Abstract
SlyA, a MarR family transcriptional regulator, controls an assortment of biological functions in several animal-pathogenic bacteria. In order to elucidate the functions of SlyA in the phytopathogen Dickeya dadantii (formerly Erwinia chrysanthemi) 3937, a slyA gene deletion mutant (denoted ΔslyA) was constructed. The mutant exhibited increased sensitivity to sodium hypochlorite, the cationic antimicrobial peptide polymyxin B, and oxidative stress. The mutant showed reduced production of pectate lyase and exopolysaccharide and an inability to form a pellicle. The mutant lacking a functional slyA gene showed a significantly reduced ability to cause maceration of potato tubers. Accordingly, the mutant exhibited significantly reduced bacterial growth and failed to hyperinduce pectate lyase production in planta. Introduction of a plasmid containing slyA into the ΔslyA mutant caused all of these phenotypes to recover to wild-type levels. These results suggest that SlyA plays an important role in virulence to plants by positively regulating the expression of multiple pathogenicity-related traits of D. dadantii 3937.
The MarR (multiple antibiotic resistance regulator) proteins are prototypical members of the MarR family of transcriptional regulators that are widely distributed in bacteria and archaea (2, 13). Members of the MarR family regulate a wide variety of cellular processes, including resistance to multiple antibiotics, organic solvents, household disinfectants, and oxidative-stress agents, which are collectively termed the multiple antibiotic resistance phenotypes (1, 2). They also regulate the synthesis of virulence factors in microbes that infect humans or plants, e.g., slyA in Salmonella enterica serovar Typhimurium (10, 30, 31a), rovA in Yersinia pseudotuberculosis (36), aphA in Vibrio cholerae (27), mgrA in Staphylococcus aureus (22), pecS in Dickeya dadantii (formerly Erwinia chrysanthemi) 3937 (46), hor in Pectobacterium carotovorum subsp. carotovorum (formerly Erwinia carotovora subsp. carotovora) (58), and hpaR in Xanthomonas campestris pv. campestris (62). It has been demonstrated that the role of MarR in controlling the expression of virulence-associated genes varies among pathogens (7, 22, 27, 29, 30, 34, 44, 45, 62). In Y. pseudotuberculosis, rovA transcription is primarily controlled by RovA itself (13, 13a, 28) and by H-NS (28), a histone-like protein that is important for the proper nucleoid packaging of the bacterial chromosome (3), in a temperature-dependent manner. Conversely, the expression of slyA in Escherichia coli is regulated by temperature but in an H-NS-independent manner (9). In S. enterica serovar Typhimurium, the expression of slyA is regulated by its own product, SlyA (57), and is induced during the stationary phase, as well as during the infection of macrophages (7). Moreover, slyA expression in S. enterica serovar Typhimurium is positively controlled by the PhoP-PhoQ two-component regulatory system in responding to magnesium starvation (40, 54).
D. dadantii 3937 is a phytopathogenic enterobacterium that attacks a wide range of economically important plant species. A variety of factors have been shown to influence the ability of D. dadantii 3937 to attack plant tissue, but pectinolytic enzymes are considered to be the major determinant of pathogenicity (41, 41a, 59a, 61). Additional factors that contribute to its pathogenicity are exopolysaccharide (8), lipopolysaccharide (53), siderophore-mediated iron transport systems (14), the type III secretion system encoded by the hrp gene cluster (5), antimicrobial peptides (18), motility (12), and proteins involved in resistance against plant defense mechanisms (12, 33). The expression of these functions is regulated in a coordinated manner in response to different stimuli, such as the presence of pectic substances, acidic pH, iron, magnesium limitation, plant extract, and the presence of antimicrobial molecules. Different regulatory proteins, such as KdgR, PecS, H-NS, PecT, Pir, CRP, Fur, and PhoP-PhoQ (8, 18, 36a, 39, 45, 46, 47), have been shown to be involved in such complex regulation. To gain further insight into the regulation of pathogenicity factors in D. dadantii 3937, we investigated the role of the MarR family transcriptional regulator SlyA in this process. Here, we show that SlyA is an important virulence factor that regulates numerous pathogenicity-related traits in D. dadantii 3937.
MATERIALS AND METHODS
Bacterial strains, plasmids, growth media, and chemicals.
Bacterial strains and plasmids and their relative characteristics are listed in Table 1. E. coli strains were cultivated in Luria-Bertani (LB) medium (1% tryptone, 0.5% yeast extract, 0.5% NaCl, pH 7.0) at 37°C. D. dadantii strains were grown in yeast extract-peptone (YP) medium (1% peptone, 0.5% yeast extract, pH 6.8) or M63 glycerol minimal medium [2.5 g of NaCl, 3 g of KH2PO4, 7 g of K2HPO4, 2 g of (NH4)2SO4, 0.5 mg of FeSO4, 1 g of MgSO4, 2 g of thiamine hydrochloride in 1 liter] and 0.2% (wt/vol) glycerol (4) at 27°C. The plant extract (potato tuber), a crude juice obtained from potato tubers and sterilized by filtration through a 0.45-μm Millipore filter, was used at a final concentration of 1% (vol/vol). The optical density (OD) of the bacterial culture was measured using a Bactomonitor BACT-500 (Intertech, Tokyo, Japan) at 660 nm. When required, antibiotics were added at the following final concentrations: ampicillin at 50 μg/ml, nalidixic acid at 50 μg/ml, kanamycin at 50 μg/ml, streptomycin at 25 μg/ml, and spectinomycin at 70 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| D. dadantii | ||
| 3937 | Wild type; Nalr | Laboratory collection |
| ΔslyA | ΔslyA mutant; Nalr | This study |
| 3937ΔslyA | pDEL-SlyA into D. dadantii 3937; Nalr Apr Stmr | This study |
| ΔslyA(pSlyA) | pSlyA into ΔslyA mutant; Nalr Apr | This study |
| MH70 | Km-inserted phoP mutant; Nalr Kmr | 18 |
| MH72 | Km-inserted phoQ mutant; Nalr Kmr | 18 |
| E. coli | ||
| DH5α | λ− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 (note the roman M15 after lacZΔ) | TaKaRa, Japan |
| CC118 | Δ(ara, leu) araD ΔlacX74 galE galK phoA20 thi-1 rps rpoB argE(Am) recA1 Spr | 19 |
| CC118 (λpir) | CC118 lysogenized with λpir phage; Spr | 19 |
| S17-1 | recA thi pro hsdR-M+RP4:2-Tc:Mu:km Tn7 λpir Smr | Biomedal |
| BL21(DE3) | F−ompT hsdSB(rB− mB−) gal dcm (DE3) | Nippon gene |
| Plasmids | ||
| pGEM-T Easy | A-T cloning vector; Apr | Promega |
| pMRS101 | R6K origin sacB marker exchange vector; mob+, Apr Stmr | 51 |
| pSlyX | 2.34-kb ApaI-SacI fragment cloned into same site of pGEM-T Easy; Nalr Apr | This study |
| pSlyY | 0.911-kb ApaI-SmaI fragment upstream from slyA ORF cloned into same site of pGEM-T Easy, Nalr, Apr | This study |
| pSlyZ | 0.999-kb SmaI-SacI fragment downstream from slyA ORF cloned into same site of pGEM-T Easy; Nalr Apr | This study |
| pSlyYZ | 1.91-kb ApaI-SacI fragment contained deletion of slyA ORF cloned into same site of pGEM-T Easy; Nalr Apr | This study |
| pDEL-SlyA | 1.75-kb ApaI-SpeI fragment containing deletion of slyA ORF cloned into same site of pMRS101, Nalr, Apr, Stmr | This study |
| pSlyA | 1.29-kb fragment containing entire slyA gene with its promoter cloned into pGEM-T Easy; Nalr Apr | This study |
| pET28a(+) | T7 expression vector; Kmr | Novagen |
| pETSlyA | 0.44-kb fragment containing entire slyA gene in pET28a(+) | This study |
| pGEM-T | A-T cloning vector; Apr | Promega |
| pGEMphoP | 0.29-kb phoP promoter fragment in pGEM-T | Promega |
Recombinant DNA techniques.
Preparation of chromosomal and plasmid DNAs, PCR, gel electrophoresis, restriction endonuclease digestion, DNA ligation, electroporation, and Southern blot hybridization were carried out as described previously (50). Nucleotide sequence analysis was done using a DNA autosequencer (Model 4000; Li-cor, Lincoln, NE). Restriction and modifying enzymes were purchased from Nippon Gene (Tokyo, Japan) and New England Biolabs (Beverly, MA).
Cloning of a slyA homologue from D. dadantii 3937.
The slyA gene of D. dadantii 3937 is located between slyB, an outer membrane lipoprotein gene, and a gene for iron-sulfur (Fe-S) protein (http://asap.ahabs.wisc.edu/annotation/php/ASAP1.htm). To clone the slyA gene, a 2.34-kb fragment containing the slyA gene, together with 911 bp upstream from the start codon and 999 bp downstream from the stop codon of the slyA gene, was amplified by PCR from genomic DNA of D. dadantii 3937 using the primer pair PS1 (5′-GGGCCCCCCAGTGCGATATGACCGAA-3′) and PS4 (5′-GAGCTCTGGTGCGTTCGACACACCA-3′). The PCR primers were designed based on the sequence of the D. dadantii 3937 slyA gene (http://www.genome.wisc.edu/tools/asap.htm). The PCR was carried out for 33 cycles under the following conditions: 94°C for 30 s for denaturation, 60°C for 1 min for annealing, and 72°C for 2 min for extension. The PCR-amplified fragment of the slyA homologue was cloned into pGEM-T Easy to generate pSlyX and confirmed by PCR.
Construction of the slyA deletion mutant.
To construct the slyA deletion mutant, the primer pair PS1 (see above) and PS2 (5′-GGGCCCGGGTTCTCCTTATAGTTAGCATACTAAGC-3′) was used to amplify a 911-bp fragment containing the upstream region of slyA, while the primer pair PS3 (5′-CCCGGGTTAATAGAAAAAGTAAATGTCTTGGCGGGC-3′) and PS4 (see above) was used to amply a 999-bp fragment containing the downstream region of slyA from pSlyX. These two fragments were purified from an agarose gel after gel electrophoresis using a QIAEX II gel extraction kit (Qiagen, Hilden, Germany), ligated, and cloned into a pGEM-T Easy plasmid to create pSlyY and pSlyZ, respectively. The plasmids pSlyY and pSlyZ were digested using ApaI and SmaI, or SmaI and SacI, respectively. The resultant fragments were subcloned into pGEM-T Easy to generate pSlyYZ. Thus, a fragment was constructed with a 438-bp deletion of the entire slyA open reading frame (ORF). To construct pDEL-SlyA, the primer pair PS1 (see above) and PS5 (5′-GGCACTAGTGGAGACGTTACAGTGCATCCGAA-3′) was used to PCR amplify a 1.75-kb fragment from pDEL-SlyYZ. After ApaI-SpeI digestion, the product was ligated into the suicide vector pMRS101 (51). This plasmid was used to create a deletion in the slyA homologue in the D. dadantii 3937 chromosome by double homologous recombination using the sucrose selection marker (sacB) as described previously (24).
Complementation of the ΔslyA mutant.
For complementation of the ΔslyA mutant, a 1.29-kb DNA fragment containing the entire slyA gene, together with 340 bp upstream from the start codon and 512 bp downstream from the stop codon, was PCR amplified from total genomic DNA of D. dadantii 3937 as the template using the primer pair PC1 (5′-AAGCTTTGTTTTCGCAGCCGCAGCGAGCGTTTTAAT-3′) and PC2 (5′-AAGCTTGATACCAAATTCCACGCCGGACAACGTGTG-3′). The resulting fragment was cloned into the multicopy vector pGEM-T Easy to generate pSlyA. This plasmid was then electroporated into the ΔslyA mutant using a Cell-Porator (set at 9.4 kV/cm, 160 μF, and 4 Ω; Bethesda Research Laboratories, Bethesda, MD). The ΔslyA mutant carrying pSlyA was then screened on a YP agar plate containing nalidixic acid and ampicillin. A confirmed representative was named ΔslyA(pSlyA) and chosen for further study.
Susceptibility to antimicrobial peptides.
Susceptibility to antimicrobial peptides was assayed as previously described (18) with a few modifications. Polymyxin B, a cationic, cyclic, amphipathic lipopeptide antibiotic, and salmon protamine, a linear antibiotic, were purchased from Sigma and dissolved in sterilized water to a final concentration of 1 mg/ml as a stock solution. Bacterial strains were grown in M63 glycerol minimal medium to stationary phase, and then 1 ml of the culture was harvested and centrifuged. The cell pellet was resuspended in sterile distilled water. A 100-μl aliquot of the serially diluted bacterial suspension (1 × 107 CFU/ml) was inoculated into M63 glycerol minimal medium containing different concentrations of polymyxin B and salmon protamine and was incubated at 27°C for 4 h with shaking. A portion of each sample was then serially diluted and plated on YP agar containing the appropriate antibiotic. The numbers of CFU with or without treatment were compared after overnight incubation, and the sensitivity was expressed as a percentage by setting the sensitivity of the strains without polymyxin B and salmon protamine to 100%. The experiments were performed in triplicate.
Susceptibility to oxidative stress.
For testing susceptibility to oxidative stress, H2O2 at 10 mM, which corresponds to a lethal concentration (45), was added to stationary-phase cells grown in M63 glycerol minimal medium, and the cell suspensions were incubated for a further 2 h at 27°C. The number of CFU was determined by serial dilution and plating onto YP agar just prior to incubation and then every 30 min over a 2-h incubation in H2O2. The survival of the H2O2-treated cells was normalized to the number of CFU at the beginning of the challenge. Similarly, determination of resistance to osmotic challenge was carried out after incubating the stationary-phase cells in the presence of 1 M NaCl. All the experiments were performed in triplicate.
Enzyme assays.
Plate assays for major extracellular enzymes, such as pectate lyases, polygalacturonases, cellulases, and proteases, were carried out as described previously (35). A spectrophotometric assay for pectate lyase activity was done as described by Haque and Tsuyumu (18). In brief, bacterial strains were grown in M63 glycerol minimal medium until an OD at 660 nm (OD660) of 1.0 was reached. One milliliter of culture was then sonicated twice for 20 s each time (Ultrasonic Disrupter UD-200; Tomy, Tokyo, Japan) on ice and centrifuged at 20,400 × g for 5 min to remove the cell debris. Pectate lyase activity in planta was determined for a homogenate of macerated tissues that were collected using a sterile spatula. The debris was removed by centrifugation (20,400 × g for 5 min), and the supernatant was used for assaying pectate lyase activity. Ten microliters of the supernatant was added to 990 μl of the reaction buffer (0.05% polygalacturonic acid [PGA], 0.1 M Tris-HCl, pH 8.5, 0.1 mM CaCl2 prewarmed to 30°C). After the solution was mixed, the increase in the OD230 was measured every 3 min (Ultrospec 3000; Pharmacia Biotech, Cambridge, England). One unit of pectate lyase activity was defined as the amount of enzyme that produced a change in absorbance of 0.001 at 230 nm over 1 min, and the specific activity was expressed as units per milliliter (U/OD660 unit).
Quantification of exopolysaccharide.
To estimate exopolysaccharide production, bacterial strains were cultured in 5 ml of YP or M63 glycerol minimal medium at 27°C with shaking at 180 rpm until an OD660 of 1.0 was reached. One milliliter of culture was then centrifuged at 1,500 × g for 10 min at 4°C. The resultant supernatant was mixed with 3 volumes of chilled 95% ethanol and centrifuged at 9,100 × g for 20 min at 4°C to precipitate the exopolysaccharide from the culture supernatant. The supernatant was discarded, and after drying, the pellet was redissolved in distilled water. To quantify the purified exopolysaccharide, a phenol-sulfuric acid method described by Hodge and Hofreiter (20) was used. In brief, 1 ml of exopolysaccharide sample was mixed with 1 ml of 5% phenol. Five milliliters of H2SO4 was then added and mixed carefully, and the mixture was incubated at room temperature for 20 min. The concentration was measured with a spectrophotometer at an absorbance of 488 nm. The amount of exopolysaccharide was determined using a standard curve for glucose solution.
Pellicle assays.
In order to study pellicle formation, bacterial strains were precultured in YP broth to an OD660 of 0.8. About 106 CFU/ml bacteria was inoculated (1:100) into salt-optimized broth plus 2% glycerol (SOBG) medium (per liter, 20 g of tryptone, 5 g of yeast extract, 0.5 g of NaCl, 2.4 g of MgSO4, 0.186 g of KCl, and 50 ml of 40% glycerol) (64) and incubated at 27°C without shaking for 48 h.
Virulence assays.
Potato tubers were purchased from a local supermarket, surface sterilized by submersion in 1% sodium hypochlorite for 10 min, and dried on a clean bench. Single colonies of each bacterial strain were inoculated into M63 glycerol minimal medium and grown to an OD660 of 0.2 before being centrifuged and resuspended in sterile distilled water. The potato tubers were inoculated with 20 μl of a suspension containing 1 × 104 CFU using a sterile micropipette tip. Each potato tuber was inoculated with the wild-type and mutant strains separately. The tubers were incubated in a moist chamber at 27°C for 36 h. Due to the small size of the potato tubers, a second experiment was carried out in which only the complemented ΔslyA(pSlyA) strain was inoculated. Twenty tubers were inoculated for each experiment. The potato tubers were then sliced in half, and the rotten tissue generated by each strain was scraped out and weighed. The masses of rot generated by each strain were compared, and the differences between the strains were assessed for statistical significance using a two-tailed t test. To estimate bacterial populations, the whole of the macerated tissue generated by the wild type and the ΔslyA and ΔslyA(pSlyA) was collected separately, and the number of bacteria per inoculation site was determined by dilution plating.
Quantitative reverse transcription-PCR.
The wild type and the ΔslyA mutant were grown in M63 glycerol minimal medium, M63 glycerol minimal medium plus 0.4% PGA, and M63 glycerol minimal medium plus 0.4% PGA plus 1% potato tuber extract to early stationary phase, and total RNA was then isolated using a Qiagen RNeasy RNA isolation kit as described by the manufacturer (Qiagen, Hilden, Germany). The RNA was quantified using a NanoDroP ND-100 spectrophotometer (NanoDroP Technologies, Wilmington, DE). After random-decamer-primed first-strand cDNA was synthesized using the Omniscript RT kit (Qiagen, Japan), real-time PCR was performed in an MX3000p Multiplex quantitative-PCR system (Stratagene) using the SYBR Premix ExTaq RT-PCR kit (TaKaRa, Japan). Primers were designed based on D. dadantii 3937 DNA sequences obtained from ASAP (http://www.genome.wisc.edu/tools/asap.htm) (14a) using Primer Quest software (http://test.idtdna.com/Scitools/Applications/Primerquest/), and the sequences are shown in Table 2. Primer specificity was assessed by using the dissociation curve protocol on the MX3000p Multiplex quantitative-PCR system (Stratagene). The efficiencies of all primer pairs were verified. The PCR amplification conditions were as follows: denaturing at 95°C for 30 s, annealing at 50°C for 60 s, and extension at 72°C for 30 s for 50 cycles. All PCR experiments were performed in triplicate, and standard deviations were calculated. The fluorescence intensity of SYBR green at each point of the annealing phase was detected, and the cycle threshold (CT) of each sample was calculated. The calculated CT data were used for quantitative analysis by the comparative CT method. For each amplification run, the calculated CT for each gene amplification was normalized to the CT of the 16S rRNA gene amplified from the corresponding sample before the difference between the wild type and the mutant was calculated using the following formula: change = 2−ΔΔCT, where ΔΔCT for gene j = (CT,j − CT,16S rRNA)mutant − (CT,j − CT,16S rRNA)wild type.
TABLE 2.
Primers used in this study
| Primer set | Sequence (5′→3′) |
|---|---|
| pelA-F | AACATTCCGGCTAACACC |
| pelA-R | GGTAACGGTATACCGGATCT |
| pelB-F | ACCAAAGGCATCACCATC |
| pelB-R | GTCACGTTGCTGTACAGG |
| pelC-F | CAACGGTTCTTCCGCTAAC |
| pelC-R | GACGTACGTTCAGACCAGA |
| pelD-F | TCAGCGTTCCGTCCAACA |
| pelD-R | TGCCAAGACCGAAGCTGT |
| pelE-F | AGCATTCCGTCCAACACC |
| pelE-R | CCGATACCGAAGCTGTACTG |
| pelI-F | GTCGTCAGCGAAAATCAGG |
| pelI-R | GCCACAATTTCCCGTGTTC |
| pelL-F | GTACAGGCGTCTTATGGG |
| pelL-R | ATGTTTCTGCCCTGACTG |
| pelX-F | CAAACCGTCGGCTCCATTC |
| pelX-R | GCTGTAATCGCCGTCCATC |
| pelZ-F | ACCACTGCAGCTTTGCCT |
| pelZ-R | GCCAGATTGTCGCTCATCCA |
| phoP-F | CCAAACCGTTCCACATCGAG |
| phoP-R | AGCGACTCCTTGCTGACTAC |
| phoQ-F | GGGTTGGTTCGTGTATGG |
| phoQ-R | TATTTCGCTGCGTGTCTG |
| 16S rRNA-F | AGAGGATGACCACCCACACT |
| 16S rRNA-R | CGCATTACACCGCTACACACCT |
Purification of the recombinant SlyA.
The slyA gene was amplified by PCR using primers SlyA_F (5′-CCGCATATGGAATTGCCGTTAGGTTCT-3′) and SlyA_R (5′-GCGGTCGACTTAAGACTGACTCTCATG-3′), which have NdeI and SalI recognition sites, respectively, at their 5′ ends, and D. dadantii 3937 genomic DNA as a template. The amplified fragment was ligated into a pGEM-T Easy vector (Promega) to construct plasmid pGEMSlyA and then subcloned into the NdeI and SalI sites of a pET28a(+) vector (Novagen, Madison, WI) to generate the expression construct pETSlyA. The construct was then transformed into E. coli strain BL21(DE3) (Nippon Gene, Japan). A culture of the recombinant E. coli BL21(DE3) strain carrying pETSlyA was grown aerobically at 37°C in LB medium with 50 μg/ml kanamycin until mid-log phase (OD600 = 0.5 to 0.6) before the addition of inducer (1 mM IPTG [isopropyl-β-d-thiogalactopyranoside]). The culture was further incubated at 37°C for 3 h, and cells were then collected by centrifugation. The harvested cells were resuspended in buffer (20 mM sodium phosphate, 500 mM NaCl, 20 mM imidazole, and 8 M urea, pH 7.6) and sonicated on ice using an Ultrasonic Disruptor UD-200 (Tomy Inc., Tokyo, Japan) with duty cycle 60 and input 4 for 5 min with 3- to 5-min intervals. The cell lysate was collected by centrifugation. The crude lysate was then filtered through a Steradisc 25 (pore size, 0.45 μm; Kurabo, Osaka, Japan) and loaded on a 1-ml HisTrap column from a HisTrap kit (GE Healthcare Biosciences AB, Uppsala, Sweden). The purification of His6-SlyA was performed according to the manufacturer's instructions with minor modifications. In brief, the bound His6-SlyA was eluted with elution buffer (20 mM sodium phosphate, 500 mM NaCl, 500 mM imidazole, and 8 M urea, pH 7.5). The eluted fractions were dialyzed against dialysis buffer (20 mM Tris-Cl, pH 7.4, 500 mM sodium chloride, and 20% glycerol). The partially purified His6-SlyA protein was then subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane in a Trans-Blot SD semidry transfer cell (Bio-Rad). The His6-SlyA protein spot was excised from the membrane and subjected to N-terminal amino acid sequencing with a protein sequencer (PPSQ-21A; Shimadzu, Japan).
Gel mobility shift assay.
The electrophoretic mobility shift assay (EMSA) was performed as described previously (4) with minor modifications. The 290-bp region (278 bp upstream and the 12-bp coding region of phoP) was amplified by PCR with phoP_F (5′-TCTAAGCTTGGCGGCATTCTCTCG-3′) and phoP_R (5′-CAGGGATCCAAGAATGCGCATGAC-3′) primers, and the amplified fragment was ligated to a pGEM-T vector (Promega) to generate plasmid pGEMphoP. After the orientation of the fragment in pGEMphoP was confirmed, the probe was prepared by PCR using primers phoP_F and T7 (5′-CCATGGCCGCGGGAT-3′) labeled with fluorescent rhodamine dye (Fasmac, Atsugi, Japan) with pGEMphoP as the template. The labeled phoP promoter fragment was purified from the agarose gel after electrophoresis using an Illustra GFX PCR DNA and Gel Purification Kit (GE Healthcare, Buckinghamshire, United Kingdom). For EMSA, 25 ng of labeled promoter fragment and an appropriate concentration of partially purified protein (50 nM, 100 nM, 200 nM, and 400 nM) were mixed and incubated in a binding buffer [4 mM Tris-Cl, pH 8.0, 0.1 mM EDTA, 50 mM KCl, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 1 μg poly(dI-dC)·poly(dI-dC), 2 μg bovine serum albumin, and 10% glycerol]. After incubation for 30 min at 27°C, the reaction mixture was loaded onto a 6% polyacrylamide nondenaturing gel in high-ionic-strength buffer (1× Tris-borate-EDTA), and electrophoresis was carried out in the same buffer for about 2 h. The gel was then visualized with a Pharos FX Plus Molecular Imager using Quantity One one-dimensional gel analysis software (Bio-Rad, Hercules, CA).
RESULTS
Generation of the ΔslyA mutant.
The slyA gene of D. dadantii 3937 was cloned into the pGEM-T Easy vector, and its nucleotide sequence was confirmed (data not shown). The sequence data showed that the 438-bp ORF of slyA encodes a protein of 145 amino acids. Homology searches revealed that the slyA ORF of D. dadantii 3937 had 85, 74, and 75% similarity to the translated products of Hor from P. carotovorum subsp. carotovorum (ID-AAD50820), SlyA of E. coli K-12 (ID-ABE-005495) and S. enterica serovar Typhimurium (ID-ABS-0083026), and RovA of Y. pseudotuberculosis (ID-ACZ-0002372), respectively, at the amino acid level. A slyA gene deletion mutant (denoted ΔslyA) was constructed as described in Materials and Methods. A mutation in the slyA gene was confirmed by PCR and DNA sequencing (data not shown) following the selection of five independent colonies exhibiting the same phenotypes, e.g., pectate lyase production and pellicle formation. When the growth rate of the ΔslyA mutant was compared with that of the wild-type strain in YP and M63 glycerol minimal media, no significant difference was observed (data not shown).
Reduced virulence of the ΔslyA mutant.
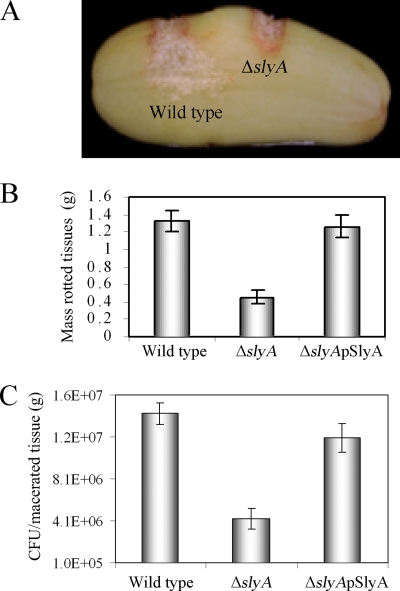
The impact of the ΔslyA mutant on the virulence of D. dadantii 3937 was evaluated in potato tubers by measuring the extent of macerated tissue 36 h postinoculation. The ΔslyA mutant was significantly reduced (threefold [P < 0.0001]) in virulence compare to the wild-type strain (Fig. 1A). When the plasmid pSlyA (with a slyA gene on a multicopy vector, pGEM-T Easy) was introduced into ΔslyA, virulence recovered to the wild-type level (Fig. 1B) (P > 0.42). Bacterial populations of the ΔslyA mutant in potatoes were found to be reduced significantly (1 order of magnitude; P < 0.0001) compared to the wild-type strain (Fig. 1C). Furthermore, when pectate lyase production levels by the wild type and the ΔslyA mutant in macerated potato tubers were compared, the specific enzymatic activity of the ΔslyA mutant was reduced to 7.12% (a 14-fold reduction) with respect to that of the wild-type strain.
FIG. 1.
Inactivation of slyA attenuates the virulence of D. dadantii 3937. The bacterial inoculum of 20 μl contained 104 CFU/ml of the wild type (D. dadantii 3937), the ΔslyA mutant, and the complementation strain [ΔslyA(pSlyA)]. The disease symptoms (A), mass of the rotted tissues (B), and bacterial populations in macerated tissue (C) were documented after 36 h of incubation at 27°C with high relative humidity. The error bars indicate standard deviations.
Failure of hyperinduction of pectate lyase in the ΔslyA mutant.
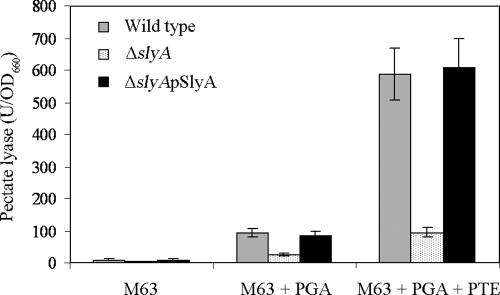
When bacteria were grown in M63 glycerol minimal medium (noninduced conditions), the basal level of pectate lyase production by the wild type was higher than that by the ΔslyA mutant (Fig. 2). Pectate lyase production was greatly induced (9.7-fold) in the wild type compared to the ΔslyA mutant when the M63 glycerol minimal medium was supplemented with 0.4% PGA (Fig. 2). Furthermore, when bacterial strains were grown in M63 glycerol minimal medium with 0.4% PGA and 1% potato tuber extract, pectate lyase synthesis was hyperinduced (61-fold) in the wild type but not in the ΔslyA mutant (Fig. 2). The ΔslyA mutant transformed with pSlyA restored pectate lyase production to the wild-type level (Fig. 2). However, the synthesis of polygalacturonases, cellulases, and proteases was not affected under the same conditions (data not shown). Thus, the SlyA homologue of D. dadantii 3937 appears to play an important role specifically in the regulation of pectate lyase, but not of the other extracellular enzymes tested.
FIG. 2.
Pectate lyase specific activity. Strains were grown in M63 glycerol minimal medium (noninduced), M63 glycerol minimal medium plus 0.4% PGA (induced), and M63 glycerol minimal medium plus 0.4% PGA plus 1% potato tuber extract (PTE) (hyperinduced) to an OD660 of 1.0 before the total pectate lyase specific activity was determined with a spectrophotometer. The mean of pectate lyase activities from five independent experiments was expressed as the specific activity (U/OD660). The error bars indicate the standard deviations.
Expression of pel genes under various growth conditions.
D. dadantii 3937 produces numerous pectate lyase isozymes encoded by pel genes. In order to identify which pel genes are controlled by slyA, the transcriptional profile of a D. dadantii 3937 mutant strain lacking a functional slyA gene was compared with that of the wild-type strain under noninduced, induced, and hyperinduced conditions by quantitative reverse transcription-PCR (Table 3). The transcription levels of pelA, pelB, pelC, pelD, pelE, pelI, and pelL were lower in the ΔslyA mutant than in the wild-type strain under noninducing conditions. However, the addition of PGA and PGA plus potato tuber extract showed different effects on the individual pel genes. The transcription levels of pelA, pelD, pelE, pelI, pelL, and pelZ showed greater induction in the presence of PGA than in M63 glycerol minimal medium (noninduced) only in the wild-type background. However, in the presence of PGA plus potato tuber extract, the transcription levels of pelD, pelI, and pelL showed hyperinduction in the wild-type but not in the ΔslyA background. Thus, the slyA homologue of D. dadantii 3937 appears to be involved specifically in the product induction (60) of all isozymes of pectate lyase and in the hyperinduction of one major and two minor pel genes.
TABLE 3.
Differential expression of pel genes under various growth conditions
| Gene name | Fold change compared to the ΔslyA mutanta
|
||
|---|---|---|---|
| M63 glycerol minimal medium | M63 glycerol minimal medium + 0.4% PGA | M63 glycerol minimal medium + 0.4% PGA + 1% potato tuber extract | |
| pelA | 0.9 ± 0.2 | 12.8 ± 1.3 | 16.5 ± 2.7 |
| pelB | 0.4 ± 0.03 | 0.7 ± 0.04 | 3.3 ± 0.7 |
| pelC | 0.6 ± 0.06 | 0.7 ± 0.04 | 2.9 ± 0.8 |
| pelD | 0.9 ± 0.08 | 13.5 ± 1.4 | 1052.7 ± 94 |
| pelE | 0.5 ± 0.02 | 1.3 ± 0.09 | 9.7 ± 0.9 |
| pelI | 0.6 ± 0.02 | 11.9 ± 1.8 | 1031.3 ± 83 |
| pelL | 0.3 ± 0.06 | 42.8 ± 3.3 | 826.7 ± 79 |
| pelZ | 0.56 ± 0.1 | 4.9 ± 0.7 | 14.3 ± 3.6 |
Expressed as the ratio of the specific gene expression level in the wild type compared to that in the ΔslyA mutant normalized to the level of expression of the 16S rRNA gene.
Stress response of the ΔslyA mutant.
Members of the MarR family in animal-pathogenic bacteria have shown resistance to multiple antibiotics, household disinfectants, and oxidative stress (1, 2, 7). When the sensitivities of the wild-type and ΔslyA mutant strains to various antibiotics, including tetracycline (0.1 μg/ml), chloramphenicol (0.1 μg/ml), erythromycin (0.01 μg/ml), and rifampin (rifampicin) (0.1 μg/ml), were compared, no significant differences were observed (data not shown). However, after 15 min of exposure to 1% sodium hypochlorite, there was an 80% reduction in the survival of the ΔslyA mutant compared to the wild-type strain (data not shown).
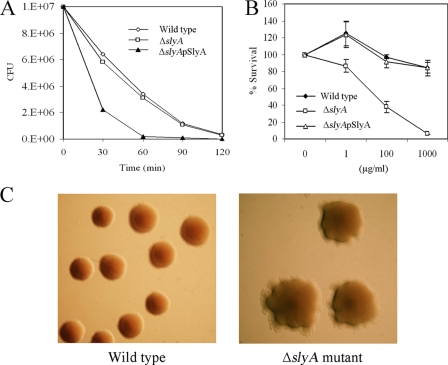
When stationary-phase cultures of the wild type and the ΔslyA mutant were diluted to 107 CFU/ml in M63 glycerol minimal medium and exposed to 10 mM H2O2 and the number of viable cells was determined as a function of time, the survival of the ΔslyA mutant was significantly lower than that of the wild-type strain (Fig. 3A). However, when plasmid pSlyA was transformed into the ΔslyA mutant, sensitivity to H2O2 returned to the wild-type level.
FIG. 3.
Sensitivity tests. (A) Sensitivities of the different bacterial strains to H2O2. The data represent one of three separate experiments, which gave similar results. (B) Sensitivity to polymyxin B, showing the mean and standard deviation of five separate experiments. (C) Colony morphologies of the wild type and the ΔslyA mutant after exposure to hydrogen peroxide. The photographs represent one of three separate experiments, which gave similar results.
A Salmonella mutant lacking a functional slyA gene was reported to be highly sensitive to polymyxin B due to alteration of cell surface properties, mainly modification of lipopolysaccharide (54). The sensitivities of D. dadantii 3937 wild type and ΔslyA to the cationic antimicrobial peptide (CAMP) polymyxin B and a linear antimicrobial peptide, salmon protamine, were compared. The ΔslyA mutant showed increased sensitivity to polymyxin B compared to the wild-type strain (Fig. 3B), but no difference was found in the sensitivities to protamine (data not shown). Resistance to polymyxin B in the ΔslyA mutant was completely restored by complementation with the slyA gene and its promoter on a multicopy vector, pGEM-T Easy, confirming that SlyA is essential for the resistance exhibited to polymyxin B in D. dadantii 3937.
SlyA homologues of S. enterica serovar Typhimurium, E. coli, and Y. pseudotuberculosis are regulated by various environmental cues, such as temperature, pH, and osmolarity (9, 36). However, sensitivity to high temperature (37°C), acidic pH (4.0 to 5.5), and high osmolarity (1 M NaCl) were indistinguishable between the wild type and the ΔslyA mutant in D. dadantii 3937, suggesting that SlyA of D. dadantii 3937 may not be involved in these forms of regulation. Although the wild type and the ΔslyA mutant had similar appearances when grown in YP and on minimal medium agar plates without treatment, ΔslyA colonies did respond to hydrogen peroxide (Fig. 3C), sodium hypochlorite, polymyxin B, high temperature, acidic pH, and high osmolarity (data not shown) by showing irregular or wrinkled colonies compared to the normal smooth and round colonies of the wild type (Fig. 3C). This result suggested that SlyA mediates alteration of the cell surface properties of D. dadantii 3937, as reported for Salmonella (54).
Reduced exopolysaccharide production.
Synthesis of exopolysaccharide is an important pathogenicity factor for many plant-pathogenic bacteria, including D. dadantii 3937 (8, 11, 29). When the wild-type and ΔslyA mutant strains were grown in M63 glycerol minimal medium until early stationary phase (OD660 = 1.0), production of exopolysaccharide by the ΔslyA mutant (0.31 ± 0.04 mg/ml) was reduced to 50% of the wild-type strain production (0.59 ± 0.07 mg/ml). Similar results were also found when the bacterial strains were grown in YP medium (data not shown). Thus, SlyA appears to be required for exopolysaccharide production in D. dadantii 3937.
It has been reported that exopolysaccharide production and motility are coregulated in many plant-associated bacteria (6, 38, 65). When the wild-type and ΔslyA mutant strains were grown overnight in YP or M63 glycerol minimal medium and the cultures were examined for motility under a phase-contrast microscope, both the wild type and the ΔslyA mutant were motile (data not shown). Conversely, the ΔslyA mutant (22 ± 0.67 mm) migrated slightly faster than the wild-type strain (19 ± 0.8 mm) through 0.3% YP soft-agar plates. However, when purified flagellin proteins from the wild type and the ΔslyA mutant were analyzed by SDS-PAGE, the amounts of flagellin protein were indistinguishable between the wild type and the ΔslyA mutant (data not shown). Thus, we are currently unable to correlate exopolysaccharide production and motility, which is considered to be one of the important virulence factors in many soft-rotting bacteria. Similarly, cytR, encoding a transcriptional repressor, was shown to negatively regulate motility by controlling the expression of genes required for flagellum biosynthesis without affecting exopolysaccharide production in P. carotovorum subsp. carotovorum (35).
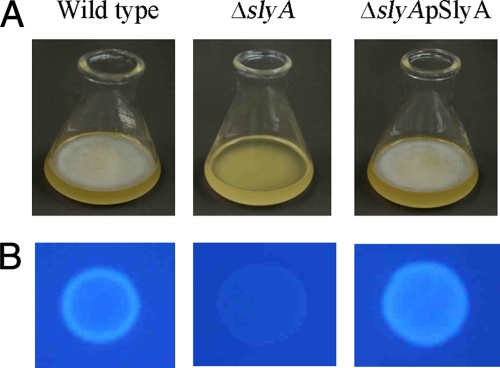
Inability to form pellicles.
After 48 h of incubation at 27°C, the wild type and the ΔslyA(pSlyA) strain formed thick and rigid pellicles (a bacterial network at the air-liquid interface that blocks the surface of the standing culture) on SOBG medium, whereas the ΔslyA mutant was unable to form a pellicle (Fig. 4A). In the enterobacterial animal and plant pathogens S. enterica serovar Typhimurium, E. coli, and D. dadantii 3937, it has been reported that cellulose contributes to pellicle formation (48, 55, 64). To determine whether cellulose is also a component of the pellicle formed by D. dadantii 3937, cells at a density of 105 CFU/ml were placed on M63 glycerol minimal medium agar plates containing the cellulose binding dye calcofluor (200 μg/ml) and incubated at 27°C for 48 h before being checked under UV light (366 nm). The wild-type and ΔslyA(pSlyA) strains induced bright fluorescence, while the ΔslyA mutant did not (Fig. 4B). Thus, the SlyA homologue may positively regulate pellicle formation, and those pellicles have been confirmed to contain cellulose in D. dadantii 3937.
FIG. 4.
(A) Pellicle formation following growth in SOBG medium without shaking at 27°C for 48 h. (B) Strains were grown in M63 glycerol minimal medium agar plates with calcofluor at 27°C for 48 h and then exposed to UV light. The photographs represent one of three separate experiments, which gave similar results.
SlyA regulates the expression of the phoP-phoQ operon.
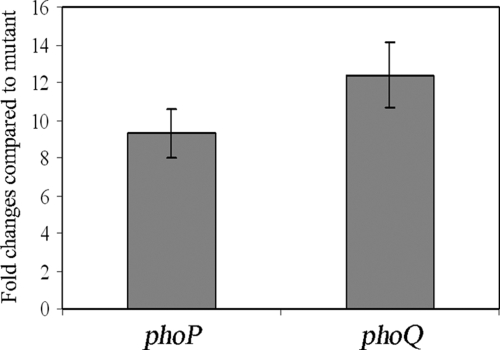
The ΔslyA mutant of D. dadantii 3937 showed phenotypes similar to those of phoP and phoQ mutants, including reduced virulence, hyperinduction of pectate lyase, and sensitivity to CAMP (18). To see whether SlyA has an effect on the transcription of phoP and phoQ, we determined the expression of these genes following growth of the wild type and the ΔslyA mutant in M63 glycerol minimal medium supplemented with low (10 μM) or high (10 mM) concentrations of magnesium. The phoP and phoQ expression levels were then analyzed by quantitative reverse transcription-PCR. The expression levels of phoP and phoQ were indistinguishable in the wild type and the ΔslyA mutant at high magnesium concentrations (data not shown). However, at low magnesium concentrations, both phoP and phoQ showed increased levels of transcription in the wild type compared to that in the ΔslyA mutant (Fig. 5). The reduced expression levels of phoP and phoQ in the ΔslyA mutant were recovered by introducing plasmid pSlyA into the ΔslyA mutant (data not shown), indicating that SlyA may play a role in regulating the transcription of the phoP-phoQ operon in D. dadantii 3937.
FIG. 5.
Effect of slyA on phoP-phoQ expression. Bacterial strains were grown in M63 glycerol minimal medium containing a low concentration (10 μM) of magnesium plus 0.4% PGA plus 1% potato tuber extract to an OD660 of 1.0 before total RNA was harvested. The expression levels of phoP and phoQ in a ΔslyA background were compared to those in the wild type by quantitative reverse transcription-PCR and normalized to the level of expression of the 16S rRNA gene. The results show the means of three replicates. The error bars indicate the standard deviations.
The SlyA protein binds to the promoter region of the phoP gene.
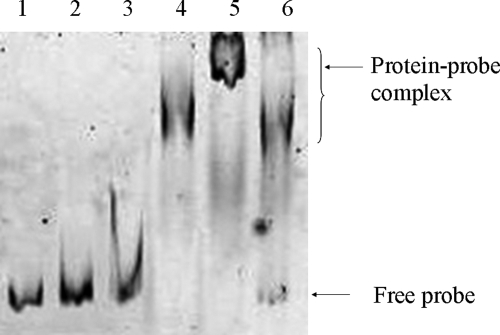
Inspection of the phoP promoter region revealed that a putative SlyA binding site (ATAGTTCACTAA; the consensus is TTAGCAAGCTAA) is present in the promoter region (76 to 65 bp upstream of the translational start site). We speculated that SlyA possibly binds to this site and regulates phoP expression. In order to determine whether SlyA physically binds or associates with the phoP promoter region, polyhistidine-tagged SlyA (His-SlyA) was purified (Fig. 6) and EMSA analysis was performed with the protein. It was revealed that His-SlyA was able to bind to a phoP promoter fragment in a concentration-dependent manner (Fig. 7). Binding was observed at a His-SlyA concentration of 200 nM. At a His-SlyA concentration of 400 nM, a binding complex with a higher molecular weight (Fig. 7, lane 5) was observed, with apparently complete binding of the probe. At 200 nM, the protein possibly formed a dimer, and at a higher concentration (400 nM), it possibly formed a tetramer. When a 50-fold excess of unlabeled promoter fragment was used, unbound labeled probe was detected at the bottom of the gel, indicating that SlyA specifically binds to the phoP promoter region (Fig. 7, lane 6).
FIG. 6.
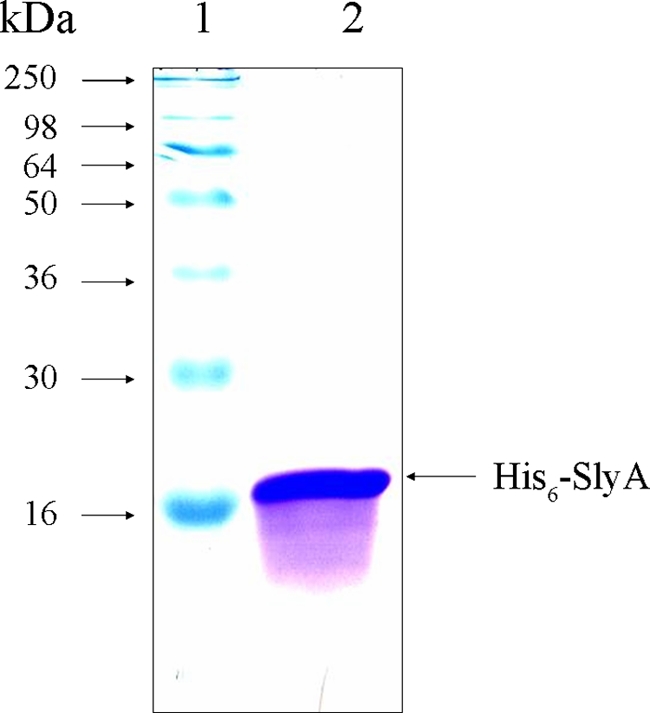
SDS-PAGE of recombinant His6-SlyA. E. coli BL21(DE3) carrying pETSlyA was induced by IPTG and purified as described in Materials and Methods. The purified protein (lane 2) was subjected to 12% SDS-PAGE. Molecular mass standards were loaded in lane 1.
FIG. 7.
EMSA using the promoter region of phoP and purified His-SlyA protein. Labeled probe (25 ng) was incubated in the absence (lane 1) or presence of increasing amounts of protein (50, 100, 200, and 400 nM) (lanes 2 to 5, respectively) or in the presence of labeled probe (25 ng), protein (400 nM), and a 50-fold excess of unlabeled probe as a specific competitor (lane 6).
DISCUSSION
In this study, we demonstrated that a mutant strain lacking a functional slyA gene in D. dadantii 3937 had pleiotropic effects: (i) diminished virulence in potato tubers; (ii) decreased survival ability in its host, potato; (iii) increased sensitivity to the CAMP polymyxin B, sodium hypochlorite, and oxidative stress; (iv) reduced exopolysaccharide production; (v) inability to form pellicles; and (vi) failure of hyperinduction of pectate lyase production while normal levels of polygalacturonases, cellulases, and proteases were observed. Changes in these phenotypes in the ΔslyA mutant were restored by introducing the slyA homologue of D. dadantii 3937 on a multicopy vector, pGEM-T Easy [ΔslyA(pSlyA)]. Thus, SlyA is a global transcriptional regulator involved in the regulation of the synthesis of a large group of virulence-associated factors in D. dadantii 3937.
SlyA was originally identified as an S. enterica serovar Typhimurium gene product by screening for cytolysin on blood agar plates and was first thought to encode a cytolysin, a cytotoxic protein that forms stable, cation-selective transmembrane pores. It was later shown to be a regulator, activating the expression of a cryptic cytolysin in E. coli K-12 (13, 34). It has been reported that Salmonella slyA mutant strains are reduced in virulence and are unable to colonize the Peyer's patches, mesenteric lymph nodes, liver, and spleen (7, 13, 30). It has also been reported that the reduced virulence of the slyA mutant of S. enterica serovar Typhimurium is, in part, due to hypersusceptibility to reactive oxygen species (7). The ΔslyA mutant of D. dadantii 3937 also had high sensitivity to oxidative stress. Thus, the increased sensitivity of the ΔslyA mutant to hydrogen peroxide might have reduced its population in planta, which ultimately resulted in lower levels of disease than with the wild type. RovA, whose amino acid sequence is 75% identical to that of D. dadantii 3937 SlyA, also plays an important role in regulating the invasion of mammalian cells by Yersinia and mediates the regulation of the invasion response to environmental signals (36).
The Hor regulator, a SlyA homologue reported by Thomson et al. (58) in the phytopathogenic bacteria P. carotovorum subsp. carotovorum, has some common features with the SlyA protein of D. dadantii 3937, as the corresponding mutant is reduced in pectate lyase production and virulence. Sensitivity to antimicrobial peptides, susceptibility to hydrogen peroxide and sodium hypochlorite, pellicle formation, and exopolysaccharide production have been described for P. carotovorum subsp. carotovorum, too (58). However, it seems that there are differences between the P. carotovorum subsp. carotovorum and D. dadantii mutants, as polygalacturonase and cellulase production is affected in the former but not in the latter.
PecS, a MarR family transcriptional regulator, is required for pathogenesis in D. dadantii 3937 (46). It is interesting that both PecS and SlyA are involved in both pectate lyase production and sensitivity to oxidative stress. Here, we observed that the ΔslyA mutant was sensitive to oxidative stress and reduced pectate lyase production, whereas the pecS mutant was more resistant to oxidative stress (45) and increased pectate lyase production (45). In addition, PecS negatively affects the expression of cellulase and flagellar biosynthesis (42, 46, 49) and also positively regulates the expression of polygalacturonase (37). However, the expression of cellulase, polygalacturonase, and flagellar biosynthesis was not regulated by SlyA of D. dadantii 3937 (data not shown). These differences in the production of plant cell-wall-degrading enzymes, sensitivity to oxidative stress, and flagellar biosynthesis may be distinguishable between PecS and SlyA.
Increased sensitivity to polymyxin B, as in the ΔslyA mutant of D. dadantii 3937, is also reported in the slyA mutant of S. enterica serovar Typhimurium. In S. enterica serovar Typhimurium, the increased sensitivity to the CAMP is due to the effects of genes regulated by the PhoP-PhoQ system, namely, pmrA-pmrB (15, 16), pagP (17), and ugtL (54). In all these cases, the gene product modifies the cell surface properties of the bacterium by changing the structure of lipid A in the bacterial lipopolysaccharide. pmrA-pmrB are responsible for adding 1 unit of 4-aminoarabinose, and pagP adds one extra acyl group to lipopolysaccharide. Such modification may lead to altered resistance to the CAMP polymyxin B (16). We speculate that a similar mechanism operates in D. dadantii 3937, as genes with high homology to pmrA (60%), pmrB (51%), and pagP (70%) are found in the D. dadantii 3937 genome database (http://asap.ahabs.wisc.edu/annotation/php/ASAP1.html). It should be noted that lipopolysaccharide plays an important role in the pathogenesis of D. dadantii 3937 and Ralstonia solanacearum (53, 59).
Exopolysaccharides are considered to be essential for bacterial growth, survival under environmental stress conditions, and virulence in many plant-pathogenic bacteria (8, 31, 32). In the case of Pseudomonas syringae, it has been reported that the exopolysaccharide alginate contributes to virulence and epiphytic fitness by facilitating colonization and/or dissemination of the bacterium in planta (65). The mutants of P. syringae pv. syringae with defective exopolysaccharide production, such as algT, aefR, and the double mutant ahlI-ahlR, exhibited increased sensitivity to hydrogen peroxide and heat shock (25, 43). In the case of D. dadantii 3937, a pecT mutant was shown to have reduced exopolysaccharide production and virulence on saintpaulia (8). We observed that the ΔslyA mutant had significantly reduced exopolysaccharide production and virulence on its host, potato, suggesting that exopolysaccharide is an important virulence factor in D. dadantii 3937. Recently, Corbett and associates (9) reported that SlyA is involved in the regulation of capsular polysaccharide (K5) in E. coli.
In contrast, pellicles are essential for the survival of bacteria under various environmental stress conditions. For examples, S. enterica serovar Typhimurium cells within pellicles showed resistance to chlorine treatment (52). Solano et al. (55) also demonstrated that cellulose is a component of the pellicle and that cellulose is required for chlorine resistance by Salmonella, since cellulose-negative mutants did not survive under low concentrations of NaOCl. However, cellulose deficiency does not affect Salmonella virulence (55). In our present study, the ΔslyA mutant was unable to form pellicles and did not produce cellulose (Fig. 4). Thus, formation of a pellicle by SlyA may play an important part in the survival of D. dadantii cells under unfavorable environmental stress conditions.
Salmonella slyA mutants exhibit phenotypes similar to those displayed by phoP and phoQ mutants (30, 54). The PhoP-PhoQ two-component regulatory system is required for transcription from one of the promoters of the slyA gene in Salmonella (38), suggesting that the phenotypes displayed by phoP and phoQ mutants could be caused by their inability to express the SlyA protein and implying that SlyA participates in the transcription of a subset of PhoP-regulated genes. It has been suggested that the PhoP protein regulates the slyA gene indirectly, because a PhoP box could not be identified in the slyA promoter (41). However, Shi et al. (54) argued that PhoP controls slyA transcription directly. Furthermore, Song and associates (56) demonstrated that SlyA fine tunes the cellular level of the PhoP-PhoQ system and participates in a positive feedback loop, which facilitates transcription of the phoPQ loci, in turn stimulating transcription of the PhoP regulon. We observed that SlyA regulates expression of phoP and phoQ in response to magnesium (Fig. 5). Furthermore, the transcription levels of genes encoding the pectate lyases PelA, PelB, PelC, PelD, and PelE, previously identified as being controlled by PhoQ (61), were found to be regulated by SlyA of D. dadantii 3937 (Table 3). These findings prompted us to speculate that SlyA may regulate the expression of the phoP gene. EMSA clearly demonstrated the specific binding of the SlyA protein to the phoP promoter region. Thus, SlyA possibly regulates the pathogenicity-related phenotypes by utilizing the PhoP regulator. Therefore, it may provide evidence for an intimate link between PhoP-PhoQ and SlyA in D. dadantii 3937.
The ability of D. dadantii 3937 to macerate plant tissue results from the actions of at least five major and five minor pel genes. A basal level of pel gene expression was observed in D. dadantii 3937 in the absence of induction substrates (21). In the presence of pectin and its derivatives from host plants, pectinase synthesis is further induced, leading to symptom development. It has been reported that during plant infection, pelD, pelE, and pelI are highly expressed in potato tubers, whereas pelA, pelB, pelC, pelL, pelZ, and pemA are moderately expressed (47). Using a Gus reporter, Jafra and associates (23) showed that pelI and pelL of D. dadantii 3937 were highly (10-fold) induced in potato tubers. Recently, Peng et al. (41) discovered that a significant increase in pelD expression occurred in potato tubers 24 h postinoculation. Minor pel genes have been shown to be induced only in plant tissue or in the presence of plant extract (26). In this study, we showed that pectate lyase production is hyperinduced in planta. Accordingly, when bacteria were grown in M63 glycerol minimal medium, along with 0.4% PGA and 1% potato tuber, transcript levels of pelD, pelI, and pelL were increased (Table 3). The roles of the pelD, pelI, and pelL genes in pathogenicity have been reported previously (23). Thus, SlyA may contribute to virulence in terms of hyperexpression of these pel genes in D. dadantii 3937.
In conclusion, our results show that the SlyA homologue of D. dadantii 3937 supports a wide variety of cellular functions, including synthesis of multiple virulence factors and stress tolerance. In many bacteria, the production of virulence factors is regulated by more than one mechanism to facilitate coordinated regulation. SlyA may operate as part of the multiple regulatory mechanism that governs virulence in D. dadantii 3937. Also, these results imply that SlyA has become adapted to counteract different but related stresses in plant- and animal-pathogenic bacteria. To gain a better understanding of the mechanisms of pathogenicity in D. dadantii 3937, a SlyA microarray analysis is under way.
Acknowledgments
We thank Ian K. Toth, Scottish Crop Research Institute (SCRI), Invergowrie, Dundee DD2 5DA, United Kingdom, for reading the manuscript and for helpful discussions.
This work was supported by grants from the Japan Society for Promotion of Science (JSPS) in the form of a grant-in-aid (no. 17108001) and in the form of postdoctoral fellowships awarded to M.M.H. (no. P05193) and M.S.K. (no. P07156).
Footnotes
Published ahead of print on 19 June 2009.
REFERENCES
- 1.Alekshun, M. N., and S. B. Levy. 1999. The mar regulon: multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 7410-413. [DOI] [PubMed] [Google Scholar]
- 2.Alekshun, M. N., S. B. Levy, T. R. Mealy, B. A. Seaton, and J. F. Head. 2001. The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 Å resolution. Nat. Struct. Biol. 8710-714. [DOI] [PubMed] [Google Scholar]
- 3.Atlung, T., and H. Ingmer. 1997. H-NS: a modular of environmentally regulated gene expression. Mol. Microbiol. 247-17. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. John Wiley and Sons, New York, NY.
- 5.Bauer, D. W., A. J. Bogdanove, S. V. Beer, and A. Collmer. 1994. Erwinia chrysanthemi hrp genes and their involvement in soft-rot pathogenesis and elicitation of the hypersensitive response. Mol. Plant-Microbe Interact. 7573-581. [DOI] [PubMed] [Google Scholar]
- 6.Brumbley, S. M., and T. P. Denny. 1990. Cloning of wild-type Pseudomonas solanacearum phcA, a gene that when mutated alters expression of multiple traits that contribute to virulence. J. Bacteriol. 1725677-5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchmeier, N., S. Bossie, C. Y. Chen, F. C. Fang, D. G. Guiney, and S. J. Libby. 1997. SlyA, a transcriptional regulator of Salmonella typhimurium, is required for resistance to oxidative stress and is expressed in the intracellular environment of macrophages. Infect. Immun. 653725-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condemine, G., A. Castillo, F. Passeri, and C. Enard. 1999. The PecT repressor coregulates synthesis of exopolysaccharides and virulence factors in Erwinia chrysanthemi. Mol. Plant-Microbe Interact. 1245-52. [DOI] [PubMed] [Google Scholar]
- 9.Corbett, D., H. J. Bennett, H. Askar, J. Green, and I. S. Roberts. 2007. SlyA and H-NS regulate transcription of the Escherichia coli K5 capsule gene cluster and expression of slyA in Escherichia coli is temperature-dependent, positively autoregulated and independent of H-NS. J. Biol. Chem. 28233326-33335. [DOI] [PubMed] [Google Scholar]
- 10.Daniels, J. J. D., I. B. Autenrieth, A. Ludwig, and W. Goebel. 1996. The gene slyA of Salmonella typhimurium is required for destruction of M cells and intracellular survival but not for invasion or colonization of the murine small intestine. Infect. Immun. 645075-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denny, T. P. 1995. Involvement of bacterial polysaccharides in plant pathogenesis. Annu. Rev. Phytopathol. 33173-197. [DOI] [PubMed] [Google Scholar]
- 12.El-Hassouni, M., J. P. Chambost, D. Expert, F. Van Gijsegem, and F. Barras. 1999. The minimal gene set member msrA, encoding peptide methionine sulfoxide reductase, is a virulence determinant of the plant pathogen Erwinia chrysanthemi. Proc. Natl. Acad. Sci. USA 96887-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellison, D. W., and V. L. Miller. 2006. Regulation of virulence by members of the MarR/SlyA family. Curr. Opin. Microbiol. 91-7. [DOI] [PubMed] [Google Scholar]
- 13a.Ellison, D. W., M. B. Lawrenz, and V. L. Miller. 2004. Invasin and beyond: regulation of Yersinia virulence by RovA. Trends Microbiol. 12296-300. [DOI] [PubMed] [Google Scholar]
- 14.Enard, C., A. Diolez, and D. Expert. 1988. Systemic virulence of Erwinia chrysanthemi 3937 requires a functional iron assimilation system. J. Bacteriol. 1702419-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Glasner, J. D., P. Liss, G. Plunkett III, A. Darling, T. Prasad, M. Rusch, A. Byrnes, M. Gilson, B. Biehl, F. R. Blattner, and N. T. Perna. 2003. ASAP, a systematic annotation package for community analysis of genomes. Nucleic Acids Res. 31147-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunn, J. S., and S. I. Miller. 1996. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J. Bacteriol. 1786857-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunn, J. S., K. B. Lim, J. Krueger, K. Kim, L. Guo, M. Hackett, and S. I. Miller. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 271171-1182. [DOI] [PubMed] [Google Scholar]
- 17.Guo, L., K. B. Lim, C. M. Poduje, M. Daniel, J. S. Gunn, M. Hackett, and S. I. Miller. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95189-198. [DOI] [PubMed] [Google Scholar]
- 18.Haque, M. M., and S. Tsuyumu. 2005. Virulence, resistance to magainin II and expression of pectate lyase are controlled by the PhoP-PhoQ two-component regulatory system responding to pH and magnesium in Erwinia chrysanthemi 3937. J. Gen. Plant Pathol. 7147-53. [Google Scholar]
- 19.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 1726557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodge, J. E., and B. T. Hofreiter. 1962. Determination of reducing sugars and carbohydrates, p. 388. In R. L. Whistler and M. L. Wolfrom (ed.), Methods in carbohydrate chemistry. Academic Press, New York, NY.
- 21.Hugouvieux-Cotte-Pattat, N., and J. Robert-Baudouy. 1992. Analysis of the regulation of the pelBC genes in Erwinia chrysanthemi 3937. Mol. Microbiol. 62363-2376. [DOI] [PubMed] [Google Scholar]
- 22.Ingavale, S., W. van Wamel, T. T. Luong, C. Y. Lee, and A. L. Cheung. 2005. Rat/MgrA, a regulator of autolysis, is a regulator of virulence genes in Staphylococcus aureus. Infect. Immun. 731423-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jafra, S., I. Figura, N. Hugouvieux-Cotte-Pattat, and E. Lojkowska. 1999. Expression of Erwinia chrysanthemi pectinase genes pelI, pelL, and pelZ during infection of potato tubers. Mol. Plant-Microbe Interact. 10845-851. [Google Scholar]
- 24.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109137-141. [DOI] [PubMed] [Google Scholar]
- 25.Keith, L. M. W., and C. L. Bender. 1999. AlgT (sigma 22) controls alginate production and tolerance to environmental stress in Pseudomonas syringae. J. Bacteriol. 1817176-7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelemu, S., and A. Collmer. 1993. Erwinia chrysanthemi EC16 produces a second set of plant-inducible pectate lyase isozymes. Appl. Environ. Microbiol. 591756-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacikova, G., W. Lin, and K. Skorupski. 2004. Vibrio cholerae AphA uses a novel mechanism for virulence gene activation that involves interaction with the LysR-type regulator AphB at the tcpPH promoter. Mol. Microbiol. 53129-142. [DOI] [PubMed] [Google Scholar]
- 28.Lawrenz, M. B., and V. L. Miller. 2007. Comparative analysis of the regulation of rovA from the pathogenic yersiniae. J. Bacteriol. 1895963-5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leigh, J. A., and D. L. Coplin. 1992. Exopolysaccharides in plant bacterial interaction. Annu. Rev. Microbiol. 46307-346. [DOI] [PubMed] [Google Scholar]
- 30.Libby, S. J., W. Goebel, A. Ludwig, N. Buchmeier, F. Bowe, F. C. Fang, D. G. Guiney, J. G. Songer, and F. Heffron. 1994. A cytolysin encoded by Salmonella is required for survival with macrophages. Proc. Natl. Acad. Sci. USA 91489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindow, S. E., and M. T. Brandl. 2003. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 691875-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.Linehan, S. A., A. Rytkonen, X. J. Yu, M. Liu, and D. W. Holden. 2005. SlyA regulates function of Salmonella pathogenicity island 2 (SPI-2) and expression of SPI-2-associated genes. Infect. Immun. 734354-4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loh, J., III, E. A. Pierson, L. S. Pierson, G. Stacey, and A. Chatterjee. 2002. Quorum sensing in plant-associated bacteria. Curr. Opin. Plant Biol. 5285-290. [DOI] [PubMed] [Google Scholar]
- 33.López-Solanilla, E., F. Garcia-Olemedo, and P. Rodríguez-Palenzuela. 1998. Inactivation of the sapA to sapF locus of Erwinia chrysanthemi reveals common features in plant and bacterial pathogenesis. Plant Cell 10917-924. [PMC free article] [PubMed] [Google Scholar]
- 34.Ludwig, A., C. Tengel, S. Bauer, A. Bubert, R. Benz, H. J. Mollenkopf, and W. Goebel. 1995. SlyA, a regulatory protein from Salmonella typhimurium, induces a haemolytic and pore-forming protein in Escherichia coli. Mol. Gen. Genet. 249474-486. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto, H., H. Muroi, M. Umehara, Y. Yoshitake, and S. Tsuyumu. 2003. Peh production, flagellum synthesis, and virulence reduced in Erwinia carotovora subsp. carotovora by mutation in a homologue of cytR. Mol. Plant-Microbe Interact. 16389-397. [DOI] [PubMed] [Google Scholar]
- 36.Nagel, G., A. Lahrz, and P. Dersch. 2001. Environmental control of invasion expression in Yersinia pseudotuberculosis is mediated by regulation of RovA, a transcriptional activator of the SlyA/Hor family. Mol. Microbiol. 411249-1269. [DOI] [PubMed] [Google Scholar]
- 36a.Nasser, W., M. Faelen, N. Hugouvieux-Cotte-Pattat, and S. Reverchon. 2001. Role of the nucleoid-associated protein H-NS in the synthesis of virulence factors in the phytopathogenic bacterium Erwinia chrysanthemi. Mol. Plant-Microbe Interact. 1410-20. [DOI] [PubMed] [Google Scholar]
- 37.Nasser, W., V. E. Shevchik, and N. Hugouvieux-Cotte-Pattat. 1999. Analysis of three clustered polygalacturonase genes in Erwinia chrysanthemi 3937 revealed an anti-repressor function for the PecS regulator. Mol. Microbiol. 34641-650. [DOI] [PubMed] [Google Scholar]
- 38.Navarre, W. W., T. A. Halsey, D. Walthers, J. Frye, M. McClelland, J. L. Potter, L. J. Kenney, J. S. Gunn, F. C. Fang, and S. J. Libby. 2005. Co-regulation of Salmonella enterica genes required for virulence and resistance to antimicrobial peptides by SlyA and PhoP/PhoQ. Mol. Microbiol. 56492-508. [DOI] [PubMed] [Google Scholar]
- 39.Nomura, K., W. Nasser, H. Kawagishi, and S. Tsuyumu. 1998. The pir gene of Erwinia chrysanthemi EC16 regulates hyperinduction of pectate lyase virulence genes in response to plant signals. Proc. Natl. Acad. Sci. USA 9514034-14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norte, V. A., M. R. Stapleton, and J. Green. 2003. PhoP-responsive expression of the Salmonella enterica serovar Typhimurium slyA gene. J. Bacteriol. 1853508-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng, Q., S. Yang, A. O. Charkowski, M.-N. Yap, D. A. Steeber, N. T. Keen, and C.-H. Yang. 2005. Population behavior analysis of dspE and pelD regulation in Erwinia chrysanthemi 3937. Mol. Plant-Microbe Interact. 19451-457. [DOI] [PubMed] [Google Scholar]
- 41a.Perombelon, M. C. M. 2002. Potato diseases caused by soft rot erwinias: an overview of pathogenesis. Plant Pathol. 511-12. [Google Scholar]
- 42.Praillet, T., W. Nasser, J. Robert-Baudouy, and S. Reverchon. 1996. Purification and functional characterization of PecS: a regulator of virulence factor synthesis in Erwinia chrysanthemi. Mol. Microbiol. 20391-402. [DOI] [PubMed] [Google Scholar]
- 43.Quinones, B., G. Dulla, and S. E. Lindow. 2005. Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. Mol. Plant-Microbe Interact. 18682-693. [DOI] [PubMed] [Google Scholar]
- 44.Revell, P. A., and V. L. Miller. 2000. A chromosomally encoded regulator is required for expression of the Yersinia enterocolitica inv gene and for virulence. Mol. Microbiol. 35677-685. [DOI] [PubMed] [Google Scholar]
- 45.Reverchon, S., C. Rouanet, D. Expert, and W. Nasser. 2002. Characterization of indigoidine biosynthetic genes in Erwinia chrysanthemi and role of this blue pigment in pathogenicity. J. Bacteriol. 184654-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reverchon, S., W. Nasser, and J. Robert-Baudouy. 1994. pecS: a locus controlling pectinase, cellulase and blue pigment production in Erwinia chrysanthemi. Mol. Microbiol. 111127-1139. [DOI] [PubMed] [Google Scholar]
- 47.Robert-Baudouy, J., W. Nasser, G. Condemine, S. Reverchon, V. E. Shevchik, and N. Hugovieux-Cotte-Pattat. 2000. Pectic enzymes of Erwinia chrysanthemi regulation and role in pathogenesis. Plant-Microbe Interact. 5221-368. [Google Scholar]
- 48.Ross, P., R. Mayer, and M. Benziman. 1991. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 55279-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rouanet, C., S. Reverchon, D. A. Rodionov, and W. Nesser. 2004. Definition of a consensus DNA-binding site for PecS, a global regulator of virulence gene expression in Erwinia chrysanthemi and identification of new members of the PecS regulon. J. Biol. Chem. 27930158-30167. [DOI] [PubMed] [Google Scholar]
- 50.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 51.Sarker, M. R., and G. R. Cornelis. 1997. An improved version of suicide vector pKNG101 for gene replacement in gram-negative bacteria. Mol. Microbiol. 23410-411. [DOI] [PubMed] [Google Scholar]
- 52.Scher, K., U. Romling, and S. Yaron. 2005. Effect of heat, acidification, and chlorination on Salmonella enterica serovar Typhimurium cells in a biofilm formed at the air-liquid interface. Appl. Environ. Microbiol. 31163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoonejans, E., D. Expert, and A. Toussaint. 1987. Characterization and virulence properties of Erwinia chrysanthemi lipopolysaccharide-defective, phiEC2-resistant mutants. J. Bacteriol. 1694011-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi, Y., T. Latifi, M. J. Cromie, and E. A. Groisman. 2004. Transcriptional control of the antimicrobial peptide resistance ugtL gene by the Salmonella PhoP and SlyA regulatory proteins. J. Biol. Chem. 27938618-38625. [DOI] [PubMed] [Google Scholar]
- 55.Solano, C., B. Garcia, J. Valle, C. Berasain, J. M. Ghigo, C. Gamazo, and I. Lasa. 2002. Genetic analysis of Salmonella Enteritidis biofilm formation: critical role of cellulose. Mol. Microbiol. 43793-808. [DOI] [PubMed] [Google Scholar]
- 56.Song, H., W. Kong, N. Weatherspoon, G. Qin, W. Tyler, J. Turk, R. Curtiss III, and Y. Shi. 2008. Modulation of the regulatory activity of bacterial two-component systems by SlyA. J. Biol. Chem. 28328158-28168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stapleton, M. R., V. A. Norte, R. C. Read, and J. Green. 2002. Interaction of the Salmonella typhimurium transcription and virulence factor SlyA with target DNA and identification of members of the SlyA regulon. J. Biol. Chem. 27717630-17637. [DOI] [PubMed] [Google Scholar]
- 58.Thomson, N. R., A. Cox, B. W. Bycroft, G. S. A. B. Stewart, P. Williams, and G. P. C. Salmond. 1997. The Rap and Hor proteins of Erwinia, Serratia and Yersinia: a novel subgroup in a growing superfamily of proteins regulating diverse physiological processes in bacterial pathogens. Mol. Microbiol. 26532-544. [DOI] [PubMed] [Google Scholar]
- 59.Titarenko, E., E. López-Solanilla, F. Garcia-Olmedo, and P. Rodriguez-Palenzuela. 1997. Mutants of Ralstonia (Pseudomonas) solanacearum sensitive to antimicrobial peptides are altered in their lipopolysaccharide structure and are avirulent in tobacco. J. Bacteriol. 1796699-6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59a.Toth, I. K., K. S. Bell, M. C. Holeva, and P. R. J. Birch. 2003. Soft rot erwinias: from genes to genomes. Mol. Plant Pathol. 417-30. [DOI] [PubMed] [Google Scholar]
- 60.Tsuyumu, S. 1977. Inducer of pectic acid lyase in Erwinia carotovora. Nature 269237-238. [DOI] [PubMed] [Google Scholar]
- 61.Venkatesh, B., L. Baburjee, H. Liu, P. Hedley, T. Fujikawa, P. Brich, I. Toth, and S. Tsuyumu. 2006. The Erwinia chrysanthemi 3937 PhoQ sensor kinase regulates several virulence determinants. J. Bacteriol. 1883088-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei, K., D. J. Tang, Y.-Q. He, J.-X. Feng, B.-L. Jiang, G.-T. Lu, B. Chen, and J.-L. Tang. 2007. hpaR, a putative MarR family transcriptional regulator, is positively controlled by HrpG and HrpX and involved in the pathogenesis, hypersensitive response, and extracellular protease production of Xanthomonas campestris pathovar campestris. J. Bacteriol. 1892055-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei, X., and W. D. Bauer. 1999. Tn5-induced and spontaneous switching of Sinorhizobium meliloti to faster swarming behavior. Appl. Environ. Microbiol. J. Bacteriol. 651228-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yap, M.-N., C.-H. Yang, J. D. Barak, C. E. Jahn, and A. O. Charkowski. 2005. The Erwinia chrysanthemi type III secretion system is required for multicellular behavior. J. Bacteriol. 187639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu, J., A. Penaloza-Vazquez, A. M. Chakrabarty, and C. L. Bender. 1999. Involvement of the exopolysaccharide alginate in the virulence and epiphytic fitness of Pseudomonas syringae pv. syringae. Mol. Microbiol. 33712-720. [DOI] [PubMed] [Google Scholar]