Abstract
The copper resistance determinant copARZ, which encodes a CPx-type copper ATPase efflux protein, a transcriptional regulator, and a putative intracellular copper chaperone, was functionally characterized for the phytopathogenic bacterium Agrobacterium tumefaciens. These genes are transcribed as an operon, and their expression is induced in response to increasing copper and silver ion concentrations in a copR-dependent fashion. Analysis of the copARZ promoter revealed a putative CopR binding box located within the spacer of the −35 and −10 promoter motifs. In vitro, purified CopR could specifically bind to the box. The inactivation of the copARZ operon or copZ reduces the level of resistance to copper but not to other metal ions. Also, the copARZ operon mutant shows increased sensitivity to the superoxide generators menadione and plumbagin. In addition, the loss of functional copZ does not affect the ability of copper ions to induce the copARZ promoter, indicating that CopZ is not involved in the copper-sensing mechanism of CopR. Altogether, the results demonstrate a crucial role for the copARZ operon as a component of the copper resistance machinery in A. tumefaciens.
Copper (Cu) is an essential trace element, serving as a cofactor for a variety of enzymes including terminal oxidases, monooxygenase, dioxygenases, and superoxide dismutases. Since Cu is a redox-active metal, an excess of the metal in aerobic cells can generate reactive oxygen species (ROS) through the Fenton-like reaction (8). ROS are cytotoxic and capable of causing damage to biological molecules including DNA, lipids, and proteins (26). Therefore, all organisms have evolved elaborate mechanisms to maintain Cu homeostasis (17, 41). Cu homeostasis is a complicated process involving Cu acquisition, sequestration, and efflux. These mechanisms are tightly regulated and are able to respond to changes in the extracellular bioavailability and intracellular demand for the metal. Active efflux is one of the key mechanisms for Cu tolerance and involves transporting excess Cu ions out of the cytoplasm and periplasm. Cu homeostasis in bacteria has been thoroughly investigated with Enterococcus hirae and Escherichia coli (41, 45). E. hirae, a gram-positive bacterium, possesses a cop operon comprised of copYZAB, which has a dominant role in Cu homeostasis (45). copA and copB encode P-type ATPases of universal ATP-driven ion pumps. CopA is responsible for Cu uptake under Cu-limiting conditions, while CopB is an efflux pump that excretes Cu when the intracellular concentration of Cu is in excess. The expression of the cop operon is modulated by cytoplasmic Cu levels through the coordinated action of the CopY transcriptional repressor and a Cu chaperone, CopZ. Under conditions of excess intracellular Cu, the Cu-bound CopZ delivers Cu ions to CopY, which displaces a structural zinc ion. Subsequent structural changes cause a dissociation of CopY from the operator site, thereby allowing the expression of the cop operon. E. coli, a gram-negative bacterium, has two Cu-regulatory systems to cope with high concentrations of Cu (41). The first system is regulated by CueR, a member of MerR family of metalloregulatory proteins. The binding of a Cu ion to CueR leads to the expression of copA, which encodes a Cu-translocating CPx-type ATPase responsible for the transport of cytosolic Cu into the periplasm. The second system is a two-component signal consisting of CusRS, which activates the expression of the divergently transcribed cusCFBA, which encodes a four-component Cu efflux pump. Both CueR and CusRS sense and respond to Cu ions primarily in the form of Cu(I). Thus, different microbes appear to have evolved distinct pathways to maintain Cu homeostasis.
Agrobacterium tumefaciens is a soil-borne plant-pathogenic bacterium causing crown gall tumors worldwide. The bacterium is also widely used as a genetic tool for delivering foreign DNA into plant cells to generate transgenic plants (15). A. tumefaciens is commonly exposed to high levels of Cu ions as a consequence of the use of Cu-based bactericides containing, viz., Cu(OH)2, CuSO4, and Cu oxychloride for plant pathogen management (23, 25). The mechanism by which the bacterium deals with Cu stress is unknown. In this study, we characterize one of the Cu resistance determinants, copARZ, located on the circular chromosome of A. tumefaciens. A disruption of the operon reduced Cu tolerance. Furthermore, the expression of the cop operon is mediated by CopR. The evidence strongly suggests that the cop operon facilitates Cu tolerance through an efflux system consisting of the efflux pump CopA CPx-type ATPase, CopR as a transcriptional regulator of the operon, and CopZ as a Cu chaperone.
MATERIALS AND METHODS
Bacterial growth conditions.
Agrobacterium tumefaciens NTL4; a pTiC58-cured derivative of C58, ΔtetC58 (30); and mutant strains were grown aerobically in Luria-Bertani (LB) medium at 30°C with continuous shaking at 150 rpm. Exponential-phase cells were used for all experiments. The metal ion induction experiments were conducted with cells treated with various concentrations of heavy-metal ions for 15 and 30 min for primer extension and enzymatic assays, respectively.
Determination of copper resistance and requirement.
Cu resistance was determined by using a plate sensitivity assay (39). Serial dilutions of exponential-phase cultures of A. tumefaciens were plated onto LB medium containing increasing concentrations of CuSO4. The surviving colonies were scored after 48 h of incubation at 30°C.
The Cu requirement was assessed as previously described (34), with some modifications. Metal ions in AB minimal medium (10) were chelated with increasing concentrations of the Cu(II) chelator cuprizone (biscyclohexanone oxalyldihydrazone; Sigma-Aldrich). Bacterial cultures in Cu(II)-chelated medium were incubated for 30 h at 30°C before the optical density at 600 nm was monitored.
Molecular biology techniques.
General molecular genetic techniques, including genomic DNA preparation, plasmid preparation, restriction endonuclease digestions, ligation, transformation into E. coli, agarose gel electrophoresis, and Southern and Northern blot analyses, were performed using standard protocols (43). A. tumefaciens was transformed by electroporation as previously described (30).
Construction of copA, copR, and copZ mutants.
A. tumefaciens copA, copR, and copZ mutants were constructed by insertional inactivation using a suicide plasmid, pKNOCK, designed for single-crossover recombination. pKNOCK containing copA, copR, or copZ gene fragments amplified by PCR was constructed. Specific pairs of oligonucleotide primers, BT1424-BT1425, BT1003-BT1004, and BT1682-BT1683 (Table 1), which were designed corresponding to the internal nucleotide coding sequences of copA, copR, and copZ of the A. tumefaciens C58 genome (22), respectively, were used to amplify a roughly 200-bp copA, copR, or copZ fragment using genomic DNA as a template. The PCR products were cloned into pGEM-T-Easy (Promega) before the EcoRI fragment was subcloned into pKNOCK-Gm (2) digested with the same enzyme. The recombinant plasmids were transferred into A. tumefaciens NTL4 using a biparental conjugation method as described previously (2). The putative mutants were selected for a gentamicin resistance phenotype, and the genotype was confirmed using Southern blot analysis probed with specific gene fragments.
TABLE 1.
List of oligonucleotide primers
| Primer | Sequence (5′→3′) |
|---|---|
| BT1003 | GCAGGATGTGCACAATCTGC |
| BT1004 | CCACTTCTGCGAGAGGGTAG |
| BT1056 | CATCACGCTGTCGCCGATGA |
| BT1057 | GCGCTCTTGGGTGACTTGAC |
| BT1424 | AGGCTCGTCGTATGTCGATG |
| BT1425 | GCAGCTGGATCGGCAGCTT |
| BT1489 | CATAAGGTCTCGAAAGGG |
| BT1490 | CGCCCGATGCCTCCGATG |
| BT1550 | CGTGATCGGTGGCTTGTAC |
| BT1551 | GCAGGTCATGCCTTCCACG |
| BT1678 | ACCGGTTGATTTGGATGG |
| BT1680 | CAAAAGGAGAAAACCATGA |
| BT1681 | GCCGCATTTCAGCCCGCC |
| BT1682 | CTGCGGCCATTGCGAAA |
| BT1683 | GCTTCGCGGATAGCCTC |
| BT1830 | TCACGCTGTCGCCGATGA |
| BT1986 | CGCCATGGACATCGGCCAG |
| BT2270 | ATGGGCACGGATGTGGAC |
| BT2330 | GCTGCGCTCACGATTGTGTGT |
Construction of pCopA, pCopR, pCopZ, and pPcopA.
The full-length genes were amplified from NTL4 genomic DNA with primers BT1489 and BT1490 for copA, BT1056 and BT1057 for copR, and BT1680 and BT1681 for copZ. The PCR products were cloned into pGEM-T-Easy and sequenced prior to subcloning into a broad-host-range plasmid, pBBR1MCS-5 (29), at ApaI and SalI sites, giving pCopA, pCopR, and pCopZ. In each case, the expression of the cloned gene was under the control of the lac promoter of the plasmid vector.
The pPcopA-containing copA promoter transcriptionally fused to a lacZ gene was constructed in a low-copy-number promoter-probe vector, pUFR027lacZ (33). The copA promoter region was amplified from NTL4 genomic DNA using primers BT1550 and BT1551. The 252-bp PCR product was cloned into pGEM-T-Easy prior to subcloning of the EcoRI fragment into pUFR027lacZ to generate pPcopA.
Complementation of the copA mutant using a mini-Tn7 transposon.
To complement the A. tumefaciens copA mutant, a mini-Tn7 vector was used to transpose the copA gene into copA mutant chromosomes. The DNA sequence spanning the copA gene and its promoter region was PCR amplified with primers BT1550 and BT1490. A 2,856-bp PCR product was ligated into pGEM-T-Easy (Promega) before an ApaI-SpeI fragment was subcloned into pUC18-mini-Tn7T-Km, a derivative of pUC18-mini-Tn7T-Gm (11), digested with the same enzymes to generate pMini-Tn7copA. The recombinant plasmid and helper plasmid pTNS (11) were cotransferred into the copA mutant using electroporation. The mini-Tn7 transposon specifically integrates into bacterial chromosomes at the Tn7 attachment (attTn7) site normally located immediately downstream of glmS, a gene encoding glucosamine-fructose-6-phosphate aminotransferase. The transformant (copA::mini-Tn7-copA) was selected by kanamycin resistance. In A. tumefaciens, a single attTn7 site was identified at glmS1 (Atu1786) (50). Since a homologous recombination between mini-Tn7-copA and the residual copA sequences at the site of the mutation may occur, the transposition of mini-Tn7-copA at the attTn7 site and the presence of the copA gene in the copA::Tn7-copA strain were confirmed by PCR analysis with primers BT2270 (located on glmS1) and Tn7R (11) and primers BT1550 and BT1490, respectively.
RT-PCR of copA-copR-copZ mRNA.
Reverse transcription (RT) of copA-copR-copZ mRNA was performed to confirm the polycistronic transcriptional organization of these genes. Total RNA was isolated from A. tumefaciens cultures grown under uninduced and metal ion-induced conditions. Purified RNA was treated with 10 U of RNase-free DNase I for 30 min. Primer BT1683 (located within copZ) (Table 1) was mixed with 1 μg of RNA, and 200 U of cloned Moloney murine leukemia virus reverse transcriptase (Promega) was added. The mixture was incubated at 42°C for 60 min. Five microliters of the mixture was added to a PCR mixture containing primers BT1683 and BT1830 (located in copA) (Table 1). PCR was performed for 25 cycles under the following conditions: denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 30 s. The PCR products were analyzed by agarose gel electrophoresis.
Primer extension.
Total RNA was isolated from uninduced and Cu-induced cultures. The experiments were performed using 32P-labeled BT1986 primers (Table 1), 5 μg of total RNA, and 200 U of Superscript II Moloney murine leukemia virus reverse transcriptase (Promega). Extension products were sized on sequencing gels next to dideoxy sequencing ladders generated using a PCR sequencing kit with labeled forward primer −pUC/M13 and pGEM-3Zf(+) as the template (Applied Biosystems).
Purification of CopR.
A PCR product containing copR, in which an NcoI site overlapping the start codon had been introduced, was generated using pCopR as a template and specific oligonucleotide primers BT1986 and BT1057. The NcoI-digested fragment was cloned into NcoI-EcoRV-digested pETBlue-2 (Novagen), yielding pETcopR.
An E. coli BL21(DE3) culture harboring pETcopR was cultivated in LB broth supplemented with 100 μM CuSO4 and incubated at 28°C. Exponential-phase cultures were induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and incubated for an additional 3 h. The cultures were harvested by centrifugation, and cell pellets were resuspended in 50 mM phosphate buffer (pH 7.0), sonicated, and then spun at 10,000 ×g for 15 min. The cleared lysate was then loaded onto an Affi-Gel heparin column (Bio-Rad), followed by extensive washing with column buffer (25 mM Tris-HCl [pH 8], 25 mM NaCl, 2 mM EDTA, 1 mM dithiothreitol [DTT]). The protein was eluted via the addition of elution buffer (25 mM Tris-HCl [pH 8], 2 mM EDTA, 1 mM DTT) containing gradient concentrations of NaCl (0 to 1 M). The eluted fraction was dialyzed against a solution containing 25 mM Tris-HCl (pH 8), 100 mM NaCl, 2 mM EDTA, and 1 mM DTT. The purity of the protein was evaluated using sodium dodecyl sulfate-polyacrylamide gels. The Cu content of purified CopR was determined using an atomic absorption spectrometer (SpectrAA 220Z; Variant, Australia). The molar ratio of CopR to Cu was 36:1, indicating that the majority of purified CopR is in the repressor apo-CopR form.
DNase I footprinting.
Primer BT2330 was end labeled with [γ-32P]dATP using T4 polynucleotide kinase (Promega). 32P-labeled DNA fragments were prepared by PCR using 32P-labeled primers BT2330 and BT1551 and pPcopA as the template to generate a 252-bp fragment spanning the copA promoter region. DNase I footprint analysis was carried out in a 50-μl reaction mixture containing 1× binding buffer (20 mM Tris-Cl [pH 7.0], 50 mM KCl, 1 mM EDTA, 5% glycerol, 50 μg/ml bovine serum albumin [BSA], 5 μg/ml calf thymus DNA, and 0.1 mM DTT), 500 ng poly(dI-dC), 20 ng labeled DNA fragment, and purified CopR at the indicated concentrations. The binding was allowed to proceed at room temperature for 15 min. Fifty microliters of solution containing 5 M CaCl2 and 10 mM MgCl2 was added to the reaction mixture prior to digestion with 0.2 units of DNase I for 30 s. Reactions were stopped by adding 700 μl stop solution (645 μl ethanol, 50 μl of 3 M sodium acetate, and 5 μl of 1 mg/ml yeast tRNA) to the mixture. The DNA was recovered by centrifugation for 15 min and resuspended in formamide loading buffer before being loaded onto 8% denatured DNA sequencing gels.
β-Galactosidase assay.
Crude bacterial lysates were prepared and protein assays were performed as previously described (12). The total protein concentration was determined for each of the cleared lysates prior to their use in enzyme assays. β-Galactosidase activity was assayed as described previously (14) and is expressed in international units (defined as the amount of enzyme capable of releasing 1 μmol p-nitrophenol generated at 25°C per min) per mg protein.
RESULTS AND DISCUSSION
Identification of cop genes in the A. tumefaciens genome.
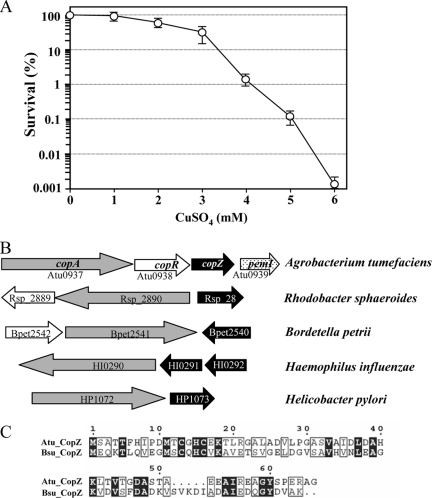
We are interested in the process governing Cu homeostasis in a soil-borne phytopathogenic bacterium, A. tumefaciens. This bacterium is often exposed to high-Cu environments, where Cu-based bactericides have been used for antibacterial and antifungal measures in crop fields. Initially, the levels of resistance of A. tumefaciens strains to Cu ions were assessed with rich medium (LB medium) using plate sensitivity assays. The presence of CuSO4 at a concentration of 2 mM slightly inhibited bacterial growth (Fig. 1A). Bacterial growth was decreased roughly 3 logs at 5 mM CuSO4 (Fig. 1A) and was totally inhibited at 7 mM CuSO4 (data not shown). The Cu resistance level of A. tumefaciens is relatively high compared to that reported previously for Xanthomonas axonopodis, where 1 mM Cu ions severely retarded bacterial growth in rich medium (49).
FIG. 1.
Copper resistance in A. tumefaciens. (A) Copper resistance was determined by using plate sensitivity assays. Serial dilutions of exponential-phase cultures of A. tumefaciens NTL4 were spread onto LB agar plates containing the indicated concentrations of CuSO4 and incubated for 48 h. The resistance level was expressed as the percentage of survival, defined as the number of CFU on plates containing CuSO4 divided by the number of CFU on plates without CuSO4. Error bars indicate the standard deviations (SD) of data from four independently performed experiments. (B) Gene organizations of the cop determinant in A. tumefaciens and particular gram-negative bacteria show the linkage between copA and copZ. Bacterial genomes were retrieved from the Kyoto Encyclopedia of Genes and Genomes (http://www.genome.jp/kegg). Gray shaded, open, and closed arrows represent the copA, copR, and copZ genes, respectively, and the arrow orientation indicates the transcription direction. (C) Alignment of deduced amino acid sequences from A. tumefaciens CopZ (Atu_CopZ) and Bacillus subtilis CopZ (Bsu_CopZ) (19).
Next, the Cu resistance mechanism in A. tumefaciens was molecularly characterized. A BLASTP (3) search of the CopA sequence against the annotated genome sequences of A. tumefaciens C58 (50) revealed the existence of copA (Atu0937), encoding a putative Cu-translocating CPx-type ATPase on the circular chromosome. An open reading frame (ORF) located immediately downstream of copA is annotated cueR (Atu0938), which encodes a MerR-type transcriptional regulator. Analysis of the intergenic sequence flanking ORFs Atu0938 (CueR) and Atu0939 (PemI) revealed a potential ORF with the same relative orientation as those of copA and cueR (see Fig. S1 in the supplemental material). This ORF, located 52 bases downstream of Atu0938, corresponding to nucleotides 928697 to 928897 of the A. tumefaciens genome (50), is predicted to encode a protein containing a heavy-metal-associated amino acid sequence motif that shares 29% identity to Bacillus subtilis CopZ (5), an intracellular Cu chaperone. The finding that these functionally related genes are located in close physical proximity suggests that copA, cueR, and copZ are expressed as a single transcription unit. Since the cueR-like ORF is located in a possible operon with copA and copZ, it is henceforth referred to as copR. The copA-copR-copZ operon organization is, to our knowledge, unique to A. tumefaciens, as shown by searches of current bacterial genome databases including Agrobacterium vitis and Agrobacterium radiobacter (Fig. 1B). However, the close linkage between copA and copR has been observed for a number of bacteria such as A. radiobacter, A. vitis, Sinorhizobium meliloti, Sinorhizobium medicae, Rhodobacter sphaeroides, Rhizobium leguminosarum, Rhizobium etli, and Bordetella petrii.
A. tumefaciens copA encodes a putative protein of 862 amino acid residues with a theoretical molecular mass of 90.1 kDa. The deduced A. tumefaciens CopA amino acid sequence shares high levels of identity to other bacterial Cu ATPases, i.e., Bacillus subtilis CopA (43%), Enterococcus hirae CopA (41%), Staphylococcus aureus CopA (41%), E. coli CopA (35%), and E. hirae CopB (31%) (20, 40, 44, 45). Cu ATPase is a member of the CPx-type ATPase superfamily, whose members are typically involved in the transport of heavy metals. P-type ATPase is generally responsible for the active transport of a variety of cations across biological membranes driven by energy from ATP hydrolysis. The direction of Cu transport mediated by the P-type ATPase in bacteria is unclear. The inactivation of copA in several bacteria reduces their ability to cope with high levels of Cu, suggesting functions in Cu export (38, 44). E. hirae contains two P-type Cu ATPase proteins encoded by copA and copB, which are involved in Cu uptake and efflux, respectively. The primary structure of A. tumefaciens CopA exhibits features that are intermediate between those of E. hirae CopA and CopB in that it contains a CXXC motif at the N terminus, as in CopA, and a histidine-rich domain at the N terminus, similarly to CopB. In gram-negative E. coli, copA mutants accumulate cytoplasmic Cu, suggesting that CopA functions as an efflux pump (38). Thus, the Cu transport function of A. tumefaciens CopA warrants further investigation.
The structure of heavy-metal P-type ATPase contains one to six CXXC metal binding motifs in addition to transmembrane helices and several conserved structural motifs (45). Topology analysis of A. tumefaciens CopA using the TMPred, HMMTOP, and TMHMM algorithms predicted that CopA contains eight transmembrane domains (data not shown). A heavy-metal binding domain GMTCXXC motif (31) near the amino terminus was previously identified. The phosphorylation domain DKTGA and the ATP binding domain GCGINDAP were found between transmembrane domains 6 and 7. The presence of CPC at transmembrane domain 6 suggests that CopA belongs to the CPx-type P-ATPase (45).
A. tumefaciens copR encodes a putative protein of 140 amino acid residues with a calculated molecular mass of 15.55 kDa. This deduced amino acid sequence shares 56% and 39% identities with CueR from Pseudomonas putida and E. coli, respectively (1, 47). The two cysteine residues essential for Cu sensing are conserved (47), suggesting a similar mechanism for the regulator to sense and respond to Cu.
The A. tumefaciens copZ homolog encodes an 66-amino-acid protein with a calculated molecular mass of 8.79 kDa. CopZ, a Cu(I) chaperone, belongs to the Atx1 family of highly conserved metallochaperone proteins containing heavy-metal-associated domains structurally similar to that at the N terminus of Cu ATPase. copZ homologs are widely distributed in a number of microbial genomes, and the majority are generally located adjacent to the gene encoding heavy-metal ATPase (27). Bacterial copA and copZ, however, are believed to have evolved independently (28). Although copZ and copA in A. tumefaciens are separated by copR, both copA and copZ are likely cotranscribed in an operon. The operonic structure of copAZ in gram-negative bacteria has been observed for Helicobacter pylori and Helicobacter felis (6). A. tumefaciens CopZ shares 27% identity with B. subtilis CopZ (Fig. 1C) (5), 25% identity with E. hirae CopZ (13), and 20% identity with H. pylori CopP (CopZ homolog) (6). The two cysteine residues of the Cu binding domain of the CXXC motif, Cys-11 and Cys-14, are also conserved in A. tumefaciens CopZ.
A BLASTP (3) search of the A. tumefaciens genome (50) identified ORFs paralogous to copA (Atu1195), copR (Atu1197), and copZ (Atu1203), located on the circular chromosome. The deduced amino acid sequences of Atu1195, Atu1197, and Atu1203 displayed 56%, 44%, and 33% identities with CopA, CopR, and CopZ, respectively. However, their role in metal homeostasis has not been determined.
The A. tumefaciens genome also contains another gene locus presumably involved in copper resistance that is located on the linear chromosome. This locus is comprised of Atu3990, Atu3991, and Atu3992, which have been annotated copC, copA, and copB, respectively (50). A BLASTP search of the deduced amino acid sequences strongly suggests that Atu3990 is a copper binding protein and that Atu3991 is homologous to the periplasmic multicopper oxidase CueO, while Atu3992 is likely an outer membrane efflux protein belonging to the TolC family. CueO, together with CopA ATPase, is known to contribute to copper resistance in E. coli and other gram-negative bacteria (24, 41). The significance of this genetic determinant with respect to copper homeostasis in A. tumefaciens is being investigated.
Expression analysis of the copARZ operon.
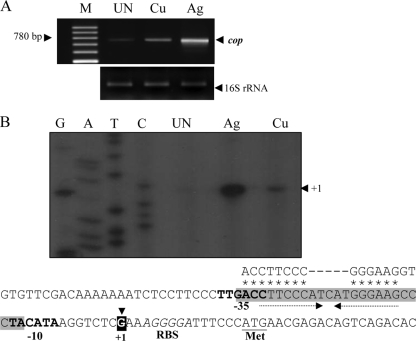
The gene organization of copARZ (Fig. 1B) suggests that they are transcribed as an operon. RT-PCR with a forward primer (BT1830) located within copA and a reverse primer (BT1683) located within copZ was performed using total RNA extracted from NTL4 cultures. As shown in Fig. 2A, a 780-bp PCR product corresponding to the expected size between primers BT1830 and BT1683 was detected in the absence of Cu and Ag ions, indicating that copA, copR, and copZ are transcribed in a single unit. However, the possibility of monocistronic transcripts of individual genes cannot be excluded by this experiment. Interestingly, RT-PCR analysis using the same primer set but with RNA samples prepared from cultures induced with either Ag or Cu ions gave high yields of PCR products based on band intensity, suggesting that the transcription of the operon could be induced by Ag and Cu ions. Densitometric analysis of the RT-PCR results show that the addition of Ag and Cu ions to the cultures induced the expression of the copARZ operon by 15-fold and 4-fold, respectively. Hence, Ag was a more potent inducer of the copA operon than Cu. The inducible expression of copARZ suggests that this operon likely has an important function in the protection of A. tumefaciens from metal ion toxicity and also implies the concerted action of CopA, CopR, and CopZ in the metal stress response.
FIG. 2.
Transcription and promoter analysis of copARZ. (A) RT-PCR analysis of copARZ was carried out with primers BT1830 and BT1683. Total RNA was extracted from NTL4 cultures cultivated under uninduced conditions (UN) and after induction with 25 μM AgNO3 (Ag) or 500 μM CuSO4 (Cu). The RT-PCR products were separated by 2% agarose gel electrophoresis. M, 100-bp molecular weight markers (Fermentas, Canada). (B) Primer extension experiments were performed using RNA isolated from uninduced (UN), AgNO3-induced (Ag), or CuSO4-induced (Cu) cultures of A. tumefaciens NTL4 with 32P-labeled primer BT1968. Extension products were sized on sequencing gels next to DNA sequence ladders (G, A, T, and C) generated using pGEM-3Zf(+) as the template and labeled forward primer pUC/M13. The arrowhead indicates the putative transcription start site (position +1). Putative −35 and −10 motifs are in boldface type. The ribosome binding site (RBS) and start codon (ATG) are in italic type and underlined, respectively. Arrows represent inverted repeat sequences. The consensus sequence of the CueR binding site (37) is aligned above the sequence line in corresponding letters, and the homologous nucleotides are marked by asterisks. The gray shaded box indicates the CopR binding box according to DNase I footprinting assays.
Analysis of the copARZ promoter.
The transcription start site of copARZ was determined with a primer extension experiment. RNA samples prepared from A. tumefaciens cultures grown under either uninduced or AgNO3- or CuSO4-induced conditions were used as templates in RT reactions. A single 57-bp primer extension product could be readily detected in RNA samples from the Ag-induced sample and, to a lesser extent, in Cu-treated cells (Fig. 2B). The putative transcription start site (position +1) was mapped according to the size of the primer extension products to the G nucleotide located 15 nucleotides upstream of the putative ATG start codon of copA (Fig. 2B). Examination of the sequence upstream of the transcriptional start site revealed two promoter sequences, TTGACC (positions −38 to −33) and TACATA (positions −13 to −8), that are separated by 19 bp. These promoter motifs closely match the −35 and −10 consensus promoter sequences for the E. coli σ70-type promoter sequences (Fig. 2B). Note that primer extension products from uninduced and metal-induced samples gave the same +1 site, indicating that the expression of the copARZ operon is driven from the same promoter under normal and metal stress conditions. Also, the primer extension results confirmed the results of RT-PCR expression analyses showing that copARZ expression could be induced by the exposure of bacterial cultures to Ag or Cu ions and that the increased level of expression of the operon in response to Ag or Cu treatments was due to the increased level of transcription of the operon.
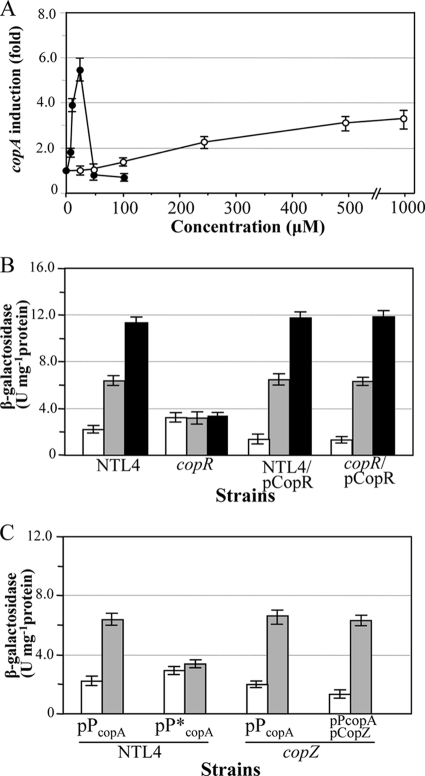
In vivo copARZ promoter analysis was performed. The copA promoter was PCR amplified, and the resultant 252-bp product was cloned and transcriptionally fused to a promoterless lacZ reporter gene in low-copy-number plasmid vector pUFR027lacZ (33), yielding pPcopA. The recombinant plasmid was transferred into A. tumefaciens NTL4 cells, and the levels of β-galactosidase were determined under induction with increasing concentrations of Cu and Ag ions. As shown in Fig. 3A, Ag is a potent inducer of the copARZ promoter. Induction by AgNO3 could be observed at 5 μM and reached a peak at 25 μM (5.5-fold). Bacterial growth was completely inhibited at 50 μM AgNO3 (data not shown). Under the conditions tested, 50 μM CuSO4 failed to induce the copARZ promoter, while a 1.4-fold induction was observed at 100 μM. Exposure of the bacteria to 250 and 500 μM CuSO4 further increased copARZ promoter activity by 2.3- and 3.2-fold, respectively. In contrast, induction with 1 mM or higher concentrations of CuSO4 resulted in only marginal increases in the promoter activity compared to the activity attained with 500 μM CuSO4 (Fig. 3A and data not shown). We also tested the promoter response to other metal ions such as Zn, Mn, Fe, Cd, Ni, and Co ions; none of these metals tested induced the copA promoter (data not shown). The induced expression of the copARZ operon in response to metal ions is therefore specific to a particular metal, e.g., Cu and Ag ions.
FIG. 3.
In vivo promoter activity determination. (A) A. tumefaciens strains bearing pPcopA that contains the copARZ promoter transcriptionally fused to the lacZ reporter gene were grown to exponential phase before being challenged with increasing concentrations of CuSO4 (open circles) or AgNO3 (closed circles). Induction was determined by dividing the β-galactosidase activity of the metal-induced culture by that of the uninduced one. (B) A. tumefaciens strains bearing pPcopA were cultivated under uninduced (open bars), 500 μM CuSO4-induced (gray shaded bars), or 25 μM AgNO3-induced (closed bars) conditions. β-Galactosidase activity is expressed as international units (U) per mg protein. NTL4, wild type; copR, copR mutant strain; NTL4/pCopR, NTL4 harboring pCopR; copR/pCopR, copR mutant strain harboring pCopR. (C) An A. tumefaciens wild-type strain (NTL4) bearing pPcopA or pP*copA in which half of the palindromic sequence in the putative CopR binding site was mutated and the copZ mutant (copZ) bearing pPcopA or both pPcopA and pCopZ were cultured under uninduced (open bars) and 500 μM CuSO4-induced (gray shaded bars) conditions. Error bars indicate the SD of data from four independently performed experiments.
In E. coli, copA expression is regulated by CueR. To test whether copR, a cueR homolog, regulates the expression of the copARZ operon, a plasmid containing a copA promoter-lacZ transcriptional fusion, pPcopA, was introduced into A. tumefaciens copR mutants. The levels of β-galactosidase in bacterial lysates prepared from cultures grown under uninduced and metal-induced conditions were monitored. The results are shown in Fig. 3B. The induction of the copA promoter by Cu or Ag ions was eliminated in the copR mutant. The loss of Cu and Ag induction in the copR mutant could be complemented by the plasmid-borne expression of copR (pCopR), as shown in Fig. 3B. This observation confirmed that the metal-inducible expression of copA is regulated by copR (Fig. 3B). CopR is a member of the transcription regulator CueR subfamily that generally represses transcription by binding to its target site, located in the vicinity of the promoter. The incorporation of either Cu(I) or Ag(I) ions causes changes in protein conformation to a form that is capable of activating the transcription of target genes (36, 47). A similar mechanism is likely to be responsible for the observed CopR-dependent metal induction of copA in A. tumefaciens. In addition, the Au(I) ion also activates E. coli CueR (46). It is noteworthy that the levels of β-galactosidase resulting from the copA promoter in the copR mutant were 1.5-fold increased (3.3 ± 0.4 U mg−1 protein) relative to the level attained with wild-type strain NTL4 (2.2 ± 0.3 U mg−1 protein) (Fig. 3B). Also, a high degree of copR expression in both NTL4 and copR mutants lowered copA promoter activities by 0.6-fold compared to the level found for NTL4 (1.3 ± 0.4 U mg−1 protein) (Fig. 3B). These evidences support the hypothesis that CueR is a transcription repressor under Cu-limiting conditions. It was previously reported that Cu(I) and Ag(I) can bind and transform CueR into its transcriptionally active form (9, 36, 47). The latter metal is an especially potent activator of CueR. The activation of CopR by Cu and Ag ions in A. tumefaciens resembles that of E. coli CueR, with the latter acting as a stronger activator of CopR (Fig. 3B).
As a member of the MerR family, CueR-regulated promoters normally have inverted repeat sequences located between positions −10 and −35 in promoter elements (19). An analysis of the nucleotide sequence of the copA promoter revealed the presence of an inverted repeat (5′-CTTCCCAT-ATGGGAAG-3′) located between the −10 and −35 motifs (Fig. 2B). The palindromic sequence exhibited a good match to the consensus sequence of the CueR binding site of E. coli (5′-ACCTTCCCnnnnnGGGAAGGT-3′) (37). This promoter feature supports the idea that copARZ transcription is mediated by the binding of CopR.
Purified CopR binds to the putative CueR binding site of the copARZ promoter.
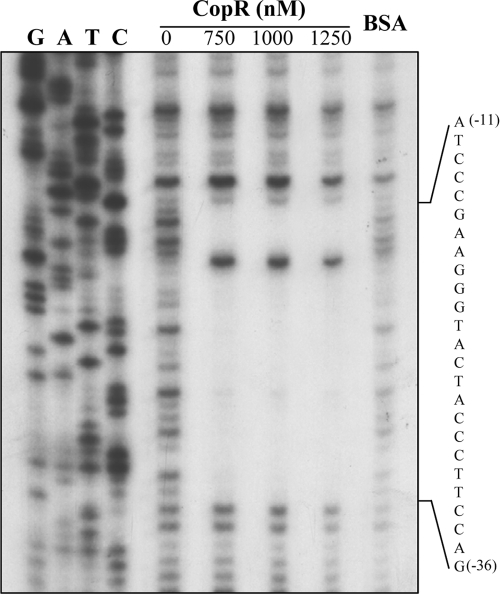
DNase I footprinting was performed to demonstrate in vitro that purified CopR binds to the copARZ promoter and localizes to the CopR binding site. Various concentrations of purified CopR protein were incubated with a radioactively labeled 252-bp DNA fragment spanning the copARZ promoter region in a binding buffer. The results shown in Fig. 4 illustrate that purified CopR specifically bound to the copARZ promoter. The DNase I-protected region is depicted in Fig. 4. CopR binding protected the copARZ promoter region from positions −36 to −11 upstream of the copA transcriptional start site. This protected region covered the putative CueR-type binding site located from positions −33 to −17. Similar to data from previous reports of other CueR proteins, an internal DNase I-hypersensitive site, indicative of promoter bending, was observed (36, 47). Without the activating metal, regulators in the MerR family commonly produce DNase I-hypersensitive sites within the footprinted region, and these hypersensitive sites are decreased in intensity when metal is bound to the regulator (35). Thus, this result suggests that the majority of our purified CopR is in an apo-CopR form, which is supported by results of atomic absorption analyses (data not shown). The results of the DNase I footprinting analysis strongly suggest that CopR regulates the copARZ promoter through the binding of the transcription factor to the palindromic binding site located between positions −10 and −35 of the copA promoter. We speculate that the binding of CopR mediates copARZ transcription in a manner similar to that of typical MerR-type regulators. Under physiological conditions, CopR binding represses the transcription of the copARZ operon. Upon exposure to elevated levels of Cu or Ag ions, the binding of these metals to CopR presumably changes the conformation of CopR from a transcriptional repressor to an activator. The importance of the palindromic sequence motifs in the putative CopR binding site was investigated by directed mutagenesis of pPcopA. Half of the palindrome sequence was changed from ATGGGAAG at positions −24 to −17 to TTTCTTTT. We expected that these mutations of the CopR binding site would reduce the binding affinity of CopR for the copARZ promoter and affect the ability of CopR to repress and activate the copARZ promoter. As shown in Fig. 3C, the uninduced levels of β-galactosidase in wild-type strain NTL4 bearing a mutated promoter fused to lacZ (pP*copA) were increased from 2.2 ± 0.3 U mg−1 protein (normal copA promoter) to 3.0 ± 0.4 U mg−1 protein (mutated copA promoter). These results are in good agreement with the observations that levels of copARZ promoter activities increased in the copR mutant strain (copR harboring pPcopA) and were repressed when copR was highly expressed (copR or NTL4 harboring pPcopA and pCopR) (Fig. 3B). These results support a role for CopR in the absence of Cu to act as a repressor of the copARZ promoter by binding to the palindrome binding site of the copARZ promoter. Furthermore, the CopR binding-site mutations substantially reduced the ability of Cu ions to induce transcription from the copARZ promoter, confirming that Cu-CopR activated the transcription of the copARZ promoter by binding at the palindrome sequence and subsequent interactions with the RNA polymerase. Hence, the palindrome sequence motifs are important for the CopR-mediated regulation of the copARZ promoter.
FIG. 4.
DNase I footprinting assay. A radioactively labeled copARZ promoter fragment was mixed with the indicated concentrations of purified CopR in binding buffer before the reaction mixture was treated with DNase I. The digested and protected DNA fragments were separated on 8% denatured DNA sequencing gels along with copARZ promoter sequence ladders (G, A, T, and C). BSA indicates that 1,500 nM BSA was added to the binding mixture instead of CopR.
CopZ is unlikely to be involved in the Cu-sensing process.
CopZ, a Cu chaperone that has attracted interest due to not only its biological functions in the transport of Cu in the Cu(I) state to Cu-utilizing enzymes and ATPase efflux pumps (4) but also its role in Cu sensing by mediating the transfer of Cu ions from the CopA Cu uptake ATPase machinery to CopY, a transcriptional regulator of the cop operon (45). Indeed, no copY-like gene could be identified in either the A. tumefaciens (22, 50) or E. coli (7) genome sequence when the gram-positive E. hirae CopY sequence (48) was used as a query to search the bacterial genome using the BLASTP algorithm (3). This finding, together with the fact that copZ is cotranscribed with copR, raised the possibility that CopZ could deliver Cu directly to CopR in the Cu-sensing process. This role for copZ was evaluated using an A. tumefaciens copZ mutant harboring pPcopA. We hypothesized that if CopZ is involved in delivering Cu ions to CopR, the lack of a functional copZ should markedly affect the ability of Cu to induce the copARZ promoter. Nonetheless, the experimental results show that the Cu induction profile of the copARZ promoter in the copZ mutant/pPcopA in response to increasing concentrations of Cu from 1 to 500 μM was identical to that of wild-type strain NTL4/pPcopA (Fig. 3C and data not shown). In A. tumefaciens, CopZ plays no role in Cu sensing by the transcriptional regulator CopR.
copARZ is a Cu resistance determinant.
The copARZ operon mutant was constructed by the insertional inactivation of the copA gene using suicide plasmid vector pKNOCK (2). The insertion of pKNOCK into the copA coding region had polar effects on copR and copZ. The copA mutant had no functional copR and copZ, as shown by the loss of copA promoter induction by RT-PCR (data not shown) and by RT-PCR analysis of copZ. This evidence also suggests that neither copR nor copZ possesses its own promoter. The physiological function of the copARZ operon in the metal resistance of A. tumefaciens was determined by using a plate sensitivity assay. The inactivation of the copARZ operon caused a 50-fold reduction in the bacterium's ability to cope with Cu toxicity (Fig. 5A). However, the Cu ion-sensitive phenotype could not be complemented with the ectopic expression of either copA alone or copARZ from a plasmid vector, pBBR1MCS. This was due to the fact that the high level of expression of these genes from multicopy plasmid pBBR1MCS severely affected bacterial growth and markedly reduced plating efficiency (data not shown). The high level of expression of Cu efflux pumps culminates in a deficiency of intracellular Cu ions. Consequently, the functions of several essential enzymes that require Cu as a cofactor such as cytochrome oxidases, the terminal enzymes of the respiratory chain, are impaired, leading to defects in bacterial growth (18). We therefore changed the strategy to use the mini-Tn7 vector (11) to transpose the copA gene containing the promoter region into the chromosome of the copA mutant. A mini-Tn7 vector mediates the specific integration of the target gene into the downstream region of the glmS gene, which encodes glucosamine fructose-6-phosphate aminotransferase. The expression of copA from its own promoter in the copA mutant (copA::mini-Tn7-copA) partially complemented the reduced-Cu-resistance phenotype (Fig. 5A). This implied that the reduced resistance observed in the copA mutant was due largely to an inactivation of copA.
FIG. 5.
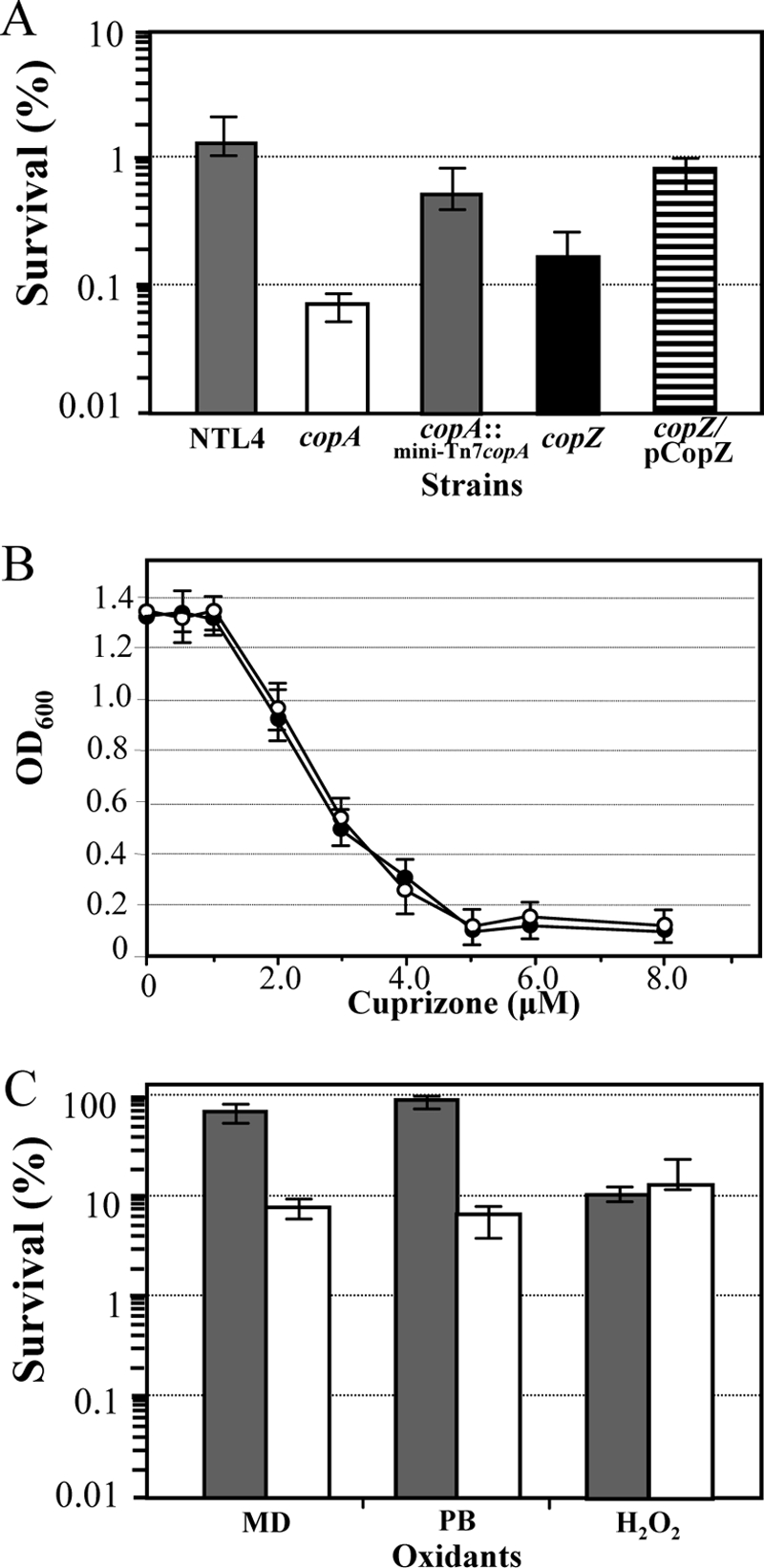
Phenotypes of A. tumefaciens cop mutants. (A) The levels of resistance to Cu ions were determined using plate sensitivity assays. A. tumefaciens strains were plated onto medium containing 5 mM CuSO4. Percent survival was determined by the number of CFU on LB agar plates containing CuSO4 divided by the number of CFU on LB agar plates multiplied by 100. (B) The Cu requirements of A. tumefaciens wild-type strain NTL4 (open circles) and the copA operon mutant (closed circles) were determined by growth in AB minimal medium supplemented with the Cu(II) chelator cuprizone. Bacterial growth was determined by measuring the optical density at 600 nm (OD600) after 30 h of incubation. (C) The levels of resistance to oxidants were determined as described above (A), but oxidants were added instead of metal ions. NTL4 (gray shaded bars) and copA operon mutant strain (open bars) cultures were plated onto medium containing 500 μM MD, 100 μM PB, or 300 μM H2O2. Error bars indicate the SD of data from four independently performed experiments.
To evaluate the function of copZ in Cu resistance, a copZ mutant was constructed using the pKNOCK system. The copZ mutant was also tested to verify that the inactivation of copZ did not have polar effects on copA and copR expression. The expression of copA and copR in the copZ mutant was confirmed by RT-PCR analysis and by Cu ion-induced copARZ promoter activity (Fig. 3C). Essentially, copZ inactivation did not significantly alter the expression of copA or copR. Analysis of the Cu ion resistance level showed that the inactivation of copZ decreased Cu ion resistance 10-fold compared that of wild-type strain NTL4 (Fig. 5A). This altered phenotype could be complemented by the expression of copZ from plasmid pCopZ. Gonzalez-Guerrero and Arguello (21) previously demonstrated that the CopZ Cu chaperone interacts with cytoplasmic Cu ions and delivers them to CopA, a Cu-transporting ATPase. Thus, A. tumefaciens CopZ possibly functions in the delivery of Cu ions to CopA. In addition, the expression of copA alone could partially restore the reduced-Cu-resistance phenotype of the copA operon mutant in which copZ expression was disrupted. This suggests that CopA, a Cu ATPase, might be able to transport Cu ions in the absence of the CopZ chaperone. Alternatively, it is possible that copZ is still expressed in the copA mutant but that the level of its transcript was too low to be detected by RT-PCR.
The reduced-Cu-resistance phenotype of the copARZ operon mutant, together with the inducible expression of the copARZ promoter upon exposure to a high-Cu environment, suggests that CopA functions as a Cu efflux pump. Next, the levels of resistance to other metal ions, including Ag, Zn, Fe, Cd, Ni, and Mn, were tested; the levels of resistance to these metals in the mutant were unchanged relative to wild-type levels (data not shown). Although the level of expression of this operon was eminently increased in response to Ag ions, it seems likely that copARZ is responsible for protection specifically against Cu toxicity. In several microorganisms, the disruption of CopA Cu(I)-translocating ATPase decreases resistance to Cu ions but not to other metal ions (20, 40). Nonetheless, Ag(I) has been shown to be transported by the Cu efflux ATPase (45, 46). Thus, the metal resistance phenotype might be dependent upon the metal-selective properties of CopA.
Cu is an essential metal ion; thus, experiments were performed to test whether an inactivation of the gene encoding the Cu efflux ATPase CopA affected the Cu requirement (34). A. tumefaciens wild-type strain NTL4 and copA operon mutant strains were cultured in AB minimal medium containing the Cu(II) chelator cuprizone. The growth inhibition patterns of wild-type strain NTL4 and the copA operon mutant are similar in that concentrations of cuprizone below 1 μM caused no adverse effects on bacterial growth, while cuprizone at concentrations higher than 5 μM completely inhibited growth (Fig. 5B). This finding indicates that a disruption of the Cu efflux ATPase has no effect on the mutant strain's Cu requirement.
The copARZ operon mutant has altered oxidative stress resistance.
Cu is a redox-active metal ion. It would therefore be expected that a lack of the CopARZ Cu efflux system would lead to increased levels of intracellular Cu ions that could affect the bacterial oxidative stress response. To test this hypothesis, the levels of resistance to H2O2 and the superoxide generators menadione (MD) and plumbagin (PB) in a copARZ operon mutant were measured using a plate sensitivity assay. The bacterial colonies forming on plates containing the indicated concentrations of oxidants were scored and compared to those of wild-type strain NTL4. The mutant was 10-fold and 20-fold more sensitive to MD and PB, respectively, than NTL4, but the level of resistance to H2O2 was unaltered (Fig. 5C). The phenotypes suggest that the inactivation of the copARZ operon renders A. tumefaciens more sensitive to oxidative stress generated from superoxide generators. Both MD and PB are redox-cycling agents capable of continuously generating intracellular superoxide anions that can be dismutated to H2O2 via an enzymatic reaction catalyzed by superoxide dismutases or chemically in the Fenton-like reaction with a redox-active metal (16). A reaction between Cu(II) and superoxide anions generates Cu(I), which can be oxidized by H2O2, producing highly reactive hydroxyl radicals (8). Thus, defects in Cu efflux systems would lead to an accumulation of intracellular Cu that could react with ROS through Fenton biochemistry to liberate the highly reactive radicals that could produce deleterious and lethal effects on bacterial cells. Nonetheless, it is controversial whether Cu ions really participate in a Fenton-like reaction in vivo (32). Thus, this hypothesis needs more experimental data to support it. However, it was previously reported that the inactivation of copA causes changes in oxidative stress resistance in Staphylococcus aureus, where a mutant lacking CopA is sensitive to H2O2 (44). The reduced ability to cope with oxidative stress in A. tumefaciens is associated with an attenuated virulence phenotype (42, 51). The copARZ operon mutant had reduced levels of resistance to both copper and superoxide generators. Hence, the mutant was tested for its ability to induce tumor formation on Nicotiana tabacum leaf explants as previously described (42). The inactivation of copARZ did not affect the ability to induce tumors on the tobacco leaf discs (data not shown), suggesting that the copARZ operon had no detectable roles in A. tumefaciens virulence on tobacco leaf discs.
The physiological functions and gene regulation of the Cu resistance determinant copARZ in A. tumefaciens were elucidated. The evidence suggests that under Cu-limiting conditions, the CopR transcriptional regulator binds to the copARZ promoter and represses the expression of the operon. This tight regulation directly benefits bacterial cells by preventing the export of essential Cu ions. Upon exposure to increased concentrations of Cu, CopR is activated and forms a transcriptional activator, thereby enhancing copARZ transcription, resulting in high levels of CopA Cu ATPase, CopR, and CopZ, a cytoplasmic Cu chaperone. CopZ presumably binds excess free Cu(I) and facilitates the transfer of the metal to CopA, a Cu exporter, to reduce intracellular Cu to lower-than-harmful concentrations. This efficient mechanism allows A. tumefaciens to survive in high-Cu environments.
Supplementary Material
Acknowledgments
The research was supported by grants from the National Center for Genetic Engineering and Biotechnology (grant BT-B-01-PG-14-5112), Chulabhorn Research Institute, Mahidol University, and from the Center of Excellence on Environmental Health, Toxicology, and Management of Chemicals. Sirikan Nawapan was supported by the Chulabhorn Graduate Institute.
We thank Supa Utamapongchai and Weerachai Tanboon for technical assistance and James M. Dubbs for a critical reading of the manuscript.
Footnotes
Published ahead of print on 5 June 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Adaikkalam, V., and S. Swarup. 2002. Molecular characterization of an operon, cueAR, encoding a putative P1-type ATPase and a MerR-type regulatory protein involved in copper homeostasis in Pseudomonas putida. Microbiology 148:2857-2867. [DOI] [PubMed] [Google Scholar]
- 2.Alexeyev, M. F. 1999. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. BioTechniques 26:824-826, 828. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., J. C. Wootton, E. M. Gertz, R. Agarwala, A. Morgulis, A. A. Schaffer, and Y. K. Yu. 2005. Protein database searches using compositionally adjusted substitution matrices. FEBS J. 272:5101-5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banci, L., I. Bertini, S. Ciofi-Baffoni, L. Gonnelli, and X. C. Su. 2003. Structural basis for the function of the N-terminal domain of the ATPase CopA from Bacillus subtilis. J. Biol. Chem. 278:50506-50513. [DOI] [PubMed] [Google Scholar]
- 5.Banci, L., I. Bertini, R. Del Conte, J. Markey, and F. J. Ruiz-Duenas. 2001. Copper trafficking: the solution structure of Bacillus subtilis CopZ. Biochemistry 40:15660-15668. [DOI] [PubMed] [Google Scholar]
- 6.Bayle, D., S. Wangler, T. Weitzenegger, W. Steinhilber, J. Volz, M. Przybylski, K. P. Schafer, G. Sachs, and K. Melchers. 1998. Properties of the P-type ATPases encoded by the copAP operons of Helicobacter pylori and Helicobacter felis. J. Bacteriol. 180:317-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 8.Bremner, I. 1998. Manifestations of copper excess. Am. J. Clin. Nutr. 67:1069S-1073S. [DOI] [PubMed] [Google Scholar]
- 9.Changela, A., K. Chen, Y. Xue, J. Holschen, C. E. Outten, T. V. O'Halloran, and A. Mondragon. 2003. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 301:1383-1387. [DOI] [PubMed] [Google Scholar]
- 10.Chilton, M. D., T. C. Currier, S. K. Farrand, A. J. Bendich, M. P. Gordon, and E. W. Nester. 1974. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc. Natl. Acad. Sci. USA 71:3672-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi, K. H., J. B. Gaynor, K. G. White, C. Lopez, C. M. Bosio, R. R. Karkhoff-Schweizer, and H. P. Schweizer. 2005. A Tn7-based broad-range bacterial cloning and expression system. Nat. Methods 2:443-448. [DOI] [PubMed] [Google Scholar]
- 12.Chuchue, T., W. Tanboon, B. Prapagdee, J. M. Dubbs, P. Vattanaviboon, and S. Mongkolsuk. 2006. ohrR and ohr are the primary sensor/regulator and protective genes against organic hydroperoxide stress in Agrobacterium tumefaciens. J. Bacteriol. 188:842-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobine, P., W. A. Wickramasinghe, M. D. Harrison, T. Weber, M. Solioz, and C. T. Dameron. 1999. The Enterococcus hirae copper chaperone CopZ delivers copper (I) to the CopY repressor. FEBS Lett. 445:27-30. [DOI] [PubMed] [Google Scholar]
- 14.Eiamphungporn, W., N. Charoenlap, P. Vattanaviboon, and S. Mongkolsuk. 2006. Agrobacterium tumefaciens soxR is involved in superoxide stress protection and also directly regulates superoxide-inducible expression of itself and a target gene. J. Bacteriol. 188:8669-8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escobar, M. A., and A. M. Dandekar. 2003. Agrobacterium tumefaciens as an agent of disease. Trends Plant Sci. 8:380-386. [DOI] [PubMed] [Google Scholar]
- 16.Farr, S. B., and T. Kogoma. 1991. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 55:561-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finney, L. A., and T. V. O'Halloran. 2003. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300:931-936. [DOI] [PubMed] [Google Scholar]
- 18.Frangipani, E., V. I. Slaveykova, C. Reimmann, and D. Haas. 2008. Adaptation of aerobically growing Pseudomonas aeruginosa to copper starvation. J. Bacteriol. 190:6706-6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaballa, A., M. Cao, and J. D. Helmann. 2003. Two MerR homologues that affect copper induction of the Bacillus subtilis copZA operon. Microbiology 149:3413-3421. [DOI] [PubMed] [Google Scholar]
- 20.Gaballa, A., and J. D. Helmann. 2003. Bacillus subtilis CPx-type ATPases: characterization of Cd, Zn, Co and Cu efflux systems. Biometals 16:497-505. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Guerrero, M., and J. M. Arguello. 2008. Mechanism of Cu+-transporting ATPases: soluble Cu+ chaperones directly transfer Cu+ to transmembrane transport sites. Proc. Natl. Acad. Sci. USA 105:5992-5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodner, B., G. Hinkle, S. Gattung, N. Miller, M. Blanchard, B. Qurollo, B. S. Goldman, Y. Cao, M. Askenazi, C. Halling, L. Mullin, K. Houmiel, J. Gordon, M. Vaudin, O. Iartchouk, A. Epp, F. Liu, C. Wollam, M. Allinger, D. Doughty, C. Scott, C. Lappas, B. Markelz, C. Flanagan, C. Crowell, J. Gurson, C. Lomo, C. Sear, G. Strub, C. Cielo, and S. Slater. 2001. Genome sequence of the plant pathogen and biotechnology agent Agrobacterium tumefaciens C58. Science 294:2323-2328. [DOI] [PubMed] [Google Scholar]
- 23.Grimm, R. 2008. Control of crown gall in Swiss apple nurseries. EPPO Bull. 17:269-272. [Google Scholar]
- 24.Hall, S. J., A. Hitchcock, C. S. Butler, and D. J. Kelly. 2008. A multicopper oxidase (Cj1516) and a CopA homologue (Cj1161) are major components of the copper homeostasis system of Campylobacter jejuni. J. Bacteriol. 190:8075-8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopkins, D. L. 2004. Management of bacterial diseases: chemical methods. Taylor & Francis, London, United Kingdom.
- 26.Imlay, J. A. 2002. How oxygen damages microbes: oxygen tolerance and obligate anaerobiosis. Adv. Microb. Physiol. 46:111-153. [DOI] [PubMed] [Google Scholar]
- 27.Jordan, I. K., D. A. Natale, and M. Y. Galperin. 2000. Copper chaperones in bacteria: association with copper-transporting ATPases. Trends Biochem. Sci. 25:480-481. [DOI] [PubMed] [Google Scholar]
- 28.Jordan, I. K., D. A. Natale, E. V. Koonin, and M. Y. Galperin. 2001. Independent evolution of heavy metal-associated domains in copper chaperones and copper-transporting ATPases. J. Mol. Evol. 53:622-633. [DOI] [PubMed] [Google Scholar]
- 29.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 30.Luo, Z. Q., T. E. Clemente, and S. K. Farrand. 2001. Construction of a derivative of Agrobacterium tumefaciens C58 that does not mutate to tetracycline resistance. Mol. Plant-Microbe Interact. 14:98-103. [DOI] [PubMed] [Google Scholar]
- 31.Lutsenko, S., K. Petrukhin, M. J. Cooper, C. T. Gilliam, and J. H. Kaplan. 1997. N-terminal domains of human copper-transporting adenosine triphosphatases (the Wilson's and Menkes disease proteins) bind copper selectively in vivo and in vitro with stoichiometry of one copper per metal-binding repeat. J. Biol. Chem. 272:18939-18944. [DOI] [PubMed] [Google Scholar]
- 32.Macomber, L., C. Rensing, and J. A. Imlay. 2007. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J. Bacteriol. 189:1616-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakjarung, K., S. Mongkolsuk, and P. Vattanaviboon. 2003. The oxyR from Agrobacterium tumefaciens: evaluation of its role in the regulation of catalase and peroxide responses. Biochem. Biophys. Res. Commun. 304:41-47. [DOI] [PubMed] [Google Scholar]
- 34.Odermatt, A., H. Suter, R. Krapf, and M. Solioz. 1993. Primary structure of two P-type ATPases involved in copper homeostasis in Enterococcus hirae. J. Biol. Chem. 268:12775-12779. [PubMed] [Google Scholar]
- 35.Outten, C. E., F. W. Outten, and T. V. O'Halloran. 1999. DNA distortion mechanism for transcriptional activation by ZntR, a Zn(II)-responsive MerR homologue in Escherichia coli. J. Biol. Chem. 274:37517-37524. [DOI] [PubMed] [Google Scholar]
- 36.Outten, F. W., C. E. Outten, J. Hale, and T. V. O'Halloran. 2000. Transcriptional activation of an Escherichia coli copper efflux regulon by the chromosomal MerR homologue, cueR. J. Biol. Chem. 275:31024-31029. [DOI] [PubMed] [Google Scholar]
- 37.Permina, E. A., A. E. Kazakov, O. V. Kalinina, and M. S. Gelfand. 2006. Comparative genomics of regulation of heavy metal resistance in eubacteria. BMC Microbiol. 6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen, C., and L. B. Moller. 2000. Control of copper homeostasis in Escherichia coli by a P-type ATPase, CopA, and a MerR-like transcriptional activator, CopR. Gene 261:289-298. [DOI] [PubMed] [Google Scholar]
- 39.Prapagdee, B., P. Vattanaviboon, and S. Mongkolsuk. 2004. The role of a bifunctional catalase-peroxidase KatA in protection of Agrobacterium tumefaciens from menadione toxicity. FEMS Microbiol. Lett. 232:217-223. [DOI] [PubMed] [Google Scholar]
- 40.Rensing, C., B. Fan, R. Sharma, B. Mitra, and B. P. Rosen. 2000. CopA: an Escherichia coli Cu(I)-translocating P-type ATPase. Proc. Natl. Acad. Sci. USA 97:652-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rensing, C., and G. Grass. 2003. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 27:197-213. [DOI] [PubMed] [Google Scholar]
- 42.Saenkham, P., W. Eiamphungporn, S. K. Farrand, P. Vattanaviboon, and S. Mongkolsuk. 2007. Multiple superoxide dismutases in Agrobacterium tumefaciens: functional analysis, gene regulation, and influence on tumorigenesis. J. Bacteriol. 189:8807-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 44.Sitthisak, S., L. Knutsson, J. W. Webb, and R. K. Jayaswal. 2007. Molecular characterization of the copper transport system in Staphylococcus aureus. Microbiology 153:4274-4283. [DOI] [PubMed] [Google Scholar]
- 45.Solioz, M., and J. V. Stoyanov. 2003. Copper homeostasis in Enterococcus hirae. FEMS Microbiol. Rev. 27:183-195. [DOI] [PubMed] [Google Scholar]
- 46.Stoyanov, J. V., and N. L. Brown. 2003. The Escherichia coli copper-responsive copA promoter is activated by gold. J. Biol. Chem. 278:1407-1410. [DOI] [PubMed] [Google Scholar]
- 47.Stoyanov, J. V., J. L. Hobman, and N. L. Brown. 2001. CueR (YbbI) of Escherichia coli is a MerR family regulator controlling expression of the copper exporter CopA. Mol. Microbiol. 39:502-511. [DOI] [PubMed] [Google Scholar]
- 48.Strausak, D., and M. Solioz. 1997. CopY is a copper-inducible repressor of the Enterococcus hirae copper ATPases. J. Biol. Chem. 272:8932-8936. [DOI] [PubMed] [Google Scholar]
- 49.Teixeira, E. C., J. C. Franco de Oliveira, M. T. Marques Novo, and M. C. Bertolini. 2008. The copper resistance operon copAB from Xanthomonas axonopodis pathovar citri: gene inactivation results in copper sensitivity. Microbiology 154:402-412. [DOI] [PubMed] [Google Scholar]
- 50.Wood, D. W., J. C. Setubal, R. Kaul, D. E. Monks, J. P. Kitajima, V. K. Okura, Y. Zhou, L. Chen, G. E. Wood, N. F. Almeida, Jr., L. Woo, Y. Chen, I. T. Paulsen, J. A. Eisen, P. D. Karp, D. Bovee, Sr., P. Chapman, J. Clendenning, G. Deatherage, W. Gillet, C. Grant, T. Kutyavin, R. Levy, M. J. Li, E. McClelland, A. Palmieri, C. Raymond, G. Rouse, C. Saenphimmachak, Z. Wu, P. Romero, D. Gordon, S. Zhang, H. Yoo, Y. Tao, P. Biddle, M. Jung, W. Krespan, M. Perry, B. Gordon-Kamm, L. Liao, S. Kim, C. Hendrick, Z. Y. Zhao, M. Dolan, F. Chumley, S. V. Tingey, J. F. Tomb, M. P. Gordon, M. V. Olson, and E. W. Nester. 2001. The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science 294:2317-2323. [DOI] [PubMed] [Google Scholar]
- 51.Xu, X. Q., and S. Q. Pan. 2000. An Agrobacterium catalase is a virulence factor involved in tumorigenesis. Mol. Microbiol. 35:407-414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.