Abstract
Lipopolysaccharide (LPS) is the first defense against changing environmental factors for many bacteria. Here, we report the first structure of the LPS from cyanobacteria based on two strains of marine Synechococcus, WH8102 and CC9311. While enteric LPS contains some of the most complex carbohydrate residues in nature, the full-length versions of these cyanobacterial LPSs have neither heptose nor 3-deoxy-d-manno-octulosonic acid (Kdo) but instead 4-linked glucose as their main saccharide component, with low levels of glucosamine and galacturonic acid also present. Matrix-assisted laser desorption ionization mass spectrometry of the intact minimal core LPS reveals triacylated and tetraacylated structures having a heterogeneous mix of both hydroxylated and nonhydroxylated fatty acids connected to the diglucosamine backbone and a predominantly glucose outer core-like region for both strains. WH8102 incorporated rhamnose in this region as well, contributing to differences in sugar composition and possibly nutritional differences between the strains. In contrast to enteric lipid A, which can be liberated from LPS by mild acid hydrolysis, lipid A from these organisms could be produced by only two novel procedures: triethylamine-assisted periodate oxidation and acetolysis. The lipid A contains odd-chain hydroxylated fatty acids, lacks phosphate, and contains a single galacturonic acid. The LPS lacks any limulus amoebocyte lysate gelation activity. The highly simplified nature of LPSs from these organisms leads us to believe that they may represent either a primordial structure or an adaptation to the relatively higher salt and potentially growth-limiting phosphate levels in marine environments.
Lipopolysaccharide (LPS) in the outer membrane layer is known to be the first line of defense against environmental factors in many gram-negative organisms, preventing lysis by complement, antimicrobial peptides and detergents (17, 21, 47). In proteobacteria, 3-deoxy-d-manno-octulosonic acid (Kdo), heptose, and phosphate are key parts of the conserved inner core of the LPS which connects the less-well-conserved outer core and sometimes an attached polysaccharide to the lipid A anchor. Why heptose is so well conserved is a mystery, but the prevalence of Kdo and phosphate may be related to the charge which they impart to the outer membrane and to their ability to bind divalent cations. The Kdo-phosphate metal binding center is capable of binding calcium with a dissociation constant (Kd) of 12 to 13 μM (28). This high-affinity binding of divalent cations is known to be necessary for the low permeability of LPS bilayers to some antibiotics (32), and it has been hypothesized that divalent cation cross-bridges may link LPS molecules on the bacterial cell surfaces of enterobacteria into a giant complex with very low membrane permeability (16).
Though the LPSs of many proteobacteria are well characterized, the LPSs from cyanobacteria are much less studied. The cell envelopes of cyanobacteria resemble those of gram-negative bacteria structurally, consisting of a cytoplasmic membrane, a peptidoglycan layer, an outer membrane containing LPS, and sometimes additional structures (9, 14). Previous chemical analyses have shown the LPS of some cyanobacteria to be devoid of phosphate, Kdo, and heptose (11, 12, 42, 43). Given the lack of Kdo in these organisms as well as the fact that the lability of the Kdo-glucosamine ketosidic linkage allows for the mild acid hydrolysis of LPS to lipid A, it is perhaps not surprising that many attempts at hydrolysis of cyanobacterial LPS to lipid A have failed (for an example, see reference 29).
Within the cyanobacteria, the genus Synechococcus represents a polyphyletic group of unicellular morphotypes. Synechococcus cells are found in both freshwater and marine environments. Organisms from group A Synechococcus and its sister taxon Prochlorococcus are extremely important primary producers in marine environments, with multiple “clades” similar to “species” described for other bacteria, dominating in different environments (3, 22). Unlike enterobacteria, which must frequently contend with an onslaught of host factors, members of the Synechococcus face grazing by protists and bacteriophages as their primary survival challenges.
The genome of Synechococcus sp. strain CC9311 has been shown to be devoid of the genes for Kdo biosynthesis, while strain WH8102 has several putative genes for Kdo biosynthesis (18, 20). This suggests that the LPS of cyanobacteria could be significantly different from that of enteric bacteria and could show species/strain variation as well. A comparison of the structures of LPS from cyanobacteria and enterobacteria would afford a unique opportunity to understand which elements of LPS structure are essential to bacterial survival and which are adaptations to the environment in which the bacteria live. To further this understanding, we present here an analysis of the LPS structure from two strains of marine Synechococcus: an open-ocean-dwelling strain having the putative genes for Kdo biosynthesis (strain WH8102; clade III) and a coastal strain lacking these genes (strain CC9311; clade I). We further present two novel methods for producing lipid A from bacteria lacking the labile Kdo ketosidic linkage.
MATERIALS AND METHODS
Cell growth and LPS purification.
Synechococcus strains CC9311 (clade I), CC9605 (clade II), WH8102 (clade III), and WH8113 (clade III) were grown under standard conditions in SN medium (40) to late log phase, harvested by centrifugation, and frozen at −80°C. A portion of the cells was retained for glycosyl composition analysis, and the rest was used for LPS isolation. For LPS isolation, cell samples from approximately 1 liter of Synechococcus culture were extracted with 50 ml 90% ethanol twice for 1 h each time at room temperature. Cells were then extracted with approximately 20 ml acetone and 20 ml diethyl ether and were air dried.
Dry cell mass was suspended in 5 ml of 10 mM Tricine, pH 8.0, with 5 mg of proteinase K overnight and harvested by ultracentrifugation (100,000 × g for 4 h). This step was found to significantly improve the LPS yield by reducing the size of the interphase in the subsequent phenol-water step. The cell pellet was resuspended in 4 ml water, heated to 65°C, and mixed with 4 ml 65°C phenol for 1 h, whereupon the samples were cooled and centrifuged to separate the layers (27). The pellet was reextracted with water two times and then with a 1:1 phenol/water ratio. These extractions were performed at 65°C for 1 h each time, and the aqueous and phenol phases were combined with the appropriate phases from the previous step.
Combined phenol phases were precipitated with 5 vol acetone and 1 vol diethyl ether at −10°C for 48 h. After centrifugation, the pellet was washed with acetone and dried. The material was then suspended in 10 mM sodium phosphate buffer, pH 7.5, with 0.1 mg/ml proteinase K, whereupon a rapid clarification was observed. Digestion was allowed to proceed overnight, and the resulting material was harvested by ultracentrifugation at 100,000 × g for 5 h. A total of 1.9 mg of material was obtained.
The combined aqueous phases were centrifuged at 100,000 × g for 4 h. The pellet was resuspended and recentrifuged and then digested as described above but with 1 mg proteinase K added for 2 h after overnight incubation. Material was harvested as described above.
Alternatively, a method similar to that of Uchida and Mizushima (33) was used with some modifications. A solvent-dried CC9311 cell pellet was suspended in 2.2 ml of water to which 0.4 ml of 100 mM Tris, pH 8.0, 0.4 ml of 0.5 M MgCl2, 1 ml of 8% Triton X-100, and 1 ml of ethanol were added. The suspension was incubated for 10 min at 100°C, and the precipitate was harvested by centrifugation for 15 min at 1,380 × g. The resulting pellet was then washed with 4 ml 10 mM Tris, pH 8.0, 10 mM MgCl2. The pellet was then suspended in 0.2 M triethylammonium EDTA, and 1 ml of 2 M NaCl and 1 ml of Triton X-100 were added. The suspension was incubated at 37°C for 60 min and centrifuged again at 1,380 × g to remove any residual material. The pellet from this stage was discarded, and 0.4 ml of 1 M MgCl2 was added for another 60-min incubation at 37°C. The suspension was then ultracentrifuged at 100,000 × g for 90 min. The pellet was resuspended in water and ultracentrifuged at 100,000 × g for 90 min prior to lyophilization.
Sodium deoxycholate-polyacrylamide gel electrophoresis (SDOC-PAGE).
Electrophoresis and staining were performed as described by Reuhs et al. (26). Alternatively, the Molecular Probes ProQ Emerald stain was used according to the instructions of the manufacturer. Images were cropped, despeckled, and adjusted for optimum contrast using GIMP 2.2.8 software (http://www.gimp.org/).
Comparative genomics.
The CyanoBase (http://bacteria.kazusa.or.jp/cyanobase/) and KEGG (http://www.genome.jp/kegg/pathway.html) databases were used to compare the annotations of CC9311, WH8102, and Escherichia coli K-12. BLAST analyses (1) were also used to compare the similarity of putative orthologs.
Glycosyl composition.
Methyl glycosides were prepared, and gas chromatography-mass spectrometry (MS) analysis of the trimethylsilyl-derivatized methyl glycosides was performed as described previously (45).
Linkage analysis by the sodium hydroxide method.
LPS samples were permethylated, depolymerized, reduced, and acetylated, and the resultant partially methylated alditol acetates were analyzed by gas chromatography-MS as described previously (31). The permethylation was repeated twice in order to aid complete methylation of the polymer.
Hakomori linkage analysis for analysis of uronic acids.
For glycosyl linkage analysis, the sample was methylated by a modification of the method of Hakomori (6). Methylated samples were reduced for 2 h at room temperature in superdeuteride, neutralized with acetic acid, dried under nitrogen, desalted by passing over an OnGuard H cation-exchange column in 50% ethanol, and then remethylated and analyzed by the sodium hydroxide method described above.
TEA-assisted periodate oxidation.
Up to 10 mg of LPS was periodate oxidized in the presence of a high concentration of triethylamine (TEA) to give lipid A. Reaction conditions were 5 mg/ml LPS, 5% (wt/vol) HIO4, 100 μl TEA, at room temperature overnight with stirring. Material was then extracted with 2.5 vol of chloroform/methanol (2:1), dried, and resuspended in 10 mg/ml sodium borohydride/1 M aqueous ammonia. After a 2-h reaction on ice, material was reextracted with chloroform/methanol (2:1) and redried. This procedure yielded 2.1 mg of material.
To confirm that periodate degradation products were not bound to lipid A, a complete Smith degradation was performed on LPS from the aqueous phase of WH8102 LPS (15).
Acetolysis.
The WH8102 phenol acetone precipitate fraction was suspended in 100 μl acetic acid/acetic anhydride/sulfuric acid (10:10:1) and incubated for 3 h at 40°C. The sample was then suspended in saturated barium methoxide. A small volume of methanol was then added to aid solubility, and the sample was incubated for 15 min, after which dry ice was added for acidification (23). After drying, the reaction mixture was suspended in water. The supernatant was taken for further analysis, and the pellet was extracted with 5 vol chloroform/methanol (2:1) to 2 parts water. The organic phase was then removed and dried before being used for analysis of the lipid A.
MALDI-TOF MS.
One to 5 μg of material was analyzed (either with or without desalting in 10 μl Dowex 50WX8 ammonium salt form in methanol) using 0.5 M 2,5-dihydroxybenzoic acid matrix. Initially, all samples were analyzed on an Applied Biosystems DE MS in the positive mode (the negative mode was completely ineffective in analyzing these samples). Subsequently, high-resolution and secondary mass spectra were obtained on an Applied Biosystems 4700 Proteomics matrix-assisted laser desorption ionization-tandem time of flight (MALDI-TOF/TOF) in the positive mode. Angiotensin, adrenocorticotropic hormone, dipalmitoyl phosphatidyl glycerol, and distearoyl phosphatidyl ethanolamine polyethylene glycol were used as standards (7).
NMR spectroscopy.
Prior to analysis, LPS samples for both CC9311 and WH8102 were treated overnight at 100°C with anhydrous hydrazine to remove fatty acids. This process was repeated once for WH8102 to ensure complete fatty acid removal. Samples were then dried under nitrogen and re-N-acetylated using 30% acetic anhydride in sodium carbonate for 1 h, followed by dialysis against deionized water and lyophilization. Samples were deuterium exchanged by lyophilization from D2O (99.9% D) (Aldrich), dissolved in 0.28 ml D2O (99.96% D) (Cambridge Isotope Laboratories), and transferred to a nuclear magnetic resonance (NMR) tube with susceptibility plugs (Shigemi). At the high concentration (∼5 mg/ml) required for NMR, solutions of the Synechococcus polysaccharides were slightly turbid, presumably because of aggregation of the saccharide. This hampered shimming, resulting in spectra with broad peaks. NMR spectra were acquired on a Varian Inova 500-MHz spectrometer at 343 K (70°C). The spectral width in the proton dimension was 3,155 Hz for all spectra, and 15,083 Hz (heteronuclear single-quantum coherence [HSQC]) and 10,054 Hz (gradient heteronuclear multiple-bond coherence) in the carbon dimension. Mixing times were 150 ms for total correlation spectroscopy and 300 ms for nuclear Overhauser effect spectroscopy. Chemical shifts were measured relative to internal acetone shifts (δH = 2.225 ppm; δC = 30.89 ppm).
Limulus amoebocyte lysate (LAL) assay.
Serial dilutions of sample were incubated with 0.03 U/ml Pyrotell reagent (Associates of Cape Cod) for 1 h at 37°C. Samples showing gelation were recorded as positive, as specified in the instructions supplied by the manufacturer and using R595 LPS controls.
RESULTS
Monosaccharide and LPS biosynthetic apparatus in Synechococcus.
The genomes of Synechococcus sp. strains WH8102 and CC9311 were examined for the presence of key LPS biosynthetic genes. Table 1 shows several important differences in the genomes of cyanobacteria and enterobacteria with respect to the biosynthesis of LPS. First, while the early steps in lipid A biosynthesis governed by the genes lpxA-lpxD are identical between Synechococcus spp. and E. coli, the lpxK gene (encoding a tetraacyldisaccharide 4′ kinase) appears to be absent in both Synechococcus strains. This gene is also known as lpxE, but we refer to it as lpxK to be consistent with the nomenclature of Raetz et al. (25). Three genes used for Kdo biosynthesis, a typical component of LPS in bacteria, are missing in strain CC9311 but not in WH8102. In addition, the biosynthesis of rhamnose was strikingly different in different Synechococcus strains. All strains had a putative pathway for rhamnose biosynthesis. However, strain WH8102 contained, while CC9311 lacked, a second rhamnose biosynthetic pathway.
TABLE 1.
LPS and related biosynthetic genes present in E. coli and Synechococcus strainsa
| Function | Present in E. coli K-12 | Predicted ortholog in Synechococcus strain WH8102 | Predicted ortholog in Synechococcus strain CC9311 |
|---|---|---|---|
| Acylation of UDP GlcNAc | Yes | SYNW0558 | SYNC_2212 |
| Condensation to (GlcNAc)2 1P | Yes | SYNW0559 | SYNC_2211 |
| Deacetylase | Yes | SYNW0556 | SYNC_2214 |
| N-Acylation | Yes | SYNW0783 | SYNC_1713 |
| Dephosphorylation of 1 phosphate | No | − | − |
| 4′ Dephosphorylation | No | − | − |
| 4′ Kinase | Yes | − | − |
| Kdo 8P synthase | Yes | SYNW0180 | − |
| Kdo synthase | Yes | SYNW0186 | − |
| CMP Kdo synthase | Yes | SYNW0184 | − |
| Rhamnose biosynthesis from GDP-mannose | Yes | SYNW0422 | SYNC_0146 |
| Rhamnose biosynthesis (misannotated as GDP-l-fucose synthetase) | − | SYNW0423 | SYNC_0145 |
| Rhamnose biosynthesis | Yes | SYNW0646-0649 | − |
−, Not found.
Glycosyl composition of whole cells of Synechococcus strains.
Because the Synechococcus genomes predicted differences in glycosyl composition, such as the absence of Kdo in CC9311, several strains of Synechococcus were analyzed for total glycosyl composition, including WH8102 and WH8113 (clade III), CC9311 (clade I), and CC9605 (clade II) after growth in SN medium (Table 2). The vast majority of saccharide in each strain is glucose, but we detected significantly different relative abundances of rhamnose. Approximately 10% of the monosaccharide composition in WH8102 and the other strains was rhamnose, except for CC9311, where it was only 1%. This suggested large differences in the use of rhamnose in CC9311 cells, consistent with missing one putative pathway for rhamnose biosynthesis. Kdo was detected in the WH8102 cells, consistent with genomic analyses, but the absolute levels are approximately 1% or less of the level found in E. coli K-12. Similarly, a very low level of heptose was detected in only one strain, WH8113. In addition, more subtle differences were found: CC9311 contained smaller amounts of mannose and possibly lacked some methylated monosaccharides found in the other strains. Chemical ionization MS of the methylated monosaccharides reveals them to be singly methylated hexoses.
TABLE 2.
Glycosyl composition (mol% of total) of whole cells of several Synechococcus strainsa
| Residue | mol% of total glycosyl composition of whole cells from indicated strain
|
|||
|---|---|---|---|---|
| WH8102 | WH8113 | CC9311 | CC9605 | |
| Kdo | 0.4-0.6 | ND | ND | ND |
| Heptose | ND | 0.2 | ND | ND |
| Glucose | 78.6-79.5 | 84.7 | 83.8 | 76.6 |
| Rhamnose | 10.4 | 7.1 | 1 | 10.0 |
| N-Acetylglucosamine | 0.7-0.9 | 0.6 | 1 | 0.5 |
| Fucose | ND | 1.2 | ND | ND |
| Mannose | 1.5-1.9 | 2.4 | TR | 3.1 |
| Galactose | 4.9-5.2 | 3.7 | ND | ND |
| Glucuronic acid | 0.7 | ND | ND | ND |
| Galacturonic acid | 1.0-2.3 | ND | 0.6 | 1.4 |
| Methylated residue 1 | + | + | ND | + |
| Methylated residue 2 | + | + | 6.7 | + |
| Methylated residue 3 | + | ND | 2.8 | + |
| Methylated residue 4 | + | ND | ND | + |
+, clearly present in small amounts; TR, trace (possibly present); ND, not detected. Ranges indicate biological replicates.
Isolation of Synechococcus LPS.
Preliminary extractions showed that the phenol-water extraction, commonly used to extract LPS from gram-negative bacteria, was particularly effective at isolating LPS from both strains of Synechococcus used here, especially when preceded by proteinase K pretreatment of the dried cell mass to remove excess protein. Though most of the LPS was extracted into the aqueous phase, careful analysis of the phenol phases revealed the presence of lower-molecular-weight LPS species. These LPSs were stained more readily by the Molecular Probes ProQ Emerald stain than by conventional alcian blue silver staining (Fig. 1). The phenomenon of poorly charged LPS staining more readily with the ProQ Emerald stain has also been witnessed in studies involving Francisella spp. (D. S. Snyder and P. Azadi, unpublished observations). While silver staining is assisted by charge-charge interactions between positively charged silver ions and negatively charged saccharides, the ProQ Emerald fluorophore uses a Schiff base mechanism to interact directly with periodate-formed aldehydes. Though it is beyond the scope of this work to prove a mechanism for this phenomenon, it is tempting to speculate that the hydrophobic fluorophore in the ProQ Emerald 300 stain may interact more strongly with other hydrophobic molecules, such as the minimal cores of these LPSs.
FIG. 1.
Isolation of LPS from Synechococcus sp. strains CC9311 and WH8102. LPS was isolated from the Synechococcus strain using the phenol-water method (27). Both the phenol and aqueous phases were analyzed for LPS by SDOC-PAGE. The gel shown at the top is stained by the ProQ Emerald stain, while the gel shown at the bottom is stained by the alcian blue silver stain method. Lane 1, E. coli O55:B5 LPS standard; lane 2, CC9311 phenol-phase LPS; lane 3, WH8102 phenol-phase LPS; lane 4, CC9311 aqueous-phase LPS; lane 5, WH8102 aqueous-phase LPS; lane 6, complete-core (Ra) LPS from E. coli EH100.
To confirm that our LPS was not contaminated with glucan, we also purified the LPS by solubilization in detergent-EDTA and precipitation with magnesium as described in Materials and Methods. LPS from this extraction procedure appeared identical to material from the phenol-water extraction by SDOC-PAGE, having bands for both minimal core (phenol-phase material) and larger polysaccharide from aqueous phase (data not shown).
Glycosyl composition of WH8102 and CC9311 LPS.
As noted above, two versions of LPS were isolated: a phenol-phase extractible minimal core and an aqueous-phase LPS of core with an attached polysaccharide. Table 3 shows the glycosyl composition of these LPS forms isolated from two strains of Synechococcus. Glucose is the major constituent of both WH8102 and CC9311 LPS. This is particularly the case of the aqueous phases, where glucose constitutes nearly 100% of the material. This is consistent with a glucan polymer being added to an LPS core in a manner analogous to the O antigen of enteric bacteria. Cellulase treatment, which is commonly used to eliminate contaminating glucans from bacterial LPS (37), and repurification by size exclusion or reverse-phase chromatography had minimal effect on the composition, further confirming sample homogeneity (data not shown). Both galacturonic acid and glucosamine are more abundant in the minimal core structures, as would be expected if they were present in the lipid A or core region of the molecule. Rhamnose is observed exclusively in the WH8102 LPS and is likewise more prevalent in the minimal core material. Neither Kdo nor heptose is detected in either LPS, despite the fact that the genes for Kdo biosynthesis are found in WH8102.
TABLE 3.
Glycosyl composition (mol% of total) of LPS from Synechococcus sp. strains WH8102 and CC9311a
| Glycosyl residue | mol% of total glycosyl composition of LPS from indicated strain
|
|||
|---|---|---|---|---|
| WH8102
|
CC9311
|
|||
| Phenol phase/minimal core | Aqueous phase | Phenol phase/minimal core | Aqueous phase | |
| Kdo | ND | ND | ND | ND |
| Heptose | ND | ND | ND | ND |
| Glucose | 55 | 100 | 48 | 97 |
| Rhamnose | 6.5 | TR | TR | ND |
| Galacturonic acid | 14 | TR | 17 | 0.7 |
| Glucosamine | 24 | TR | 32 | 1.7 |
| Mannose | ND | TR | 1.4 | 0.9 |
| Xylose | ND | ND | 1.5 | ND |
TR, trace (possibly present); ND, not detected.
Linkage analysis.
We have used two different methods of linkage analysis to determine how the monosaccharides of WH8102 and CC9311 LPS are linked. Initially, the sodium hydroxide method was used to prepare partially methylated alditol acetate derivatives of WH8102 and CC9311 LPS samples. The linkage analysis showed 4-linked glucose to be the predominant linkage in both strains (Table 4). Branching is observed in each strain, with significant levels of terminal glucose seen in both cases. Several doubly acetylated species are observed at low levels, and these can be the result of either undermethylation or actual linkage. Because of the difficulty in determining response factors for the different molecular species, we have used NMR to determine the branch points of the polysaccharide (see below).
TABLE 4.
Hakomori linkage analysis of Synechococcus sp. LPSa
| Residue | % of total analyzed residue for indicated strain
|
||
|---|---|---|---|
| WH8102
|
CC9311 aqueous phase | ||
| Minimal core/phenol phase | Aqueous phase | ||
| Terminal glucopyranose | 37 | 17 | 12 |
| Terminal galacturonic acid | 19 | ||
| 2,3,4-Linked rhamnopyranose | 5 | ||
| 4-Linked glucopyranose | 21 | 40 | 58 |
| 2,6-Linked glucopyranose | 19 | ||
| 2-Linked glucopyranose | 2 | ||
| 3-Linked glucopyranose | 5 | ||
| 6-Linked glucopyranose | 3 | 2 | |
| 4,6-Linked glucopyranose | 12 | 20 | |
| 3,4-Linked glucopyranose | 16 | 3 | |
| 2,4-Linked glucopyranose | 2 | 4 | |
| 3,4,6-Linked glucopyranose | 4 | ||
Residues are expressed as a percentage of total analyzed. Percentages are area percentages and not necessarily quantitative for exact molar ratios. For clarity, residues comprising <2% of the total area have been omitted.
Looking at the minimal core LPS of WH8102 with the Hakomori linkage method, which detects uronic acid linkages, reveals a considerable level of terminal galacturonic acid. This is consistent with the residue either being on a side chain or being connected to lipid A in place of the 4′ phosphate, as shown in the LPS from Rhizobium spp. (24). Such a linkage is inconsistent with the residue being a direct replacement for Kdo, as this would require linkage to the rest of the chain through a second carbon. A higher level of terminal glucose is also observed, consistent with a shorter, branched saccharide, still consisting of glucose, as the minimal core. Analysis of the minimal core CC9311 LPS gave results similar to WH8102, containing primarily 4-linked glucose with trace levels of terminal glucose, but because of the weak signals, area percentages cannot be reliably determined. No multiply linked residues were observed for the CC9311 minimal core (data not shown).
MALDI-TOF MS analysis of lipid A produced by periodate oxidation.
If the LPS from Synechococcus spp. does indeed lack Kdo, one would expect difficulty in producing lipid A by mild-acid hydrolysis, as cleavage of the labile ketosidic linkage between Kdo and glucosamine is necessary for this reaction to work. Indeed, our efforts at hydrolysis have been universally unsuccessful, even when 0.1 M hydrochloric acid was used in place of acetic acid. To circumvent this difficulty, we have utilized TEA-assisted periodate oxidation to degrade the saccharide portion of the molecule. MALDI MS of the resulting material shows the pattern we would expect for an acylated diglucosamine backbone with no phosphates. As shown in Fig. 2 and Table 5, three main groupings of ions are observed corresponding to masses predicted for tetraacyl, triacyl, and diacyl species. As an example, a major ion at 1,055 m/z is observed for both CC9311 and WH8102. This is the expected mass of the sodium adduct of a species having two glucosamines, one β-hydroxymyristic acid, one β-hydroxystearic acid, and one myristic acid. Tandem MS of this ion gives an ion at 801 m/z, equivalent to the loss of three hydroxystearic acids, for both strains. Additionally, the spectrum of WH8102 shows an ion at 829 m/z, consistent with the loss of β-hydroxymyristic acid (Fig. 3).
FIG. 2.
(A) Primary mass spectra of periodate oxidation-derived lipid A from Synechococcus strain WH8102 positive-ion primary mass spectrum. (B) Corresponding spectrum for CC9311.
TABLE 5.
Structure assignments of periodate-derived lipid A and minimal core ions by MALDIb
| Molecule | Ion m/z | Fatty acidsa | No. of monosaccharides | Adduct |
|---|---|---|---|---|
| Lipid A | 1,309 | 3OH C14, 3OH C15, C14, C18 | 2 GlcN | M+Na |
| 1,069 | 3OH C14, 3OH C17, C14 | 2 GlcN | M+Na | |
| 1,055 | 3OH C14, 3OH C16, C14 | 2 GlcN | M+Na | |
| 1,027 | 2(3OH C14), C14 | 2 GlcN | M+Na | |
| CC9311 LPS | 2,479 | 3OH C14, 3OH C17, C18:1, C14 | 2 GlcN, 6 Glc, 1 GalA | M+Na |
| 2,471 | 3OH C16, 3OH C16, C18:1, C14 | 2 GlcN, 6 Glc, 1 GalA | M+H | |
| 2,457 | 3OH C14, 3OH C17, C18:1, C14 | 2 GlcN, 6 Glc, 1 GalA | M+H | |
| 2,443 | 3OH C14, 3OH C16, C18:1, C14 | 2 GlcN, 6 Glc, 1 GalA | M+H | |
| WH8102 LPS | 2,429 | 3OH C14, 3OH C16, C14, C18 | 2 GlcN, 5 Glc, 1 GalA, 1 Rha | M+H |
| 2,415 | 3OH C14, 3OH C15, C14, C18 | 2 GlcN, 5 Glc, 1 GalA, 1 Rha | M+H | |
| 2,401 | 3OH C14, 3OH C16, C14, C16 | 2 GlcN, 5 Glc, 1 GalA, 1 Rha | M+H | |
| 2,387 | 3OH C14, 3OH C15, C14, C16 | 2 GlcN, 5 Glc, 1 GalA, 1 Rha | M+H |
For the sake of brevity, only one potential set of fatty acids is shown per ion.
For LPS ions, the lowest-mass ion in each cluster is listed to be consistent with nominal mass predictions.
FIG. 3.
Secondary mass spectra. (A) Secondary MS from the ion at 847 m/z of the lipid A of WH8102 subjected to full Smith degradation. (B) Secondary spectrum of the ion at 1,056 m/z from WH8102 lipid A. Loss of 3OH C16 and 3OH C14 fatty acids is evident. (C) Secondary mass spectrum of the ion at 1,056 m/z for CC9311 lipid A.
The masses of the major ions in the primary mass spectra are consistently 14 and 28 mass units apart, consistent with the addition or removal of a −CH2 group (e.g., replacement of C15OH by C14OH or C16OH yields a change of 14 mass units). As an example, the ion at 1,027 m/z is separated from the ion at 1,055 by 28 mass units. This is as expected for the replacement of β-hydroxystearic acid with β-hydroxymyristic acid.
Representative compositions of the major ion species are shown in Table 5. For simplicity, we have represented only the highest- and lowest-mass ions in this table. There are, for example, a large number of combinations possible for the ion at 1,294 m/z, which is consistent with the fatty acid composition we have observed. All fatty acid assignments are consistent with fatty acids produced upon both hydrolysis and methanolysis of LPS from these strains (data not shown). Note that while positive-ion MALDI was effective in analyzing the lipid A species observed here, negative-ion MALDI showed few or no ions, consistent with a lack of negatively charged phosphate on the molecule.
Follow-up degradation with HCl in the Smith degradation procedure shows ions consistent with deacylated versions of the ions described above, indicating that there are no periodate degradation artifacts associated with lipid A. Tandem MS of the ion at 847 m/z of the WH8102 lipid A subjected to complete Smith degradation shows losses of both the 3OH myristic and stearic acids as well as an ion at 663 m/z, consistent with the sodium adduct of an oxonium ion having C16 and 3OH C15 fatty acids (Fig. 3).
MALDI-TOF MS analysis of lipid A produced by acetolysis.
Acetolysis is now an underutilized method, commonly employed in the days before NMR became a facile method of saccharide analysis, to remove 6-linked species from a saccharide (reviewed in reference 23). Assuming that the linkage between lipid A and the saccharide core is a 6-linkage, as in enteric LPS, one would expect such a cleavage to result in the diglucosamine backbone being separated from the saccharide core. The mass spectral analysis of the organic-phase extract of the acetolysis of WH8102 LPS is shown in Fig. 4 and Table 6. The spectrum reveals several species differing from the triacylated forms of the periodate-derived lipid A by the mass of a galacturonic acid (typically, galacturonic acid minus sodium as the acetolysis-derived material has virtually no sodium adducts). As an example, the ion at 1,209 m/z differs from the major periodate-derived ion at 1,055 m/z by 154 mass units, a gain of 176 mass units (galacturonic acid) and a loss of 22 mass units (sodium replaced by hydrogen). Since periodate oxidation would be expected to eliminate galacturonic acid as well as the saccharide backbone, these results are consistent with the presence of galacturonic acid on lipid A. In addition, several ions consistent with the loss of methanol and acetic acid are observed, presumably from the harsh acidic conditions used in this procedure. For example, the ion at 1,177 m/z is consistent with the loss of methanol from the ion at 1,209 m/z, while the ion at 1,149 m/z is consistent with the loss of acetic acid. Similarly, we detected more extensive deacylation than in the periodate oxidation procedure, which is expected from heating the material in concentrated acetic/sulfuric acid.
FIG. 4.
MALDI MS of lipid A from acetolysis. Lipid A derived from WH8102 LPS by acetolysis was analyzed by MALDI MS in the positive-ion mode. While the harsher conditions used in acetolysis result in a frequent loss of methanol and acetate from the ions involved, many of the main ions differ from the periodate-derived species by the addition of one galacturonic acid minus the sodium typically seen in the periodate; i.e., the ion at 1,209 m/z differs from the major ion at 1,055 m/z, as seen in Fig. 2A by 154 AMU.
TABLE 6.
Primary mass assignments of lipid A ions from acetolysis of WH8102a
| Ion m/z | Fatty acids | No. of monosaccharides |
|---|---|---|
| 1,209 | 3OH C14, 3OH C16, C14 | 2 GlcN, 1 GalA |
| 4(3OH C14), C16 | 2 GlcN, 1 GalA | |
| 2(3OH C15), C14 | 2 GlcN, 1 GalA | |
| 2(3OH C16), C12 | 2 GlcN, 1 GalA | |
| 1,181 | 2(3OH C14), C14 | 2 GlcN, 1 GalA |
| 3OH C14, 3OH C16, C12 | 2 GlcN, 1 GalA | |
| 2(3OH C15), C12 | 2 GlcN, 1 GalA | |
| 984 | C16OH, C14 | 2 GlcN, 1 GalA |
| C14OH, C16 | 2 GlcN, 1 GalA | |
| 955 | C14OH, C14 | 2 GlcN, 1 GalA |
| C16OH, C12 | 2 GlcN, 1 GalA |
Note that this list is not necessarily exhaustive. All adducts are M+H+.
MALDI-TOF MS analysis of intact minimal core.
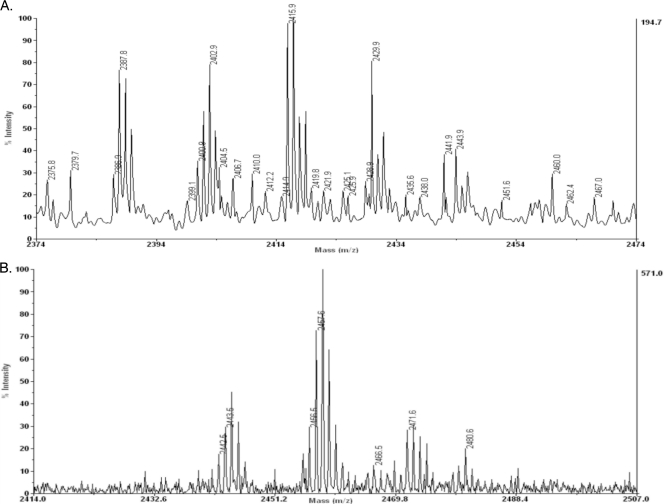
Having determined the mass of lipid A from WH8102 and CC9311, we determined the mass spectrum of the intact minimal cores which we isolated from the phenol phase of our extractions. As shown in Fig. 5, the structures are consistent with the addition of a predominantly glucose core to lipid A. For example, the ion at 2,415 m/z is consistent with the H+ ion formed from the addition of five glucose, one rhamnose, and one galacturonic acid residue to the ion at 1,309 m/z in the lipid A spectrum. In WH8102, the masses are consistent with addition of one rhamnose and five glucose residues to the galacturonic acid-substituted lipid A. The species observed are tetraacylated or triacylated, consistent with the highest levels of acylation in the lipid A, with even chain fatty acids being the most abundant. In CC9311, we detected an ion at 2,457 m/z, which corresponds to the addition of six glucose residues to the galacturonic acid-substituted lipid A. In this case, the acylation appears to be exclusively tetraacyl with higher levels of odd-chain fatty acids. Consistent with these results, 31P-labeled NMR shows a lack of phosphate on the LPS (data not shown). Note also that MALDI data were acquired in the positive-ion mode but minimal signal was observed in the negative mode, as would be expected for a compound lacking phosphate. Our putative structure for the CC9311 minimal core is shown in Fig. 6. Because the placement of the rhamnose in the WH8102 minimal core is not known, we have not suggested an exact structure for this core.
FIG. 5.
MALDI MS of phenol-phase minimal cores. WH8102 (A) and CC9311 (B) LPSs were analyzed by MALDI MS in the positive-ion mode. Note that the two groups of peaks in the top panel are separated from one another by the difference of one 3OH C14 or 3OH C16 fatty acid, while major peaks within the groups are separated by 28 AMU, consistent with the addition of fatty acids differing by 2 carbon units. Compositions of major ions are described in Table 5.
FIG. 6.
Putative structure of Synechococcus minimal core LPS. Structures represent one of a variety of the acylated lipid A structures found in these organisms.
NMR analysis of the polysaccharides released by hydrazinolysis.
The combination of 1- and 2-dimensional NMR experiments of the WH8102 polysaccharide allowed elucidation of the structure and assignment of the NMR signals. Due to the lower sample amount, the spectrum of the CC9311 polysaccharide produced weaker signals; however, no discernible differences with WH8102 were detected (Table 7).
TABLE 7.
Chemical shift assignments of WH8102 polysaccharide liberated from aqueous-phase LPS by hydrazinolysis
| Residue | Nucleus | Chemical shift (ppm)
|
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| 4-α-Glcp | 1H | 5.37 | 3.65 | 3.97 | 3.64 | 3.85 | 3.89/3.85 |
| 13C | 100.6 | 72.4 | 74.2 | 78.5 | 72.4 | 61.6 | |
| α-Glcp | 1H | 5.37 | 3.62 | 3.73 | 3.45 | 3.71 | 3.87/3.79 |
| 13C | 101.0 | 72.4 | 73.8 | 70.5 | 72.4 | 61.6 | |
| 2,4-α-Glcp | 1H | 4.97 | 3.61 | 4.03 | 3.65 | 3.88 | 3.89/3.85 |
| 13C | 99.6 | 79.9 | 74.2 | 78.5 | 72.4 | 61.6 | |
The 1-dimensional proton spectrum of WH8102 showed two anomeric signals in a 12:1 ratio. Correlation spectroscopy and total correlation spectroscopy revealed that the major signal, at 5.37 ppm, was composed of two overlapping signals belonging to the anomeric protons of two different sugar residues. According to HSQC, one of these residues had proton and carbon chemical shifts that were consistent with those of 4-linked α-glucopyranose, and the other residue's chemical shifts were consistent with those of terminal α-glucopyranose. H-4 of the terminal glucose residue resonated at 3.45 ppm and was separated from the rest of the carbohydrate ring proton signals so that it was possible to quantitate this residue in relation to the 4-linked residue. The ratio of these two sugars was determined to be about 8:1. The minor anomeric signal resonated at 4.97 ppm and by analysis of the 2-dimensional spectrum was found to originate from a 2,4-linked α-glucopyranosyl residue.
Nuclear Overhauser effect spectroscopy showed interresidue cross peaks between H-1 of 4-Glc to H-4 of 4-Glc, between H-1 of 2,4-Glc and H-3 and H-4 of 4-Glc, and between H-1 of terminal Glc and H-2 of 2,4-Glc. These data suggested that the terminal residues are attached to the 2,4-linked residues. Both of these residues are present at roughly equimolar concentrations, each of them at ∼10% of the total residues present. The NMR data indicated that the polysaccharides from both strains are polymers of α-1,4-linked glucopyranose, with 10% of the backbone residues being further substituted at O-2 by terminal α-glucopyranose.
Because linkage analysis of CC9311 gave a larger area peak for 4,6-linked than 2,4-linked glucose, we acquired a multiplicity-edited HSQC spectrum (CH and CH3 positive; CH2 negative), trying to find a pair of methylene signals around 70 ppm, but no negative signals were detected at this chemical shift. We conclude that the peak observed for 4,6-linked glucose is an artifact of undermethylation.
Biological activity of Synechococcus LPS.
As a standard surrogate for inflammatory activity, the LAL gelation assay was carried out with WH8102 LPS and derivatives. WH8102 LPS was tested both before and after an extraction procedure known to remove inflammatory molecules often copurified with LPS (8). The WH8102 LPS had activity neither before nor after this extraction, whereas the strain R595 LPS [Salmonella enterica subsp. enterica serotype Minnesota R595 (Re)] control had activity at a concentration of as little as 3 pg/ml.
DISCUSSION
Both Synechococcus sp. strains CC9311 and WH8102 appear to produce similar, very simplified LPS structures lacking typical components of bacterial LPS, such as Kdo, heptose, and phosphate. Though both strains of bacteria add an α1,4-linked glucose chain to their core and use predominantly glucose in the core, a few interesting differences in structures of CC9311 and WH8102 can be discerned. First, we observe a significantly higher level of incorporation of odd-chain fatty acids as evidenced by the increased prevalence of mass spectral peaks 14 mass units different from the nearest peak in the CC9311 LPS and lipid A mass spectral profiles. Second, the WH8102 minimal core has a single rhamnose, while the CC9311 has exclusively glucose. This difference may help explain the larger amount of rhamnose detected in WH8102 whole cells. While these differences are not unexpected for two highly genetically divergent strains adapted to different marine environments (coastal versus oligotrophic open ocean), the differences between these structures and the canonical LPS structure are worth noting. Such differences may have consequences for differences in phage recognition that have been noted for cyanobacteria in general (46), and specifically marine Synechococcus strains (39), or potentially grazer recognition. Species/strain differences in LPS (particularly rhamnose content) may also impart different nutritional qualities to Synechococcus strains that may be important for heterotrophic grazers that consume them.
One of the most striking differences between the structure observed here and enteric LPS is the lack of both Kdo and phosphate. These residues have often been found to be lacking in Synechococcus and Synechocystis spp., and the lack of Kdo has been found to correlate with the lack of LPS hydrolyzability when attempts to produce lipid A have been made (41). Though the lack of LPS hydrolyzability can also be imparted by the linkage of lipid A to the rare sugar d-glycero-d-talo-oct-2-ulopyranosylate (KO) (10, 36), we do not believe this to be the case here. In the few cases where KO has been observed, Kdo has always been found in the LPS as well. We can find neither Kdo in the LPS nor, in the case of CC9311, the genes necessary for its biosynthesis. Also, and most importantly, our mass spectra of the minimal core are inconsistent with the presence of this residue. Furthermore, frequently missing in cyanobacteria is heptose, which in Synechococcus is replaced by glucose, resulting in a core structure which is biosynthetically cheap and simple to produce. Similarly, while the LPS from a number of different organisms (i.e., Francisella, Rhizobium, and Marinomonas spp. and Bacteroides fragilis) (13, 24, 35, 44) have been shown to be deficient in or devoid of phosphate, this is typically done through dephosphorylation of lipid A using gene products from lpxE and or lpxF (25). With WH8102 and CC9311, we actually observe a lack of the 4′ kinase, implying that lipid A is made without phosphorylation.
As expected for an LPS molecule lacking phosphate and Kdo, WH8102 LPS is completely negative in the LAL assay. Previous studies of LPS from freshwater filamentous cyanobacteria in the genera Anabaena and Oscillatoria have shown low levels of activity in the LAL assay in comparison to enteric LPS (11). Since these LPSs were found to lack phosphate and have variable levels of Kdo, this would seem to be contradictory to our findings. One possible explanation for this contradiction is that there is an inflammatory non-LPS component present in the LPS of the genera Anabaena and Oscillatoria. Such copurifying molecules have been observed in enteric LPS (8).
In place of phosphate, both WH8102 and CC9311 use galacturonic acid to place a negative charge on their lipid A. This modification allows the bacteria to keep a negative charge on their outer membrane. It is tempting to speculate that the replacement of phosphate with the less-charged galacturonic acid may also reflect a decreased need to bind calcium or magnesium tightly, as these ions are comparatively abundant in seawater. Similarly, the lack of phosphorylation could be most beneficial to cyanobacteria by allowing them to survive on lower levels of phosphate. Productivity in some areas of the marine environment are thought to be phosphate limited (reviewed in reference 19), including the Sargasso Sea (2), where WH8102 was originally isolated (38). Indeed, several strains of marine bacteria have been shown to incorporate sulfate into their membrane lipids, reducing their dependence on this often limiting nutrient (34).
An interesting question is whether the simplified structure of these cyanobacterial LPSs can be rationalized strictly in terms of adaptation to their low-phosphate, high-salinity environment. In support of this, substitution of galacturonic acid for phosphate in the core region of Klebsiella sp. LPS has been observed. This occurs in environmental isolates, and this modification has been thought to give the bacteria an advantage in low-phosphate conditions (5). Similarly, galacturonic acid can replace the 4′ phosphate of Rhizobium sp. LPS, yet this substitution is performed on a lipid A which has been phosphorylated and then dephosphorylated (4, 25). In Synechococcus, the core contains no charged residues and we do not detect the 4′ kinase for lipid A. Furthermore, we are left wondering why Kdo and heptose would be eliminated from the structure of both cyanobacterial LPSs if the only purpose is to conserve phosphate, especially since CC9311 is a representative of clade I, often found in phosphate-replete coastal environments. Last, the lack of Kdo, phosphate, and heptose is by no means universal in marine bacteria, and many other bacterial species are known to have a more canonical type of LPS (41), leading us to wonder why these cyanobacteria would so dramatically revise the biosynthesis of this molecule. Though we cannot rule out adaptation to the environment as a reason for the simplification of LPS structure, we believe alternate explanations might also be important.
Since enteric LPS has been far better studied than cyanobacterial LPS, it is natural to think of the canonical structure as a “default” structure. In nature, however, cyanobacterial LPSs are both far more abundant and far more ancient than their enteric cousins. Cyanobacteria are among the most ancient of Earth's organisms, and fossils of these photosynthetic bacteria indicate a striking resemblance between current species and ones extant over 2 billion years ago (30). If one were to look for a primordial LPS from which other LPSs were derived, one would expect to find it in an ancient organism, for it to be simple in structure, and for it to come from an organism lacking biosynthetic genes for production of more complex structures (as opposed to a streamlined structure which would be expected to have genes for making such structures and removing them). Though gene swapping and adaptation to phages through alterations in LPS structure make assigning an evolutionary tree for LPS biosynthesis difficult, we find it interesting that these relatively simple structures, lacking Kdo, heptose, and phosphate, are common in the set of cyanobacterial LPSs for which we have any chemical composition data (41, 43). On this basis, we propose that the simple cyanobacterial LPS structures reported here may represent the LPS from which other types have evolved.
Acknowledgments
Funds for this project were provided by the UC Academic Senate and the NSF (OCE 0648175) to B.P. and B.B. Support to P.A. and the Complex Carbohydrate Research Center was provided by the Department of Energy (DE-7G02-93ER20097).
We thank Andrew Kropinski (Public Health Agency of Canada) for helpful and insightful comments on the manuscript. We also thank Christian Heiss for performing the NMR analysis.
Footnotes
Published ahead of print on 6 July 2009.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotner, J., and R. Metzel. 1992. Uptake of dissolved inorganic and organic phosphorus compounds by phytoplankton and bacterioplankton. Limnol. Oceanogr. 37232-243. [Google Scholar]
- 3.Ferris, M. J., and B. Palenik. 1998. Niche adaptation in ocean cyanobacteria. Nature 396226-228. [Google Scholar]
- 4.Forsberg, L. S., and R. W. Carlson. 1998. The structures of the lipopolysaccharides from Rhizobium etli strains CE358 and CE359—the complete structure of the core region of R. etli lipopolysaccharides. J. Biol. Chem. 2732747-2757. [DOI] [PubMed] [Google Scholar]
- 5.Frirdich, E., and C. Whitfield. 2005. Lipopolysaccharide inner core oligosaccharide structure and outer membrane stability in human pathogens belonging to the Enterobacteriaceae. J. Endotoxin Res. 11133-144. [DOI] [PubMed] [Google Scholar]
- 6.Hakomori, S. 1964. A rapid permethylation of glycolipid and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J. Biochem. 55205-208. [PubMed] [Google Scholar]
- 7.Harvey, D. J. 2003. Matrix-assisted laser desorption/ionization mass spectrometry of carbohydrates and glycoconjugates. Int. J. Mass Spectrom. 2261-35. [Google Scholar]
- 8.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165618-622. [DOI] [PubMed] [Google Scholar]
- 9.Hoiczyk, E., and A. Hansel. 2000. Cyanobacterial cell walls: news from an unusual prokaryotic envelope. J. Bacteriol. 1821191-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isshiki, Y., K. Kawahara, and U. Zähringer. 1998. Isolation and characterisation of disodium(4-amino-4-deoxy-βl-arabinopyranosyl)-(1 to 8)-(d-glycero-d-talo-oct-2-ulopyranosylonate)-(2 to 4)-(methyl 3-deoxy-d-manno-oct-2-ulopyranosid)onate from the lipopolysaccharide of Burkholderia cepacia. Carbohydr. Res. 31321-27. [DOI] [PubMed] [Google Scholar]
- 11.Keleti, G., and J. L. Sykora. 1982. Production and properties of cyanobacterial endotoxins. Appl. Environ. Microbiol. 43104-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keleti, G., J. L. Sykora, E. C. Lippy, and M. A. Shapiro. 1979. Composition and biological properties of lipopolysaccharides isolated from Schizothrix calcicola (Ag.) Gomont (Cyanobacteria). Appl. Environ. Microbiol. 38471-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krasikova, I. N., N. V. Kapustina, V. V. Isakov, A. S. Dmitrenok, P. S. Dmitrenok, N. M. Gorshkova, and T. F. Solov'eva. 2004. Detailed structure of lipid A isolated from lipopolysaccharide from the marine proteobacterium Marinomonas vaga ATCC 27119T. Eur. J. Biochem. 2712895-2904. [DOI] [PubMed] [Google Scholar]
- 14.McCarren, J., J. Heuser, R. Roth, N. Yamada, M. Martone, and B. Brahamsha. 2005. Inactivation of swmA results in the loss of an outer cell layer in a swimming Synechococcus strain. J. Bacteriol. 187224-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNeil, M., M. Daffe, and P. J. Brennan. 1991. Location of the mycolyl ester substituents in the cell walls of mycobacteria. J. Biol. Chem. 26613217-13233. [PubMed] [Google Scholar]
- 16.Nikaido, H., and M. Vaara. 1985. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 491-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nixdorff, K., J. Gmeiner, and H. H. Martin. 1978. Interaction of lipopolysaccharide with detergents and its possible role in the detergent resistance of the outer membrane of Gram-negative bacteria. Biochim. Biophys. Acta 51087-98. [DOI] [PubMed] [Google Scholar]
- 18.Palenik, B., B. Brahamsha, F. W. Larimer, M. Land, L. Hauser, P. Chain, J. Lamerdin, W. Regala, E. E. Allen, J. McCarren, I. Paulsen, A. Dufresne, F. Partensky, E. A. Webb, and J. Waterbury. 2003. The genome of a motile marine Synechococcus. Nature 4241037-1042. [DOI] [PubMed] [Google Scholar]
- 19.Palenik, B., and S. T. Dyhrman. 1998. Recent progress in understanding the regulation of marine primary productivity by phosphorus, p. 26-38. In J. P. Lynch and J. Deikman (ed.), Phosphorus in plant biology: regulatory roles in molecular, cellular, organismic, and ecosystem processes. American Society of Plant Physiologists, Rockville, MD.
- 20.Palenik, B., Q. Ren, C. L. Dupont, G. S. Myers, J. F. Heidelberg, J. H. Badger, R. Madupu, W. C. Nelson, L. M. Brinkac, R. J. Dodson, A. S. Durkin, S. C. Daugherty, S. A. Sullivan, H. Khouri, Y. Mohamoud, R. Halpin, and I. T. Paulsen. 2006. The genome of Synechococcus CC9311: insights into adaptation to a coastal environment. Proc. Natl. Acad. Sci. USA 10313555-13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papo, N., and Y. Shai. 2005. A molecular mechanism for lipopolysaccharide protection of gram-negative bacteria from antimicrobial peptides. J. Biol. Chem. 28010378-10387. [DOI] [PubMed] [Google Scholar]
- 22.Partensky, F., J. Blanchot, and D. Vaulot (ed.). 1999. Prochlorococcus and Synechococcus in oceanic waters: a review. Bulletin de l'Institut Oceanographique, Monaco, Monaco.
- 23.Pazur, J. 1994. Neutral polysaccharides, p. 90-92. In M. F. Chaplin and J. F. Kennedy (ed.), Carbohydrate analysis, a practical approach, 2nd ed. Oxford University Press, New York, NY.
- 24.Que, N. L., A. A. Ribeiro, and C. R. Raetz. 2000. Two-dimensional NMR spectroscopy and structures of six lipid A species from Rhizobium etli CE3. J. Biol. Chem. 27528017-28027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raetz, C. R. H., C. M. Reynolds, M. S. Trent, and R. E. Bishop. 2007. Lipid A modification systems in gram-negative bacteria. Annu. Rev. Biochem. 76295-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reuhs, B. L., D. P. Geller, J. S. Kim, J. E. Fox, V. S. K. Kolli, and S. G. Pueppke. 1998. Sinorhizobium fredii and Sinorhizobium meliloti produce structurally conserved lipopolysaccharides and strain-specific K antigens. Appl. Environ. Microbiol. 644930-4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridley, B. L., B. S. Jeyaretnam, and R. W. Carlson. 2000. The type and yield of lipopolysaccharide from symbiotically deficient Rhizobium lipopolysaccharide mutants vary depending on the extraction method. Glycobiology 101013-1023. [DOI] [PubMed] [Google Scholar]
- 28.Schindler, M., and M. J. Osborn. 1979. Cation binding to LPS. Biochemistry 184425-4430. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt, W., G. Drews, J. Weckesser, and I. Fromme. 1980. Characterization of the lipopolysaccharides from eight strains of the cyanobacterium Synechococcus. Arch. Microbiol. 127209-215. [Google Scholar]
- 30.Schopf, J. W. 2006. Fossil evidence of archaean life. Phil. Trans. R. Soc. Lond. B 361869-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder, D. S., D. Gibson, C. Heiss, W. Kay, and P. Azadi. 2006. Structure of a capsular polysaccharide isolated from Salmonella enteritidis. Carbohydr. Res. 3412388-2397. [DOI] [PubMed] [Google Scholar]
- 32.Snyder, D. S., and T. J. McIntosh. 2000. The lipopolysaccharide barrier: correlation of antibiotic susceptibility with antibiotic permeability and fluorescent probe binding kinetics. Biochemistry 3911777-11787. [DOI] [PubMed] [Google Scholar]
- 33.Uchida, K., and S. Mizushima. 1987. A simple method for isolation of lipopolysaccharides from Pseudomonas aeruginosa and some other bacterial strains. Agric. Biol. Chem. 513107-3114. [Google Scholar]
- 34.Van Mooy, B. A. S., G. Rocap, H. F. Fredricks, C. T. Evans, and A. H. Devol. 2006. Sulfolipids dramatically decrease phosphorus demand by picocyanobacteria in oligotrophic marine environments. Proc. Natl. Acad. Sci. USA 1038607-8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinogradov, E., M. B. Perry, and J. W. Conlan. 2002. Structural analysis of Francisella tularensis lipopolysaccharide. Eur. J. Biochem. 2696112-6118. [DOI] [PubMed] [Google Scholar]
- 36.Vinogradov, E. V., K. Bock, B. O. Petersen, O. Holst, and H. Brade. 1997. The structure of the carbohydrate backbone of the LPS from Acinetobacter strain ATCC 17905. Eur. J. Biochem. 243122-127. [DOI] [PubMed] [Google Scholar]
- 37.Volk, W. 1968. Isolation of a d-galacturonic acid 1-phosphate from hydrolysates of cell wall lipopolysaccharide extracted from Xanthomonas campestris. J. Bacteriol. 95782-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waterbury, J. B., and R. Rippka. 1989. Subsection I. Order Chroococcales Wettstein 1924, emend. Rippka et al., 1979, p. 1728-1746. In J. T. Staley, M. P. Bryant, N. Pfennig, and J. B. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 3. Williams and Wilkins, Baltimore, MD. [Google Scholar]
- 39.Waterbury, J. B., and F. W. Valois. 1993. Resistance to co-occurring phages enables marine Synechococcus communities to coexist with cyanophages abundant in seawater. Appl. Environ. Microbiol. 593393-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waterbury, J. B., and J. M. Willey. 1988. Isolation and growth of marine planktonic cyanobacteria. Methods Enzymol. 167100-105. [Google Scholar]
- 41.Weckesser, J., G. Drews, and H. Mayer. 1979. Lipopolysaccharides of photosynthetic prokaryotes. Annu. Rev. Microbiol. 33215-239. [DOI] [PubMed] [Google Scholar]
- 42.Weckesser, J., and U. J. Jurgens. 1988. Cell walls and external layers. Methods Enzymol. 167173-188. [Google Scholar]
- 43.Weckesser, J., A. Katz, G. Drews, H. Mayer, and I. Fromme. 1974. Lipopolysaccharide containing l-acofriose in the filamentous blue-green alga Anabaena variabilis. J. Bacteriol. 120672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wientraub, A., U. Zähringer, H.-W. Wollenweber, U. Seydel, and E. T. Rietschel. 1989. Structural characterization of the lipid A component of Bacteroides fragilis strain NCTC 9343 lipopolysaccharide. Eur. J. Biochem. 183425-431. [DOI] [PubMed] [Google Scholar]
- 45.Wozniak, D. J., T. J. O. Wyckoff, M. Starkey, R. Keyser, P. Azadi, G. O'Toole, and M. R. Parsek. 2003. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA 1007907-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu, X., I. Khudyakov, and C. P. Wolk. 1997. Lipopolysaccharide dependence of cyanophage sensitivity and aerobic nitrogen fixation in Anabaena sp. strain PCC 7120. J. Bacteriol. 1792884-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zimmermann, W., and A. Rosselet. 1977. Function of outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob. Agents Chemother. 12368-372. [DOI] [PMC free article] [PubMed] [Google Scholar]