNemar P valueb |
|---|---|---|---|---|---|
| 2 | 97.71 (96.53-98.58) | 99.02 (98.08-99.58) | 87.13 (79.00-92.96) | 0.881 (0.830-0.931) | 0.3833 |
| 2.6 | 97.82 (96.66-98.67) | 98.53 (97.45-99.24) | 92.08 (84.99-96.52) | 0.891 (0.843-0.938) | 0.5034 |
| 3.3 | 97.61 (96.40-98.49) | 98.04 (96.84-98.88) | 94.06 (87.52-97.79) | 0.883 (0.835-0.931) | 0.0525 |
| 3.9 | 96.74 (95.37-97.79) | 96.94 (95.52-98.01) | 95.05 (88.82-98.37) | 0.847 (0.793-0.900) | 0.0003 |
n = 919.
Test of dissymmetry in concordance.
FIG. 4.
Deming's regression of the anti-HBs in-house assay and AUSAB RIA with CVs of 11.86% for both the RIA and anti-HBs in-house assay. UL, upper limit; LL, lower limit.
TABLE 8.
Bioequivalence of the slope for the anti-HBs in-house assay compared to the AUSAB RIAa
| Equivalence range lower specification | Equivalence range upper specification | Equivalence |
|---|---|---|
| 0.50 | 2.00 | Yes |
| 0.67 | 1.50 | Yes |
| 0.80 | 1.25 | Yes |
| 0.90 | 1.11 | Yes |
Shown are the results of Deming regression (ultrastructural model) with CVs of 11.86% for the RIA and the anti-HBS in-house assay. The regression represents a test of bioequivalence on the slope.
Analytical range.
The lower limit of the analytical range usually corresponds to the LOQ of the assay. However we elected to use the cutoff for positivity in order to increase confidence in the assay. Thus, the lower limit of the analytical range was set at 3.3 mIU/ml. The upper analytical range was fixed at 187.0 mIU/ml, the level at which the accuracy is still effective (see “The calibration curve and its fitting algorithm”).
Clinical validation of the assay.
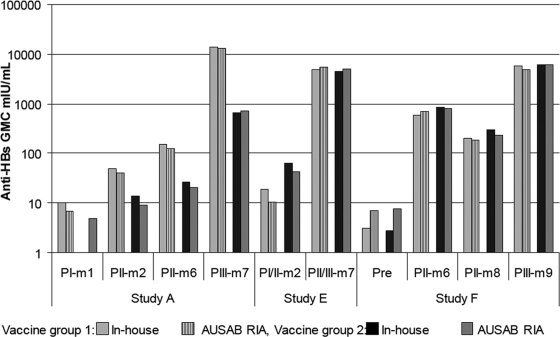
Seroprotection rates observed with the anti-HBs in-house and reference assays in seven clinical trials are summarized in Table 9. Figure 5 shows anti-HBs GMCs using the in-house and reference assays in three studies representative of different age groups.
TABLE 9.
Seroprotection rates per time point and study for the anti-HBs in-house assay versus the AUSAB RIA and EIA reference assays
| Study | No. of subjects/groupa | Time pointb | % Seroprotection in:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine group 1
|
Vaccine group 2
|
Vaccine group 3
|
||||||||
| In-house assay | RIA | EIA | In-house assay | RIA | EIA | In-house assay | EIA | |||
| A | 72 | PI, mo 1 | 15.3 | 20.8 | 0.0 | 2.7 | ||||
| 72 | PII, mo 2 | 80.6 | 81.9 | 23.6 | 26.4 | |||||
| 72 | PII, mo 6 | 95.8 | 93.1 | 59.7 | 56.9 | |||||
| 72 | PIII, mo 7 | 98.6 | 98.6 | 81.9 | 83.3 | |||||
| B | 51 | PI, mo 1 | 47.1 | 49.0 | 63.6 | 63.6 | ||||
| 51 | PII, mo 2 | 60.8 | 60.8 | 81.8 | 81.8 | |||||
| 50 | PII, mo 6 | 48.0 | 42.0 | 85.2 | 81.5 | |||||
| 47 | PIII, mo 7 | 72.3 | 72.3 | 96.2 | 96.2 | |||||
| C | 100 | PI, mo 1 | 2.0 | 2.0 | 9.7 | 12.5 | ||||
| 72 | PII, mo 2 | 80.6 | 68.1 | 68.1 | 69.4 | |||||
| 72 | PII, mo 6 | 93.1 | 93.1 | 95.8 | 95.8 | |||||
| 72 | PIII, mo 7 | 98.6 | 97.2 | 100.0 | 100.0 | |||||
| D | 72 | PIII, mo 7 | 97.2 | 95.8 | 97.2 | 97.2 | 97.2 | 97.2 | ||
| E | 134 | PI/II, mo 2 | 53.0 | 40.3 | 41.8 | 90.4 | 86.8 | 86.8 | ||
| 145 | PII/III, mo 7 | 99.3 | 98.6 | 99.3 | 100.0 | 100.0 | 100.0 | |||
| F | 50 | Pre | 13.8 | 16.9 | 12.2 | 12.2 | ||||
| 50 | PII, mo 6 | 98.0 | 98.0 | 96.0 | 96.0 | |||||
| 50 | PII, mo 8 | 94.0 | 94.0 | 100.0 | 100.0 | |||||
| 50 | PIII, mo 9 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| G | 34 | Pre, mo 1.5 | 52.9 | 49.0 | 59.6 | 68.4 | ||||
| 47 | PIII, mo 7 | 100.0 | 100.0 | 95.9 | 95.0 | |||||
| 53 | PIII, mo 15 | 96.6 | 96.6 | 93.0 | 96.0 | |||||
| 57 | PIV, mo 6 | 100.0 | 100.0 | 98.3 | 98.3 | |||||
If the number between the two groups was different, the lowest number was taken.
Pre, sample taken prior to vaccination; PI to PIV, postdose months 1 to 4, respectively; mo 1, mo 2, etc., study month 1, study month 2, etc.
FIG. 5.
GMCs (mIU/ml) at each blood sampling time point in study A (elderly patients), study E (adolescents), and study F (infants) for the anti-HBs in-house assay versus reference assays. PI to PIV repesent postdose months 1 to 4, respectively, and m1, m2, etc., represent study months 1, 2, etc.
Study B investigated immune responses to HBV vaccination in nonresponder subjects who had received at least four doses of the Engerix HBV vaccine and was included in order to verify that those nonresponders would not have been considered as protected by the anti-HBs in-house assay, which would be an expression of a lack of specificity of the new assay. Out of 96 subjects assessed as seronegative at the study start with the AUSAB RIA, 9 were assessed as seropositive with the anti-HBs in-house assay. Accordingly, the specificity was calculated at 90.6% at the study start (data not shown). However, out of these nine samples, seven were confirmed as seropositive with the AUSAB EIA. At this stage, this apparent suboptimal specificity could be linked to a better sensitivity of the anti-HBs in-house assay and AUSAB EIA than that of the AUSAB RIA. These nonresponders then participated in a new trial (mentioned as study B in Table 9). The final analysis performed on study B after the complete vaccine course showed similar seroprotection rates and similar geometric mean antibody titers (GMTs) determined with either the anti-HBs in-house assay or the AUSAB RIA. Overall, the specificity of the anti-HBs in-house assay compared to the AUSAB RIA determined on a normal population was found to be 94.06% (see “Clinical cutoff and correlation with AUSAB RIA”), which was considered good.
In the reanalysis of seven studies in which different recombinant HBsAg-containing vaccines were used, a very good agreement in terms of seroprotection rate was observed after a full schedule and also 1 month postvaccination. Antibody GMCs were similar across the in-house and reference assays, with a trend toward overestimation using the in-house assay in the lower concentration range. Importantly, there was no evidence that the use of the anti-HBs in-house assay would lead to different clinical conclusions compared to the reference assays.
DISCUSSION
The new anti-HBs in-house assay has been developed to replace use of a commercial assay soon to be removed from the market. The performance characteristics of the new in-house assay have been thoroughly tested and are acceptable. Clinical application of the in-house assay has been documented by reanalysis of 4,180 samples from individuals of all ages participating in clinical trials of recombinant HBsAg-containing vaccines. In these studies, seroprotection rates and anti-HBs GMCs for individual studies were similar using either the in-house assay or the reference assays. In all studies, the immunogenicity conclusions determined by the reference assay were confirmed using the in-house assay.
The specificity of the anti-HBs in-house assay determined in study B was 90.6%, but this figure was calculated at the study start and in nonresponder subjects having received at least four doses of hepatitis B vaccine. After the full schedule, seroprotection rates and GMTs were similar for the anti-HBs in-house assay and the reference assay.
In conclusion, the new in-house anti-HBs antibody assay has been technically and clinically validated and displays adequate performance characteristics. There is no indication that use of the new assay would lead to different clinical conclusions from the currently used reference methods.
Acknowledgments
The assay was developed and supported by GlaxoSmithKline Biologicals. On behalf of GlaxoSmithKline Biologicals, we thank Joanne Wolter, Veronique Delpire, Vidya Virajith, and Manjula K for assistance with manuscript preparation, as well as ensuring manuscript coordination.
All authors of this article are employed by the commercial entity which has developed the in-house assay. J. Jacquet is also a shareholder of GlaxoSmithKline Biologicals.
Footnotes
Published ahead of print on 24 June 2009.
REFERENCES
- 1.Barker, L. F., D. Lorenz, S. C. Rastogi, J. S. Finlayson, and E. B. Seligmann. 1977. Study of a proposed international reference preparation for anti-hepatitis B immunoglobulin. Expert Committee on Biological Standardization technical report series. World Health Organization, Geneva Switzerland.
- 2.Braggio, S., R. J. Barnaby, P. Grossi, and M. Cugola. 1996. A strategy for validation of bioanalytical methods. J. Pharm. Biomed. Anal. 14375-388. [DOI] [PubMed] [Google Scholar]
- 3.Findlay, J. W. A., W. C. Smith, J. W. Lee, G. D. Nordblom, I. Das, B. S. DeSilva, M. N. Khan, and R. R. Bowsher. 2000. Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J. Pharm. Biomed. Anal. 211249-1273. [DOI] [PubMed] [Google Scholar]
- 4.Hauser, P., P. Voet, E. Simoen, H. C. Thomas, J. Pêtre, M. De Wilde, and J. Stephenne. 1987. Immunological properties of recombinant HBsAg produced in yeast. Postgrad. Med. J. 6383-91. [PubMed] [Google Scholar]
- 5.Keating, G. M., and S. Noble. 2003. Recombinant hepatitis B vaccine (Engerix™-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs 631021-1051. [DOI] [PubMed] [Google Scholar]
- 6.Miller, K. J., R. R. Bowsher, A. Celniker, J. Gibbons, S. Gupta, J. W. Lee, S. J. Swanson, W. C. Smith, and R. S. Weiner. 2001. Workshop on bioanalytical methods validation for macromolecules: summary report. Pharmacol. Res. 181373-1383. [DOI] [PubMed] [Google Scholar]
- 7.Nakane, P. K., and A. Kawaoi. 1974. Peroxidase-labeled antibody. A new method of conjugation. J. Histochem. Cytochem. 221084-1091. [DOI] [PubMed] [Google Scholar]
- 8.Pêtre, J., F. Van Wijnendaele, B. De Neys, K. Conrath, O. Van Opstal, P. Hauser, T. Rutgers, T. Cabezon, C. Capiau, N. Harford, et al. 1987. Development of a hepatitis B vaccine from transformed yeast cells. Postgrad. Med. J. 63(Suppl. 2)73-81. [PubMed] [Google Scholar]
- 9.Plikaytis, B. D., S. H. Turner, L. L. Gheesling, and G. M. Carlone. 1991. Comparisons of standard curve-fitting methods to quantitate Neisseria meningitidis group A polysaccharide antibody level by enzyme-linked-imunosorbent assay. J. Clin. Microbiol. 291439-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodbard, D., and G. R. Frazier. 1975. Statistical analysis of radioligand assay data. Methods Enzymol. 373-22. [DOI] [PubMed] [Google Scholar]
- 11.Soulier, J. P., and A. M. Couroucé-Pauty. 1973. New determinants of hepatitis B antigen (Au or HB antigen). Vox Sang. 25212-234. [DOI] [PubMed] [Google Scholar]
- 12.Tan, C. Y., and B. Iglewicz. 1999. Measurement-methods comparisons and linear statistical relationship. Technometrics 41192-201. [Google Scholar]
- 13.U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation, and Research Center for Veterinary Medicine. 2001. Guidance for industry. Bioanalytical method validation. U.S. Department of Health and Human Services, Washington, D.C.
- 14.U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research. 2001. Guidance for industry. Statistical approaches to establish bioequivalence. U.S. Department of Health and Human Services, Washington, D.C.
- 15.Vnek, J., and A. M. Prince. 1976. Large-scale purification of hepatitis B surface antigen. J. Clin. Microbiol. 3626-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. 2004. Hepatitis B vaccines W.H.O. position paper. Wkly. Epidemiol. Rep. 79255-263. [Google Scholar]