Abstract
Meiosis is the developmental program by which sexually reproducing diploid organisms generate haploid gametes. In yeast, meiosis is followed by spore morphogenesis. When Schizosaccharomyces pombe diploid cells undergo meiosis, they differentiate into asci containing four haploid ascospores that are highly resistant to environmental stress. The formation of the ascospore wall requires the activity of several enzymes involved in the biosynthesis and modification of its components, such as α- and β-glucan synthases. Once the spores are completely mature, the wall of the ascus undergoes an endolytic process that results in the release of ascospores from the ascus, allowing their dispersal into the environment. This process requires the activity of the endo-α-1,3-glucanase Agn2. Here, we focus on the characterization of the endo-β-1,3-glucanase Eng2, which is also required for ascospore release from the ascus. Although Eng2 is present during the mitotic cycle, the protein accumulates after meiosis II. The expression of eng2+ is required for the efficient release of ascospores, as shown by placing eng2+ under the control of a repressible promoter. Furthermore, a point mutation that destroys the catalytic activity of the protein results in a phenotype similar to that of the mutant strain. Finally, we demonstrate that exogenous addition of purified Eng2 releases the ascospores from asci generated by an eng2Δ mutant. We propose that Eng2 would act together with Agn2 to completely hydrolyze the ascus wall, thereby assisting in the release of ascospores in S. pombe.
Cells of the fission yeast Schizosaccharomyces pombe have a rod-like shape and grow at the poles. S. pombe cells are stable in the haploid state and proliferate asexually until there is a shortage of nutrients. When cells are starved, especially of nitrogen, a sexual development program is triggered, and hence, cells from the opposite mating types conjugate to form zygotes. These immediately undergo meiosis, giving rise to four haploid zygotic ascospores (34).
Spore formation is a complex differentiation program in which two sequential processes, meiosis and ascospore formation, occur in a coordinate fashion. During meiosis, the recently formed diploid nucleus undergoes a round of DNA replication, followed by two successive nuclear divisions generating four haploid nuclei (34). The morphogenetic program that leads to the formation of ascospores starts during meiosis II, when the four spindle-pole bodies (SPBs) differentiate into a sporulation-specific shape and change into a multilayered structure (15). These modified SPBs serve as the nucleation points for the fusion of membrane vesicles, resulting in the formation of a double-membrane structure, known as the forespore membrane, which engulfs each nuclear lobe and isolates the four haploid nuclei (15, 33, 34). Following this, the cell wall of the ascospore is synthesized de novo within the lumen of the forespore membrane through the deposition of successive layers of cell wall material mediated by the action of specific synthases. The synthesis of spore cell wall material requires the activity of several sporulation-specific enzymes, such as the α-glucan synthase subunits Mok12, Mok13, and Mok14, paralogues of the vegetative α-glucan synthase Mok1 (14, 16, 34). Additionally, the biosynthesis of the spore wall β-1,3-glucan is carried out by a specific β-1,3-glucan synthase complex, whose catalytic subunit is encoded by bgs2+ (20, 22). Synthesis of the ascospore cell wall also requires the activity of other enzymes, such as the chitin synthase Chs1, the putative chitin deacetylase Cda1, and the β-glucanosyl transferase Gas4 (2, 8, 29). Interestingly, all these genes are induced during the sporulation process, and most of them belong to the middle and late groups, which contain genes induced during meiosis I and II and spore formation, respectively (27). Furthermore, the expression of most of these genes involved in spore wall assembly requires the meiosis-specific transcription factor Mei4 (28).
The final step in the sporulation process is the hydrolysis of the ascus wall surrounding the ascospores for release of the meiotic products into the environment, allowing their dispersal. The ascus wall is the cell wall of vegetative cells that fuse to form a diploid and is thus mainly composed of α- and β-glucans (17, 21). Recently, it has been shown that the α-1,3-glucanase Agn2 functions late in sporulation and that it is necessary for the hydrolysis of the α-1,3-glucan of the ascus wall for release of the ascospores (6, 7). Agn2 lacks a signal sequence for secretion, and the protein localizes to the epiplasm, the material surrounding the ascospores inside the ascus wall (6). Interestingly, the S. pombe genome also contains an endo-β-1,3-glucanase, named Eng2, which lacks a secretion signal and whose expression is induced during the sporulation process (27, 36). This suggests that it might also be involved in the sporulation process. Expression of agn2+ and eng2+ during sporulation is also dependent on the Mei4 transcription factor (28).
Here, we demonstrate that Eng2 is also necessary for the endolysis of the S. pombe ascus wall. Eng2 has a pattern of induction and localization similar to that reported for Agn2. The expression of eng2+ and the catalytic activity of the protein are required for the efficient dispersal of ascospores. Moreover, the exogenous addition of purified S. pombe Eng2, but not Saccharomyces cerevisiae Eng2, was able to complement the defect of eng2Δ mutants. These results indicate that Agn2 and Eng2 form a pair of hydrolytic enzymes whose concerted action is essential for spore release from the ascus.
MATERIALS AND METHODS
Strains, growth conditions, and genetic manipulations.
The S. pombe strains used in this study are listed in Table 1. Yeast cells were grown on YES medium or minimal medium (EMM) with appropriate supplements (30). For conjugation and sporulation assays, haploid cells of opposite mating types were induced to conjugate and sporulate on EMM-N solid medium (EMM without the nitrogen source). The regulated expression of eng2+ during sporulation was achieved as previously described (7). Diploid cells first were grown to the exponential phase in EMM containing 1% ammonium chloride (EMM-AC). To induce sporulation synchronously, cells were then shifted to EMM containing 0.5% (wt/vol) sodium glutamate and 0.5% glucose (EMM-SG). Yeast transformations were performed with the lithium acetate method (19). For overexpression experiments using the nmt1+ promoter, cells were grown in YES medium up to the logarithmic phase. Then, cells were harvested, washed three times with EMM-N, and inoculated in fresh medium with or without thiamine (15 mM).
TABLE 1.
Yeast strains used in this study
| Strain | Genotype or description | Source |
|---|---|---|
| OL23 | ura4-D18 h− | Laboratory stock |
| OL24 | ura4-D18 eng2::ura4 h− | Laboratory stock |
| OL176 | ade6-M210 leu1-32 ura4-D18 h− | Laboratory stock |
| OL177 | ade6-M216 leu1-32 ura4-D18 h+ | Laboratory stock |
| OL757 | ade6-M210 leu1-32 ura4-D18 eng1::kanMX4 h− | Laboratory stock |
| OL759 | ade6-M210 leu1-32 ura4-D18 eng2::kanMX4 h− | Laboratory stock |
| OL763 | ade6-M210 leu1-32 ura4-D18 agn2::kanMX4 h− | Laboratory stock |
| OL771 | ade6-M216 leu1-32 ura4-D18 eng1::kanMX4 h+ | Laboratory stock |
| OL773 | ade6-M216 leu1-32 ura4-D18 eng2::kanMX4 h+ | Laboratory stock |
| OL777 | ade6-M216 leu1-32 ura4-D18 agn2::kanMX4 h+ | Laboratory stock |
| OL896 | ura4-D18 eng2-GFP:Kanrh− | This work |
| OL937 | ura4-D18 agn2-GFP:Kanrh− | This work |
| OL958 | ura4-D18 P41nmt1-HA3-eng2:Kanrh− | This work |
| OL952 | ade6-M210 eng2-GFP:Kanrura4-D18 leu1-32 h− | This work |
| OL953 | ade6-M216 eng2-GFP:Kanrura4-D18 leu1-32 h+ | This work |
| OL954 | ade6-M210 agn2-GFP:Kanrura4-D18 leu1-32 h+ | This work |
| OL955 | ade6-M216 agn2-GFP:Kanrura4-D18 leu1-32 h− | This work |
| OL946 | OL176/OL177 | This work |
| OL948 | OL757/OL771 | This work |
| OL950 | OL759/OL773 | This work |
| OL959 | OL763/OL777 | This work |
| OL961 | OL952/OL953 | This work |
| OL962 | OL954/OL955 | This work |
Construction of null mutants and green fluorescent protein (GFP)-tagged strains.
The oligonucleotides used in this study are listed in Table 2 (oligonucleotide pairs used in each experiment are indicated in parentheses). The entire coding sequences of eng1+ (SPAC821.09), eng2+ (SPAC23D3.10c), and agn2+ (SPBC646.06c) were deleted to create the null mutants by replacing the coding sequences by the ura4+ or kanMX4 cassette (which confers resistance to the G418 antibiotic). The deletion cassettes were constructed using the recombinant PCR approach described by Wach (35). To accomplish this, DNA fragments of 300 to 500 bp corresponding to the 5′ and 3′ flanking regions of each gene were PCR amplified using specific oligonucleotide pairs. The resulting fragments were then fused by recombinant PCR to the kanMX4 cassette or to the ura4+ gene.
TABLE 2.
Oligonucleotides used in this study
| Primer location and no. | Sequence (5′-3′) |
|---|---|
| eng2+-GFP | |
| 1391 | CCTTTACTGCTGATAAAATTGATAACGGAGCTAGTAAAACCTGGTACTTAGCTATGGCTGCTGGTATGGGTGGATCACCAGCAGCAGCAGCAGCAGCAGCAGCACGGATCCCCGGGTTAATTAA |
| 1392 | AACAATGCAGAAGCGAAAAAGTAATTTTCTGTCTTTAATATTTATGGAAAACTTCGAAGTAAGCCAACTTGAATAAGCAGGAATTCGAGCTCGTTTAAAC |
| agn2+-GFP | |
| 1465 | GGGTATGGTCCATTAAATATTCTTGGTAACAATTCTGTTGTGCTATACAACTTCAACTTCTGCACCACTAGGATATCCTGGCGGATCCCCGGGTTAATTAA |
| 1466 | TTGATACCACTTCGTTGGAGTTTGTTACGGTCAGTTGATCAGCAGCCCAAGTGTCAAGCGGTATCGAACTTTCAGGTTTATGAATTCGAGCTCGTTTAAAC |
| HA3-eng2+ | |
| 1393 | TGTTACTTTCGCTAAGTTATTTAAGACAAATAATTGAGTGTTGTTTCATTTTTTAGTTAGTTCCAAATTTTTGAGGTGGCGAATTCGAGCTCGTTTAAAC |
| 1394 | CCAAGAGGAGGAGATGGGTGTGCTCTTGATGGAAAAACCGGATTGATAGGTCCAGTATAGATTGGTACTAAAACATCCATTGCTGCTGCTGCTGCTGCTGCTGCGCACTGAGCAGCGTAATCTG |
| pJED12 | |
| 366 | GGGGGGGTCGACTTTTAATGTTTGAAGGCC |
| 367 | GGGGGGGAGCTCGAAAGCAGCTTCCAT |
| pJED13 | |
| 743 | GAAAGTTGAAGTCGACTGAGGTTG |
| 744 | CAACCTCAGTCGACTTCAACTTTC |
| 1440 | CACTGGGGTTATCATATTTACGC |
| 1445 | CGTGAATAGTGAACTCTACAATTTACGGC |
The C-terminally GFP-tagged (eng2-GFP and agn2-GFP) strains were constructed by direct chromosome integration of PCR fragments, generated using the pFA6a-GFP-kanMX6 plasmid as a template and specific oligonucleotides (1391/1392 for eng2-GFP and 1465/1466 for agn2-GFP) (3). The amplified fragments contained the GFP-coding region fused in-frame to the last codon of the eng2+ and agn2+ genes and the kanMX6 cassette, which was used to select for transformants. The N-terminally three-hemagglutinin (HA3)-tagged (HA3-eng2) strain under the control of the repressible nmt1+ promoter was constructed by direct chromosome integration of PCR fragments generated using the pFA6a-3HA-kanMX6 plasmid as a template and specific oligonucleotides (1393/1394) (3). Correct integration of the DNA fragment was verified by PCR.
Plasmid pJED12 carries the wild-type eng2+ gene cloned under the control of its own promoter. Oligonucleotides 366/367, which generated SalI/SacI sites at the ends, were used for PCR amplification, and SalI/SacI restriction sites were used to clone the fragment into the pAU-KS vector. pJED13 carries the eng2(E537A) allele. Site-directed mutagenesis was accomplished by recombinant PCR according to the protocol described by Wach (35). The DNA fragment containing the desired mutation was amplified using a pair of oligonucleotides that generated the mutation substitution (743/744) and two external primers (1440/1445). The amplified fragment (968 bp) containing the mutation was then cloned into plasmid pJED12 (BamHI-SpeI).
Ascospore release.
For ascospore release experiments, diploid strains were sporulated for 7 days. Mature asci were washed, resuspended in 300 μl of 50 mM acetate buffer, pH 5.5, and incubated with 0.8 μg of purified S. pombe Eng2 or purified S. cerevisiae Eng2 (25) at 37°C for 1 h. The percentage of ascospore release was calculated as the number of free spores versus the total number of mature spores counted.
Extract preparation, electrophoresis, and immunoblotting.
Sporulating cells were collected by centrifugation; a small aliquot was used to assess the sporulation process by microscopy. Pellets were boiled for 10 min, quickly frozen (in dry ice), and stored at −80°C. Total cell extracts of sporulating cells were prepared by breaking the cells or the spores with glass beads in lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 20 mM PMSF, and 1% Triton X-100).
For immunoblotting, 60 μg of protein extract was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% gels. Protein transfer, blotting, and chemiluminescence detection were performed using standard procedures. Anti-GFP (JL-8 Living Colors; Clontech) or antiactin (ICN Biomedicals) antibodies were used.
Microscopy techniques.
For light microscopy, cells were stained with DAPI (4′,6-diamino-2-phenylindole) for DNA visualization. Samples were viewed using a Leica DMRXA microscope equipped for bright-field and Nomarski optics and epifluorescence and were photographed with a Hamamatsu ORCA-ER camera. To estimate the proportion of cells in meiosis I, meiosis II, or sporulation, the percentages of cells with one, two, or four nuclei observed after DAPI staining and the percentage of asci with mature spores observed under Nomarski microscopy were determined.
β-Glucanase activity assay and substrates.
Activity was detected using reduced laminarin (Sigma) as a substrate. Laminarin was reduced by treatment with NaBH4 in 50 mM NaOH, dialyzed against acetate buffer (50 mM, pH 5.5), and freeze dried. Determination of β-glucanase activity was performed at 37°C for different incubation times with 50 mM acetate buffer, pH 5.5, containing 0.64 mg/ml of laminarin and 0.1 to 0.6 mg of protein extract. Activity was determined by measuring the amount of reducing sugars released from the substrate with the p-amino-hydroxybenzoic acid hydrazide method (12). After enzyme incubation, an aliquot of 50 μl was added to a solution of 950 μl of 50 mM sodium sulfite, 250 mM NaOH, 25 mM sodium citrate, 10 mM CaCl2 containing 1 g p-amino-hydroxybenzoic acid hydrazide per 100 ml. Following a 10 min boiling period, reduced sugars were quantified at 405 nm, using glucose as a standard. One unit of activity was defined as the amount of enzyme that catalyzed the release of reducing sugar groups equivalent to 1 μmol of glucose per h, and specific activity was expressed as U per milligram of protein.
RESULTS
Eng2 is induced during ascospore formation.
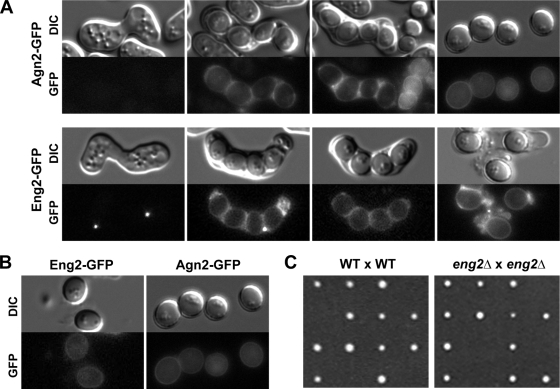
In its genome, S. pombe contains two genes, eng1+ and eng2+, that code for proteins belonging to glycosyl hydrolase family 81 (GH81). While Eng1 is necessary for controlled dissolution of the primary septum during cell separation at the end of mitosis (23), no function has been described for Eng2. Genome-wide transcription-profiling experiments on synchronously sporulating S. pombe cells have revealed that the expression of eng2+, which belongs to the middle group of genes (27), is induced during this process. This induction pattern is similar to that of the endo-α-1,3-glucanase Agn2, which is required for hydrolysis of the ascus wall before spore release (7), suggesting that Eng2 might perform its function during the sporulation process. To confirm that this induction pattern was accompanied by an increase in Eng2 protein levels, eng2+ was tagged with GFP to analyze protein levels along the sporulation process; the fusion protein was detected during vegetative growth (Fig. 1A). A diploid strain carrying Eng2-GFP was induced to sporulate, and samples were collected at different times after induction and analyzed using anti-GFP antibodies. The results indicated that Eng2 was strongly induced along the sporulation process, maximum accumulation coinciding with the end of meiosis II and spore formation (9 h after induction of sporulation). As a control, a strain bearing Agn2-GFP was used, and a similar pattern was observed, although Agn2-GFP was detected earlier than Eng2 (Fig. 1B). Thus, although Eng2 is present during vegetative growth, its synthesis is strongly induced during sporulation, with an induction profile slightly slower than that observed for Agn2.
FIG. 1.
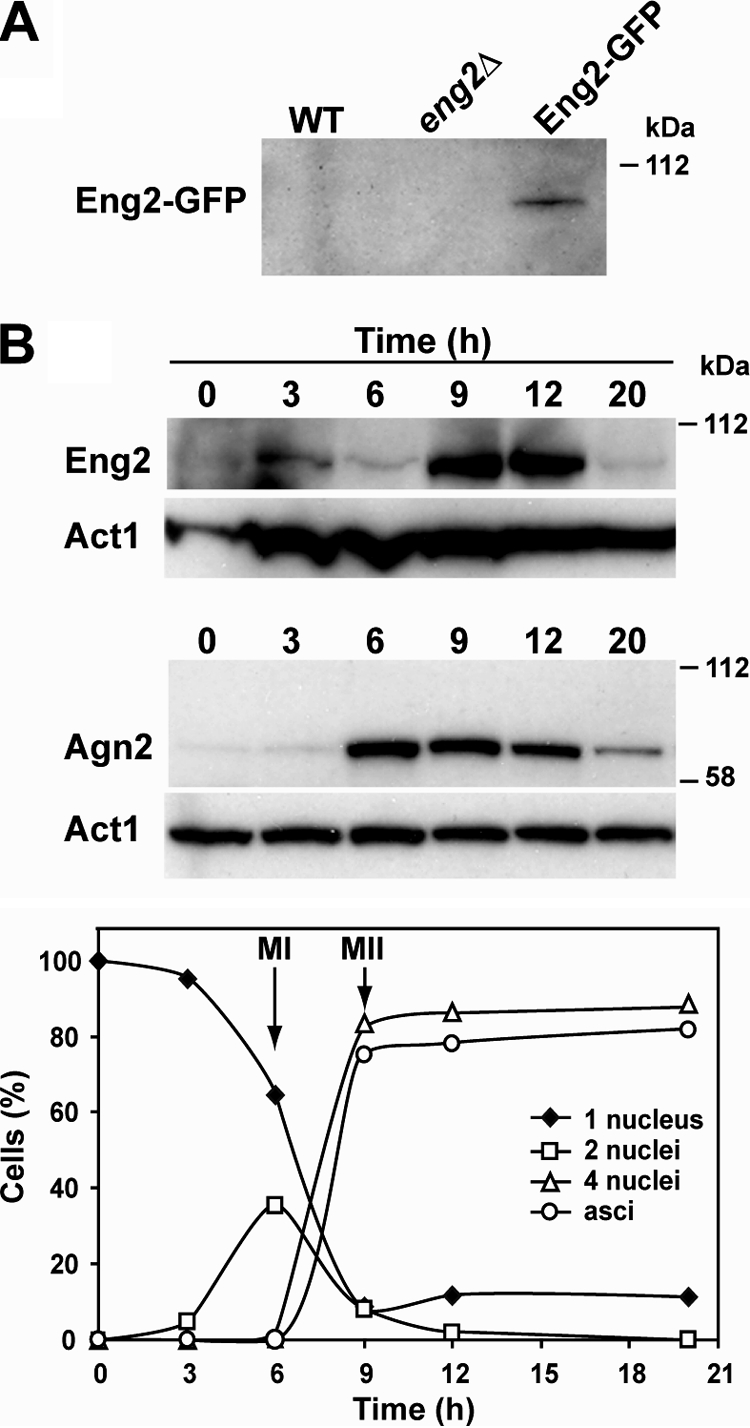
Eng2 accumulates during sporulation. (A) Western blot analysis of Eng2-GFP during vegetative growth. Samples were collected from exponentially growing cultures of wild-type (WT; OL23), eng2Δ mutant (OL24), and eng2-GFP (OL896) strains to prepare protein extracts. Anti-GFP antibody was used. (B) Western blot analysis of Eng2-GFP and Agn2-GFP during sporulation. The diploid strains OL961 and OL962 were induced to sporulate. Samples were collected at the indicated times after the induction of sporulation to prepare protein extracts. Anti-GFP antibody was used. Actin was used as a loading control. Meiotic progression was followed by DAPI staining of nuclei, and sporulation was checked by microscopic observation of asci. The percentages of mononucleate, binucleate, and tetranucleate cells and spores at each time point for the strain carrying Eng2-GFP are shown in the graph.
Endo-β-1,3-glucanase activity during sporulation depends on eng2+.
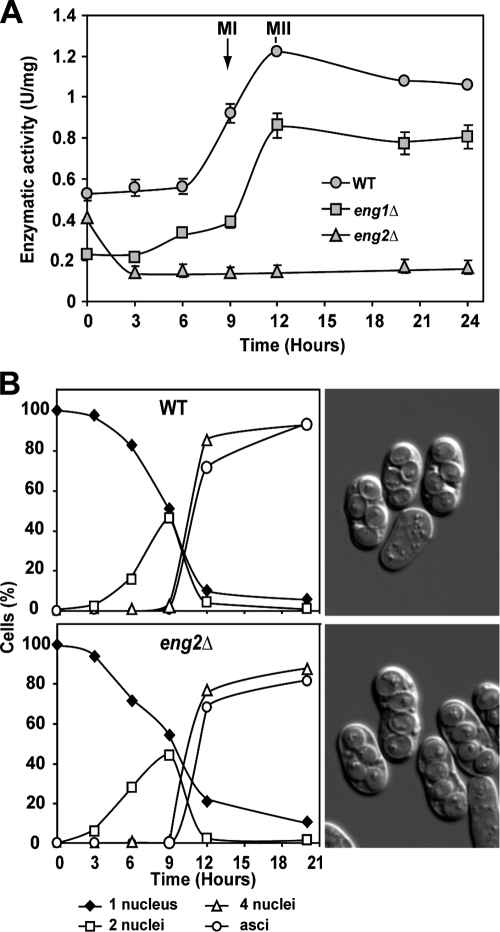
Previously, we have shown that Eng2 is a glucanase with high specificity for β-1,3-glucans, acting with an endo-hydrolytic mode of action (25). To analyze whether the increase in Eng2 protein levels during sporulation might result in an increase in β-1,3-glucanase activity, the activity of sporulating cells was assayed using laminarin as a substrate. As shown in Fig. 2A, β-1,3-glucanase activity gradually increased in wild-type sporulating cells, reached a peak at the moment that the culture completely sporulated (12 h), and thereafter decreased slowly, in good agreement with the results obtained by Western analysis (Fig. 1B). Since S. pombe contains two proteins belonging to the GH81 family encoded by the eng1+ and eng2+ genes (23, 25), homozygous eng2Δ/eng2Δ and eng1Δ/eng1Δ diploids were constructed to confirm that the increase in β-1,3-glucanase activity during sporulation was indeed due to eng2+. We observed that the basal level of β-1,3-glucanase activity of the eng2Δ/eng2Δ strain did not increase along sporulation, which can be attributed to the activity of the Eng1 glucanase (Fig. 2A). In contrast, eng1Δ/eng1Δ diploids had low levels of glucanase activity during vegetative growth (time zero) that increased over time, with kinetics identical to that of wild-type diploids, although the amplitude of the increase was smaller. These results therefore indicate that the bulk of β-1,3-glucanase activity produced during sporulation corresponds to Eng2.
FIG. 2.
β-1,3-Glucanase activity during sporulation. (A) The diploid strains OL946 (WT), OL948 (eng1Δ/eng1Δ), and OL950 (eng2Δ/eng2Δ) were grown on EMM-AC and then transferred to EMM-SG to induce sporulation. Samples were collected at the indicated times after the induction of sporulation to prepare protein extracts and to assay β-1,3-glucanase activity, using laminarin as substrate. Activity is represented as units/mg protein. Values are means of results from three independent measurements, and standard deviations are shown. MI, meiosis I; MII, meiosis II. (B) Meiotic progression of the wild-type (WT) and eng2Δ/eng2Δ strains. Aliquots of the culture were stained with DAPI, and the percentages of mononucleate, binucleate, and tetranucleate cells and spores at each time-point are shown. Images show mature spores after 24 h of incubation in sporulation medium.
eng2+ functions after ascospore formation.
To analyze the moment at which Eng2 exerts its function during sporulation, we first tested the possibility that Eng2 might play a role during mating or sporulation. Wild-type, eng1Δ, or eng2Δ cells of opposite mating types were allowed to mate and sporulate at 25°C for 3 days. Mating and sporulation efficiency levels were monitored by microscopic inspection of the cultures. The percentages of mature spores in eng2Δ/eng2Δ and eng1Δ/eng1Δ crosses were similar to those found for wild-type haploid strains, indicating that eng1+ and eng2+ are not essential genes for conjugation and subsequent sporulation.
Progression through meiosis was also analyzed for wild-type and homozygous eng2Δ/eng2Δ mutant strains. The kinetics of the appearance of bi- and tetranucleate cells was measured for wild-type and eng2Δ mutant strains incubated in sporulation medium for different time intervals. Microscopic inspection of the DAPI-stained cells indicated that bi- and tetranucleate cells were present in the eng2Δ mutant. When progression through meiosis was quantified, the results indicated that the kinetics of appearance of bi- and tetranucleate cells in the eng2Δ mutant was almost identical to that of the wild-type strain (Fig. 2B). Mature spores started to appear after 9 h of incubation and accounted for about 70% of the culture at 12 h of incubation in the wild-type strain, and a similar value was observed for the eng2Δ mutant. Microscopic inspection of spores incubated for 24 h in sporulation medium suggested that the spores produced by the eng2Δ mutant were fully mature (Fig. 2B), although the spatial arrangement of the mutant spores had a slight tendency to be linear rather than the typical diamond shape. Together, these results indicate that eng2+ is not required for DNA duplication, meiotic segregation, or spore formation.
Eng2 is required for endolysis of the ascus wall.
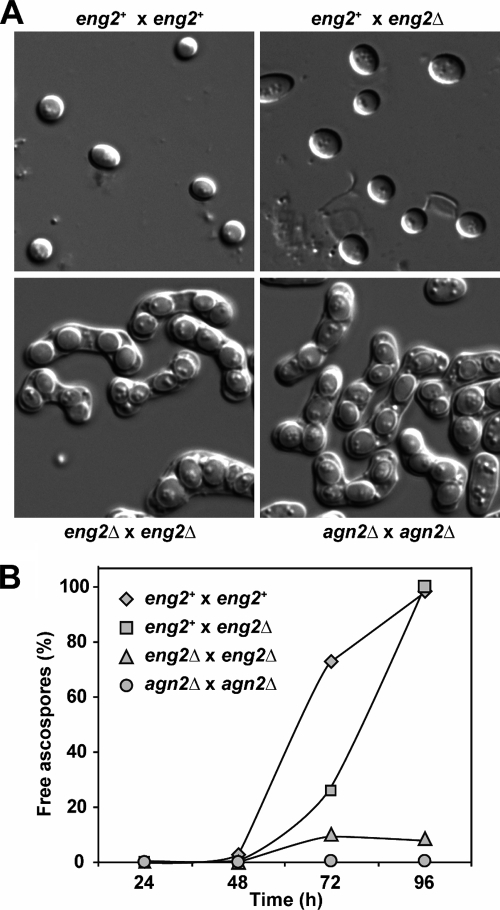
It has been reported that the endo-α-1,3-glucanase Agn2 is directly involved in endolysis of the ascus wall and that Agn2 is necessary for the release of ascospores into the medium (6, 7). agn2+ expression is also induced during sporulation, and the protein lacks a signal sequence for entry into the secretory pathway, localizing to the cytoplasm of the ascus. Since Eng2 also lacks a signal sequence for secretion, we analyzed whether Eng2 might play a similar role in the endolysis of the ascus wall after sporulation at the time of ascospore release. To test this idea, eng2Δ/eng2Δ diploid cells were transferred to sporulation medium and incubated for long periods of time. As controls, we used isogenic wild-type and agn2Δ/agn2Δ diploid strains. The three strains formed similar percentages of asci containing four ascospores within 24 to 48 h after the start of induction. In the wild-type tetrads, the ascus walls started to lyse, releasing the individual ascospores over time, a maximum of free spores (>95%) being reached at 96 h after induction (Fig. 3). In contrast, the ascus walls remained intact in most agn2Δ/agn2Δ and eng2Δ/eng2Δ tetrads. The defect in spore release was slightly more prominent in agn2Δ/agn2Δ cells than in eng2Δ/eng2Δ mutants (1% free spores versus 8%, respectively), suggesting that Agn2 might play a more relevant role than Eng2 in hydrolysis of the ascus wall (Fig. 3B).
FIG. 3.
Eng2 participates in ascus wall hydrolysis following sporulation. (A) Microscopic appearance of sporulated cultures obtained from crosses between wild-type haploid (OL176/OL177), eng2+/eng2Δ (OL176/OL773), eng2Δ/eng2Δ (OL759/OL773), and agn2Δ/agn2Δ (OL763/OL777) strains. Cultures were incubated for 96 h before the images were taken. (B) Quantification of ascospore release from the asci indicated in sporulated cultures from the same crosses. At the indicated time intervals, the percentages of free ascospores were determined by light microscopy. At least 150 cells were counted for each time point.
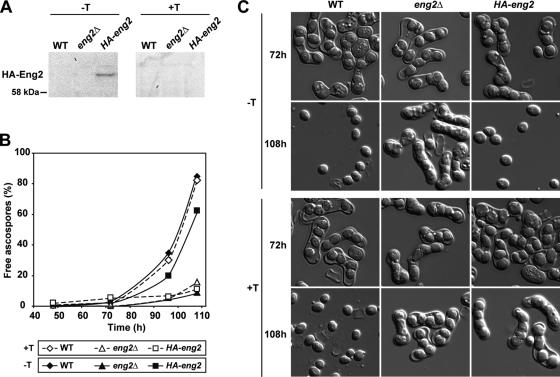
To confirm that eng2+ expression was required for proper ascospore dispersal, the eng2+ open reading frame was placed under the control of the nmt1+ thiamine-repressible promoter at its chromosomal locus. The HA epitope was also introduced at the N terminus in order to monitor protein levels. Subsequently, we created a heterozygous diploid strain carrying the Pnmt1-eng2 allele and the eng2Δ allele (Pnmt1-eng2/eng2Δ) and transferred the cells to sporulation medium. As controls for the experiment, we used a heterozygous wild type (eng2+/eng2Δ) and the eng2/eng2 mutant. Haploid strains were grown on YES medium to repress the expression of the Pnmt1-eng2 allele and then spotted on EMM-N plates, with or without thiamine, to allow mating and sporulation. Eng2 protein levels were analyzed after 24 h to confirm that no protein was produced under repressing conditions (Fig. 4A). The sporulation efficiencies of the three strains were similar in both media (data not shown). When free spores were analyzed over time, we found that the wild-type strain released similar numbers of spores under both repressing and inducing media (Fig. 4B and C). In the presence of thiamine (promoter off), the Pnmt1-eng2/eng2Δ strain produced asci with four ascospores that remained encapsulated by the ascus wall, like the eng2Δ/eng2Δ diploids. In contrast, under inducing conditions, most Pnmt1-eng2/eng2Δ ascus walls lysed to release free ascospores to a degree comparable to that of the heterozygous wild type (Fig. 4B and C). Together, these experiments indicate that Eng2 is also involved in endolysis of the ascus wall, like the α-1,3-glucanase Agn2.
FIG. 4.
Expression of eng2+ is essential for the release of ascospores from asci. The haploid eng2Δ strain carrying Pnmt1-eng2 (OL958) and the haploid strains from the opposite mating type, OL176 (WT) and OL773 (eng2Δ), were grown on YES medium to mid-log phase. Equal numbers of cells were collected and spotted onto EMM-N plates with (+T) and without (−T) thiamine to induce mating and sporulation. At the indicated times, aliquots were collected for microscopic inspection and quantification of the percentage of free spores. The crosses were eng2+/eng2Δ (WT), eng2Δ/eng2Δ (eng2Δ), and eng2Δ/eng2Δ plus Pnmt1-eng2 (HA-eng2). (A) Western analysis of Eng2-HA after 24 of incubation in EMM-N medium. (B) Percentages of free spores. (C) Sample images of the different strains grown in the presence and absence of thiamine.
Eng2 enzymatic activity is necessary for spore release.
GH81 proteins share a conserved region of around 650 amino acids in which the catalytic domain is included (25). Within this domain, two perfectly conserved Glu residues (E550 or E554) have been proposed as putative nucleophiles of the active site of the Aspergillus fumigatus Engl1 endoglucanase, while the proton donor would be D475 (31). These conserved residues are also required for the activity of S. cerevisiae Eng2 and soybean glucan-binding elicitor protein, since point mutations abolish catalytic activity without affecting protein levels (10, 25). To test whether hydrolysis of the ascus wall requires the enzymatic activity of Eng2, a point mutant in which one of the conserved Glu residues had been replaced by Ala was constructed [eng2(E537A) allele] (Fig. 5A). The plasmid was introduced into an eng2Δ mutant, and the resulting strain was crossed with an eng2Δ mutant of the opposite mating type. When the efficiency of spore release was analyzed, we found that it was almost identical to that of the eng2Δ/eng2Δ mutant (around 5% free spores after 96 h) (Fig. 5B), while eng2Δ/eng2Δ mutants carrying a plasmid with the wild-type eng2+ gene were similar to the wild-type control strain in this respect (>70% free spores). Thus, the endo-β-1,3-glucanase activity of Eng2 is essential for efficient spore release.
FIG. 5.
Eng2 catalytic activity is required for ascus endolysis. (A) Schematic representation of Eng2. The gray rectangle indicates the common region present in GH81 proteins that contains the putative catalytic domain of the protein (black rectangle). The white circle marks the position of the two perfectly conserved Glu residues, which have been proposed to act as putative nucleophiles (asterisks). E537 was mutated to Ala. (B) Quantification of ascospore release from the asci in sporulated cultures. At the indicated time intervals, the percentages of free ascospores were determined by light microscopy. At least 150 cells were counted for each time point. The crosses were OL176/OL177 (WT), OL759/OL773 (eng2Δ), OL759/OL773 carrying pJED12 (eng2Δ/peng2+), and OL759/OL773 carrying pJED13 [eng2Δ/peng2(E537A)].
Eng2 is directly involved in endolysis of the ascus wall.
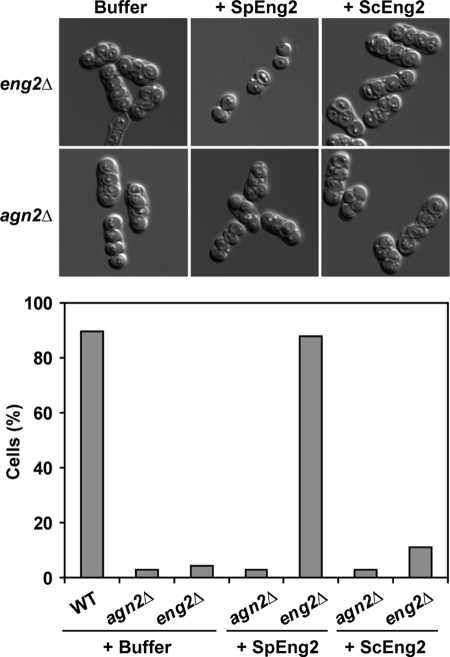
To corroborate the cellular function of Eng2, eng2Δ/eng2Δ diploid cells were induced to sporulate. When they had completely developed and matured, they were incubated with purified Eng2 from S. pombe and the percentage of ascospores released was determined by microscopic inspection (Fig. 6). As controls, we used purified S. cerevisiae Eng2 and an agn2Δ/agn2Δ diploid. The results indicated that the S. pombe Eng2 was able to complement the defect of the eng2Δ/eng2Δ mutant and hydrolyze the remnants of the ascus walls to release free ascospores, but Eng2 failed to complement the defect of the agn2Δ/agn2Δ mutant. Interestingly, S. cerevisiae Eng2 was largely deficient in complementing the defect of the eng2Δ/eng2Δ mutant, releasing hardly any ascospores. This failure to complement the phenotype of eng2Δ/eng2Δ mutants was not due to the absence of enzymatic activity, since both proteins were seen to have similar glucanase activities when laminarin was used as a substrate (58 mU/mg for S. pombe Eng2 versus 66 mU/mg for S. cerevisiae Eng2). This demonstrates directly that Eng2 is able to restore the endolysis defect of the eng2Δ/eng2Δ mutant, presumably by hydrolyzing the β-1,3-glucan present in the ascus wall. Furthermore, it demonstrates that the S. pombe and S. cerevisiae proteins have different specificities for their substrates in vivo.
FIG. 6.
Exogenous addition of purified Eng2 results in ascospore release. eng2Δ/eng2Δ (OL759/OL773) or agn2Δ/agn2Δ (OL763/OL777) diploid cells were allowed to sporulate for 7 days. After spore formation, the asci were incubated for 60 min at 37°C with buffer or 0.05 units of purified S. pombe Eng2 (SpEng2) or S. cerevisiae Eng2 (ScEng2). The percentage of ascospores released in each culture was examined by light microscopy. At least 100 cells were counted. WT, wild type.
Eng2 localizes to the epiplasm.
The α-glucanase Agn2 lacks a signal sequence for entry into the secretory pathway, and Agn2 localizes to the cytoplasm of the ascus, the epiplasm (6). Since Eng2 also lacks a conventional signal for secretion, it is possible that it might have a similar cytoplasmic localization. To test this, we used the Eng2-GFP construct to monitor Eng2 localization along the sporulation process. Microscopic observation of sporulating cells revealed that Eng2-GFP localized to the epiplasm when the spores had already matured (Fig. 7A), in a pattern similar to that of Agn2-GFP. However, some differences were observed during the early stages of spore development. While Agn2-GFP was not observed in sporulating cells in which the spores had not formed, Eng2-GFP appeared concentrated as two intense dots (one in each cell). This difference could be due to the fact that Eng2 is also present in vegetative cells, where it localizes as a dot in the cells. However, the significance of this dot and its function are currently unknown.
FIG. 7.
Eng2 localizes to the cytoplasm of the ascus. (A) Haploid Eng2-GFP (OL952) or Agn2-GFP (OL954) cells were allowed to mate on sporulation plates with cells from the opposite mating type carrying the same tagged proteins (strain OL953 or OL955, respectively), and the resulting zygotic asci were examined using fluorescence microscopy. Bar, 10 μm. (B) Details of free spores. (C) Germination of spores from the wild-type (WT) and eng2Δ/eng2Δ crosses. DIC, differential interface contrast.
We also found that a faint signal for Agn2-GFP and Eng2-GFP could be observed at the periphery of the released spores (Fig. 7B), raising the possibility that these two enzymes might have a function in spore wall degradation during germination. To test this possibility, we analyzed the spore germination and viability of eng2Δ/eng2Δ mutants in comparison with those of the wild-type strain. The results indicated that similar numbers of spores were able to germinate in the two strains (>70%) (Fig. 6C), ruling out the possibility that Eng2 is required for spore germination.
DISCUSSION
S. pombe contains two proteins belonging to the GH81 family encoded by the eng1+ and eng2+ genes. Both proteins have been shown to have endo-glucanase activity, specifically hydrolyzing β-1,3-glucan chains (25), like most of the members of this family described so far (4, 11, 12, 31). Even though they display the same enzymatic activity, Eng1 and Eng2 function at different moments of the life cycle of fission yeast. Thus, whereas Eng1 is involved in the controlled dissolution of the linear β-1,3-glucan of the primary septum during the last step of the cell cycle, i.e., cell separation (23), in the present study, we demonstrate that not only eng2+ expression but also the catalytic activity of Eng2 is required for endolysis of the ascus wall, the last step in the sexual cycle. The ascus wall is the cell wall of mating haploid cells or the cell wall of a sporulating diploid cell and is therefore expected to have a composition similar to that of vegetative cells, consisting mainly of α-1,3-glucan and β-1,3-glucan (17, 21). In light of its high substrate specificity, it is very likely that Eng2 is required for the degradation of the β-1,3-glucans of the ascus wall prior to spore release.
Interestingly, S. pombe contains another pair of hydrolytic enzymes that appear to function at similar times of the life cycle and have functions complementary to those of Eng1 and Eng2. These are the α-1,3-glucanases Agn1 and Agn2, belonging to the GH71 family. The β-glucanase Eng1 and the α-glucanase Agn1 fulfill their function during the last step of the vegetative cell cycle, i.e., controlled dissolution of the primary septum and the cylinder of cell wall that surrounds it, termed the septum edging (6, 13, 23). The complementary action of these two enzymes is necessary for the efficient degradation of the linear β-1,3-glucan of the primary septum and the α- and β-glucans of septum edging, allowing the two daughter cells to become two independent entities. The two genes, eng1+ and agn1+, show a periodic pattern of expression during the cell cycle, with a peak at the end of mitosis, and their transcription is controlled by the transcription factor Ace2 (1, 5, 32). Both Eng1 and Agn1 contain a signal sequence for entry into the secretory pathway, and they are transported to the septum region, where they initially localize as a ring that surrounds the septum in a process that is dependent on septins and the exocyst (1, 23, 26).
Similarly, the β-glucanase Eng2 and the α-glucanase Agn2 form another pair of complementary enzymes with some shared characteristics, and they function during the final step of the S. pombe sexual cycle. The expression of eng2+ and agn2+, belonging to the middle group of genes, is highly upregulated during the sporulation process, and their products are mainly involved in spore morphogenesis (27). Additionally, Eng2 lacks a signal peptide for entry into the secretory pathway and therefore localizes intracellularly to the cytosol of the diploid cell, as has been described for Agn2 (6). Since the ascus wall corresponds to the cell wall of the diploid cell or to the cell wall of conjugating haploids, this wall is expected to have a composition similar to that of the vegetative cell wall. The fact that the deletion of either of these enzymes produces a similar defect in spore release suggests that both α-1,3-glucan and β-1,3-glucan must be hydrolyzed for ascospores to be released efficiently, and this is achieved by the concerted action of Agn2 and Eng2. Both of these enzymes localize to the cytosol of the cell, but they exert their function at the extracellular side of the plasma membrane of the ascus. Since the synthesis of the spore wall requires a modification in the vesicular traffic to target the secretion of the components of the biosynthesis machinery to the forespore membrane, the absence of a secretory signal sequence might be essential for Eng2 and Agn2 to localize correctly and fulfill their cellular function. When the spore cell wall is synthesized, the inner layer of the forespore membrane becomes the spore plasma membrane, whereas the outer layer autolyzes. It is possible that a similar degradation occurs with the plasma membrane of the ascus, allowing Eng2 and Agn2 to access their substrates, as has been previously proposed (7). Interestingly, the genomes of other yeasts, such as S. cerevisiae and Candida albicans, contain a pair of endo-β-1,3-glucanases, one of which lacks a signal peptide (4, 9). Whether the cytoplasmic β-glucanase plays a role during other moments of the life cycle remains to be investigated.
Another interesting issue is the differences in the in vivo substrate specificities of the GH81 proteins. We have shown that purified Eng2 from S. pombe fully complements the defect of an eng2Δ/eng2Δ mutant, while S. cerevisiae Eng2 is largely deficient in this process. Although the two proteins are found to have similar enzymatic activities when assayed in vitro, the present results could be an indication that they have different substrate specificities in vivo and that the β-1,3-glucans of the S. pombe spore wall are inefficiently recognized and cleaved by S. cerevisiae Eng2. Alternatively, this difference could reflect the differences between the biological properties of S. cerevisiae and S. pombe, since asci are not autolyzed before spore germination in budding yeast. Additionally, the two S. pombe proteins of this family, Eng1 and Eng2, also seem to have different in vivo substrates. In vitro, both proteins are able to degrade β-1,3-glucans (25). However, in vivo, their substrates must be different, since Eng1 acts specifically on the primary septum, which is rich in linear chains of β-1,3-glucan (18), while Eng2 should act on the β-1,3-glucans of the cell wall. These differences could be due to the fact that at the C terminus, Eng1 contains three repeats of a sequence acting as a carbohydrate-binding domain that are necessary for its correct localization to the septum region and that might provide strong specificity for the linear β-glucan chains whereas Eng2 lacks this region (24).
Acknowledgments
We thank Ana Belen Martín-Cuadrado for Eng2 protein purification, members of the laboratory for help and support, and Nick Skinner for language revision.
This work was supported by grants from Ministerio de Ciencia y Tecnología (BFU2004-00778 and BFU2007-60390).
Footnotes
Published ahead of print on 19 June 2009.
REFERENCES
- 1.Alonso-Nuñez, M. L., H. An, A. B. Martín-Cuadrado, S. Mehta, C. Petit, M. Sipiczki, F. del Rey, K. L. Gould, and C. R. Vázquez de Aldana. 2005. Ace2p controls the expression of genes required for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell 162003-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arellano, M., H. Cartagena-Lirola, M. A. Nasser Hajibagheri, A. Durán, and M. H. Valdivieso. 2000. Proper ascospore maturation requires the chs1+ chitin synthase gene in Schizosaccharomyces pombe. Mol. Microbiol. 3579-89. [DOI] [PubMed] [Google Scholar]
- 3.Bähler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie, A. B. Steever, A. Wach, P. Philippsen, and J. R. Pringle. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14943-951. [DOI] [PubMed] [Google Scholar]
- 4.Baladrón, V., S. Ufano, E. Dueñas, A. B. Martín-Cuadrado, F. del Rey, and C. R. Vázquez de Aldana. 2002. Eng1p, an endo-1,3-β-glucanase localized at the daughter side of the septum, is involved in cell separation in Saccharomyces cerevisiae. Eukaryot. Cell 1774-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dekker, N., A. de Haan, and F. Hochstenbach. 2006. Transcription regulation of the α-glucanase gene agn1 by cell separation transcription factor Ace2p in fission yeast. FEBS Lett. 5803099-3106. [DOI] [PubMed] [Google Scholar]
- 6.Dekker, N., D. Speijer, C. H. Grün, M. van den Berg, A. de Haan, and F. Hochstenbach. 2004. Role of the α-glucanase Agn1p in fission-yeast cell separation. Mol. Biol. Cell 153903-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dekker, N., J. van Rijssel, B. Distel, and F. Hochstenbach. 2007. Role of the α-glucanase Agn2p in ascus-wall endolysis following sporulation in fission yeast. Yeast 24279-288. [DOI] [PubMed] [Google Scholar]
- 8.de Medina-Redondo, M., Y. Arnáiz-Pita, T. Fontaine, F. del Rey, J. P. Latgé, and C. R. Vázquez de Aldana. 2008. The β-1,3-glucanosyltransferase gas4p is essential for ascospore wall maturation and spore viability in Schizosaccharomyces pombe. Mol. Microbiol. 681283-1299. [DOI] [PubMed] [Google Scholar]
- 9.Esteban, P. F., I. Ríos, R. García, E. Dueñas, J. Plá, M. Sánchez, C. R. Vázquez de Aldana, and F. del Rey. 2005. Characterization of the CaENG1 gene encoding an endo-1,3-β-glucanase involved in cell separation in Candida albicans. Curr. Microbiol. 51385-392. [DOI] [PubMed] [Google Scholar]
- 10.Fliegmann, J., A. Mithofer, G. Wanner, and J. Ebel. 2004. An ancient enzyme domain hidden in the putative β-glucan elicitor receptor of soybean may play an active part in the perception of pathogen-associated molecular patterns during broad host resistance. J. Biol. Chem. 2791132-1140. [DOI] [PubMed] [Google Scholar]
- 11.Fliegmann, J., E. Montel, A. Djulic, S. Cottaz, H. Driguez, and J. Ebel. 2005. Catalytic properties of the bifunctional soybean β-glucan-binding protein, a member of family 81 glycoside hydrolases. FEBS Lett. 5796647-6652. [DOI] [PubMed] [Google Scholar]
- 12.Fontaine, T., R. P. Hartland, A. Beauvais, M. Diaquin, and J. P. Latgé. 1997. Purification and characterization of an endo-1,3-β-glucanase from Aspergillus fumigatus. Eur. J. Biochem. 243315-321. [DOI] [PubMed] [Google Scholar]
- 13.García, I., D. Jiménez, V. Martín, A. Durán, and Y. Sánchez. 2005. The α-glucanase Agn1p is required for cell separation in Schizosaccharomyces pombe. Biol. Cell 97569-576. [DOI] [PubMed] [Google Scholar]
- 14.Garcia, I., V. Tajadura, V. Martin, T. Toda, and Y. Sanchez. 2006. Synthesis of α-glucans in fission yeast spores is carried out by three α-glucan synthase paralogues, Mok12p, Mok13p and Mok14p. Mol. Microbiol. 59836-853. [DOI] [PubMed] [Google Scholar]
- 15.Hirata, A., and C. Shimoda. 1994. Structural modification of spindle pole bodies during meiosis II is essential for the normal formation of ascospores in Schizosaccharomyces pombe: ultrastructural analysis of spo mutants. Yeast 10173-183. [DOI] [PubMed] [Google Scholar]
- 16.Hochstenbach, F., F. M. Klis, H. van den Ende, E. van Donselaar, P. J. Peters, and R. D. Klausner. 1998. Identification of a putative α-glucan synthase essential for cell wall construction and morphogenesis in fission yeast. Proc. Natl. Acad. Sci. USA 959161-9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horisberger, M., and M. Rouver-Vauthey. 1985. Cell wall architecture of the fission yeast Schizosaccharomyces pombe. Experientia 41748-750. [Google Scholar]
- 18.Humbel, B. M., M. Konomi, T. Takagi, N. Kamasawa, S. A. Ishijima, and M. Osumi. 2001. In situ localization of β-glucans in the cell wall of Schizosaccharomyces pombe. Yeast 18433-444. [DOI] [PubMed] [Google Scholar]
- 19.Ito, H., K. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cation. J. Bacteriol. 153163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, J., X. Tang, H. Wang, and M. Balasubramanian. 2000. Bgs2p, a 1,3-β-glucan synthase subunit, is essential for maturation of ascospore wall in Schizosaccharomyces pombe. FEBS Lett. 478105-108. [DOI] [PubMed] [Google Scholar]
- 21.Manners, D. J., and M. T. Meyer. 1977. The molecular structures of some glucans from the cell wall of Schizosaccharomyces pombe. Carbohydr. Res. 57189-203. [Google Scholar]
- 22.Martín, V., J. C. Ribas, E. Carnero, A. Durán, and Y. Sánchez. 2000. bgs2+, a sporulation-specific glucan synthase homologue is required for proper ascospore wall maturation in fission yeast. Mol. Microbiol. 38308-321. [DOI] [PubMed] [Google Scholar]
- 23.Martín-Cuadrado, A. B., E. Dueñas, M. Sipiczki, C. R. Vázquez de Aldana, and F. del Rey. 2003. The endo-β-1,3-glucanase Eng1p is required for dissolution of the primary septum during cell separation in Schizosaccharomyces pombe. J. Cell Sci. 1161689-1698. [DOI] [PubMed] [Google Scholar]
- 24.Martín-Cuadrado, A. B., J. Encinar del Dedo, M. de Medina-Redondo, T. Fontaine, F. del Rey, J. P. Latgé, and C. R. Vázquez de Aldana. 2008. The Schizosaccharomyces pombe endo-1,3-β-glucanase Eng1 contains a novel carbohydrate binding module required for septum localization. Mol. Microbiol. 69188-200. [DOI] [PubMed] [Google Scholar]
- 25.Martín-Cuadrado, A. B., T. Fontaine, P. F. Esteban, J. Encinar del Dedo, M. de Medina-Redondo, F. del Rey, J. P. Latgé, and C. R. Vázquez de Aldana. 2008. Characterization of the endo-β-1,3-glucanase activity of S. cerevisiae Eng2 and other members of the GH81 family. Fungal Genet. Biol. 45542-553. [DOI] [PubMed] [Google Scholar]
- 26.Martín-Cuadrado, A. B., J. L. Morrell, M. Konomi, H. An, C. Petit, M. Osumi, M. Balasubramanian, K. L. Gould, F. del Rey, and C. R. Vázquez de Aldana. 2005. Role of septins and the exocyst complex in the function of hydrolytic enzymes responsible for fission yeast cell separation. Mol. Biol. Cell 164867-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mata, J., R. Lyne, G. Burns, and J. Bahler. 2002. The transcriptional program of meiosis and sporulation in fission yeast. Nat. Genet. 32143-147. [DOI] [PubMed] [Google Scholar]
- 28.Mata, J., A. Wilbrey, and J. Bahler. 2007. Transcriptional regulatory network for sexual differentiation in fission yeast. Genome Biol. 8R217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuo, Y., K. Tanaka, H. Matsuda, and M. Kawamukai. 2005. cda1+, encoding chitin deacetylase is required for proper spore formation in Schizosaccharomyces pombe. FEBS Lett. 5792737-2743. [DOI] [PubMed] [Google Scholar]
- 30.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetics analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194795-823. [DOI] [PubMed] [Google Scholar]
- 31.Mouyna, I., J. Sarfati, P. Recco, T. Fontaine, B. Henrissat, and J. P. Latgé. 2002. Molecular characterization of a cell wall-associated β(1-3)endoglucanase of Aspergillus fumigatus. Med. Mycol. 40455-464. [DOI] [PubMed] [Google Scholar]
- 32.Rustici, G., J. Mata, K. Kivinen, P. Lió, C. J. Penkett, G. Burns, J. Hayles, A. Brazma, P. Nurse, and J. Bähler. 2004. Periodic gene expression program of the fission yeast cell cycle. Nat. Genet. 36809-817. [DOI] [PubMed] [Google Scholar]
- 33.Shimoda, C. 2004. Forespore membrane assembly in yeast: coordinating SPBs and membrane trafficking. J. Cell Sci. 117389-396. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka, K., and A. Hirata. 1982. Ascospore development in the fission yeasts Schizosaccharomyces pombe and S. japonicus. J. Cell Sci. 56263-279. [DOI] [PubMed] [Google Scholar]
- 35.Wach, A. 1996. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in Saccharomyces cerevisiae. Yeast 12259-265. [DOI] [PubMed] [Google Scholar]
- 36.Wood, V., R. Gwilliam, M. A. Rajandream, M. Lyne, R. Lyne, A. Stewart, J. Sgouros, N. Peat, J. Hayles, S. Baker, D. Basham, S. Bowman, K. Brooks, D. Brown, S. Brown, T. Chillingworth, C. Churcher, M. Collins, R. Connor, A. Cronin, P. Davis, T. Feltwell, A. Fraser, S. Gentles, A. Goble, N. Hamlin, D. Harris, J. Hidalgo, G. Hodgson, S. Holroyd, T. Hornsby, S. Howarth, E. J. Huckle, S. Hunt, K. Jagels, K. James, L. Jones, M. Jones, S. Leather, S. McDonald, J. McLean, P. Mooney, S. Moule, K. Mungall, L. Murphy, D. Niblett, C. Odell, K. Oliver, S. O'Neil, D. Pearson, M. A. Quail, E. Rabbinowitsch, K. Rutherford, S. Rutter, D. Saunders, K. Seeger, S. Sharp, J. Skelton, M. Simmonds, R. Squares, S. Squares, K. Stevens, K. Taylor, R. G. Taylor, A. Tivey, S. Walsh, T. Warren, S. Whitehead, J. Woodward, G. Volckaert, R. Aert, J. Robben, B. Grymonprez, I. Weltjens, E. Vanstreels, M. Rieger, M. Schafer, S. Muller-Auer, C. Gabel, M. Fuchs, C. Fritzc, E. Holzer, D. Moestl, H. Hilbert, K. Borzym, I. Langer, A. Beck, H. Lehrach, R. Reinhardt, T. M. Pohl, P. Eger, W. Zimmermann, H. Wedler, R. Wambutt, B. Purnelle, A. Goffeau, E. Cadieu, S. Dreano, S. Gloux, V. Lelaure, S. Mottier, F. Galibert, S. J. Aves, Z. Xiang, C. Hunt, K. Moore, S. M. Hurst, M. Lucas, M. Rochet, C. Gaillardin, V. A. Tallada, A. Garzon, G. Thode, R. R. Daga, L. Cruzado, J. Jimenez, M. Sanchez, F. del Rey, J. Benito, A. Dominguez, J. L. Revuelta, S. Moreno, J. Armstrong, S. L. Forsburg, L. Cerrutti, T. Lowe, W. R. McCombie, I. Paulsen, J. Potashkin, G. V. Shpakovski, D. Ussery, B. G. Barrell, and P. Nurse. 2002. The genome sequence of Schizosaccharomyces pombe. Nature 415871-880. [DOI] [PubMed] [Google Scholar]