Abstract
Negative feedback regulation, mediated through repressor binding site O3, which overlaps the lacI gene, could explain the robustness of the weak expression of Lac repressor. Significant autorepression of Lac repressor has never been ruled out. In the work presented here, the degree of autoregulation of Lac repressor was determined. It is negligible.
The lac operon is one of the classical model systems for transcriptional regulation (9). Expression of the tricistronic lac mRNA is negatively controlled (repressed) by the operon-specific Lac repressor (LacR) and positively controlled (activated) by the global regulator CAP (17). In recent years, the lac operon has become a focus and testing ground for systems biology analyses (19). Such modeling can only deliver meaningful results when it incorporates all relevant features of the system. The textbook picture of the lac operon has sustained substantial change over the years (9), and despite the wealth of information, many questions are still unanswered.
One of the traditional views that have become questionable holds that the lacI gene, encoding LacR, is constitutively expressed at low levels (in wild-type [wt] cells, LacR is present at about 10 tetramers per genome [4]). It was established in the 1960s that Lac repressor expression is not coordinately controlled with the three genes of the lac operon (5). This, however, did not exclude autoregulation of LacR altogether. Indirect methods suggested that LacR autoregulation cannot be large. Gilbert and Müller-Hill did not find an obvious difference between the yields of LacR purified from uninduced and induced cultures. Their recoveries, however, were not reproducible enough to detect a small severalfold difference (4). An in vivo experiment with an Escherichia coli strain that is temperature-sensitive for the production of LacR actually indicated a twofold inducibility of LacR expression, but the mutation used was not well enough characterized to allow conclusive interpretation of the data (11). The expression of the lacI gene is low but robust: there appears to be little stochastic fluctuation of LacR (12). Feedback regulation is a mechanism that would suppress such fluctuations (7). It was found that the third lac operator, O3, which lies upstream of the lac promoter and overlaps with the last nucleotides of the lacI gene, is not a pseudooperator (15, 16). It contributes through DNA loop formation to repression at the first lac operator, O1 (Fig. 1). It was later reported that transcription of lacI stalls at an occupied O3 and that the incompletely translated protein is tagged for degradation through the tmRNA pathway (1). LacR expression thus seemed subject to negative feedback regulation, the extent of which could not, however, be determined from these data. This circumstantial evidence was so convincing that it has been stated as a fact that LacR is autorepressed (1).
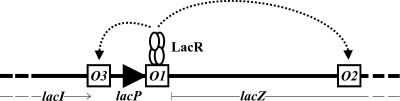
FIG. 1.
The organization of the lac operators. Not drawn to scale. The first lac operator, O1, lies immediately downstream of the lac promoter, lacP, and is the site of lac operon repression through LacR. Tetrameric LacR bound to O1 also binds to either O2, in the coding region of lacZ, or O3, upstream of the lac promoter, leading to the formation of DNA loops (indicated by dotted arrows). The open reading frames (ORFs) of the lacI and lacZ genes are indicated by the thin lines below. The end of the lacI ORF is marked with an arrowhead, and the beginning of the lacZ ORF is marked with a short vertical line. Part of O3 overlaps with the last bases of lacI, which codes for LacR.
Even an induction of two- or threefold, small compared to that of the lac promoter, would invalidate conclusions drawn from induction studies and quantitative modeling ignorant of it. To obtain data sufficiently accurate to detect an effect in this range, a direct assay of LacR levels in cell extracts seemed most appropriate. The traditional method for LacR determination is equilibrium dialysis with radioactive inducer (2, 4). Because inducer binding is relatively weak—the equilibrium dissociation constant (Kd) is ∼7 μM for isopropyl-β-d-thiogalactopyranoside (IPTG) (13)—and repressor concentration in wt cells is low (∼10 nM), it can only be used for strains overexpressing LacR or for preparations enriched in repressor (4, 10). On the other hand, the affinity of repressor to operator is high (Kd of ∼10 pM in vitro for symmetric “ideal” lac operator) (13, 18, 20), which suggests stoichiometric titration of lac operator as an alternative approach.
Autorepression was measured by comparing LacR levels of bacteria growing under repressed (LacR binds to its operators) versus induced (the operators are free of LacR) conditions, analogous to repression measurements of β-galactosidase. Three derivatives of lac deletion strain BMH8117 (3), carrying different episomes, were used: (i) the episome from CSH23 (8), containing the wt lac operon; (ii) an episome containing a lac operon with an inactivated O2 (3); and (iii) the episome from CSH37 (8), containing a lac operon with an Oc (severely impaired O1, leading to constitutive lac expression). While the first episome reflects the wt situation, the second will exhibit stronger loop formation between O1 and O3, since O2 no longer competes with O3 for interaction with O1 (Fig. 1), and the third has reduced occupancy of O3 through reduced loop formation.
Cultures of the three strains were grown at 37°C in minimal medium A (8) with 0.4% glycerol, from which two subcultures were inoculated, one without and one with 0.2 mM IPTG. Cells were harvested at an optical density at 600 nm of 0.8 to 1.0. Aliquots were used for determining specific β-galactosidase activities (8), and the rest for the preparation of crude extracts, as follows. Pellets from 25 ml of culture were resuspended in 400 μl BB+ (10 mM Tris-HCl, pH 7.2, 3 mM Mg-acetate, 0.1 mM EDTA, 3% dimethyl sulfoxide, 0.1 mM dithiothreitol) and sonicated, insoluble material removed by centrifugation, and the supernatants dialyzed four times for 2 h against a 100-fold excess of BB+; all steps were performed at 4°C or on ice. Protein concentrations were determined with the method of Warburg and Christian (21) after Layne (6).
Because of the low abundance of LacR, the total protein concentration in the binding reaction mixtures had to be higher than usual (14). Therefore, the linear range of operator binding by LacR as a function of protein concentration was determined first. An extract of BMH8117 carrying the wt lac episome and a radiolabeled 257-bp DNA fragment containing the “ideal” lac operator (13) were used. All binding reactions were done on ice for 15 min in volumes of 10 or 20 μl BB+ with 1 nM of the DNA fragment. The products of the binding reaction were assayed with band shift assays as described previously (13). Figure 2A shows the quantitative evaluation. Percent bound operator is plotted against protein concentration. Binding is initially linear and starts to be inhibited at protein concentrations above 1 mg/ml. Figure 2B shows the linear portion of the binding curve with the corresponding linear regression line. The correlation coefficient is 0.996. LacR quantitations were performed in this range (0.33 to 0.55 mg/ml).
FIG. 2.
(A) Operator binding by Lac repressor as a function of the concentration of total soluble protein. A crude extract of strain StAAa1 (lac deletion strain BMH8117 carrying an episome containing the wt lac operon) was used. (B) The initial, linear portion of the binding curve with the corresponding linear regression line.
Table 1 gives the repression values (the quotient of specific activity in the presence of IPTG [induced] and specific activity in the absence of IPTG [repressed]) of β-galactosidase, as well as the analogous repression values of LacR.
TABLE 1.
Repression values of β-galactosidase and Lac repressora
| Strain | Pertinent genotype | Repression of β-galactosidaseb | Repression of Lac repressorc |
|---|---|---|---|
| StAAa1 | Δlac F′ lac+ | 1,940 (60) | 1.06 (0.09) |
| StAAa2 | Δlac F′ lacO2 | 450 (8) | 1.17 (0.10) |
| StAAa3 | Δlac F′ lacOc | 2.7 (0.1) | 0.88 (0.14) |
All repression values are the means(± standard errors) of six determinations.
Repression is defined as specific activity of cells grown in the presence of 0.2 mM IPTG divided by specific activity of cells grown in the absence of IPTG.
Repression is defined as fmole LacR/μg soluble protein of cells grown in the presence of 0.2 mM IPTG divided by fmole LacR/μg soluble protein of cells grown in the absence of IPTG. A repression value of 1 indicates no repression.
Repression of the episomal wt lac operon is close to 2,000-fold. Inactivation of O2 leads to the expected three- to fivefold drop in repression (3, 16), and the Oc mutation nearly abolishes repression. LacR expression itself is, however, not detectably repressed in any of the three strains. While the tmRNA pathway prevents the accumulation of truncated LacR in the cell (1), its effect on LacR expression is apparently small. Autorepression of LacR expression is at most about 10%. It thus appears that the classical picture prevails. There seems to be no biologically meaningful autoregulation of LacR. If there is a mechanism that ensures robust expression of LacR, it has to be sought elsewhere.
Acknowledgments
I thank Benno Müller-Hill for providing me with the lacO2 episome and Alexandros Kiupakis for critically reading the manuscript.
Footnotes
Published ahead of print on 5 June 2009.
REFERENCES
- 1.Abo, T., T. Inada, K. Ogawa, and H. Aiba. 2000. SsrA-mediated tagging and proteolysis of LacI and its role in the regulation of lac operon. EMBO J. 19:3762-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkley, M. D., A. D. Riggs, A. Jobe, and S. Burgeois. 1975. Interaction of effecting ligands with lac repressor and repressor-operator complex. Biochemistry 14:1700-1712. [DOI] [PubMed] [Google Scholar]
- 3.Eismann, E., B. von Wilcken-Bergmann, and B. Müller-Hill. 1987. Specific destruction of the second lac operator decreases repression of the lac operon in Escherichia coli fivefold. J. Mol. Biol. 195:949-952. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert, W., and B. Müller-Hill. 1966. Isolation of the Lac repressor. Proc. Natl. Acad. Sci. USA 56:1891-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacob, F., and J. Monod. 1961. Genetic regulatory mechanisms in the synthesis of proteins. J. Mol. Biol. 3:318-356. [DOI] [PubMed] [Google Scholar]
- 6.Layne, E. 1957. Spectrophotometric and turbidimetric methods for measuring proteins. Methods Enzymol. 3:117-454. [Google Scholar]
- 7.Longo, D., and J. Hasty. 2006. Dynamics of single-cell gene expression. Mol. Syst. Biol. 2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 9.Müller-Hill, B. 1996. The lac operon: a short history of a genetic paradigm. Walter de Gruyter, Berlin, Germany.
- 10.Müller-Hill, B., L. Crapo, and W. Gilbert. 1968. Mutants that make more lac repressor. Proc. Natl. Acad. Sci. USA 59:1259-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novick, A., J. M. McCoy, and J. R. Sadler. 1965. The Non-inducibility of repressor formation. J. Mol. Biol. 12:328-330. [DOI] [PubMed] [Google Scholar]
- 12.Novick, A., and M. Weiner. 1957. Enzyme induction as an all-or-none phenomenon. Proc. Natl. Acad. Sci. USA 43:553-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oehler, S., S. Alberti, and B. Müller-Hill. 2006. Induction of the lac promoter in the absence of DNA loops and the stoichiometry of induction. Nucleic Acids Res. 34:606-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oehler, S., R. Alex, and A. Barker. 1999. Is nitrocellulose filter binding really a universal assay for protein-DNA interactions? Anal. Biochem. 268:330-336. [DOI] [PubMed] [Google Scholar]
- 15.Oehler, S., M. Amouyal, P. Kolkhof, B. von Wilcken-Bergmann, and B. Müller-Hill. 1994. Quality and position of the three lac operators of E. coli define efficiency of repression. EMBO J. 13:3348-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oehler, S., E. R. Eismann, H. Kramer, and B. Müller-Hill. 1990. The three operators of the lac operon cooperate in repression. EMBO J. 9:973-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reznikoff, W. S. 1992. The lactose operon-controlling elements: a complex paradigm. Mol. Microbiol. 6:2419-2422. [DOI] [PubMed] [Google Scholar]
- 18.Sadler, J. R., H. Sasmor, and J. L. Betz. 1983. A perfectly symmetric lac operator binds the lac repressor very tightly. Proc. Natl. Acad. Sci. USA 80:6785-6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santillan, M., and M. C. Mackey. 2008. Quantitative approaches to the study of bistability in the lac operon of Escherichia coli. J. R. Soc. Interface 5(Suppl. 1):S29-S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simons, A., D. Tils, B. von Wilcken-Bergmann, and B. Müller-Hill. 1984. Possible ideal lac operator: Escherichia coli lac operator-like sequences from eukaryotic genomes lack the central G X C pair. Proc. Natl. Acad. Sci. USA 81:1624-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warburg, O., and W. Christian. 1942. Isolierung und Kristallisation des Gärungsfermentes Enolase. Biochemische Zeitschrift 310:384-421. [Google Scholar]