Abstract
Porphyromonas gingivalis synthesizes two lipopolysaccharides (LPSs), O-LPS and A-LPS. Here, we elucidate the structure of the core oligosaccharide (OS) of O-LPS from two mutants of P. gingivalis W50, ΔPG1051 (WaaL, O-antigen ligase) and ΔPG1142 (O-antigen polymerase), which synthesize R-type LPS (core devoid of O antigen) and SR-type LPS (core plus one repeating unit of O antigen), respectively. Structural analyses were performed using one-dimensional and two-dimensional nuclear magnetic resonance spectroscopy in combination with composition and methylation analysis. The outer core OS of O-LPS occurs in two glycoforms: an “uncapped core,” which is devoid of O polysaccharide (O-PS), and a “capped core,” which contains the site of O-PS attachment. The inner core region lacks l(d)-glycero-d(l)-manno-heptosyl residues and is linked to the outer core via 3-deoxy-d-manno-octulosonic acid, which is attached to a glycerol residue in the outer core via a monophosphodiester bridge. The outer region of the “uncapped core” is attached to the glycerol and is composed of a linear α-(1→3)-linked d-Man OS containing four or five mannopyranosyl residues, one-half of which are modified by phosphoethanolamine at position 6. An amino sugar, α-d-allosamine, is attached to the glycerol at position 3. In the “capped core,” there is a three- to five-residue extension of α-(1→3)-linked Man residues glycosylating the outer core at the nonreducing terminal residue. β-d-GalNAc from the O-PS repeating unit is attached to the nonreducing terminal Man at position 3. The core OS of P. gingivalis O-LPS is therefore a highly unusual structure, and it is the basis for further investigation of the mechanism of assembly of the outer membrane of this important periodontal bacterium.
Porphyromonas gingivalis is a gram-negative anaerobe which is strongly implicated in the etiology of periodontal disease. Several putative virulence factors are produced by this organism. These virulence factors include the cysteine proteases Arg-gingipains (Rgps) and Lys-gingipain (Kgp) specific for Arg-X and Lys-X peptide bonds, respectively, which are capable of degrading several host proteins (56), and lipopolysaccharide (LPS), which has the potential to cause an inflammatory response in the periodontal tissues of the host. These factors are important antigens in patients with periodontal disease and may account for a considerable proportion of the immune response directed against P. gingivalis (58).
LPS is a major constituent of the outer membrane of gram-negative bacteria and facilitates interactions with the external environment. It consists of three regions: a hydrophobic lipid A embedded in the outer leaflet of the outer membrane, a core oligosaccharide (OS), and the O-polysaccharide (O-PS) side chain composed of several repeating units. The hydrophobic lipid A serves as an anchor for the LPS and consists of β-1,6-linked d-glucosamine disaccharide, which is usually phosphorylated at the 1 and/or 4′ positions and N and/or O acylated at positions 2, 3, 2′, and 3′ with various amounts of fatty acids. The rest of the LPS molecule projects from the surface. The core region is attached to lipid A and is composed of ∼10 sugars in most bacteria studied to date and can be further subdivided into an inner core and an outer core. The inner core usually contains l(d)-glycero-d-(l)-manno-heptose and 3-deoxy-d-manno-octulosonic acid (Kdo) residues, whereas the outer core is usually composed of hexoses. Attached to the outer core are the repeating units of O antigen (O-PS), which vary in composition, stereochemistry, and the sequence of O-glycosidic linkages between bacterial strains and thereby give rise to O-serotype specificity within bacterial species. Attachment of O antigen to core lipid A results in “smooth” LPS (S-type LPS), whereas LPS lacking O antigen is “rough” LPS (R-type LPS). Attachment of one repeating unit of O-PS to core lipid A results in SR-LPS (core-plus-one repeating unit) (41, 47, 48). In addition, the outer core OS region can be either “uncapped” or “capped.” The “uncapped” core OS is devoid of O-PS repeating units, whereas the “capped” core OS contains attached O-PS repeating units (47, 53) due to modifications in the outer core region.
P. gingivalis W50 was originally thought to synthesize a single LPS composed of a tetrasaccharide repeating unit in the O-PS, [→6)-α-d-Glcp-(1→4)-α-l-Rhap-(1→3)-β-d-GalNAc-(1→3)-α-d-Galp-(1→], which is modified by phosphoethanolamine (PEA) at position 2 of Rha in a nonstoichiometric manner (43). However, a second LPS in this organism, namely A-LPS (49), which has a phosphorylated mannan-containing anionic polysaccharide (A-PS), was identified in our laboratory. The A-PS repeating unit is built up of a phosphorylated branched d-Man-containing oligomer composed of an α1→6-linked d-mannose backbone to which α1→2-linked d-Man side chains of different lengths (one or two residues) are attached at position 2. One of the side chains contains Manα1→2-Manα-1-phosphate linked via phosphorus to a backbone Man residue at position O-2. Although A-LPS is predominantly composed of α-d-mannose residues, it cannot be referred to as a homopolymer due to the presence of Manα1→2Manα1-phosphate-containing OS side chains forming a nonglycosidic linkage between the backbone α-mannose and side chains. Hence, it is likely that the synthesis of A-PS (A-LPS) occurs via a “wzy-dependent” pathway in which repeating units formed on the cytoplasmic face of the inner membrane are polymerized at the periplasmic face following transport or flipping across the cytoplasmic membrane. A-LPS cross-reacts with monoclonal antibody (MAb) 1B5 raised against one of the isoforms of Arg-gingipains, a family of differentially glycosylated cysteine proteases (14, 19). Deglycosylation of the cross-reacting Rgps with anhydrous trifluoromethane sulfonic acid abolishes their immunoreactivity to MAb 1B5, indicating that this antibody recognizes a carbohydrate-containing epitope also present in A-LPS (14, 44). Hence, there appear to be common elements in the biosynthesis of A-LPS and the Arg-gingipains of this organism.
Inactivation of P. gingivalis waaL (PG1051, O-antigen ligase) abolishes the synthesis of both O-LPS and A-LPS (49). Hence, the WaaL O-antigen ligase appears to have dual specificity and is capable of ligating both O-PS and A-PS chains to core lipid A. The dual specificity of WaaL in the final step of LPS biosynthesis has also been demonstrated in the synthesis of Escherichia coli O-LPS and MLPS (38) and for Pseudomonas aeruginosa A-band and B-band LPSs (1).
However, the linkage between O-PS and A-PS and core OS has not been identified in P. gingivalis. In this paper, we describe a structural investigation of the core OS of O-LPS in which we used R-LPS prepared from ΔPG1051 (49) and ΔPG1142 (putative O-antigen polymerase), which we hypothesized would synthesize an SR-LPS (core plus one repeating unit) (60). The putative O-antigen polymerase encoded at PG1142 (42) is a phenylalanine-rich membrane protein consisting of 347 amino acids which shows 46% similarity over 297 amino acids to EpsK of Lactobacillus delbrueckii subsp. bulgaricus. EpsK is proposed to be a polymerase on the basis of homology and topological similarity to the O-antigen polymerase (Wzy) of E. coli and is required for the synthesis of an exopolysaccharide composed of Gal, Glc, and Rha (5:1:1) containing repeating units in L. delbrueckii (32). Application of one-dimensional (1D) and two-dimensional (2D) nuclear magnetic resonance (NMR) spectroscopy and methylation and monosaccharide analyses using gas chromatography-mass spectrometry (GC-MS) to purified core-containing OSs isolated from LPS from ΔPG1051 and ΔPG1142 mutants enabled us to solve the LPS core structure of an oral gram-negative bacterium for the first time.
MATERIALS AND METHODS
Bacterial growth conditions.
P. gingivalis strain W50 and ΔPG1051 and ΔPG1142 mutants were grown either on blood agar plates containing 5% defibrinated horse blood or in brain heart infusion broth supplemented with hemin (5 μg ml−1) in an anaerobic atmosphere consisting of 80% N2, 10% H2, and 10% CO2 (2). The cultures were harvested, and the cell pellets were washed with phosphate-buffered saline and freeze-dried.
P. gingivalis mutants.
The P. gingivalis ΔPG1051 (waaL) mutant strain and the complemented strain cPG1051 were isolated as described previously (49). The P. gingivalis ΔPG1142 mutant strain (putative O-antigen polymerase, wzy) was generated as follows.
Primer pairs were used to PCR amplify fragments corresponding to the 5′ (primers F1 and R1) and 3′ (primers F2 and R2) flanking regions of PG1142 that included SstI and XbaI sites, respectively (see Table S1 in the supplemental material). The two amplicons were ligated to an SstI-XbaI-restricted erm cassette (17, 21) and reamplified using F1 and R2. The amplicon, a chimera consisting of the 5′ end of PG1142, erm, and the 3′ end of PG1142, was used to electrotransform P. gingivalis W50, which conferred clindamycin resistance (3, 50). Following screening by PCR (to confirm legitimate chromosomal integration), one strain was chosen and designated the ΔPG1142 mutant.
Complementation of PG1142.
Primers PG1142EF2 and PG1142ER1 incorporating NotI restriction sites (see Table S1 in the supplemental material) were designed to amplify a 1,637-bp (cPG1142) region of the P. gingivalis W50 genome which also includes an additional 575-bp upstream sequence of the open reading frame containing the potential transcription apparatus (42). The amplicon was cloned into the NotI restriction site of pUCET1 (13, 49) to construct a recombinant plasmid containing ermF-cPG1142-tetQ-ermAM (pEA3) with all of the genes in the same direction. Following linearization of pEA3 with the BamHI restriction enzyme, the ermF and ermAM components were targeted in order to replace the erm cassette in the P. gingivalis ΔPG1142 mutant via electrotransformation, allelic exchange mutagenesis, and selection on blood agar plates containing tetracycline (1 μg ml−1). Six colonies showing resistance to tetracycline were assessed by using PCR, gingipain activities, and growth characteristics and were found to be identical (49). A representative isolate, P. gingivalis cPG1142, was chosen for isolation of LPS.
SDS-PAGE and Western blotting of LPS.
LPS from cells of P. gingivalis W50 and complemented strains cPG1051 and cPG1142 grown in brain heart infusion broth for 24 h was purified on an analytical scale using 2 ml of cultures using an LPS purification kit (Intron Biotechnology, South Korea), subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and Western blotting, and probed with MAb 1B5 as described previously (49). LPS could not be purified from the ΔPG1051 and ΔPG1142 mutants using this protocol, and the following procedure was used to purify LPS from these two mutant strains.
Isolation of LPS.
LPS of the P. gingivalis waaL (ΔPG1051) mutant strain was prepared using 4.2 g of freeze-dried cells and the procedure of Westphal and Jann (59), with some modifications (55). The water-phenol extract was exhaustively dialyzed against distilled water, the cell debris was removed by centrifugation, and the supernatant was freeze-dried. The freeze-dried residue was dissolved in Tris-HCl buffer (pH 8.0) and digested with RNase, DNase, and proteinase K, which was followed by dialysis and freeze-drying. The residue was dissolved in 60 ml of 10 mM Tris-HCl buffer (pH 8.0) and subjected to ultracentrifugation (105,000 × g) at 4°C for 4 h to pellet all of the LPS. The pellet was resuspended in water, and the supernatant obtained after ultracentrifugation was both dialyzed against distilled water and freeze-dried.
LPS of the P. gingivalis wzy (ΔPG1142) mutant strain was isolated using the procedure of Darveau and Hancock (15), followed by dialysis and freeze-drying. This procedure yields only O-LPS and no A-LPS probably due to the conditions employed during LPS isolation, which involve exposure of cell lysates to pH ∼9.5 for long periods for the removal of peptidoglycan, heating at 85°C to denature SDS-resistant proteins, and subsequent digestion with pronase. A-LPS is very sensitive to both high pH (unpublished observations) and low pH (14, 44).
Isolation of core OS(s).
LPS (45 mg) from the ΔPG1051 mutant strain was suspended in 6 ml of aqueous acetic acid (2%, vol/vol) and heated at 100°C for 2 h. Insoluble lipid A and undegraded LPS were removed by ultracentrifugation (105,000 × g) at 14°C for 3 h. The supernatant was freeze-dried, and the residue was fractionated by gel filtration chromatography using a Sephadex G-50 column (inside diameter, 2.5 cm; length, 90 cm) equilibrated in 50 mM pyridinium acetate buffer (pH 4.7). The column effluent was monitored for changes in the refractive index with a Knauer Wellchrom K-2400 RI detector (Wissenschaftliche Geratebau Dr. Ing. Herbert Knauer GmbH, Berlin, Germany). The core-containing fractions were pooled, freeze-dried, and rechromatographed using a Fractogel TSK HW-40(S) column (inside diameter, 2 cm; length, 80 cm) equilibrated in 0.05% (vol/vol) acetic acid. Appropriate fractions were pooled and freeze-dried, yielding 10.4 mg of core OS, which was used for structural analysis. LPS (100 mg) from the ΔPG1142 mutant strain was treated with 2.5 ml of 2% aqueous acetic acid at 100°C for 4 h. The reaction mixture was centrifuged in a microcentrifuge, and the supernatant was freeze-dried. It was then subjected to gel filtration chromatography using the Fractogel TSK HW-40(S) column as described above.
Monosaccharide composition and methylation analysis.
The monosaccharide composition of the core-containing OSs was determined by methanolysis, followed by analysis of the methyl glycosides as O-trimethylsilyl ethers by GC-MS (5, 26).
Methylation analysis of the native and de-O-phosphorylated material was performed by using the procedure of Kvernheim (31), followed by hydrolysis with 0.5 M trifluoroacetic acid for 1.5 h at 120°C. Methyl esters were reduced with NaBD4 (22°C, 4 h) and acetylated with pyridine-acetic anhydride (1:1, vol/vol) at 100°C for 1 h. Partially methylated alditol acetates were analyzed by GC-MS. The absolute configurations of the monosaccharides were determined as described by Gerwig et al. (20). The allosamine standard was kindly provided by Shohei Sakuda (The University of Tokyo).
De-O-phosphorylation of LPS.
LPS (20 mg) from the P. gingivalis waaL (ΔPG1051) mutant was de-O-phosphorylated by treatment with 0.5 ml of 48% aqueous H2F2 (24 h, 4°C). The reaction mixture was freeze-dried using a KOH trap, water-insoluble lipid material was removed by centrifugation (100,000 × g, 14°C, 30 min), and the supernatant was fractionated on a Fractogel TSK HW-40(S) column as described above to obtain de-O-phosphorylated OS1051 (OS1051-DF) (3 mg).
NMR spectroscopy.
Spectra were obtained using a Bruker AV600 spectrometer with standard Bruker software. OS samples were dissolved in D2O (99.90 atom% D; Aldrich), lyophilized twice, and finally dissolved in 0.52 ml of deuterium oxide (99.96 atom% D; Aldrich). 1D and 2D NMR spectra of the OSs were acquired at 25°C using acetone as an internal standard (δH 2.225; δC 31.45) and ortho-phosphoric acid as an external standard (δP 0.00), and assignments were determined by using the following homo- and heteronuclear chemical shift correlation techniques: double-quantum filtered correlation spectroscopy in phase-sensitive mode (DQF-COSY) (46), total correlation spectroscopy (TOCSY) (7), rotating-frame Overhauser enhancement spectroscopy (ROESY) (8), heteronuclear single-quantum correlation spectroscopy (HSQC) (1H detected, 13C decoupled via single coherence) (12), heteronuclear multiple-quantum correlation with additional TOCSY transfer (1H-{13C, 31P} HMQC-TOCSY) (29), and heteronuclear multiple-bond correlation spectroscopy (1H-{13C} HMBC) (9).
RESULTS
SDS-PAGE of LPSs.
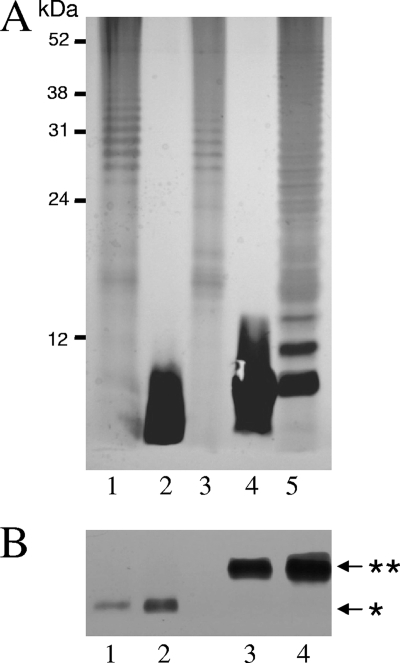
LPSs isolated from P. gingivalis parent strain W50 (10 μg), the ΔPG1051 (5 μg) and ΔPG1142 (5 μg) mutant strains, and complemented strains cPG1051(10 μg) and cPG1142 (10 μg) were subjected to SDS-PAGE and silver staining (Fig. 1A). LPSs from parent W50 strain and the two complemented strains showed the characteristic laddering pattern of S-type LPS, whereas LPSs from the ΔPG1051 and ΔPG1142 mutant strains showed strong silver staining only in the lower-molecular-weight region of the gel. LPS from the ΔPG1051 strain appeared to have greater mobility than LPS from the ΔPG1142 strain, which may reflect the core and core-plus-one repeating unit structures of these two LPSs (Fig. 1A). We therefore performed another SDS-PAGE experiment using smaller amounts (1 μg and 2 μg) of the LPSs from the ΔPG1051 and ΔPG1142 mutants (Fig. 1B) in order to more accurately determine the migration patterns of these molecules. This analysis revealed two well-resolved species probably corresponding to an R-LPS lacking any O-PS or A-PS attached to the lipid A core OS in the LPS from the ΔPG1051 strain and an SR-LPS or core-plus-one repeating unit in the LPS from the ΔPG1142 strain.
FIG. 1.
SDS-PAGE and silver staining of LPS from P. gingivalis. LPSs from P. gingivalis parent strain W50 and complemented strains cPG1051 and cPG1142 were prepared using the phenol guanidine isothiocyanate method (Intron Biotechnology, South Korea) and were included for comparison. Bands were identified by silver staining. (A) Lane 1, P. gingivalis W50 LPS (10 μg); lane 2, ΔPG1051 strain LPS (5 μg); lane 3, cPG1051 LPS (10 μg); lane 4, ΔPG1142 strain LPS (5 μg); lane 5, cPG1142 LPS (10 μg). (B) Lanes 1 and 2, ΔPG1051 strain LPS (1 μg and 2 μg, respectively); lanes 3 and 4, ΔPG1142 strain LPS (1 μg and 2 μg, respectively). The arrows indicate the positions of core (*) and core-plus-one (**) repeating units in the two mutants.
Neither the ΔPG1051 strain LPS (49) nor the ΔPG1142 strain LPS showed immunoreactivity with MAb 1B5, indicating that A-LPS was not present in either preparation (not shown).
Monosaccharide analysis of core OSs.
The monosaccharide compositions of the core OSs of the ΔPG1051 (OS1051) and ΔPG1142 (OS1142) mutant strains are shown in Table 1. Mannose was the major component of OS1051, along with an unidentified amino sugar and minor amounts of Kdo, whereas in OS1142 rhamnose, galactose, glucose, and N-acetylgalactosamine were present in addition to the components found in OS1051. None of the core-containing OSs analyzed contained heptose residues.
TABLE 1.
Monosaccharide composition of OS1051 and OS1142a
| OS | Monosaccharide composition (molar ratio)
|
||||||
|---|---|---|---|---|---|---|---|
| Rha | Man | Gal | Glc | Kdo | HexN(Ac)b | GalNAcb | |
| OS1051 | NFc | 67.3 | NF | NF | 1.0 | 4.0 | NF |
| OS1142 | 10.2 | 18.4 | 12.8 | 15.6 | 1.0 | 2.2 | 4.8 |
OSs were subjected to methanolysis, which was followed by GC-MS analysis of the trimethylsilyl derivatives of the corresponding methyl glycosides. Dulcitol was used as an internal standard.
The reduced amounts of N-acetylhexosamines are due to their resistance to acid hydrolysis. HexN(Ac), N-acetylallosamine.
NF, not found.
Heptose is a highly conserved and common constituent of the core OS of LPS; the fact that it was not observed in the P. gingivalis W50 core OS suggests that the core region of O-LPS in this organism has an unusual structure.
Methylation analysis of core OSs.
Methylation analysis of OS1051, OS1142, and OS1051-DF (Table 2) showed the presence of terminal mannose, a terminal amino sugar, and 3-linked mannose. In addition, OS1051-DF contained 3,6-linked mannose and 2,3-di-O-acetyl-1-O-methylglycerol.
TABLE 2.
Methylation analysis data for OS1051, OS1051-DF, and OS1142
| Component | Peak area |
|---|---|
| OS1051 | |
| 1,5-Di-O-acetyl-1-deuterio-2,3,4,6-tetra-O-methylmannitol | 1.7 |
| 1,3,5-Tri-O-acetyl-1-deuterio-2,4,6-tri-O-methylmannitol | 3.2 |
| 1,5-Di-O-acetyl-2-(acetylmethylamino)-2-deoxy-1-deuterio-3,4,6-tri-O-methylallositola | 0.4 |
| OS1051-DF | |
| 2,3-Di-O-acetyl-1-O-methylglycerol | 5.4 |
| 1,5-Di-O-acetyl-1-deuterio-2,3,4,6-tetra-O-methylmannitol | 8.3 |
| 1,3,5-Tri-O-acetyl-1-deuterio-2,4,6-tri-O-methylmannitol | 34.5 |
| 1,3,5,6-Tetra-O-acetyl-1-deuterio-2-O-methylmannitol | 12.1 |
| 1,5-Di-O-acetyl-2-(acetylmethylamino)-2-deoxy-1-deuterio-3,4,6-tri-O-methylallositola | 4.8 |
| OS1142 | |
| 1,4,5-Tri-O-acetyl-6-deoxy-1-deuterio-2,3-di-O-methylrhamnitol | 4.5 |
| 1,3,5-Tri-O-acetyl-1-deuterio-2,4,6-tri-O-methylmannitol | 14.6 |
| 1,5,6-Tri-O-acetyl-1-deuterio-2,3,4-tri-O-methylglucitol | 31.8 |
| 1,5-Di-O-acetyl-1-deuterio-2,3,4,6-tetra-O-methylgalactitol | 33.8 |
| 1,3,5-Tri-O-acetyl-2-(acetylmethylamino)-2-deoxy-1-deuterio-4,6-di-O-methyl-2-deoxygalactitola | 1.2 |
The reduced amounts of partially methylated derivatives of N-acetylhexosaminitols are due to their resistance to acid hydrolysis.
Analysis of OS1051.
The sequence and nature of glycosyl residues of OS1051 were established by high-resolution 1D and 2D NMR. The 1H-NMR spectrum of OS1051 showed resonances in the low-field region (δH 5.05 to 5.21), which were attributed to the anomeric protons from seven major sugar residues, which were designated residues A to G in order of decreasing chemical shifts (Table 3). The presence of low-intensity signals in the high-field region for H-3ax and H-3eq of the Kdo residue at δH 2.12 and 2.33, respectively, may be due to the presence of Kdo at the reducing end in multiple, mainly anhydro forms, possibly as a result of decarboxylation during mild acid hydrolysis (37). There were no signals in the δH 1.2 to 2.3 region characteristic of methyl protons of O-acetyl groups, N-acetamido groups, and 6-deoxyhexoses.
TABLE 3.
1H and 13C NMR data for OS1051a
| Residue | Parameter | H-1/C-1 | H-2/C-2 | H-3/C-3 | H-4/C-4 | H-5/C-5 | H-6/C-6 | H-6′/C-6 |
|---|---|---|---|---|---|---|---|---|
| A (2H) | δH | 5.21 | 3.29 | 3.91 | 3.51 | 3.60 | 3.75 | 3.90 |
| δC | 95.33 | 54.20 | 71.34 | 69.40 | 72.90 | 60.05 | 60.05 | |
| J (Hz) | 3.34 (J1,2) | 3.33 (J2,3) | 3.37 (J3,4) | 9.80 (J4.5) | 2.30 (J5,6) | 5.2 (J5,6′) | 12.2 (J6,6′) | |
| B (1H) | δH | 5.17 | 3.26 | 3.87 | 3.50 | 3.60 | 3.75 | 3.90 |
| δC | 95.28 | 53.93 | 70.72 | 69.40 | 72.90 | 60.05 | 60.05 | |
| J (Hz) | 3.34 (J1,2) | 3.33 (J2,3) | 3.37 (J3,4) | 9.80 (J4,5) | 2.30 (J5,6) | 5.2 (J5,6′) | 12.2 (J6,6′) | |
| C (2H) | δH | 5.17 | 4.09 | 3.89 | 3.78 | 3.82 | 3.88 | 3.80 |
| δC | 102.46 | 69.83 | 78.02b | 66.20 | 71.53 | 60.53 | 60.53 | |
| D (2H) | δH | 5.13 | 4.09 | 3.91 | 3.80 | 3.80 | 3.88 | 3.80 |
| δC | 102.48 | 70.08 | 78.50 | 66.20 | 71.12 | 60.82 | 60.82 | |
| E (1H) | δH | 5.11 | 4.08 | 3.90 | 3.78 | 3.76 | 3.88 | 3.88 |
| δC | 98.56 | 70.08 | 71.51 | 66.90 | 69.10 | 60.50 | 60.50 | |
| F (1H) | δH | 5.10 | 4.07 | 3.92 | 3.77 | 3.69 | 4.05 | 3.98 |
| δC | 98.57 | 69.89 | 78.00 | 66.87 | 73.67 | 65.58 | 65.58 | |
| G (2H) | δH | 5.05 | 4.01 | 3.90 | 3.77 | 3.64 | 4.05 | 3.98 |
| δC | 96.85 | 70.08 | 78.01 | 66.87 | 73.67 | 65.82 | 65.82 | |
| α-Kdo-5P | δH | 2.12 (H-3ax) | 2.33 (H-3eq) | 4.13 | 4.31 | NFc | NF | |
| δC | 174.5 | 31.80 | 31.80 | 67.92 | 74.10 |
The data for Gro-I are as follows: H-3, 4.21 ppm; H-3′, 3.63 ppm; H-2, 3.91 ppm; H-1, 3.98 ppm; H-1′, 4.02 ppm; C-3, 65.18 ppm; C-2, 71.55 ppm; and C-1, 64.50 ppm. The data for Gro-II are as follows: H-3, 4.18 ppm; H-3′, 3.68 ppm; H-2, 3.89 ppm; H-1, 3.98 ppm; H-1′, 4.02 ppm; C-3, 65.18 ppm; C-2, 71.55 ppm; and C-1, 64.50 ppm. The chemical shifts for PEA are as follows: P(O)CH2CH2NH2, δH 4.13 and δC 61.92; and P(O)CH2CH2NH2, δH 3.29 and δC 39.99. The coupling constants are not shown, but when they were measured, they were in agreement with expected values.
Bold type indicates the positions of substituted carbons.
NF, not found.
Data from a combined analysis of 2D DQF-COSY, TOCSY, 1H-{13C} HMBC, and 1H-{13C} HSQC spectra allowed us to identify the spin systems for each sugar residue in OS1051 (Table 3).
Residue A.
Residue A was determined to be an α-2-amino sugar based on the value of the 3J1,2 vicinal coupling constant, 3.34 Hz (position for H-1 at δH 5.21 and position for C-2 at δC 54.20), which was characteristic of carbon adjacent to nitrogen (25) (Table 3). The values for vicinal coupling constants were found to be specific for the residue having an equational orientation for H-3 (i.e., 3J2,3 ∼ 3J3,4 value of 3.3 Hz) resulting in the appearance of the signal for H-2 as a clear triplet. Taking these data into account, together with the J4,5 value of 9.80 Hz and the 1JC-1,H-1 value of 172.0 Hz (10), the spin system of this residue was fully assigned and shown to have an α-allo configuration.
Residue B.
On the basis of the positions of the proton and carbon signals (i.e., position for C-2 at δC 53.93 [Table 3]) and values for 1JC-1,H-1 and 3Jn,(n+1) that were the same as those for residue A, it was concluded that residue B is an α-2-amino sugar also having an α-allo configuration.
Residues C to G.
The position of H-1 within the δH 5.17 to 5.05 region of residues C to G, their relatively low 3J1,2 values (∼1.5 Hz each), and their 1J C-1,H-1 values (172 to 175 Hz) (10), together with monosaccharide analysis data, indicate that these residues have an α-manno configuration. The positions of the signals for H-3 and C-3 of residues C to G were obtained from 2D 1H-1H and 1H-13C NMR experiments (Table 3). The signals for C-3 for residues C, D, F, and G had resonances in the δC 78.01 to 78.50 region, which is characteristic of 3-linked α-mannose-containing disaccharides (11). The results of an analysis of the proton and carbon chemical shifts for the spin system of residue E suggest that this residue is a nonsubstituted α-mannopyranose. Evidence for this conclusion is based on the small 3J1,2 and 3J2,3 coupling constants and the positions of C-2 to C-6 (Table 3), which are characteristic of nonglycosylated hexopyranoses (11). From the methylation analysis and NMR spectral data, we concluded that residues C, D, F, and G are glycosylated at position 3, whereas residue E appears to be a terminal sugar. In addition, there were signals in the NMR spectra of OS1051 that belonged to two three-carbon-containing spin systems which have been assigned as two sets of trisubstituted glycerol residues, designated Gro-I and Gro-II (Table 3). The methylation analysis of OS1051 did not reveal partially methylated acetate derivatives of glycerol residues. However, following dephosphorylation, 2,3-di-O-acetyl-1-O-methylglycerol was identified during the methylation analysis of OS1051-DF (Table 2). This suggests that both Gro-I and Gro-II residues are phosphorylated at position C-1 or at position C-3 (27).
The combined 2D ROESY and HMBC data for OS1051 (Table 4) showed interresidual cross-peaks (between transglycosidic protons and some neighboring protons) and intraresidual cross-peaks (H-1/H-2 for α-linked sugars, H-1/H-3 and H-1/H-5 for β-linked sugars [34]) which allowed us to establish the sequence of sugars in OS1051.
TABLE 4.
1H-1H ROESY data for OS1051a
| Residue | δH | Intra- and interresidual NOEs |
|---|---|---|
| A | 5.21 | 3.30 (H-2A), 3.63 (H-3 Gro-I), 4.21 (H-3 Gro-I) |
| B | 5.17 | 3.26 (H-2B), 3.64 (H-3′ Gro-II), 4.18 (H-3 Gro-II) |
| C | 5.17 | 4.09 (H-2C), 3.90 (H-3D/H-2 Gro-I) |
| D | 5.13 | 4.08 (H-2D), 3.89 (H-3C/H-2 Gro-II) |
| E | 5.11 | 4.08 (H-2E), 3.92 (H-3F) |
| F | 5.10 | 4.07 (H-2F), 3.90 (H-3G) |
| G | 5.05 | 4.014 (H-2G), 3.91 (H-3D) |
Bold type indicates interresidual NOE contacts.
For residue A, in addition to an intraresidual cross-peak at δH/H 5.21/3.30 (H-1A/H-2A), the following interresidual correlations were observed at δH/H 5.21/3.63 and 5.21/4.21, which have been assigned as H-3′ Gro-I and H-3 Gro-I, respectively, and indicate that residue A is attached to the glycerol residue Gro-I at position 3 (Tables 3 and 4). Residue B is attached to the glycerol residue Gro-II at position 3 in an analogous manner.
For residues C and D, the nuclear Overhauser effect (NOE) cross-peaks in the δH 4.08 to 4.09 region were assigned as H-2 of the same sugars, and interresidual cross-peaks were observed at δH 3.89 and δH 3.91, indicating that these residues are α-(1→3) linked and that residue C is attached to residue D at O-3 or vice versa (Table 3). This conclusion was arrived at due to the presence of C-H multibond (three-bond) interresidual connectivities at δC/H 102.46/3.91 and 102.48/3.89 as C-1/H-3 cross-peaks which were found to correspond to C-1C/H-3D and C-1D/H-3C, respectively, in the HMBC spectrum of OS1051 (Table 5).
TABLE 5.
2D 1H-{13C} HMBC data for OS1051
| Residue | Parameter | Intra- and interresidual H/C and C/H connectivities (δH/C and δC/H) |
|---|---|---|
| C | H-1 at δH 5.17 | 71.33 (C-5C) |
| C-1 at δC 102.46 | 3.91 (H-3D) | |
| D | H-1 at δH 5.13 | 71.12 (C-5D), 78.02 (C-3C), 71.55 (C-2 Gro-I) |
| C-1 at δC 102.48 | 3.89 (H-3C), 3.89-3.90 (H-2 Gro-I) | |
| F | H-1 at δH 5.10 | 73.67 (C-5F) |
| G | H-1 at δH 5.05 | 73.67 (C-5G) |
| Kdo | H-5 at δH 4.31 | 64.5 (C-1 Gro-I/C-1 Gro-II) |
The existence of the D(1→)C fragment was confirmed by interpretation of the cross-peak at δH/C 5.13/78.02 as H-1D/C-3C. However, there were two cross-peaks at δH/C 5.13/71.55 and at δC/H 102.50/3.89 to 3.91 corresponding to H-1D/C-2 Gro-I and C-1D/H-2 Gro-I, respectively, which allowed us to suggest that there is an additional D(1→2)Gro-I fragment in OS1051. In addition, the correlation at δH/H 5.17/3.90 in the ROESY spectrum could be interpreted as a result of superimposition of the H-1C/H-3D (see above) and H-1C/H-2 Gro-II cross-peaks. Integration of signals in the anomeric region of the 1H NMR spectrum showed the presence of two overlapped protons for residue D, and taking into account the assignment of the HMBC and ROESY interresidual cross-peaks (see above), this may be accounted for by the presence of two subglycosidic fragments, one of which contains D(1→3)C(1→2)Gro-II and one of which contains D(1→2)Gro-I.
For residue E, an interresidual NOE cross-peak at δH 5.11/3.92 observed in the ROESY spectrum was assigned as H-1E/H-3F, resulting in the conclusion that residue E is linked to residue F at position 3 (Table 4). The spin systems of residues F and G were assigned on the basis of HMBC spectral data. The positions of the chemical shifts for H-5 and C-5 of these α-mannose residues resulted from the presence of intraresidual C-1/H-5 three-bond correlations at δC/H 98.57/3.69 and 96.85/3.64, respectively, and the presence of cross-peaks at δC 73.67 correlating with their H-1 protons at δH 5.10 and 5.05. The interresidual NOE cross-peaks at δH/H 5.10/3.90 and 5.05/3.91 were due to H-1F/H-3G and H-1G/H-3D (Table 4) and indicated that residues F and G are linked to residues G and D at position 3, respectively.
31P NMR analysis of OS1051.
The 31P NMR spectrum of OS1051 (Fig. 2) had four peaks at δP 1.55, 1.48, 1.45, and 1.41 which were characteristic of signals for monophosphodiesters (between 2 and −2 ppm) (36). The observed cross-peaks in a 1H-{31P} HMQC NMR experiment indicated that phosphorus signals at δP 1.48 to 1.41 correlate with proton resonances at δH 4.13 and 3.29 which are specific to the methylene protons of phosphoethanolamine (27) (Table 3). From the correlations between the phosphorus resonances at δP 1.48 to 1.41 and the protons at δH 4.05 and 3.98 which belong to H-6 and H-6′ of residues F and G, we concluded that the phosphate groups are linked to these residues at C-6 via monophosphodiester linkage.
FIG. 2.
1D 31P NMR spectrum of OS1051.
For residues F and G, this conclusion was supported by the values for the 3JC,P vicinal coupling constants for C-5 (7.15 Hz and 7.30 Hz) and for C-6 (3.70 Hz and 3.68 Hz), respectively, derived from 1H-13C HSQC spectral data (45). In addition, the low-field chemical shifts for C-6 of both residue F and residue G at δC 65.58 and 65.82, respectively (δC 61.50 for C-6 of α-mannopyranoses not substituted at O-6 [25]), were in agreement with the conclusion that α-mannopyranoses were substituted with a phosphate group at position C-6 (45). Taking the data described above into account, we concluded that residues F and G have a PEA residue at position C-6.
The signal at δP 1.55 correlates with signals at δH 3.98 and 4.02, which have been assigned as H-1 and H-1′ of both residues of glycerol Gro-I and Gro-II (Table 3). The position of C-1 of both glycerol residues was at δC at 64.50, which is in accordance with the position in monophosphorylated glycerol (27) and confirmed that there could be two types of core glycoforms in which each of the glycerol residues carries a phosphate at position C-1.
In addition, combined analysis of 2D 1H-1H TOCSY, DQF-COSY, and 1H-{13C/31P}-heterocorrelated NMR spectral data indicated that the phosphate group bridges glycerol and Kdo residues by a diester linkage. Although the signals of H-3 to H-5 for an α-Kdo residue were relatively low-intensity signals, the presence of a cross-peak in the HMBC spectrum at δH/C 4.31/64.5 indicated that there is linkage between H-5 of Kdo and C-1 of glycerol (Table 5) (39).
Hence, the data obtained suggest that the outer core-containing OS1051 consists of two oligomers, each of which comprises α-(1→3)-linked mannopyranosyl residues (residues C to G), glycerol-1-phosphate (Gro-I-1P, Gro-II-1P), and α-2-amino-2-deoxy-allo-pyranose (residues A and B), as follows: E(1→3)F(1→3)G(1→3)D(1→2)[A(1→3)]Gro-I-1P→5)Kdo and E(1→3)F(1→3)G(1→3)D(1→3)C(1→2)[B(1→3)]Gro-II-1P→5)Kdo.
Analysis of OS1142.
Mild acidic degradation of LPS isolated from the ΔPG1142 (O-antigen polymerase) mutant strain gave two main OS-containing fractions. The 1H NMR spectrum of the low-molecular-weight fraction was identical to that of core-containing fraction OS1051, whereas the 1H NMR spectrum of the higher-molecular-weight fraction, OS1142, showed signals belonging to the residues of O-PS (43) in addition to the signals assigned to the core components. Residues in OS1142 were designated residues A to E in order of decreasing chemical shifts of their anomeric signals at δH 5.13, 5.04, 4.99, 4.88, and 4.70, and their spin systems were assigned based on a comparative analysis of 1H-{13C} HMQC-TOCSY spectral data (not shown) and NMR spectral data for O-PS (43) (Table 6). The signal at δH 4.70 was identified as H-1 of 3-substituted β-GalNAc (residue E). The resonances for H-6 of methyl groups at δH 1.36 and 1.32 represented by doublets with vicinal coupling constants of 6.20 Hz and 6.30 Hz, respectively, corresponded to 4-linked α-rhamnose residue B substituted with PEA at position 2 and to 4-linked α-rhamnose residue D without PEA, respectively. The positions of their H-4 and C-4 localized at δH 3.49 and δC 82.70, respectively, were in accordance with the positions found in O-PS (43). The positions of the resonances for H-1, H-2, and H-3 at δH 4.99 (3J1,2, 4.2 Hz), 3.83, and 3.89 of residue C were characteristic of a nonsubstituted α-galactopyranose residue (57). In addition, the signal for C-1 of the α-galactopyranose residue at δC 98.24 was in accordance with the signal for position C-1 for α-Gal at the nonreducing terminus of an α-Gal (1→6)-α-Glc disaccharide (11). Therefore, residue C was assigned as a nonsubstituted α-galactopyranose. The spin system of the residue where the position of H-1 overlapped with the position of residue B at δH 5.04 was assigned as α-(1→6)-linked glucopyranose, and this was in agreement with 1H and 13C NMR data obtained for O-PS (43). The proton anomeric signal at δH 5.13 was found to belong to the spin system of residue A with an α-manno configuration based on analysis of the vicinal coupling constants and proton and carbon chemical shifts (Table 6).
TABLE 6.
1H-1H ROESY data for OS1142a
| Residue | δH | Intra- and interresidual NOEs observedb |
|---|---|---|
| A | 5.13 [→3)α-Man] | 3.73 (H-4A), 3.87, 3.92, 4.07-4.09, 4.17 (H-2A) |
| B | 5.04 [→6)α-Glc + →4)α-Rha (PEA)] | 3.49 (H-4D, H-4B), 3.57 [H-2 →6)α-Glc], 3.80 (H-3E), 3.89 (H-3C), 4.05 (H-4E) |
| C | 4.99 (t-α-Gal) | 3.71 [H-6 →6)α-Glc], 3.83 (H-2C), 3.89 (H-3C), 3.99 [H-6′ →6)α-Glc] |
| D | 4.88 [→4)α-Rha] | 3.80 (H-3E), 3.84 (H-2D), 4.05 (H-4E) |
| E | 4.70 [→3)β-GalNAc] | 3.71 (H-5E), 3.80 (H-3E), 4.07 (H-3A), 4.16 (H-2A) |
The 1H and 13C chemical shifts for residue A are as follows (13C NMR data for α-mannnopyranose are in parentheses [32]): H-1/C-1δH/C 5.13/103.56 (95.30), H-2/C-2 δH/C 4.17/67.80 (72.00), H-3/C-3 δH/C 4.06/78.40 (71.50), and H-4/C-4 δH/C 3.77/66.40 (67.9).
Bold type indicates interresidual NOE contacts.
The linkages for residues B to E, which are part of the O-PS, were determined from analysis of the 2D 1H-1H ROESY spectrum of OS1142 (Table 6 and Fig. 3). The following interresidual NOE cross-peaks observed at δH/H 4.70/4.07 and 4.07/4.16 were assigned as H-1E/H-3A and H-1E/H-2A, respectively.
FIG. 3.
Part of the 1H-1H ROESY spectrum of OS1142. The NOE contacts between H-1 of β-d-GalNAc and H-2 and H-3 of α-d-Man are indicated by dotted lines.
Further analysis of the ROESY NMR data for interresidual connectivities between anomeric protons of glycosylating sugars and protons adjacent to the C-O fragment of the glycosylated residue showed that residue D (α-rhamnose without PEA) is linked to β-GalNAc via position 3 (H-1D/H-3E at δH/H 4.88/3.80). Moreover, the presence of an additional NOE cross-peak at δH/H 4.88/4.05 (H-1D/H-4E) confirmed that residue E having a galacto configuration is glycosylated at position 3 (34).
The linkage between residue C (terminal nonreducing α-galactopyranose) and residue B (α-glucopyranose) was established from NOE contacts at δH/H 4.99/3.71 and 4.99/3.99 corresponding to H-1C/H-6B and H-1C/H-6′B. Analysis of the interresidual NOE at δH/H 5.04/3.80 revealed that α-rhamnopyranose carrying PEA was linked to a β-GalNAc residue at position 3. Despite the overlapping anomeric resonances of α-rhamnopyranose linked to PEA at position C-2 and α-glucopyranose which appeared in a 1H NMR spectrum as a signal with double intensity, the NOE cross-peak at δH/H 5.04/3.49 was assigned as H-1B/H-4D and H-1B/H-4B due to superimposed signals for H-4 of residue B (→4)Rha (PEA) and for H-4 of residue D (→4)Rha, indicating that α-glucopyranose was linked to both α-Rha residues at position 4. The combined analysis of the ROESY and HMQC-TOCSY spectral data for OS1142 (Table 6) suggested that residue E is linked to residue A at position 3 due to the presence of H-1E/H-2A and H-1E/H-3A NOE cross-peaks at δH/H 4.70/4.16 and 4.70/4.07, respectively, and positions for C-2 and C-3 of residue A were shown to have resonances at δC 67.80 and 78.40, which is characteristic of resonances for α-(1→3)-linked mannopyranoses (11). Therefore, taking the data described above and the fact that no NOE contacts between β-GalNAc (residue E) and α-galactopyranose (residue C) were found into account, it was concluded that there are two types of single O-PS repeating units linked to α-mannose (residue A) at position 3 via residue E in OS1142. The first type is represented by the sequence α-d-Galp-(1→6)-α-d-Glcp-(1→4)-α-l-Rhap[2←PEA]-(1→3)-β-d- GalNAcp-(1→3)-α-d-Manp, whereas the second type of O-PS repeating unit does not contain the PEA residue attached to α-l-Rha at position O-2 [i.e., α-d-Galp-(1→6)-α-d-Glcp-(1→4)-α-l-Rhap-(1→3)-β-d-GalNAcp-(1→3)-α-d-Manp].
Since residue A (α-Man) was found to be glycosylated by residue E (2-acetamido-2-deoxy-β-d-galacto-hexopyranose) at position O-3, a comparison of the 13C NMR chemical shifts at C-2 and C-4 (β effects of glycosylation) and at C-3 (α effects of glycosylation) on glycosylated residue A with those of nonglycosylated α-manno-hexopyranose independently allowed us to determine the absolute configuration of residue A (l or d) (33). The relatively large positive α-effect on C-3 (6.9 ppm) and the relatively large negative (by module) β-effects on C-2 and C-4 (−4.2 ppm and −1.5 ppm, respectively) of glycosylated sugar residue A (Table 6) indicated that both residues A and E should have the same absolute configuration (d-d or l-l). As β-GalNAc (residue E) has a d absolute configuration (43), this confirms that the 3-linked α-mannopyranosyl residue A to which one O-PS repeating unit is attached also has a d configuration.
The presence of additional interresidual NOE cross-peaks at δH 4.07 to 4.09, 3.92, and 3.87 correlating with H-1 of residue A at δH 5.13 and a relatively high value for the integrated area under the anomeric signal of residue A in the 1H NMR spectrum of OS1142 led us to conclude that residue A is linked to an extended sequence consisting of between three and five additional α-(1→3)-linked mannose residues. These residues are attached to each of the main outer core glycoforms at position O-3 of the nonreducing terminal α-mannopyranose (position for H-2 and H-3 of residue C at δH 4.08 and 3.86 and for H-2 and H-3 of residue D at δH 4.09 and 3.91 for OS1051 [Table 3]). Hence, it was concluded that two different types of O-specific polysaccharide repeating units are attached to α-(1→3)-linked mannose-containing glycoforms at position O-3 of the nonreducing α-mannopyranose (see above). As a result, we propose a structure for the outer core region of P. gingivalis in which an O-PS repeating unit is attached via the β-d-GalNAc residue to position O-3 of the nonreducing terminal α-D-mannopyranose residue which is part of an α-(1→3)-mannose-containing motif forming the “capped core” glycoform (Fig. 4).
FIG. 4.
Proposed structure of the outer core OS present in P. gingivalis O-LPS. “Uncapped core” OS is devoid of O-antigen and additional α-(1→3)-linked d-Man residues. “Capped core” OS provides the site of O-antigen attachment through a terminal α-d-Man residue. The O-antigen repeating unit is shown in the shaded area.
DISCUSSION
While the importance of the proinflammatory properties of the LPS of P. gingivalis in periodontal disease has been recognized for some time, it is only recently that the complexity of the structural isoforms of this molecule in this organism has been acknowledged (4, 6, 51). The structural variation includes significant changes in the lipid A structure in terms of the degree of acylation and phosphorylation and the presence of two types of repeating units (49). The degree to which these structural modifications influence biological activity is under investigation. However, we believe that an important goal is elucidation of the full structure of the LPS(s) of P. gingivalis, together with an understanding of the biosynthetic machinery and mechanisms of variation.
P. gingivalis W50 has been shown to synthesize two LPSs, O-LPS and A-LPS (49), although it is not known whether the repeating units of these LPS molecules are linked to the same core OS at different sites or to two different core OSs. In this work we aimed to solve the structure of the outer core region of O-LPS as a prelude to complete structural analyses of both molecules. Analysis of the core of S-type LPS is complicated by the high molar percentage of the O-antigen repeats. In order to enrich for the core-containing species, we isolated R-type LPS from an O-antigen ligase (WaaL) mutant previously described by Rangarajan et al. (49) which lacks the repeating units. LPS from the ΔPG1051(WaaL ligase) mutant was isolated by phenol extraction, and SDS-PAGE analysis showed that it was characteristic of an R-type LPS (Fig. 1). Only one type of core OS was isolated from the P. gingivalis ΔPG1051 strain, suggesting that (i) only one core lipid A is synthesized and both O-PS and A-PS are attached independently to this moiety, (ii) the core OS to which A-PS is attached is present at much lower and hence undetectable levels, reflecting the relative amounts of A-LPS and O-LPS in P. gingivalis W50, or (iii) the A-LPS core OS from the ΔPG1051 strain is degraded or destroyed by acid treatment during preparation. The yield of A-LPS from P. gingivalis W50 is approximately 5 to 10% of the yield of O-LPS obtained from the same amount of P. gingivalis W50 cells.
Purification of A-LPS involves hot phenol extraction of P. gingivalis cells and affinity chromatography on concanavalin A columns, followed by ion-exchange chromatography (49). It is likely that exposure of P. gingivalis cells to hot phenol (70°C) causes some degradation of A-LPS. But the number of subsequent steps involved is similar to the number of steps used for isolation of O-LPS. Hence, it seems that P. gingivalis produces or synthesizes a smaller amount of A-LPS than of O-LPS.
Monosaccharide analysis of OS1051 showed that mannose was the main component (Table 1), whereas the components of the O-antigen (namely, l-rhamnose, d-galactose, d-glucose, and 2-acetamido-2-deoxy-d-galactose) were absent, confirming that PG1051 acts as an O-antigen ligase. Methylation analysis revealed the presence of terminal mannose, 3-substituted mannose, and terminal 2-acetamido-2-deoxyallositol, whereas no traces of 2-O-substituted or 2,6-di-O-substituted mannoses, both of which are components of A-PS, were found (Table 2).
Methylation analysis of OS1051-DF revealed the presence of the same linkage sugars as those in OS1051; in addition, 3,6-di-O-substituted mannose and 2,3-di-O-substituted glycerol were present. These data were in accordance with 2D 1H-{31P} heterocorrelation HMQC NMR experimental data which suggested that the core OS contains α-(1→3)-linked mannopyranosyl residues, at least one-half of which are phosphorylated at position O-6 by PEA (Fig. 4). There is uncertainty concerning the phosphorylation pattern of Kdo, which may be due to phosphate migration since Kdo is in the inner core region (22). Another factor is that there may be more Kdo residues than are usually found in many other bacterial LPSs (24). The observations made by Kumada et al. (30) that Kdo from LPS of P. gingivalis strain 381 could carry phosphates at positions C-4, C-7, and/or C-8 and is linked to the core OS region via position 5 indicate that the full structure of the inner core region, including confirmation of the configurations of glycosidic linkages of other Kdo residues, requires further study.
The identification of α-d-allosamine carrying a free amino group and glycosylating the glycerol residue in the core region of P. gingivalis LPS is intriguing since this is the first example of the presence of such an unusual component in the LPS of a gram-negative bacterium (23), although N-acetyl-β-d-allosamine has been characterized in Streptomyces sp. as part of a pseudotrisaccharide, “allosamidin” (54). Hence, the studies on the core OS from PG1051 confirmed that this a highly unusual LPS core which appears to lack heptosyl residues and contains the rare amino sugar α-d-allosamine.
Analysis of the LPS isolated from the ΔPG1142 strain demonstrated that this strain contained an SR-LPS composed of a core OS plus one repeating unit of O-PS (Fig. 1). This analysis strongly suggested that PG1142 acts as the sole polymerase capable of polymerizing the O-antigen repeating unit in P. gingivalis. Detailed investigation of OSs released from LPS of the ΔPG1142 strain showed the presence of a lower-molecular-weight OS, whose structure was identical to that of OS1051, and a higher-molecular-weight OS1142 species. Structural analysis of the latter species revealed that residue A of α-d-mannopyranose is glycosylated by β-GalNAc at position O-3, as shown by the low-field chemical shift of C-3 for residue A at δC 78.40, which is characteristic for α-(1→3)-linked mannopyranoses. Taking into account the data described above and the finding that the O-PS repeating unit of O-LPS consists of the linear tetrasaccharide →3)-β-d-GalNAcp-(1→3)-α-d-Galp-(1→6)-α-d-Glcp-(1→4)-α-l-Rhap[(2←60%PEA)]-(1→, in which α-d-galactopyranose is glycosylated by N-acetyl-β-d-galactosamine at position O-3 (43), the identification of a terminal α-d-Gal instead of 3-linked α-d-Gal in OS1142 strongly suggests that (i) one O-PS repeating unit is attached to the α-mannose residue and (ii) the structure of the “chemical” repeating unit of O-LPS of P. gingivalis W50 is as follows: (→3)-α-d-Galp-(1→6)-α-d-Glcp-(1→4)-α-l-Rhap[(2←60%PEA)]- (1→3)-β-d-GalNAcp-(1→. Interestingly, the methylation analysis of OS1142 showed an increase in the amount of 3-linked mannose compared to that in OS1051 (Table 2). Furthermore, the observation of additional NOE cross-peaks in the ROESY spectrum of OS1142 (Table 6) at δH 5.13 (H-1A)/4.07 to 4.09 (δH 5.13 [H-1A/3.92]) and δH 5.13 (H-1A/3.87) indicates that the O-PS repeating unit is attached to an extended α-(1→3)-linked mannose-containing fragment, which in turn is linked to the α-mannose at the nonreducing terminal residue of the outer core region at position O-3.
The core regions of LPSs of gram-negative bacteria contain a vast variety of glycoforms (23). The inner core motif in most variants comprises linear or branched sequences of l(d)-glycero-d(l)-manno-heptosyl residues linked to up to four residues of Kdo, and these heptoses are often decorated with noncarbohydrate substituents, such as phosphate in E. coli K-12 (40), PEA in Haemophilius influenzae strain R2846 (35), and pyrophosphate-PEA and carbamoyl in P. aeruginosa serotypes O5 and O3, respectively (28). The absence of heptosyl or phosphorylated heptosyl residues in the core of LPS of P. gingivalis is not unprecedented as there are examples of LPSs that lack heptosyl residues in the inner core glycoforms; for example, Rhizobium etli strains CE358 and CE359 (18) and Pseudomonas cichorii (Pseudomonadaceae RNA group 1) (16) produce LPS lacking heptosyl residues and thereby form outer core glycoforms linked to 3-deoxy-d-manno-2-octulosonic acid directly.
The structural analysis of OS1051 and OS1142 showed the presence of two different outer core-containing OSs. OS1142 differs from OS1051 by up to five additional α-(1→3)-linked Man residues attached to the nonreducing terminal residue of α-mannose at position 3 of the main outer core glycoform, forming a “capped core” (Fig. 4). The existence of the extended α-(1→3)-linked mannose-containing motif in the “capped core” in OS1142 may allow or account for the attachment of a single unit of O-PS. This is analogous to the existence of two glycoforms of core OS in P. aeruginosa which differ by linkage of an l-Rha to different d-Glc residues in the outer core region. One of the glycoforms consists of l-Rha attached via (1→3) linkage to β-d-Glc of the core side chain and acts as the acceptor molecule for the covalent attachment of A- or B-band O antigen (47) and is known as the “capped core.” The other core glycoform contains l-Rha linked to the backbone α-d-Glc residue at position 6 and is not substituted by O antigen but by β-d-Glc, forming the “uncapped-core” (52, 53). Poon et al. (47) showed that MigA, an α-1,6-rhamnosyltransferase, and WapR, an α-1,3-rhamnosyltransferase, are responsible for attaching l-Rha residues to the different d-Glc residues in the outer core OS in P. aeruginosa and could play an important role in regulating the relative amounts of “uncapped core” and “capped core” glycoforms, respectively, on the cell surface.
In the case of P. gingivalis, the longer core OS to which a single repeating unit of O-PS is attached in OS1142 could be the “capped core” generated by addition of extra α-(1→3)-linked Man residues to the shorter “uncapped core” by a mannosyltransferase, or the “uncapped core” could be generated from the “capped core” by the action of α-1,3-mannosidase. Genes encoding a putative α-mannosyltransferase (PG0129) and an α-1,3 mannosidase (PG1711) are both present in the genome of P. gingivalis W83 and may be involved in these conversions. These findings will be the basis of future investigations. Furthermore, ongoing studies in our laboratory to generate P. gingivalis mutant strains which synthesize only S-type A-LPS or SR-type A-LPS should enable us to identify the site of attachment of A-PS repeating units to the core OS.
Supplementary Material
Acknowledgments
This investigation was supported by Medical Research Council (UK) grant G0501478 and by Research Advisory Board of the Barts and The London Charity grant RAB06/PJ/14.
Footnotes
Published ahead of print on 12 June 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Abeyrathne, P. D., C. Daniels, K. K. Poon, M. J. Matewish, and J. S. Lam. 2005. Functional characterization of WaaL, a ligase associated with linking O-antigen polysaccharide to the core of Pseudomonas aeruginosa lipopolysaccharide. J. Bacteriol. 187:3002-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aduse-Opoku, J., J. Muir, J. M. Slaney, M. Rangarajan, and M. A. Curtis. 1995. Characterization, genetic analysis, and expression of a protease antigen (PrpRI) of Porphyromonas gingivalis W50. Infect. Immun. 63:4744-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aduse-Opoku, J., J. M. Slaney, A. Hashim, A. Gallagher, R. P. Gallagher, M. Rangarajan, K. Boutaga, M. L. Laine, A. J. Van Winkelhoff, and M. A. Curtis. 2006. Identification and characterization of the capsular polysaccharide (K-antigen) locus of Porphyromonas gingivalis. Infect. Immun. 74:449-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Qutub, M. N., P. H. Braham, L. M. Karimi-Naser, X. Liu, C. A. Genco, and R. P. Darveau. 2006. Hemin-dependent modulation of the lipid A structure of Porphyromonas gingivalis lipopolysaccharide. Infect. Immun. 74:4474-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altman, E., M. B. Perry, and J. R. Brisson. 1989. Structure of the lipopolysaccharide antigenic O-chain produced by Actinobacillus pleuropneumoniae serotype 4 (ATCC 33378). Carbohydr. Res. 191:295-303. [DOI] [PubMed] [Google Scholar]
- 6.Bainbridge, B. W., L. Karimi-Naser, R. Reife, F. Blethen, R. K. Ernst, and R. P. Darveau. 2008. Acyl chain specificity of the acyltransferases LpxA and LpxD and substrate availability contribute to lipid A fatty acid heterogeneity in Porphyromonas gingivalis. J. Bacteriol. 190:4549-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bax, A., and D. G. Davis. 1985. Mlev-17-based two-dimensional homonuclear magnetization transfer spectroscopy. J. Magn. Reson. 65:355-360. [Google Scholar]
- 8.Bax, A., and D. G. Davis. 1985. Practical aspects of two-dimensional transverse NOE spectroscopy. J. Magn. Reson. 63:207-213. [Google Scholar]
- 9.Bax, A., and M. F. Summers. 1986. H-1 and C-13 assignments from sensitivity-enhanced detection of heteronuclear multiple-bond connectivity by 2D multiple quantum NMR. J. Am. Chem. Soc. 108:2093-2094. [Google Scholar]
- 10.Bock, K., and C. Pedersen. 1974. Study of C-13H coupling-constants in hexopyranoses. J. Chem. Soc. Perkin Trans. 2:293-299. [Google Scholar]
- 11.Bock, K., C. Pedersen, and H. Pedersen. 1984. C-13 nuclear magnetic-resonance data for oligosaccharides. Adv. Carbohydr. Chem. Biochem. 42:193-225. [Google Scholar]
- 12.Bodenhausen, G., and D. J. Ruben. 1980. Natural abundance N-15 NMR by enhanced heteronuclear spectroscopy. Chem. Phys. Lett. 69:185-189. [Google Scholar]
- 13.Bury, C. L. 2004. Analysis of multiple product formation from a single gene from the anaerobic bacterium Porphyromonas gingivalis. Ph.D. thesis. University of London, London, United Kingdom.
- 14.Curtis, M. A., A. Thickett, J. M. Slaney, M. Rangarajan, J. Aduse-Opoku, P. Shepherd, N. Paramonov, and E. F. Hounsell. 1999. Variable carbohydrate modifications to the catalytic chains of the RgpA and RgpB proteases of Porphyromonas gingivalis W50. Infect. Immun. 67:3816-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darveau, R. P., and R. E. Hancock. 1983. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J. Bacteriol. 155:831-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Castro, C., A. Molinaro, R. Nunziata, R. Lanzetta, M. Parrilli, and O. Holst. 2004. A novel core region, lacking heptose and phosphate, of the lipopolysaccharide from the gram-negative bacterium Pseudomonas cichorii (Pseudomonadaceae RNA group 1). Eur. J. Org. Chem. 2004:2427-2435. [Google Scholar]
- 17.Fletcher, H. M., H. A. Schenkein, R. M. Morgan, K. A. Bailey, C. R. Berry, and F. L. Macrina. 1995. Virulence of a Porphyromonas gingivalis W83 mutant defective in the prtH gene. Infect. Immun. 63:1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsberg, L. S., and R. W. Carlson. 1998. The structures of the lipopolysaccharides from Rhizobium etli strains CE358 and CE359. The complete structure of the core region of R. etli lipopolysaccharides. J. Biol. Chem. 273:2747-2757. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher, A., J. Aduse-Opoku, M. Rangarajan, J. M. Slaney, and M. A. Curtis. 2003. Glycosylation of the Arg-gingipains of Porphyromonas gingivalis and comparison with glycoconjugate structure and synthesis in other bacteria. Curr. Protein Pept. Sci. 4:427-441. [DOI] [PubMed] [Google Scholar]
- 20.Gerwig, G. J., J. P. Kamerling, and J. F. G. Vliegenthart. 1978. Determination of d and l configuration of neutral monosaccharides by high-resolution capillary GLC. Carbohydr. Res. 62:349-357. [Google Scholar]
- 21.Haake, S. K., S. C. Yoder, G. Attarian, and K. Podkaminer. 2000. Native plasmids of Fusobacterium nucleatum: characterization and use in development of genetic systems. J. Bacteriol. 182:1176-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helander, I. M., B. Lindner, H. Brade, K. Altmann, A. A. Lindberg, E. T. Rietschel, and U. Zahringer. 1988. Chemical structure of the lipopolysaccharide of Haemophilus influenzae strain I-69 Rd−/b+. Description of a novel deep-rough chemotype. Eur. J. Biochem. 177:483-492. [DOI] [PubMed] [Google Scholar]
- 23.Holst, O. 1999. Chemical structure of the core region of lipopolysaccharides, p. 115-154. In H. Brade, S. M. Opal, S. N. Vogel, and D. C. Morrison (ed.), Endotoxin in health and disease. Marcel Dekker, New York, NY.
- 24.Holst, O., and H. Brade. 1992. Chemical structure of the core region of lipopolysaccharide, p. 135-170. In D. C. Morrison and J. L. Ryan (ed.), Bacterial endotoxic lipopolysaccharide, vol. 1. Molecular biochemistry and cellular biology. CRC Press, Boca Raton, FL. [Google Scholar]
- 25.Jansson, P. E., L. Kenne, and G. Widmalm. 1989. Computer-assisted structural analysis of polysaccharides with an extended version of CASPER using 1H- and 13C-NMR data. Carbohydr. Res. 188:169-191. [DOI] [PubMed] [Google Scholar]
- 26.Kakehi, K., and S. Honda. 1989. Analysis of carbohydrates by GLC and MS. CRC Press, Boca Raton, FL.
- 27.Kogan, G., D. Uhrin, J. R. Brisson, and H. J. Jennings. 1997. Structural basis of the Neisseria meningitidis immunotypes including the L4 and L7 immunotypes. Carbohydr. Res. 298:191-199. [DOI] [PubMed] [Google Scholar]
- 28.Kooistra, O., G. Bedoux, L. Brecker, B. Lindner, C. P. Sanchez, D. Haras, and U. Zahringer. 2003. Structure of a highly phosphorylated lipopolysaccharide core in the ΔalgC mutants derived from Pseudomonas aeruginosa wild-type strains PAO1 (serogroup O5) and PAC1R (serogroup O3). Carbohydr. Res. 338:2667-2677. [DOI] [PubMed] [Google Scholar]
- 29.Kover, K. E., V. J. Hruby, and D. Uhrin. 1997. Sensitivity- and gradient-enhanced heteronuclear coupled/decoupled HSQC-TOCSY experiments for measuring long-range heteronuclear coupling constants. J. Magn. Reson. 129:125-129. [DOI] [PubMed] [Google Scholar]
- 30.Kumada, H., S. Kondo, T. Umemoto, and K. Hisatsune. 1993. Chemical structure of the 2-keto-3-deoxyoctonate region of lipopolysaccharide isolated from Porphyromonas (Bacteroides) gingivalis. FEMS Microbiol. Lett. 108:75-79. [DOI] [PubMed] [Google Scholar]
- 31.Kvernheim, A. L. 1987. Methylation analysis of polysaccharides with butyllithium in dimethylsulfoxide. Acta Chem. Scand. Ser. B Org. Chem. Biochem. 41:150-152. [Google Scholar]
- 32.Lamothe, G. T., L. Jolly, B. Mollet, and F. Stingele. 2002. Genetic and biochemical characterization of exopolysaccharide biosynthesis by Lactobacillus delbrueckii subsp. bulgaricus. Arch. Microbiol. 178:218-228. [DOI] [PubMed] [Google Scholar]
- 33.Lipkind, G. M., A. S. Shashkov, Y. A. Knirel, E. V. Vinogradov, and N. K. Kochetkov. 1988. A computer-assisted structural analysis of regular polysaccharides on the basis of 13C-NMR data. Carbohydr. Res. 175:59-75. [DOI] [PubMed] [Google Scholar]
- 34.Lipkind, G. M., A. S. Shashkov, S. S. Mamyan, and N. K. Kochetkov. 1988. The nuclear Overhauser effect and structural factors determining the conformations of disaccharide glycosides. Carbohydr. Res. 181:1-12. [Google Scholar]
- 35.Lundstrom, S. L., J. Li, M. E. Deadman, D. W. Hood, E. R. Moxon, and E. K. Schweda. 2008. Structural analysis of the lipopolysaccharide from nontypeable Haemophilus influenzae strain R2846. Biochemistry 47:6025-6038. [DOI] [PubMed] [Google Scholar]
- 36.McGroarty, E. J. 1990. Biophysical aspects and function of endotoxin, p. 85-94. In A. Nowortny, J. J. Spitzer, and E. J. Ziegler (ed.), Cellular and molecular aspects of endotoxin reactions. Excerpta Medica, Amsterdam, The Netherlands.
- 37.McNicholas, P. A., M. Batley, and J. W. Redmond. 1987. The reactions of 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) in dilute acid. Carbohydr. Res. 165:17-22. [DOI] [PubMed] [Google Scholar]
- 38.Meredith, T. C., U. Mamat, Z. Kaczynski, B. Lindner, O. Holst, and R. W. Woodard. 2007. Modification of lipopolysaccharide with colanic acid (M-antigen) repeats in Escherichia coli. J. Biol. Chem. 282:7790-7798. [DOI] [PubMed] [Google Scholar]
- 39.Muller-Loennies, S., L. Brade, and H. Brade. 2002. Chemical structure and immunoreactivity of the lipopolysaccharide of the deep rough mutant I-69 Rd−/b+ of Haemophilus influenzae. Eur. J. Biochem. 269:1237-1242. [DOI] [PubMed] [Google Scholar]
- 40.Muller-Loennies, S., B. Lindner, and H. Brade. 2003. Structural analysis of oligosaccharides from lipopolysaccharide (LPS) of Escherichia coli K12 strain W3100 reveals a link between inner and outer core LPS biosynthesis. J. Biol. Chem. 278:34090-34101. [DOI] [PubMed] [Google Scholar]
- 41.Nakhamchik, A., C. Wilde, and D. A. Rowe-Magnus. 2007. Identification of a Wzy polymerase required for group IV capsular polysaccharide and lipopolysaccharide biosynthesis in Vibrio vulnificus. Infect. Immun. 75:5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson, K. E., R. D. Fleischmann, R. T. DeBoy, I. T. Paulsen, D. E. Fouts, J. A. Eisen, S. C. Daugherty, R. J. Dodson, A. S. Durkin, M. Gwinn, D. H. Haft, J. F. Kolonay, W. C. Nelson, T. Mason, L. Tallon, J. Gray, D. Granger, H. Tettelin, H. Dong, J. L. Galvin, M. J. Duncan, F. E. Dewhirst, and C. M. Fraser. 2003. Complete genome sequence of the oral pathogenic bacterium Porphyromonas gingivalis strain W83. J. Bacteriol. 185:5591-5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paramonov, N., D. Bailey, M. Rangarajan, A. Hashim, G. Kelly, M. A. Curtis, and E. F. Hounsell. 2001. Structural analysis of the polysaccharide from the lipopolysaccharide of Porphyromonas gingivalis strain W50. Eur. J. Biochem. 268:4698-4707. [DOI] [PubMed] [Google Scholar]
- 44.Paramonov, N., M. Rangarajan, A. Hashim, A. Gallagher, J. Aduse-Opoku, J. M. Slaney, E. Hounsell, and M. A. Curtis. 2005. Structural analysis of a novel anionic polysaccharide from Porphyromonas gingivalis strain W50 related to Arg-gingipain glycans. Mol. Microbiol. 58:847-863. [DOI] [PubMed] [Google Scholar]
- 45.Parolis, L. A., J. O. Duus, H. Parolis, M. Meldal, and K. Bock. 1996. The extracellular polysaccharide of Pichia (Hansenula) holstii NRRL Y-2448: the structure of the phosphomannan backbone. Carbohydr. Res. 293:101-117. [DOI] [PubMed] [Google Scholar]
- 46.Piantini, U., O. W. Sorensen, and R. R. Ernst. 1982. Multiple quantum filters for elucidating NMR coupling networks. J. Am. Chem. Soc. 104:6800-6801. [Google Scholar]
- 47.Poon, K. K., E. L. Westman, E. Vinogradov, S. Jin, and J. S. Lam. 2008. Functional characterization of MigA and WapR: putative rhamnosyltransferases involved in outer core oligosaccharide biosynthesis of Pseudomonas aeruginosa. J. Bacteriol. 190:1857-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raetz, C. R. 1990. Biochemistry of endotoxins. Annu. Rev. Biochem. 59:129-170. [DOI] [PubMed] [Google Scholar]
- 49.Rangarajan, M., J. Aduse-Opoku, N. Paramonov, A. Hashim, N. Bostanci, O. P. Fraser, E. Tarelli, and M. A. Curtis. 2008. Identification of a second lipopolysaccharide in Porphyromonas gingivalis W50. J. Bacteriol. 190:2920-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rangarajan, M., J. Aduse-Opoku, J. M. Slaney, K. A. Young, and M. A. Curtis. 1997. The prpR1 and prR2 arginine-specific protease genes of Porphyromonas gingivalis W50 produce five biochemically distinct enzymes. Mol. Microbiol. 23:955-965. [DOI] [PubMed] [Google Scholar]
- 51.Reife, R. A., S. R. Coats, M. Al-Qutub, D. M. Dixon, P. A. Braham, R. J. Billharz, W. N. Howald, and R. P. Darveau. 2006. Porphyromonas gingivalis lipopolysaccharide lipid A heterogeneity: differential activities of tetra- and penta-acylated lipid A structures on E-selectin expression and TLR4 recognition. Cell. Microbiol. 8:857-868. [DOI] [PubMed] [Google Scholar]
- 52.Sadovskaya, I., J. R. Brisson, J. S. Lam, J. C. Richards, and E. Altman. 1998. Structural elucidation of the lipopolysaccharide core regions of the wild-type strain PAO1 and O-chain-deficient mutant strains AK1401 and AK1012 from Pseudomonas aeruginosa serotype O5. Eur. J. Biochem. 255:673-684. [DOI] [PubMed] [Google Scholar]
- 53.Sadovskaya, I., J. R. Brisson, P. Thibault, J. C. Richards, J. S. Lam, and E. Altman. 2000. Structural characterization of the outer core and the O-chain linkage region of lipopolysaccharide from Pseudomonas aeruginosa serotype O5. Eur. J. Biochem. 267:1640-1650. [DOI] [PubMed] [Google Scholar]
- 54.Sakuda, S., Z. Y. Zhou, H. Takao, and Y. Yamada. 1996. Mechanism of the cyclopentane ring formation of allosamizoline, an aminocyclitol derivative of the chitinase inhibitor allosamidin. Tetrahedron Lett. 37:5711-5714. [DOI] [PubMed] [Google Scholar]
- 55.Severn, W. B., R. F. Kelly, J. C. Richards, and C. Whitfield. 1996. Structure of the core oligosaccharide in the serotype O8 lipopolysaccharide from Klebsiella pneumoniae. J. Bacteriol. 178:1731-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheets, S. M., A. G. Robles-Price, R. M. McKenzie, C. A. Casiano, and H. M. Fletcher. 2008. Gingipain-dependent interactions with the host are important for survival of Porphyromonas gingivalis. Front. Biosci. 13:3215-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sims, I. M., D. J. Craik, and A. Bacic. 1997. Structural characterisation of galactoglucomannan secreted by suspension-cultured cells of Nicotiana plumbaginifolia. Carbohydr. Res. 303:79-92. [DOI] [PubMed] [Google Scholar]
- 58.Slaney, J. M., M. Rangarajan, J. Aduse-Opoku, S. Fawell, I. Darby, D. Kinane, and M. A. Curtis. 2002. Recognition of the carbohydrate modifications to the RgpA protease of Porphyromonas gingivalis by periodontal patient serum IgG. J. Periodontal Res. 37:215-222. [DOI] [PubMed] [Google Scholar]
- 59.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure, p. 83-91. In R. L. Whistler (ed.), Methods in carbohydrate chemistry. Academic Press, New York, NY.
- 60.Whitfield, C., and K. Larue. 2008. Stop and go: regulation of chain length in the biosynthesis of bacterial polysaccharides. Nat. Struct. Mol. Biol. 15:121-123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.