Abstract
Regulatory small RNAs (sRNAs) in bacterial genomes have become a focus of research over the past 8 years. Whereas more than 100 such sRNAs have been found in Escherichia coli, relatively little is known about sRNAs in gram-positive bacteria. Using a computational approach, we identified two sRNAs in intergenic regions of the Bacillus subtilis genome, SR1 and SR2 (renamed BsrF). Recently, we demonstrated that SR1 inhibits the translation initiation of the transcriptional activator AhrC. Here, we describe detection of BsrF, its expression profile, and its regulation by CodY. Furthermore, we mapped the secondary structure of BsrF. BsrF is expressed in complex and minimal media in all growth phases in B. subtilis and, with a similar expression profile, also in Bacillus amyloliquefaciens. Neither overexpression nor deletion of bsrF affected the growth of B. subtilis. BsrF was found to be long-lived in complex and minimal media. Analysis of 13 putative transcription factor binding sites upstream of bsrF revealed only an effect for CodY. Here, we showed by using Northern blotting, lacZ reporter gene fusions, in vitro transcription, and DNase I footprinting that the transcription of bsrF is activated by CodY in the presence of branched-chain amino acids and GTP. Furthermore, BsrF transcription was increased 1.5- to 2-fold by glucose in the presence of branched-chain amino acids, and this increase was independent of the known glucose-dependent regulators. BsrF is the second target for which transcriptional activation by CodY has been discovered.
Bacterial small RNAs (sRNAs) play important roles as stress regulators in metabolic processes (for a review, see reference 42) or virulence gene regulators (for a review, see reference 45). Over the past 8 years, more than 100 sRNAs have been discovered in Escherichia coli, and it is estimated that 200 to 300 such RNAs are present in an average bacterial genome (19). We distinguish base-pairing sRNAs (antisense RNAs) and sRNAs that act by protein binding (for a review, see reference 5). Many trans-encoded antisense RNAs have multiple targets. The majority of them block translation initiation, whereas others affect the stability of their target mRNAs (for a review, see reference 5).
Meanwhile, a few systematic searches have been performed for gram-positive bacteria. In Bacillus subtilis, 24 sRNAs are known (1, 24, 35, 39, 40), and targets for 5 of them have been identified; RatA controls the toxin TxpA (39), SR1 controls the transcription activator AhrC (17) (see below), FsrA regulates sdhCAB, citB, yvfW, and leuCD (11), and BsrA and BsrB are two 6S RNAs (1). In Staphylococcus aureus, in addition to RNAIII (3), 12 novel sRNAs from pathogenicity islands have been detected (33). Furthermore, in Listeria monocytogenes, three Hfq-binding sRNAs with unknown functions (8) and nine novel sRNAs in intergenic regions have been identified (27). In 2007, five sRNAs controlled by the response regulator CiaR were found in Streptococcus pneumoniae (14). However, identification of mRNA targets of the previously discovered bacterial sRNAs is rather difficult and has been successful in only a few cases. Recently, all currently known chromosome-encoded sRNAs for which targets have been identified were summarized (5).
The majority of sRNAs from E. coli bind the abundant RNA chaperone Hfq (47, 49). Thus, Hfq is required for stabilization of the sRNAs and/or to promote complex formation with the target RNAs. However, for sRNAs from gram-positive bacteria, the putative function of Hfq is still elusive (5).
Recently, we have demonstrated that SR1, which was found by using a computational approach and subsequent Northern blotting (24), acts by base pairing with its primary target, ahrC mRNA encoding the transcriptional activator of the rocABC and rocDEF arginine catabolic operons (17). Seven complementary regions between SR1 and ahrC were identified, and the most 5′ region, region G, is located 97 nucleotides (nt) downstream from the ahrC ribosome binding site. SR1 inhibits translation initiation of ahrC mRNA by a novel mechanism: induction of structural changes downstream from the ribosome binding site (18). Interestingly, SR1 is expressed under gluconeogenic conditions and repressed under glycolytic conditions, and the repression is mediated mainly by CcpN and, to a minor extent, by CcpA (24, 25).
CodY was first identified as a repressor of the B. subtilis dipeptide permease (dppABCDE) operon and turned out to be a critical regulator in the control of the expression of stationary-phase genes and the initiation of sporulation. Meanwhile, it has been found that CodY regulates more than 100 genes in B. subtilis, the products of which are involved in adaptation to poor growth conditions (for a review, see reference 41). CodY homologues have been found to be encoded in almost all low-G+C-content gram-positive bacteria, including six Bacillus species and eight Streptococcus species, and in some cases they have been purified (41). CodY appears to play the global role in B. subtilis and its relatives that Lrp plays in gram-negative bacteria, but it has an even broader role as it regulates anabolic, catabolic, differentiation, and virulence pathways. In the majority of cases, CodY acts as a transcriptional repressor in the logarithmic growth phase. It has been demonstrated that B. subtilis CodY binds two ligands, GTP (34) and branched-chain amino acids (BCAA) (37), which, to different extents, increase the repression activity of CodY. So far, the B. subtilis acetate kinase (ackA) gene is the only known gene for which CodY (in concert with CcpA) acts as a transcriptional activator (38).
Here, we report the properties of a novel sRNA, SR2 (30), in B. subtilis. SR2 was found by our group by using a combination of computational search and Northern blotting that previously also identified SR1. Recently, SR2 was also found using an independent approach by Saito et al. and was designated BsrF (35). Therefore, we use the designation BsrF below. BsrF is expressed in complex and minimal media from early logarithmic to late stationary growth, but the expression decreases when cells start to sporulate. Neither overexpression nor deletion of bsrF affected the growth of B. subtilis. Interestingly, the bsrF gene is also expressed in Bacillus amyloliquefaciens. Half-life measurements showed that BsrF is very stable. Secondary structure probing of BsrF identified a branched stem-loop structure and the transcription terminator. Of 13 transcription factors for which hypothetical binding sites were found upstream of bsrF, only CodY proved to have an effect. We demonstrate here that in the presence of BCAA, CodY activates bsrF transcription by binding 329 bp upstream of the bsrF transcription start site. Consequently, bsrF is the second gene for which an activating effect of CodY has been found. Furthermore, in the presence of BCAA, glucose activated BsrF transcription 1.5- to 2-fold, and this activation was independent of CcpA, CcpN, CcpC, GlcR, and CggR.
MATERIALS AND METHODS
Enzymes and chemicals.
The chemicals used were the highest purity available. Taq DNA polymerase was purchased from Roche, and Firepol polymerase was purchased from Solis Biodyne, Estonia. DNase I was purchased from Ambion, and the Thermoscript reverse transcription system was purchased from Invitrogen.
Strains, media, and growth conditions.
E. coli strain DH5α, B. subtilis strains DB104 (21), PS251 codY::(erm::spc) trpC2 (2), and QB5407 (ccpA::spc) (10), and B. amyloliquefaciens FZB42 were used. TY medium was used as a complex medium for E. coli and Bacillus (17). Spizizen and CSE media were used as minimal media for B. subtilis (17). SP medium (8 g nutrient broth, 0.25 g MgSO4 · 7 H2O, 1 g KCl, 1 ml CaCl2 [0.5 M], 1 ml MnCl2 [10 mM], and 2 ml ammonium iron citrate [2.2 mg/ml] dissolved in 1 liter) was used as the sporulation medium.
Isolation of chromosomal DNA from B. subtilis.
Chromosomal DNA was isolated from B. subtilis strains as described previously (24).
Primer extension.
Primer extension experiments were carried out as described previously (24) using total RNA from B. subtilis strain DB104 and 5′-labeled oligodeoxyribonucleotide SB847.
Construction of plasmids for bsrF knockout and overexpression strains.
To obtain a bsrF knockout strain, plasmid pINT5 was constructed in the following way. First, DNA fragments upstream and downstream from the bsrF gene were amplified by PCR using chromosomal DNA of B. subtilis DB104 and oligodeoxyribonucleotides SB813 (see Table S1 in the supplemental material) and SB814, as well as oligodeoxyribonucleotides SB815 and SB816, and, after digestion with the appropriate restriction enzymes, were inserted as BamHI/EcoRI or SalGI/PstI fragments into the corresponding pUC19 vectors cleaved with the same pair of enzymes. This approach resulted in pFRONT5 and pBACK5. Next, plasmid pCBACK5 was constructed by jointly cloning the CAT-EcoRI/SalI fragment of vector pCAT (24) and the BACK-SalGI/PstI fragment of vector pBACK5 into the pUC19 EcoRI/PstI vector. Subsequently, the EcoRI/PstI fragment of pCBACK was jointly cloned with the BamHI/EcoRI fragment of pFRONT5 into the pUC19 BamHI/PstI vector, resulting in integration vector pINT5. All fragments were confirmed by sequencing.
Plasmid pWSR2, which was used for inducible overexpression of bsrF in B. subtilis, was constructed as follows. The promoterless bsrF gene was amplified with oligodeoxyribonucleotides SB1063 and SB1064 using chromosomal DNA of B. subtilis DB104 as the template, digested with HindIII, and inserted into the pUC19 HindIII-vector, resulting in plasmid pUCSR2. The insert was confirmed by sequencing. Subsequently, the pUCSR2 HindIII fragment was inserted into the unique HindIII site of vector pWH353 (12) downstream of the tet promoter, yielding plasmid pWSR2, and the correct insert orientation was verified by sequencing. Plasmid pUCBSR2, which was used for constitutive overexpression of bsrF in B. subtilis under its own promoter, was obtained by cloning a BamHI/HindIII fragment obtained by performing PCR with chromosomal DNA and primers SB832 and SB833 into the BamHI/HindIII-digested vector pUCB2.
Construction of plasmids for transcriptional lacZ fusions and determination of β-galactosidase activity.
To construct plasmid pACG6 containing the largest bsrF-lacZ transcriptional fusion (415 bp upstream of bsrF transcriptional start site), a PCR was performed with chromosomal DNA of strain DB104 and oligodeoxyribonucleotides SB1233 and SB868. The resulting fragment was digested with EcoRI and BamHI and inserted into the pAC6 (43) vector cleaved with the same enzymes. All other pACG derivatives comprising progressively shorter upstream regions were constructed in the same way and are listed in Table 1.
TABLE 1.
Plasmids used in this study
| Plasmid | Description | Reference |
|---|---|---|
| pUC19 | E. coli cloning vector, Apr, multiple cloning site | 36 |
| pUCB2 | Shuttle vector for pUC19 and pUB110, Kmr Pmr | Brantl, unpublished data |
| pCAT | pUC19 with 1.4-kb CAT gene | 24 |
| pAC6 | Vector for integration of transcriptional lacZ fusions | 42 |
| pWH353 | E. coli-B. subtilis shuttle vector, Tet system, Kmr | 12 |
| pWSR2 | pWH353 with bsrF gene under tet-inducible promoter | This study |
| pUCBSR2 | pUCB2 with bsrF gene under its own promoter | This study |
| pFRONT5 | pUC19 with 800 bp upstream of bsrF | This study |
| pBACK5 | pUC19 with 500 bp downstream of bsrF | This study |
| pCBACK5 | pBACK5 with CAT gene from pCAT | This study |
| pINT5 | Vector for replacement of bsrF by CAT gene | This study |
| pACG6 | pAC6 with 415 bp upstream of bsrF transcription start site | This study |
| pACG9 | pAC6 with 375 bp upstream of bsrF transcription start site | This study |
| pACG10 | pAC6 with 302 bp upstream of bsrF transcription start site | This study |
| pACG12 | pAC6 with 71 bp upstream of bsrF transcription start site | This study |
| pACG13 | pAC6 with 89 bp upstream of bsrF transcription start site | This study |
| pACG16 | pAC6 with 58 bp upstream of bsrF transcription start site | This study |
| pACG17 | pAC6 with 48 bp upstream of bsrF transcription start site | This study |
| pACG4 | pACG6 with 35 bp upstream of bsrF transcription start site | This study |
| pACG11 | pACG6 with two point mutations in CodY binding site | This study |
| pACG15 | pACG6 with one point mutation in CodY binding site | This study |
| pACG18 | pACG6 with two point mutations in CodY binding site | This study |
| pACG19 | pACG6 with two point mutations in CodY binding site | This study |
Transcriptional fusions containing mutations in the CodY binding site were obtained by using a two-step PCR as described below for pACG11. First, two PCRs with primer pairs SB1233/SB1260 and SB1259/SB868 were carried out, and subsequently, the joined, gel-purified fragments were subjected to a second PCR with outer primers SB1233 and SB868. The resulting fragment was digested with EcoRI and BamHI and inserted into the EcoRI/BamHI-digested pAC6 vector. The other codY mutant pAC6 derivatives pACG15, pACG18, and pACG19 (Table 1) were constructed in the same way. All pAC6 derivatives were linearized with ScaI and inserted into the amyE locus of the B. subtilis DB104 chromosome by double crossing over. The resulting integrant strains were used for determination of β-galactosidase activities as described previously (4).
Preparation of total RNA, RNA gel electrophoresis, and Northern blotting.
Preparation of total RNA, RNA gel electrophoresis on 1.5% agarose gels and 6% denaturing polyacrylamide gels, and Northern blotting were carried out as described previously (17).
Purification of CodY and DNase I footprinting.
C-terminally six-histidine-tagged CodY was purified from E. coli overexpression strain KS272(pKT1) as described previously (22). DNase I footprinting was performed as described previously (25). GTP and each of the BCAA isoleucine, leucine, and valine were added prior to DNase I at final concentrations of 2 mM and 10 mM, respectively. Both a PCR fragment obtained with primers SB1233 and SB868 and a fragment obtained by annealing of oligodeoxyribonucleotide SB1306 and SB1307 were used for DNase I treatment.
In vitro transcription with B. subtilis RNA polymerase.
In vitro transcription with purified B. subtilis RNA polymerase was performed as described previously (6). Dried gels were analyzed and subjected to phosphorimaging (Fujix BAS 1000).
In vitro transcription with T7 RNA polymerase and RNA secondary structure probing.
In vitro transcription with T7 RNA polymerase and partial digestion of in vitro-synthesized, 5′-end-labeled BsrF with RNases T1, T2, and V were carried out as described previously (16).
RESULTS
A combination of computer prediction and Northern blotting identified the novel sRNA SR2 (BsrF) in an intergenic region of the B. subtilis genome.
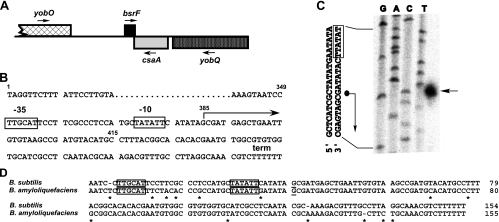
Previously, we used a computational approach to predict noncoding sRNAs in intergenic regions of the B. subtilis genome (24). Briefly, all potential transcription terminators in the B. subtilis genome were predicted, and subsequently, 50 to 450 bp upstream of these terminators promoters were predicted based on both consensus promoter sequences and data in the DTBS promoter database. Finally, all predicted sequences were evaluated by using different criteria (24), which yielded 25 candidate sRNAs. Oligonucleotides were designed to confirm these RNAs in Northern blots. Using this approach, two novel sRNAs were discovered, which were designated SR1 (sRNA 1) and SR2 (sRNA 2; now BsrF). SR1 (205 nt) was described previously (24) and subsequently was characterized in some detail (17, 18). BsrF was found later and is approximately 110 nt long (see below). The bsrF gene is located in the intergenic region between the uncharacterized gene yobO and csaA (Fig. 1A). The bsrF gene and csaA overlap by 30 bp in their terminator regions (Fig. 1). The bsrF promoter is a typical σA promoter and comprises −35 and −10 boxes that are close to the consensus and separated by a 17-bp GC-rich spacer (Fig. 1B). As shown in Fig. 1C, the 5′ end of BsrF was mapped. A search for potential open reading frames (ORFs) in the BsrF gene revealed an ORF consisting of 24 codons. However, this ORF lacks an appropriately located Shine-Delgarno sequence, and no homolog of its deduced protein product was found in the GenBank database. Therefore, BsrF is probably a noncoding sRNA. A search for BsrF homologues in other gram-positive bacteria yielded results only for B. amyloliquefaciens (Fig. 1D).
FIG. 1.
Location of the bsrF gene. (A) Schematic diagram of the location of the bsrF gene on the B. subtilis chromosome. The direction of transcription is indicated by arrows. Genes transcribed from the plus strand are indicated above the line. (B) Sequence of the bsrF gene. −35 and −10 boxes of the bsrF promoter are indicated. The start site and direction of transcription are indicated by an arrow, and the transcription termination site is indicated by term. The transcription start site is at position 2078305 in the B. subtilis genome. The small numbers indicate the positions of nucleotides referred to in Fig. 8. (C) Mapping of the 5′ end of BsrF. The results of a sequencing reaction with pUCBSR2 (containing the entire bsrF gene under its own promoter) and primer SB847 are shown in the left four lanes. The lane on the right shows the results of a primer extension reaction with total RNA of B. subtilis DB104 and 5′-labeled primer SB847. The arrow indicates the transcriptional start site of BsrF. Part of the sequence is shown on the left. The dot and the arrow indicate the mapped transcriptional start site. (D) Alignment of the bsrF genes of B. subtilis and B. amyloliquefaciens. Promoter boxes are indicated by boxes, and the transcription start site is underlined.
BsrF is expressed in complex and minimal media throughout growth, but the expression decreases when cells start to sporulate.
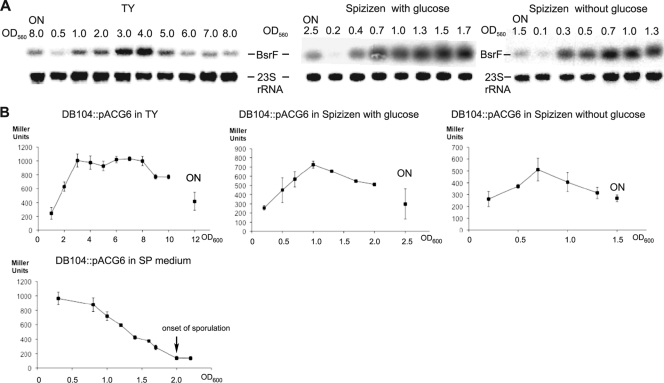
To investigate the expression profile of BsrF, strain DB104 was grown at 37°C in either complex TY or Spizizen minimal medium with or without glucose, and total RNA having different optical densities was prepared and subjected to Northern blotting. As shown in Fig. 2A, the level of expression of BsrF is very low in the early logarithmic growth phase, increases at the end of the logarithmic growth phase, remains high during stationary phase, and decreases slightly at late stationary phase. Even after overnight cultivation (late stationary phase), BsrF is significantly expressed in all three media. These data were substantiated by measurement of β-galactosidase activity using all three media from early logarithmic phase until late stationary phase and plasmid pACG6 comprising a transcriptional bsrF-lacZ fusion with 415 bp upstream of the transcription start site, which was integrated into the amyE locus of strain DB104 (Fig. 2B). To analyze the effect of sporulation on bsrF expression, DB104::pACG6 was cultivated in SP sporulation medium for 15 h. The β-galactosidase activities showed that BsrF transcription decreases progressively when cells start to sporulate and, after 15 h, is about one-eighth the initial value (Fig. 2B).
FIG. 2.
Expression profile of BsrF. (A) Expression of BsrF in complex and minimal media as monitored by Northern blotting. B. subtilis strain DB104 was grown in different media, and aliquots were removed at the OD560 indicated, immediately frozen in liquid nitrogen, and later used for preparation of total RNA. RNA was treated with glyoxal, separated on 2% agarose gels, blotted onto a nylon membrane, and hybridized with an [α-32P]dATP-labeled BsrF-specific DNA probe. Autoradiograms of the corresponding gels are shown. ON, overnight cultivation (∼15 h). For correction of loading errors, filters were reprobed with [γ-32P]ATP-labeled oligonucleotide SB747 specific for 23S rRNA. (B) Expression of BsrF in complex, minimal, and sporulation media as monitored by measurement of the β-galactosidase activity. B. subtilis strain DB104::pACG6 was grown in different media, and at the OD600 indicated, samples were withdrawn and used for measurement of the β-galactosidase activity. The data are the averages ± standard deviations for three independent measurements. ON, overnight cultivation.
Neither bsrF knockout nor overexpression affect the growth of B. subtilis.
To find out whether BsrF is essential in B. subtilis, a bsrF knockout strain was constructed. Vector pINT5 carrying the CAT gene flanked by the upstream and downstream regions of the bsrF wild-type gene was constructed as described in Materials and Methods and integrated into the chromosome of B. subtilis DB104. Successful integration by double crossing over was confirmed by PCR and Northern blotting. The growth curves of the ΔbsrF strain grown in TY medium and in minimal medium with or without glucose at 37°C were identical to the growth curves of the wild-type strain (see Fig. S1 in the supplemental material).
To analyze whether overexpression of bsrF has significant physiological effects, two overexpression strains were constructed, one for inducible overexpression under control of the tetracycline repressor (pWSR2) and the other for constitutive expression of bsrF under control of its own promoter (pUCBSR2) in B. subtilis. B. subtilis ΔbsrF strains were transformed with one of these vectors and, as controls, with the empty vectors. The growth curves were identical whether SR2 was overexpressed from multicopy vectors or not (see Fig. S1 in the supplemental material).
These data indicate that neither knockout nor overexpression of bsrF affects the growth of B. subtilis.
BsrF is long-lived and not stabilized by Hfq.
To determine the half-life of BsrF, B. subtilis strain DB104 was grown in TY or Spizizen minimal medium at 37°C until the beginning of the stationary growth phase, rifampin (rifampicin) was added to a final concentration of 100 μg/ml, time samples were taken, and total RNA was prepared and subjected to Northern blotting. Quantification of the corresponding gels (Fig. 3) yielded half-lives of BsrF of ∼23 min in Spizizen medium and ∼55 min in TY medium. Determination of the half-life in CSE minimal medium at an optical density of 2.0 resulted in a value of ∼30 min (not shown). The half-life of BsrF in the hfq knockout strain DB104 (Δhfq::cat) in Spizizen medium was ∼28 min, indicating that BsrF is not stabilized by Hfq (Fig. 3A). However, Fig. 3C shows that BsrF binds Hfq with very low affinity. In summary, we concluded that BsrF is a long-lived RNA.
FIG. 3.
Determination of the BsrF half-lives in different media. Half-lives were determined as described in Materials and Methods. Samples were taken at the times indicated after rifampin addition or without rifampin. Reprobing was performed with [γ-32P]ATP-labeled oligonucleotide SB767 specific for 5S rRNA. Autoradiograms of the Northern blots are shown. (A) Determination of the BsrF half-life in Spizizen minimal medium without glucose in the presence and absence of Hfq. (B) Determination of the BsrF half-life in TY medium (calculation performed as described above). (C) BsrF binds Hfq. Purified B. subtilis Hfq was added to 5′-end-labeled in vitro-synthesized BsrF and incubated for 15 min at 37°C in TMN buffer, and the complex was separated on a 6% native polyacrylamide gel as described previously (18). t1/2, half-life.
The BsrF secondary structure includes a long stem-loop with a branched top and a terminator stem-loop.
Since computer-predicted RNA structures often deviate from experimentally determined RNA structures (e.g., RNAI/RNAII of pT181 [7]), the secondary structure of BsrF was probed using limited digestion with structure-specific RNases in vitro. BsrF was 5′ end labeled, gel purified, and treated with RNase T1 (which cleaves 3′ of unpaired G residues), RNase T2 (which cleaves unpaired nucleotides with a slight preference for A residues), and RNase V (which cleaves double-stranded or stacked regions). Figure 4 shows the results of the gel analysis and a diagram of the secondary structure derived from the cleavage data. The experimentally determined BsrF structure has in its 5′ half a long stem-loop branched terminally into two small stem-loops and, at the 3′ end, the transcriptional terminator. Two alternative structures for the 5′ terminus can be derived from the experimental data, which might indicate that there is folding equilibrium in vivo.
FIG. 4.
Secondary structure of BsrF. (A) Secondary structure probing of BsrF with RNases. Purified, 5′-end-labeled BsrF was subjected to limited cleavage with the RNases indicated at the top. The digested RNAs were separated on an 8% denaturing gel. Autoradiograms are shown. The following RNase concentrations used were: RNase T1, 10−2 U/μl (1:50); RNase T2, 10−1 U/μl (1:500); and RNase V1, 10−1 U/μl (1:10). Lane C, control without RNase treatment; lane L, alkaline ladder. undil., undiluted. (B) Proposed secondary structure of BsrF. Two structures consistent with the cleavage data shown in panel A are shown. Major and minor cuts are indicated by symbols. The main stem-loops L1, L2, and L3 and the terminator stem-loop T are indicated.
Of 13 transcription factors analyzed, only CodY had an effect on bsrF transcription.
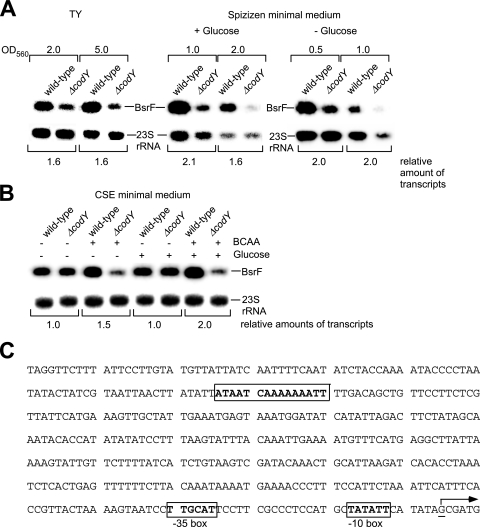
A computational search for transcription factor binding sites upstream of the bsrF −35 box revealed 13 putative binding sites with up to five mismatches compared with the published consensus sequences (see Fig. S2 in the supplemental material). Knockout strains for all of these factors were used for preparation of chromosomal DNA to construct isogenic B. subtilis DB104 knockout strains. DB104 and the isogenic knockout strains were grown under appropriate conditions until logarithmic or stationary growth phase, and total RNA was prepared and subjected to Northern blotting. A difference was observed in only one case. The expression of bsrF was increased about twofold in both TY and Spizizen minimal media in the presence of CodY (Fig. 5A). A putative CodY binding site is located 329 bp upstream of the BsrF transcription start site (Fig. 5C). Since Fur, MntR, AhrC, RocR, LevR, and PerR were among the suspected transcription factors, the effects of Fe2+, Mn2+, arginine, fructose, sucrose, and H2O2 on the levels of BsrF were analyzed; however, no alteration of the BsrF expression was found. Furthermore, no effect of anaerobic conditions on BsrF expression was detected (data not shown).
FIG. 5.
CodY affects the amount of BsrF. (A) Effect of CodY on the expression of bsrF in TY and Spizizen minimal media. B. subtilis DB104 was grown to the OD560 indicated, and total RNA was prepared, treated with glyoxal, separated on 2% agarose gels, and subjected to Northern blotting. Autoradiograms of Northern blots are shown. (B) Effect of CodY, glucose, BCAA, and glucose plus BCAA on the expression of bsrF in CSE medium. Cultures were grown until the OD600 was 0.3 (log phase), and samples were prepared and treated as described above for panel A. An autoradiogram of a Northern blot is shown. (C) Sequence of the bsrF upstream region with a putative CodY binding site. The CodY site is enclosed in a box, and the transcription start site is indicated by an underlined G residue and an arrow. −35 and −10 boxes of pBsrF are indicated.
BsrF transcription is activated twofold in the presence of glucose, but this effect is independent of both CcpA and CcpN.
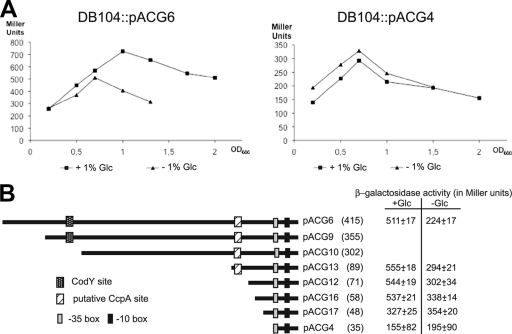
Transcriptional bsrF-lacZ fusions with progressively shorter pBsrF upstream regions containing or lacking the corresponding putative transcription factor binding sites were constructed and integrated into the amyE locus of the B. subtilis DB104 genome to monitor hypothetical factors influencing bsrF expression (Fig. 6B). With pACG6 we observed approximately 1.5- to 2-fold activation of BsrF transcription in Spizizen minimal medium in the presence of glucose, whereas no activation was found with pACG4 lacking a pBsrF upstream sequence (Fig. 6A). No effect of other sugars, like fructose or sucrose, was observed. Since the measured activation is small, a statistical significance test was performed, which confirmed that there was a significant increase in β-galactosidase activity in the presence of glucose (confidence level, 96.5%). To narrow down the bsrF upstream region responsible for the glucose effect, pACG13 and pACG12 comprising and lacking a putative CcpA binding site were assayed for β-galactosidase activity, but there were no differences, which is consistent with the absence of an effect of CcpA in the Northern blots (see above). To further narrow down the binding region of a potential glucose-dependent regulator, pACG16 and pACG17 (Fig. 6B) were analyzed. Whereas pACG16 comprising 23 bp upstream of the −35 box of pBsrF still displayed the glucose effect, pACG17 containing 13 bp did not. Since no CcpN binding site was detected upstream of pBsrF and knockout of ccpN [strain DB104(ΔccpN::phleo) (24)] did not influence BsrF transcription (measured with pACG6), we concluded that the observed glucose effect was independent of both CcpA and CcpN. Furthermore, we assayed the effects of knockouts of CcpC, GlcR, and CggR, which also were found to be not involved in the activation effect of glucose.
FIG. 6.
BsrF transcription is activated in the presence of glucose. (A) Effect of glucose on the bsrF expression depending on a sequence upstream of pBsrF. B. subtilis strains DB104::pACG6 and DB104::pACG4 were grown in Spizizen medium with and without glucose, and at the OD600 indicated, samples were withdrawn and used for β-galactosidase measurement. The data are averages of three independent determinations. (B) Influence of the putative CcpA binding site on the activating effect of glucose on bsrF expression. Diagrams of all constructed pACG derivatives are shown. The numbers of base pairs upstream of the bsrF transcription start site are indicated in parentheses. The β-galactosidase activities in Spizizen medium with and without glucose at an OD600 of 1.3 are indicated on the right. The values are averages for three independent determinations. The −35 and −10 boxes of the bsrF promoter, the CodY binding site, and the hypothetical CcpA binding site are indicated.
CodY activates the transcription of bsrF in vivo and in vitro in the presence of BCAA and GTP.
Since the results of the Northern blotting showed that CodY had a clear activating effect on the expression of bsrF in cells grown in TY and Spizizen media, we analyzed this effect using Northern blots with cells grown in CSE medium with and without BCAA. As shown in Fig. 5B, the amount of BsrF was largest in cells grown in CSE with glucose and BCAA, and under these conditions about threefold more BsrF was observed in the wild-type than in the ΔcodY strain. In CSE with BCAA but without glucose, slightly less BsrF was detected. To further analyze the BCAA effect, we used a combination of bsrF-lacZ transcriptional fusions and in vitro transcription with B. subtilis RNA polymerase.
To investigate the influence of BCAA on the expression of bsrF, DB104::pACG6 and DB104::pACG6(ΔcodY::spc) were grown in CSE medium with 0.1% glucose in the presence and absence of BCAA (isoleucine, leucine, and valine, 10 mM each). In this experiment, a threefold activating effect of these BCAA in the presence of CodY was observed (Table 2). When isoleucine alone was added instead of all three BCAA, a 2.4-fold activating effect on BsrF was found, indicating that isoleucine is responsible for 73% of the BCAA effect. By contrast, leucine or valine alone had only an 1.5-fold activating effect. Interestingly, a 1.5-fold difference in β-galactosidase activity between DB104 grown with glucose and DB104 grown without glucose was detectable in the presence of BCAA, but not in their absence. This is in agreement with the observation that glucose has an activating effect in Spizizen medium which also contains BCAA (Fig. 6). There were no differences in the β-galactosidase activities of the codY knockout strain grown in CSE medium in the presence of glucose with and without BCAA (Table 2). Since it has been found that CodY binds GTP, a possible effect of GTP on bsrF expression was analyzed. To this end, DB104::pACG6 was grown in CSE medium with glucose and BCAA until the optical density at 560 nm (OD560) was 0.3 (logarithmic phase), and samples were removed before and 30 min after addition of mycophenolic acid dissolved in methanol. Mycophenolic acid inhibits IMP dehydrogenase and thus the synthesis of guanine nucleotides, and, therefore, it decreases the cellular GTP pool. Control samples were generated by addition of methanol and incubation for 30 min. Inhibition of GTP synthesis resulted in 1.5-fold-lower β-galactosidase activities in the presence of BCAA, whereas no effect was observed when the cultures were grown in CSE medium without BCAA (Table 2). No influence of GTP was detectable in the ΔcodY strain.
TABLE 2.
Influence of BCAA, GTP, and glucose and CodY on BsrF transcriptiona
| Medium | β-Galactosidase activity (Miller units)
|
|
|---|---|---|
| DB104::pACG6 | DB104::pACG6(ΔcodY::spc) | |
| CSE | 305 ± 8 | 308 ± 5 |
| CSE + BCAA | 606 ± 16 | 318 ± 30 |
| CSE + Glc | 303 ± 21 | 310 ± 25 |
| CSE + Glc + BCAA | 909 ± 7 | 301 ± 33 |
| CSE + Glc + isoleucine | 744 ± 10 | 305 ± 24 |
| CSE + Glc + leucine | 445 ± 12 | 310 ± 15 |
| CSE + Glc + valine | 436 ± 15 | 301 ± 12 |
| CSE + Glc +/− GTP | 324 ± 22/312 ± 30 | 304 ± 10/310 ± 16 |
| CSE + Glc + BCAA +/− GTP | 903 ± 50/639 ± 27 | 308 ± 21/306 ± 27 |
| CSE + Glc +/− methanol | 316 ± 15/321 ± 22 | ND |
| CSE + Glc + BCAA +/− methanol | 896 ± 43/907 ± 59 | ND |
All values are averages of at least three independent determinations with five different integrants obtained from cultures grown until the OD600 was 0.3 (logarithmic growth phase). The influence of GTP was measured 30 min after addition of mycophenolic acid (a guanine nucleotide synthesis inhibitor) dissolved in methanol (final concentration, 100 μM) or methanol alone to cultures. The concentration of each BCAA (isoleucine, leucine, and valine) added was 10 mM, and the concentration of glucose (Glc) was 0.1 %. ND, not determined. In cases where no glucose was added, the carbon sources in the CSE medium were succinate and glutamate.
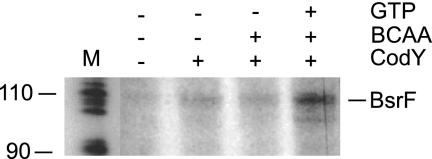
To confirm our in vivo data in vitro, in vitro transcription experiments with purified native B. subtilis RNA polymerase were performed. As shown in Fig. 7, in the absence of CodY, almost no transcript could be detected. By contrast, in the presence of CodY, as well as in the presence CodY and BCAA, a weak transcript that had the expected size (∼110 nt) was produced, and its intensity increased in the presence of CodY, BCAA, and GTP.
FIG. 7.
In vitro transcription with B. subtilis RNA polymerase. In vitro transcription in the presence or absence of CodY was performed with B. subtilis RNA polymerase. BCAA and GTP were added at concentrations of 10 mM and 2 mM, respectively, where indicated. The template was prepared by performing PCR with oligonucleotides SB1233 and SB1064 and comprises the same bsrF upstream region as pACG6.
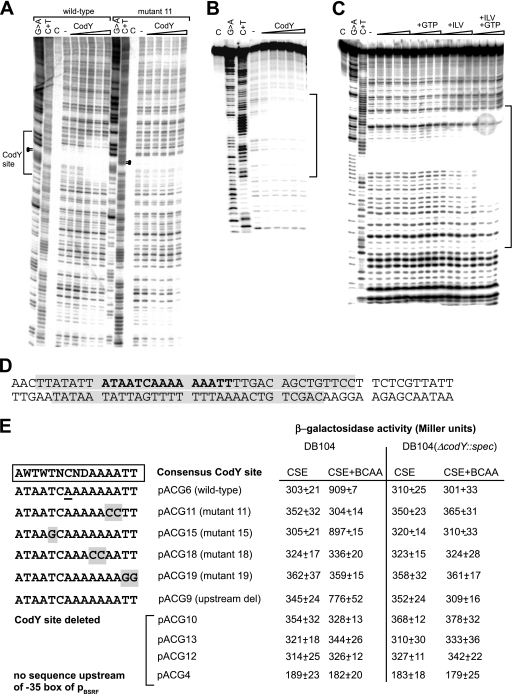
DNase I footprinting confirms the predicted CodY binding site.
To delineate the CodY binding site within the bsrF upstream region in vitro, DNase I footprinting assays were carried out with increasing CodY concentrations in the presence of 10 mM BCAA and 2 mM GTP. For these experiments, the DNA fragments obtained by PCR with primers SB1233 and SB868 and plasmids pACG6 (wild-type CodY site) and pACG11 (mutated CodY site) were used. As shown in Fig. 8A and B, in the wild-type fragment, one clearly protected region is visible at the predicted CodY binding site. No further CodY sites upstream or downstream of this site were observed. This region was partially protected with 1.4 μM CodY and fully protected with 2.2 μM CodY and is located 329 bp upstream of the bsrF transcription start site (Fig. 8D). By contrast, the mutated DNA fragment from pACG11 carrying two C residues at the 3′ end of the poly(A) stretch did not produce any footprint (Fig. 8A). This finding correlates well with the failure of pACG11 to produce any differences in the β-galactosidase assays between DB104 and DB104(ΔcodY::spc) (Fig. 8E). DNase I footprinting performed with an 89-bp DNA fragment generated by annealing two oligonucleotides with the CodY site in its center in the presence or absence of BCAA and/or GTP corroborated the requirement for both BCAA and GTP for the activating effect of CodY (Fig. 8C). Without both effector molecules, no footprint was obtained. An effect of GTP was visible only in the presence of BCAA, whereas BCAA alone had a weak effect.
FIG. 8.
Interaction of CodY with the bsrF promoter region. (A and B) DNase I footprinting analysis of the interaction of CodY and the bsrF promoter. DNase I footprinting with increasing amounts of purified CodY-His6 (1.0 μM, 1.4 μM, 1.8 μM, 2.2 μM, and 2.6 μM) (indicated by triangles) was performed in the presence of 2 mM GTP and 10 mM BCAA as described in Materials and Methods. Lane C, control without DNase; lane −, control with DNase but without CodY. The binding site is indicated. (A) Coding strand DNA fragment comprising nt 1 to 176 (Fig. 5C). Dots indicate residues replaced in mutant 11. (B) Noncoding strand (DNA fragment comprising nt 57 to 145 [see Fig. 5C]). (C) Analysis in the presence and absence of CodY, BCAA, and GTP (DNA fragment as in panel B). The triangles indicate increasing amounts of CodY (as described above for panel A). The bracket indicates the region protected by CodY. (D) Sequences on the coding and noncoding strands examined. Protected regions are shaded. The predicted CodY consensus binding site is indicated by bold type. (E) Overview of the mutants with mutations in the CodY binding site. Mutated nucleotides are shaded. The corresponding pACG derivatives and the measured β-galactosidase activities are indicated on the right. Mutated pACG derivatives pACG11, pACG15, pACG18, and pACG19 contain the same region upstream of the CodY site as pACG6, whereas pACG9, pACG10, pACG12, pACG13, and pACG4 contain shortened upstream regions (see Fig. 6). For measurement of β-galactosidase activity, B. subtilis strains were grown in CSE medium with 0.1% glucose with or without BCAA (as indicated) until the OD600 was 0.3. All of the values are averages of at least three independent determinations with five different integrants.
A mutational analysis reveals the sequence requirements of the CodY binding site for the activating effect on BsrF.
To confirm the location of the CodY binding site in vivo, the pACG derivatives pACG9 (25 bp upstream of putative CodY binding site) and pACG10 (13 bp downstream of putative CodY site) were constructed and, along with the previously constructed deletion mutants pACG12, pACG13, and pACG4 (Fig. 6), used for measurement of β-galactosidase activity in DB104 and DB104(ΔcodY::spc) in CSE medium with glucose in the presence and absence of BCAA. The results substantiated the two- to threefold activating effect of CodY on bsrF transcription (Fig. 8E). To further analyze the sequence requirements of the CodY binding site, pACG11, pACG15, pACG18, and pACG19 having four different mutations in the predicted CodY binding site were constructed (Fig. 8E). β-Galactosidase measurements for DB104 and DB104(ΔcodY::spc) revealed that mutations in the 3′ terminal A7 tract of the CodY binding site and 3′ terminal two T residues eliminated the ability of CodY to exert its activating effect in the presence of BCAA, whereas a T-G exchange at position 5 did not influence transcription activation by CodY (Fig. 8E).
BsrF is also expressed in B. amyloliquefaciens and has an expression pattern similar to that in B. subtilis.
As shown in Fig. 1D, an sRNA highly homologous to BsrF is encoded in the B. amyloliquefaciens genome. Since the promoter elements were identical to those of the B. subtilis bsrF gene, we set out to analyze the expression of this predicted sRNA. B. amyloliquefaciens FZB42 was grown in TY medium for 10 h, samples were taken at different optical densities, and total RNA was prepared and subjected to Northern blotting with the B. subtilis BsrF probe. As shown in Fig. 9, BsrF is expressed in this species, and, as in B. subtilis, the level of BsrF expression is low in early log phase but increases when cells reach late log phase and slightly decreases during stationary growth. Since the upstream region of the B. amyloliquefaciens bsrF gene is completely different from that of B. subtilis, we searched for a putative CodY binding site. One putative site, which had more mismatches with the consensus than the B. subtilis site, was found 100 bp upstream of the −35 box. Strain FZB42 was grown in CSE medium with glucose in the presence and absence of BCAA, samples were removed at an OD560 of 1.0, and RNA was prepared and analyzed by Northern blotting. As shown in Fig. 9, an approximately threefold-larger amount of BsrF was observed in the presence of BCAA, suggesting that CodY might be also involved in the expression of bsrF in B. amyloliquefaciens.
FIG. 9.
Expression of the bsrF gene in B. amyloliquefaciens. B. amyloliquefaciens strain FZB42 was grown in TY or CSE medium, and aliquots were removed at hourly intervals at the OD560 indicated, immediately frozen in liquid nitrogen, and later used for preparation of total RNA. Northern blotting was performed as described in the legend to Fig. 2 with the same BsrF probe. Autoradiograms of the corresponding gels are shown. ON, overnight cultivation (∼15 h). For correction of loading errors, filters were reprobed with [γ-32P]ATP-labeled oligonucleotide SB1319 specific for B. amyloliquefaciens 23S rRNA.
DISCUSSION
The majority of all chromosomally encoded sRNAs are expressed only under specific environmental stress conditions. Many different classes of regulators, including global regulators, have been found to be responsible for this inducible expression. For instance, expression of E. coli MicF sRNA is governed by a regulatory network including HU, H-NS, Lrp, OmpR, SoxS, MarA, and Rob (9). E. coli RyhB and B. subtilis FsrA involved in iron metabolism are regulated by the Fur repressor (11, 28), RprA is regulated by the RcsC/RcsD/RcsB phosphorelay (26), and OmrA and OmrB are regulated by the EnvZ/OmpR two-component system (13). MicA and RybB regulating outer membrane proteins are dependent on the alternative sigma factor σE (20, 32, 44, 46) associated with membrane stress. Recently, a novel Listeria sRNA that is under the control of the stress sigma factor σB was found (31).
Here, we report the properties of a novel sRNA, SR2 (30), identified by our group using the same approach that was used previously for SR1 (24). SR2 was also found independently by Saito et al., who detected six novel sRNAs in intergenic regions of B. subtilis and designated BsrF (35). Therefore, the designation BsrF was used in this study. Our sequence alignment revealed a highly homologous sRNA in B. amyloliquefaciens (Fig. 1D), and Northern blotting showed that bsrF is also expressed in this species with a profile similar to that in B. subtilis (Fig. 9). We show here that BsrF is highly expressed in all growth phases and that significant amounts are also present after overnight cultivation (15 h). When cells sporulate, BsrF expression decreases about eightfold (Fig. 2B).
The lack of growth defects upon deletion or constitutive or inducible overexpression of the bsrF gene indicates that BsrF, like many other sRNAs, is involved in fine-tuning of gene expression. Surprisingly, the BsrF half-life is very long, and Hfq was found to be not responsible for stabilizing BsrF. Hfq bound BsrF with a very low affinity. Therefore, it is rather unlikely that Hfq promotes the interaction of BsrF with its target(s). To overcome the limitations of a very long-lived sRNA in gene regulation, other factors might cooperate with BsrF to regulate metabolic processes or the stress response.
Furthermore, we demonstrated by using a combination of Northern blotting, lacZ-reporter gene fusions, in vitro transcription, and DNase I footprinting that the transcription of BsrF is activated by the global regulator CodY. Thus, the bsrF gene is the second gene for which an activating effect of CodY has been found (the first gene is ackA [38]). The influence of BCAA and GTP on the CodY effect was analyzed both in vivo and in vitro. Whereas BCAA increased the activating effect of CodY about threefold in vivo, the effect of GTP was smaller; in the presence of BCAA, but not in the absence of BCAA, a 1.5-fold increase in the activation effect of CodY was observed in the presence of GTP. The in vitro transcription assay and the DNase I footprinting analysis corroborated the requirement for both GTP and BCAA. This is in contrast to the majority of CodY-regulated operons, including the ilvB operon, in which GTP and BCAA were shown to act additively to increase the CodY affinity for DNA and to repress transcription (15). By contrast, relative insensitivity to GTP levels was found for ackA, and BCAA alone were observed to be sufficient for the activating effect of CodY (38). Shivers et al. concluded that BCAA and GTP may have independent effects and that promoters differ in their responsiveness to GTP and BCAA pools. This is in good agreement with our observations, which showed that a twofold activating effect of CodY on BsrF also occurred in the stationary phase in TY and Spizizen media, when the sizes of GTP pools are small (Fig. 5A). Interestingly, isoleucine was found to be responsible for 73% of the activating effect of all three BCAA (Table 2). Similar results were obtained for the ilvB operon, where isoleucine mimicked the effect of all three BCAA (37). The DNase I footprinting data show that binding of CodY is weak, which may explain the finding that it had only a threefold effect on BsrF transcription in vivo.
In the presence of glucose, BsrF transcription increases about 1.5- to 2-fold, whereas other sugars, like sucrose or fructose, had no effect. Interestingly, the activating effect of glucose found in late log phase (Fig. 6A) was observed only in the presence of BCAA, and none of the known glucose-dependent regulators was responsible for this effect. The region bound by a hypothetical glucose-dependent regulator could be narrowed down to 23 bp upstream of the −35 box of pBsrF (Fig. 6B). Two alternative explanations for the BCAA dependence of this glucose effect can be imagined. On the one hand, a glucose-dependent regulator that binds to a region immediately upstream of pBsrF could cooperate with BCAA-bound CodY, resulting in the threefold activating effect. Such regulatory loops have been found in a number of systems (for a review, see reference 48). Alternatively, the regulator itself could be subject to transcriptional activation by CodY in the presence of BCAA. For ackA, the first gene for which activation of transcription by CodY was demonstrated (38), an interaction of CodY and CcpA was found. However, in this case, the CodY and CcpA binding sites were in close proximity, and CodY bound at two positions, one at positions −61 to −100 upstream of packA and the other at positions −20 to 14, and the cre2 site was found between them. Our DNase I footprinting experiments showed that CodY protects positions −329 to −301 upstream of the BsrF transcriptional start site. Therefore, a regulatory loop involving CodY interacting with the glucose-dependent regulator is conceivable.
Recently, a thorough analysis of all hitherto known CodY binding sites in B. subtilis (2) showed broad variation in positions, particularly in the 5′ half of the site, and the consensus sequence 5′-AATTTTCWGAAAATT was derived. Taking into account all of the variations in the original data of Belitsy and Sonenshein (2), we modified this consensus to the more relaxed 5′-AWTWTNCNDAAAATT, which allowed us to find the CodY site upstream of bsrF. A mutational analysis of our CodY binding site demonstrated that the A7 stretch and the two 3′-terminal T residues are the regions most important for CodY binding. By contrast, the T5-G5 exchange did not affect CodY activation at pBsrF (Fig. 8E). Attempts to improve the CodY effect on BsrF by mutating the 5′ half of the site toward the consensus, however, failed (not shown.)
Currently, experiments are under way to identify target genes of BsrF. Using Western blotting, we ruled out the possibility that BsrF acts as a cis-encoded antisense RNA to regulate csaA, which directly overlaps the 3′ end of bsrF. The regulation of bsrF expression by CodY makes it likely that targets might be discovered that are known to be regulated by CodY but for which no direct CodY binding site has been found, like some of the targets detected in a combined chromatin immunoprecipitation and transcriptome analysis (29). So far, our first microarray analysis revealed a decreased amount of the cydABCD mRNA in the ΔbsrF strain, suggesting that BsrF might be involved in the regulation of cytochrome synthesis.
Supplementary Material
Acknowledgments
We are grateful to A. L. Sonenshein, Boston, MA, for providing the codY mutant and overexpression strains, J. Stülke, Göttingen, Germany, for providing the ccpA, ccpC, glcR, and cggR knockout strains, and R. Borriss, Berlin, Germany, for providing B. amyloliquefaciens strain FZB42. We thank M. Salas, Madrid, Spain, for providing the purified B. subtilis RNA polymerase. R. Godfrey is acknowledged for determination of the BsrF half-life in minimal medium.
This work was supported by grant BR1552/7-1 from the Deutsche Forschungsgemeinschaft (to S.B.).
Footnotes
Published ahead of print on 19 June 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Barrick, J. E., N. Sudarsan, Z. Weinberg, W. L. Ruzzo, and R. R. Breaker. 2005. 6S RNA is a widespread regulator of eubacterial RNA polymerase that resembles an open promoter. RNA 11744-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belitsky, B. R., and A. L. Sonenshein. 2008. Genetic and biochemical analysis of CodY binding sites in Bacillus subtilis. J. Bacteriol. 1901224-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boisset, S., T. Geissmann, E. Huntzinger, P. Fechter, N. Bendridi, M. Possedko, C. Chevalier, A. C. Helfer, Y. Benito, A. Jacquier, C. Gaspin, F. Vandenesch, and P. Romby. 2007. Staphylococcus aureus RNAIII coordinately represses the synthesis of virulence factors and the transcription regulator Rot by an antisense mechanism. Genes Dev. 211353-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brantl, S. 1994. The copR gene product of plasmid pIP501 acts as a transcriptional repressor at the essential repR promotor. Mol. Microbiol. 14473-483. [DOI] [PubMed] [Google Scholar]
- 5.Brantl, S. 2009. Bacterial chromosome-encoded small regulatory RNAs. Future Microbiol. 485-103. [DOI] [PubMed] [Google Scholar]
- 6.Brantl, S., B. Nuez, and D. Behnke. 1992. In vitro and in vivo analysis of transcription within the replication region of plasmid pIP501. Mol. Gen. Genet. 234105-112. [DOI] [PubMed] [Google Scholar]
- 7.Brantl, S., and E. G. H. Wagner. 2000. Antisense-RNA mediated transcriptional attenuation: an in vitro study of plasmid pT181. Mol. Microbiol. 351469-1482. [DOI] [PubMed] [Google Scholar]
- 8.Christiansen, J. K., J. S. Nielsen, T. Ebersbach, P. Valentin-Hansen, L. Søgaard-Andersen, and B. H. Kallipolitis. 2006. Identification of small Hfq-binding RNAs in Listeria monocytogenes. RNA 121383-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delihas, N., and S. Forst. 2001. MicF: an antisense RNA gene involved in response of Escherichia coli to global stress factors. J. Mol. Biol. 3131-12. [DOI] [PubMed] [Google Scholar]
- 10.Faires, N., S. Tobisch, S. Bachem, I. Martin-Verstraete, M. Hecker, and J. Stülke. 1999. The catabolite control protein CcpA controls ammonium assimilation in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 1141-148. [PubMed] [Google Scholar]
- 11.Gaballa, A., H. Antelmann, C. Aguilar, S.-K. Khakh, K.-B. Song, G. T. Smaldone, and J. D. Helmann. 2008. The Bacillus subtilis iron-sparing response is mediated by a Fur-regulated small RNA and three small, basic proteins. Proc. Natl. Acad. Sci. USA 10511927-11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geissendörfer, M., and W. Hillen. 1990. Regulated expression of heterologous genes in Bacillus subtilis using the Tn10 encoded tet regulatory elements. Appl. Microbiol. Biotechnol. 33657-663. [DOI] [PubMed] [Google Scholar]
- 13.Guillier, M., S. Gottesman, and G. Storz. 2006. Modulating the outer membrane with small RNAs. Genes Dev. 202338-2348. [DOI] [PubMed] [Google Scholar]
- 14.Halfmann, A., M. Kovács, R. Hakenbeck, and R. Brückner. 2007. Identification of the genes directly controlled by the response regulator CiaR in Streptococcus pneumoniae: five out of 15 promoters drive expression of small non-coding RNAs. Mol. Microbiol. 66110-126. [DOI] [PubMed] [Google Scholar]
- 15.Handke, L. D., R. P. Shivers, and A. L. Sonenshein. 2008. Interaction of Bacillus subtilis CodY with GTP. J. Bacteriol. 190798-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidrich, N., and S. Brantl. 2003. Antisense-RNA mediated transcriptional attenuation: importance of a U-turn loop structure in the target RNA of plasmid pIP501 for efficient inhibition by the antisense RNA. J. Mol. Biol. 333917-929. [DOI] [PubMed] [Google Scholar]
- 17.Heidrich, N., A. Chinali, U. Gerth, and S. Brantl. 2006. The small untranslated RNA SR1 from the B. subtilis genome is involved in the regulation of arginine catabolism. Mol. Microbiol. 62520-536. [DOI] [PubMed] [Google Scholar]
- 18.Heidrich, N., I. Moll, and S. Brantl. 2007. In vitro analysis of the interaction between the small RNA SR1 and its primary target ahrC mRNA. Nucleic Acids Res. 35331-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershberg, R., S. Altuvia, and H. Margalit. 2003. A survey of small RNA-encoding genes in Escherichia coli. Nucleic Acids Res. 311813-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansen, J., A. A. Rasmussen, M. Overgaard, and P. Valentin-Hansen. 2006. Conserved small non-coding RNAs that belong to the σE regulon: role in down-regulation of outer membrane proteins J. Mol. Biol. 3641-8. [DOI] [PubMed] [Google Scholar]
- 21.Kawamura, F., and R. H. Doi. 1984. Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J. Bacteriol. 160442-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, H.-J., S.-I. Kim, M. Ratnayake-Lecamwasam, K. Tachikawa, K., A. L. Sonenshein, and M. Strauch. 2003. Complex regulation of the Bacillus subtilis aconitase gene. J. Bacteriol. 1851672-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, J. M., S. Zhang, S. Saha, S. Santa Anna, C. Jiang, C., and J. Perkins. 2001. RNA expression analysis using an antisense Bacillus subtilis genome array. J. Bacteriol. 1837371-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Licht, A., S. Preis, and S. Brantl. 2005. Implication of CcpN in the regulation of a novel untranslated RNA (SR1) in B. subtilis. Mol. Microbiol. 58189-206. [DOI] [PubMed] [Google Scholar]
- 25.Licht, A., and S. Brantl. 2006. Transcriptional repressor CcpN from Bacillus subtilis compensates asymmetric contact distribution by cooperative binding. J. Mol. Biol. 364434-448. [DOI] [PubMed] [Google Scholar]
- 26.Majdalani, N., D. Hernandez, and S. Gottesman. 2002. Regulation and mode of action of the second small RNA activator of RpoS translation, RprA. Mol. Microbiol. 46813-826. [DOI] [PubMed] [Google Scholar]
- 27.Mandin, P., F. Repoila, M. Vergassola, T. Geissmann, and P. Cossart. 2007. Identification of new nonconding RNAs in Listeria monocytogenes and prediction of mRNA targets. Nucleic Acids Res. 35962-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massé, E., and S. Gottesman. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA 994620-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molle, V., Y. Nakaura, R. P. Shivers, H. Yamaguchi, R. Losick, Y. Fujita, and A. L. Sonenshein. 2003. Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis. J. Bacteriol. 1851911-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narberhaus, F., and J. Vogel. 2007. Sensory and regulatory RNAs in prokaryotes: a new German research focus. RNA Biol. 4160-164. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen, J. S., A. S. Olsen, M. Bonde, P. Valentin-Hansen, and B. H. Kallipolitis. 2008. Identification of a sigma B-dependent small noncoding RNA in Listeria monocytogenes. J. Bacteriol. 1906264-6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papenfort, K., V. Pfeiffer, F. Mika, S. Lucchini, J. C. D. Hinton, and J. Vogel. 2006. σE-dependent small RNAs of Salmonella respond to membrane stress by accelerating global omp mRNA decay. Mol. Microbiol. 621674-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pichon, C., and B. Felden. 2005. Small RNA genes expressed from Staphyloccoccus aureus genomic and pathogenicity islands with specific expression among pathogenic strains. Proc. Natl. Acad. Sci. USA 10214249-14254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratnayake-Lecamwasam, M., P. Serror, K.-W. Wong, and A. L. Sonenshein. 2001. Bacillus subtilis CodY represses early-stationary phase genes by sensing GTP levels. Genes Dev. 151093-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito, S., H. Kakeshita, and K. Nakamura. 2009. Novel small RNA-encoding genes in the intergenic regions of Bacillus subtilis. Gene 4282-8. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 37.Shivers, R. P., and A. L. Sonenshein. 2004. Activation of the Bacillus subtilis global regulator CodY by direct interaction with branched chain amino acids. Mol. Microbiol. 53599-611. [DOI] [PubMed] [Google Scholar]
- 38.Shivers, R. P., S. S. Dineen, and A. L. Sonenshein. 2006. Positive regulation of Bacillus subtilis ackA by CodY and CcpA: establishing a potential hierarchy in carbon flow. Mol. Microbiol. 62811-822. [DOI] [PubMed] [Google Scholar]
- 39.Silvaggi, J. M., J. B. Perkins, and R. Losick. 2005. Small untranslated RNA antitoxin in Bacillus subtilis. J. Bacteriol. 1876641-6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silvaggi, J. M., J. B. Perkins, and R. Losick. 2006. Genes for small, noncoding RNAs under sporulation control in Bacillus subtilis. J. Bacteriol. 188532-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonenshein, A. L. 2005. CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr. Opin. Microbiol. 8203-207. [DOI] [PubMed] [Google Scholar]
- 42.Storz, G., S. Altuvia, and K. M. Wassarman. 2005. An abundance of RNA regulators. Annu. Rev. Biochem. 74199-217. [DOI] [PubMed] [Google Scholar]
- 43.Stülke, J., I. Martin-Verstraete, M. Zagorec, M. Rose, A. Klier, and G. Rapoport. 1997. Induction of the Bacillus subtilis ptsGHI operon by glucose is controlled by a novel antiterminator, GlcT. Mol. Microbiol. 2565-78. [DOI] [PubMed] [Google Scholar]
- 44.Thompson, K. M., V. A. Rhodius, and S. Gottesman. 2007. σE regulates and is regulated by a small RNA in Escherichia coli. J. Bacteriol. 1894243-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toledo-Arana, A., F. Repoila, and P. Cossart. 2007. Small noncoding RNAs controlling pathogenesis. Curr. Opin. Microbiol. 10182-188. [DOI] [PubMed] [Google Scholar]
- 46.Udekwu, K., and E. G. H. Wagner. 2007. Sigma E controls biogenesis of the antisense RNA MicA. Nucleic Acids Res. 351279-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valentin-Hansen, P., M. Eriksen, and C. Udesen. 2004. The bacterial Sm-like protein Hfq: a key player in RNA transactions. Mol. Microbiol. 511525-1533. [DOI] [PubMed] [Google Scholar]
- 48.Xu, H., and T. R. Hoover. 2001. Transcriptional regulation at a distance in bacteria. Curr. Opin. Microbiol. 4138-144. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, A., K. M. Wassarman, J. Ortega, A. C. Steven, and G. Storz. 2002. The Sm-like Hfq protein increases OxyS RNA interaction with target mRNAs. Mol. Cell 911-22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.