Abstract
In gammaproteobacteria the Hfq protein shows a great variation in size, especially in its C-terminal part. Extremely large Hfq proteins consisting of almost 200 amino acid residues and more are found within the gammaproteobacterial family Moraxellaceae. The difference in size compared to other Hfq proteins is due to a glycine-rich domain near the C-terminal end of the protein. Acinetobacter baylyi, a nonpathogenic soil bacterium and member of the Moraxellaceae encodes a large 174-amino-acid Hfq homologue containing the unique and repetitive amino acid pattern GGGFGGQ within the glycine-rich domain. Despite the presence of the C-terminal extension, A. baylyi Hfq complemented an Escherichia coli hfq mutant in vivo. By using polyclonal anti-Hfq antibodies, we detected the large A. baylyi Hfq that corresponds to its annotated size indicating the expression and stability of the full protein. Deletion of the complete A. baylyi hfq open reading frame resulted in severe reduction of growth. In addition, a deletion or overexpression of Hfq was accompanied by the loss of cell chain assembly. The glycine-rich domain was not responsible for growth and cell phenotypes. hfq gene localization in A. baylyi is strictly conserved within the mutL-miaA-hfq operon, and we show that hfq expression starts within the preceding miaA gene or further upstream.
The bacterial Hfq protein was originally discovered as a necessary host factor for replication of the Qβ phage RNA plus-strand in Escherichia coli by melting its 3′ end and permitting accessibility of phage Qβ replicase (10). Deletion of hfq by insertion of an omega interposon into the E. coli genome leads to pleiotropic phenotypes, depending on the insertion site of the interposon (35). Hfq is now regarded as global RNA chaperone involved in posttranscriptional regulation, e.g., by stimulation or protection of mRNA decay and translation or promotion of duplex formation between small regulatory RNAs and their target mRNAs by unfolding of both molecules (3, 12, 41). During the process of mRNA degradation a combination of endonucleolytic cleavages catalyzed by RNase E or III followed by exonucleolytic cleavage through polynucleotide phosphorylase and RNase II occur. Hfq-mediated protection against mRNA degradation appears if Hfq, for example, binds to the poly(A) tail of rpsO mRNA, encoding for the S15 protein of the 30S ribosomal subunit, which leads to decreased sensitivity for polynucleotide phosphorylase and RNase II, respectively (9). In addition, the process of translation protects mRNAs from degradation, e.g., 30S ribosome binding to the 5′-untranslated region of ompA mRNA protects against RNase E recognition. However, in the presence of Hfq, ompA mRNA translation is repressed, and mRNA decay is stimulated because Hfq interferes with ribosome binding (41). Hfq-mediated duplex formation between a small RNA (sRNA) and a target mRNA was observed, e.g., for the Hfq binding sRNAs SgrS and RyhB. Both of these sRNAs lead to translation inhibition and RNase E-dependent degradation of their mRNA targets ptsG (SgrS) and sodB (RyhB), respectively (18, 20). Conducted BLAST searches of microbial genomes highlight Hfq as a conserved protein, Hfq homologues can be found in at least half of bacterial genomes (32). The N termini of Hfq proteins especially share a high similarity with the Sm1 motif of eukaryotic Sm proteins, which bind numerous RNAs as heteroheptamers (21, 27). Sm1 is connected to a second motif called Sm2 by a region of variable length and sequence in eukaryotic cells and forms a protein structure called Sm fold consisting of an α-helix, followed by a strongly bent five-stranded β-sheet that is responsible for RNA binding and protein-protein interaction (17, 36). Crystals of the C-terminally truncated Hfq protein from E. coli or the full-length Hfq protein from Staphylococcus aureus revealed indeed a homohexameric ring-shaped structure. Despite Sm2 lacking sequence homology to eukaryotic Sm proteins, both proteins adopt the same Sm-fold structure (26, 27). Cocrystallization of S. aureus Hfq with a hepta-oligoribonucleotide (AU5G) revealed that RNA binding is located within the Sm motif especially in β-sheets 2 to 5 (27). Based on this result, the assumption was made that the Hfq C terminus is not necessary for sRNA-mRNA interaction. In fact, a C-terminal 37-amino-acid truncated E. coli Hfq protein is able to bind sRNAs but is defective in mRNA binding (40). Interestingly, the C termini of Hfq proteins from different bacteria vary considerably in length and sequence. The longest C-terminal extensions are found in beta- and gammaproteobacteria. However, it is not known what the function of the Hfq C terminus is and why it is extremely elongated in a few organisms.
In the present study, we describe the Acinetobacter baylyi hfq gene and its encoded protein, which is almost twice the size of other gammaproteobacterial Hfqs due to an elongated C terminus (Fig. 1 and 2). A. baylyi is a gram-negative, nonpathogenic, and strictly aerobic gammaproteobacterium whose genome is completely sequenced and annotated (2). The A. baylyi Hfq protein contains an unusual glycine-rich domain near its C-terminal end consisting of the repetitive amino acid patterns GGGFGGQ and GGFGGQ. hfq is located downstream the gene miaA (encoding a tRNA modification enzyme) on the same DNA strand and upstream of surA (encoding a protein folding enzyme) on the opposite DNA strand.
FIG. 1.
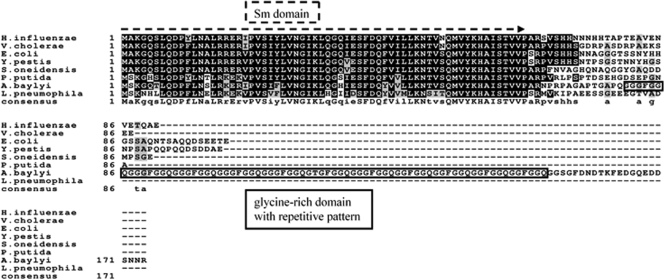
Multiple sequence alignment of the A. baylyi Hfq amino acid sequence with seven gammaproteobacterial Hfq homologues by CLUSTAL W and visualized with BoxShade 3.2. Capital letters in the consensus sequence indicate conserved amino acids appearing in all aligned sequences. Lowercase letters indicate conserved amino acids appearing in at least two sequences. Numbers indicate the amino acid positions. Amino acids shaded gray have the same polarity as the black-shaded ones, whereas amino acids with a white background differ in their polarity from the black- and gray-shaded amino acids. The Sm domain is marked by a dotted arrow and is encoded by the first 64 amino acids in all compared sequences. The repetitive glycine-rich amino acid pattern of the A. baylyi Hfq sequence starts at amino acid 80 (glycine), ends at amino acid 152 (glutamine), and is outlined by a black box.
FIG. 2.
Comparison of Hfq amino acid sequences from different Moraxellaceae by CLUSTAL W and visualized with BoxShade 3.2. Capital letters in the consensus sequence indicate conserved amino acids appearing in all aligned sequences. Lowercase letters indicate amino acids in at least two sequences. Amino acids shaded gray share the same polarity as the black-shaded ones, whereas amino acids with a white background differ in their polarity to black- and gray-shaded amino acids. Numbers indicate the amino acid position in the protein.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in the present study are listed in Table 1. The E. coli strains MC4100 AM111 hfq1::Ω and MC4100 AM112 hfq2::Ω have been described elsewhere (22) and were grown in Luria-Bertani (LB) medium supplemented with 50 μg of kanamycin/ml with aeration at 37°C. E. coli strain MC4100 AM111/pRK415 hfqA. baylyi was cultivated in LB medium supplemented with 12 μg of tetracycline/ml and 50 μg of kanamycin/ml. Cultivation of all Acinetobacter strains was carried out in minimal medium (33) with aeration at 30°C. If needed, 100 μg of spectinomycin/ml, 6 μg of tetracycline/ml, or 6 μg of kanamycin/ml was added to the medium.
TABLE 1.
Bacterial strains
| Strain | Relevant characteristicsa | Source or reference |
|---|---|---|
| A. baylyi | ||
| ADP1 | Wild type (strain BD413, ATCC 33305) | 38 |
| ADP1 Δhfq | hfq ORF replaced by Ω-interposon; Spr | This study |
| ADP1 ΔmiaA | Ω-interposon 55 bp downstream of miaA 5′ end; Spr | This study |
| ADP1 ΔmutL | mutL ORF replaced by Km-integration cassette; Kmr | 6 |
| ADP1 ΔHfq73aa | Mutated hfq gene (chromosomal deletion of amino acids 80 to 152) | This study |
| ADP1/pRK415 hfq | Overexpression of hfq; Tcr | This study |
| ADP1 Δhfq/pRK415 hfq | Complementation of hfq deletion in trans; Spr Tcr | This study |
| E. coli | ||
| MC4100 AM111 hfq1::Ω | Ω-interposon inserted into hfq BclI site; Kmr | 22, 35 |
| MC4100 AM112 hfq2::Ω | Ω-interposon inserted into hfq KpnI site; Kmr | 22, 35 |
| MC4100 AM111/pRK415A. baylyi hfqPlac | Expression of A. baylyi hfq controlled by its natural promoter; Kmr Tcr | This study |
| MC4100 AM111/pRK415A. baylyiPlac hfq | Expression of A. baylyi hfq controlled by the lac promoter of pRK415; Kmr Tcr | This study |
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ−thi-1 gyrA96 relA1 | 13 |
Tcr, tetracycline resistance; Ampr, ampicillin resistance; Spr, spectinomycin resistance; Kmr, kanamycin resistance.
Plasmid and strain construction.
Inactivation of the A. baylyi hfq gene was done by deletion of the complete open reading frame (ORF) and replacement with the Ω-interposon of plasmid pHP45Ω designed to terminate both transcriptional and translational activities and encoding spectinomycin sensitivity (25). The DNA regions next to A. baylyi hfq were amplified via PCR using the primers 241, 242, 243, and 244 (Table 2) and A. baylyi chromosomal DNA as a template. PCR products were purified with the PCR clean-up gel extraction kit (Macherey-Nagel, Düren, Germany) and subsequently cloned by using their artificial BamHI, natural AflIII, and XhoI recognition sites into cloning vector pBSKII+ (Stratagene, Amsterdam, The Netherlands), creating plasmid pBSKII+ Δhfq, and used for transformation of E. coli DH5α cells (13). The Ω-interposon was inserted into the created BamHI-site of pBSK+ Δhfq, and finally the complete fragment Δhfq Ω was cut after plasmid preparation from E. coli DH5α pBSK+ Δhfq Ω with AflIII/XhoI and transformed into the A. baylyi genome using natural competence (33). The specific integration of the construct was confirmed by whole-cell PCR with the primers 241 and 244, which anneal to the insert-flanking sequences (Fig. 3 and Table 2).
TABLE 2.
Oligonucleotides
| Primer | Sequence (5′-3′)a | Restriction site(s) |
|---|---|---|
| 4 | ATCAACAACGCACCACT | |
| 5 | ACCCAAGTCAAGCTGAT | |
| 241 | AGGAAACATTGGCACAATTTCAAAC | |
| 242 | CATGGATCCCTTTAGACATTTTATAACTCC | BamHI |
| 243 | CATGGATCCCAATCGTTAATCCTAAAACC | BamHI |
| 244 | ACGAATAAGTTCACAGTTGCTGCAA | |
| 266 | GGAGAATTCAAGCTTTGTAGATCCTGTTGCGGGAG | EcoRI, HindIII |
| 267 | GGACTGCAGTTGCAGAGTTACCTTCTGAAC | PstI |
| 289 | GGTGGTTCAGGCTTTGACAACGATACTAAATTTGA | |
| 247 | TAGTGAACCATTCCAGACTCAGTTTGG | |
| 291 | AAAGCCTGAACCACCTTGTGGAGCACCAGTAGGCG | |
| 246 | CAGGCTCAAGAAGACTTGCGAAACTC | |
| 342 | CTACTGCAAGCGGGAAGGATCCTTTGGCGT | BamHI |
| 344 | ACGCCAAAGGATCCTTCCCGCTTGCAGTAG | BamHI |
| 343 | CGACGGCCGTTAACGATTGTTAGAATCGTC | |
| 301 | TCAAAAGATTCAATGTGGCCTTG |
Restriction sites are indicated in boldface.
FIG. 3.
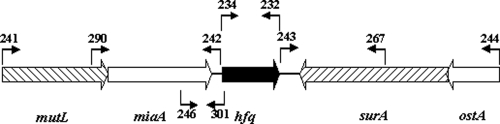
Schematic representation of the genomic localization of A. baylyi hfq and primer sites for hfq manipulation, MiaA, Hfq, and SurA Northern blot detection and hfq RT-PCR. Primer sites are indicated by arrows and numbers (see Table 2).
Complementation of the hfq deletion and overproduction of Hfq in A. baylyi were carried out in trans from plasmid pRK415 (19). The hfq expression construct was created via primer 266 containing an EcoRI recognition site and primer 267 containing a PstI recognition site (see Table 2). The purified PCR product was cut with EcoRI and PstI and cloned into pRK415. To ensure expression from the natural hfq promoter and avoid additional expression from the lac promoter of the vector, the integration was made such that the vector promoter was downstream of the cloned hfq gene. Plasmid pRK415 hfq Plac was conjugated (16) with the help of plasmid pRK2013 (8) and successfully established via tetracycline resistance in A. baylyi Δhfq and wild-type cells. Positive clones were confirmed and distinguished from E. coli donor cells containing plasmid pRK415 hfq by whole-cell PCR with the primers 4 and 5 (Table 2) annealing in the qui-pob operon of A. baylyi. The qui-pob operon encodes enzymes of the aromatic degradation pathway and is missing in E. coli. In addition, growth on A. baylyi minimal medium was applied because E. coli does not grow on this medium. The presence of the Δhfq mutation was confirmed by whole-cell PCR with the primers 241 and 244.
Deletion of the 73-amino-acid repetitive glycine-rich domain in the A. baylyi hfq gene was achieved by amplifying two PCR products with the primer pairs 289 and 247 (289/247) and 291/246. Both DNA fragments were used as templates for an overlap extension PCR with the primer pair 247/246. The resulting hfq construct was missing the internal hfq glycine-rich domain and was transformed into A. baylyi Δhfq cells. Positive clones expressing an HfqΔ73aa protein were selected based on spectinomycin sensitivity. Confirmation of the clones was done by whole-cell PCR.
Deletion of miaA was performed by insertion of the Ω-interposon near the 5′ end of the ORF. To this end, a BamHI recognition site had to be established in the A. baylyi miaA gene. Two DNA fragments were created by using the primer pairs 342/343 and 344/241 by PCR. Both products were included as a template in an overlap extension PCR leading to the insertion of a BamHI site 52 bases downstream of the miaA 5′ end without interruption of the reading frame. This construct was amplified with the primer pair 241/343, cut with SalI/EagI, cloned in pBSK+II, and established in E. coli cells. Positive clones were selected via ampicillin resistance and blue-white screening. The Ω-interposon was cut out of pHP45Ω with BamHI and ligated with plasmid pBSK+ miaA cut with the same enzyme. Positive clones were selected by ampicillin and spectinomycin resistance, and the complete miaA deletion construct was cut by PvuI/SalI (both are natural enzyme recognition sites of the A. baylyi hfq genomic region), gel purified, and transformed into A. baylyi wild-type cells. Positive clones were selected based on spectinomycin resistance and confirmed by whole-cell PCR.
Complementation of an hfq deletion in E. coli Δhfq strains by A. baylyi hfq was fulfilled by transfer of plasmid pRK415 hfqA. baylyi into E. coli MC4100 strain AM111 hfq1::Ω and screening for positive clones by tetracycline and kanamycin resistance. Cloning of hfq with the HindIII recognition site of primer 266 resulted in a plasmid expressing hfq from the pRK415 lac promoter. This plasmid was also established in E. coli MC4100 strain AM111 hfq1::Ω.
RNA isolation and Northern blot analysis.
Total RNA was isolated by a procedure described previously (24). The RNA quality and concentration were determined from the ratio of the optical density at 260 nm (OD280) to that at 280 nm. Then, 15 μg of purified RNA was heat denatured for 5 min at 65°C and separated on a 1.2% formaldehyde-agarose gel in 1× RNA loading dye (1 ml of 5× RNA loading dye was composed of 8 μl of 500 mM EDTA, 200 μl of 100% glycerol, 72 μl of 37% [vol/vol] formaldehyde, 308 μl of formamide, 400 μl of 10× running buffer [200 mM morpholinepropanesulfonic acid, 50 mM sodium acetate, 5 mM EDTA; pH 7], 2 μl of saturated bromophenol blue, and 10 μl of deionized water) and transferred to a Hybond-N+ nylon membrane (Amersham Biosciences, Freiburg, Germany) by capillary blotting in 10× SSC (1.5 M sodium chloride, 0.15 mM sodium citrate) overnight. The membrane was dried at room temperature, and the RNA was covalently bound by a UV cross-linker (Amersham Biosciences) for 90 s at 1,200 μJ/cm2. Specific RNA detection was performed by the digoxigenin (DIG) labeling and detection system (Roche Applied Sciences, Mannheim, Germany).
Western blot analysis.
To obtain A. baylyi total protein crude extract, 1 ml of cells was treated for 15 min on ice with 25 μl of 2% deoxycholic acid. Afterward, the total protein was precipitated with 30 μl of 40% trichloroacetic acid, centrifuged 5 min at 14,000 × g, and dissolved in deionized water. Separation of total protein was carried out with a 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel, and the protein concentration was measured by using a Bradford assay. Then, 20 μg of the total protein was applied per lane, after dilution with 1 volume of SDS loading dye (12% SDS [wt/vol], 6% β-mercaptoethanol [vol/vol], 30% glycerol [vol/vol], 0.05% Coomassie brilliant blue G-250 [wt/vol], 150 mM Tris-HCl; pH 7) and incubation at 65°C for 5 min before loading. After separation, the protein was transferred onto a nitrocellulose membrane by using an electroblotter (Serva Electrophoresis, Heidelberg, Germany) at 200 mA for 2 h. The membrane was blocked in 5% skim milk powder for 1 h at room temperature before incubation with anti-Hfq antibodies (raised against Pseudomonas putida Hfq and kindly provided by Gerhard Burchardt, University of Greifswald, Greifswald, Germany) diluted 1:1,000 for 1 h at room temperature. Detection of specific Hfq-anti-Hfq interaction was performed with a Vectastain ABC kit (Vector Laboratories, Peterborough, England).
RT-PCR analysis.
RNA of A. baylyi grown until early stationary phase was isolated and treated with DNase I (Roche Applied Sciences) to remove any DNA contamination. Then, 1 μg of DNA-free RNA was denatured at 70°C for 5 min together with 2 pmol of primer 301, which is complementary to A. baylyi hfq and anneals 119 bases downstream of the hfq 5′ end. Reverse transcription (RT) was performed with 100 U of Moloney murine leukemia virus reverse transcriptase RNase Minus Point Mutant (Promega, Mannheim, Germany) in a total volume of 25 μl at 45°C for 1 h. Finally, 1 μl of the created cDNA was used as a template in a PCR with the primers 301 and 246 (see Fig. 3 and Table 2). As controls, the reaction was separately done without the addition of reverse transcriptase on the one hand (a negative control for the RT reaction) and A. baylyi chromosomal DNA as cDNA template on the other hand (a positive control for the PCR).
RESULTS
In silico identification of an unusually large Hfq protein encoded by A. baylyi.
A. baylyi strain ADP1 encodes an unusually large Hfq protein (174 amino acids) with highly significant homology between amino acid residues 1 and 66 to those of other gammaproteobacterial Hfqs, including the Hfq Sm motifs (Fig. 1). In contrast, the C-terminal end of A. baylyi Hfq is more than three times larger and contains a hydrophobic glycine-rich domain. Detailed BLAST searches identified this domain as special Hfq feature within the Moraxellaceae family. Whereas Hfq proteins of the genus Acinetobacter show the strictly repetitive amino acid patterns GGGFGGQ and GGFGGQ (starting at positions 81 and 126 of A. baylyi Hfq [Fig. 1]), Hfq homologues of the genera Psychrobacter and Moraxella contain no obvious patterns compared to A. baylyi Hfq within their glycine-rich Hfq domains (Fig. 2, consensus line). A PHI-BLAST (pattern hit initiated) search revealed GGGFGGQ also within DNAJ/DNAJ-like chaperones of different bacteria, which contain a glycine-rich domain and are involved in protein folding, protein transport, and response to cell stress (42). We also observed this pattern in DNA-binding proteins, e.g., in DNA polymerase III from Frankia sp.
A. baylyi Hfq complements an E. coli hfq deletion.
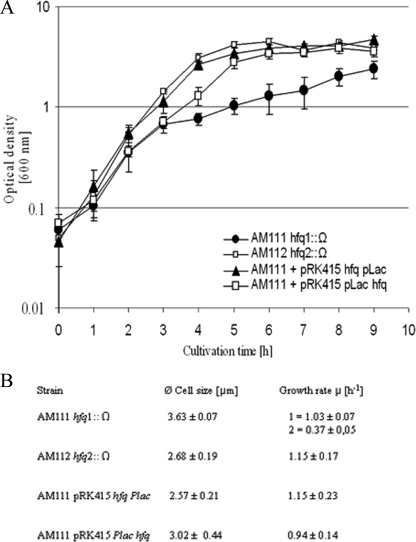
A. baylyi Hfq functionality was investigated by complementation of growth and cell phenotype effects of E. coli Δhfq strains. A. baylyi Hfq was expressed in trans and controlled by the natural hfq promoter (pRK415 hfq) or by the lac promoter (pRK415 Plac hfq), respectively. Since A. baylyi does not contain a lac operon, the use of IPTG (isopropyl-β-d-thiogalactopyranoside) was not necessary. We know from previous work that this promoter functions in A. baylyi. Both plasmids were transformed into the E. coli MC4100 strain AM111 hfq1::Ω, which possesses an Ω-interposon in the hfq BclI recognition site (117 bases downstream of the hfq start codon) and shows effects on growth and cell size. Strain E. coli MC4100 AM112 hfq2::Ω contained the Ω-interposon at the hfq KpnI site (232 bases downstream of the hfq start codon) and showed no phenotype (35). The latter strain was used as a reference strain in the present study. Although restoring normal growth by complementation of strain AM111 hfq1::Ω with the E. coli hfq gene was not possible (35), strains expressing A. baylyi Hfq instead showed the same growth behavior and cell size like the reference strain in LB complex medium (Fig. 4). Hfq controlled by its own promoter and expressed from the low-copy-number plasmid pRK415 (a derivate of RK2) is sufficient to restore the original growth behavior, whereas additional expression from the lac promoter results in a slightly retarded growth and elongated cells compared to the reference strain. These observations indicate that the concentration of Hfq must be well balanced to perform its natural function. We never observed biphasic growth behavior of AM111 hfq1::Ω complemented with A. baylyi hfq. However, strain AM111 hfq1::Ω showed a biphasic growth in LB medium supplemented with 171 mM sodium chloride, which has been described before (35). This biphasic growth behavior was characterized as salt-dependent and not observed in LB medium supplemented with 0, 0.085, or 0.5 M sodium chloride (35).
FIG. 4.
Growth comparison of E. coli hfq mutants with or without a plasmid expressing A. baylyi Hfq. (A) Growth curves of cells grown in LB medium supplemented with 170 mM NaCl and antibiotics. Strains were grown overnight, diluted 1/100, and incubated at 37°C with aeration, and growth was monitored by measuring the absorbance at 600 nm every hour. (B) Cell size and growth rate comparison of E. coli hfq mutants with or without a plasmid expressing A. baylyi Hfq. Thirty cells collected from cultures were measured at an OD600 of 1. For E. coli MC4100 AM111 hfq1::Ω showing a biphasic growth, the growth rates were determined at OD600 of 0.4 (growth rate 1) and an OD600 of 0.8 (growth rate 2).
Growth effects of hfq manipulation in A. baylyi.
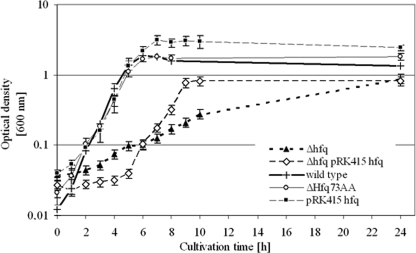
The deletion of A. baylyi hfq was done by replacing the complete ORF with an Ω-interposon and complemented by expression of hfq in trans from the low-copy plasmid pRK415 or by replacing the Ω-interposon with a partially deleted (Δ73 amino acid [Δ73aa] = glycine-rich domain) A. baylyi hfq gene. Overexpression of Hfq was carried out by transfer of the plasmid pRK415 hfq into A. baylyi wild-type cells (Fig. 5). The deletion of hfq led to a strong decrease in the growth rate (A. baylyi wild type, μ = 0.92 h−1; A. baylyi Δhfq, μ = 0.12 h−1) and a lower final OD of the culture than in wild-type cells. Restoring the original growth behavior with hfq expression in trans from the low-copy replicating plasmid pRK415 and controlled by its own promoter was not completely possible and resulted in a strain starting exponential growth after a long lag phase and reaching a lower final OD. Establishing pRK415 without hfq in A. baylyi had no effect on the growth rate, but cultures reached a higher final OD compared to wild-type cells (OD600 = 1.7 for wild-type cells and OD600 = 2.7 for pRK415-containing cells [data not shown]). In contrast, chromosomal complementation of the deletion with a smaller A. baylyi hfq gene resulted in restoring the original growth behavior of A. baylyi, indicating that the C-terminal part, which is deleted in this strain (Δ73aa) is not required for normal growth. Transfer of an hfq expressing plasmid controlled by the lac promoter into A. baylyi hfq mutant cells was not successful, perhaps due to the constitutive hfq expression, which led to a toxic Hfq accumulation. Interestingly, overexpression of Hfq regulated by its own promoter in addition to the chromosomal wild-type hfq allele led to the same growth rate but to a higher final OD of the culture.
FIG. 5.
Growth of A. baylyi strains manipulated in the mutL-miaA-hfq operon in minimal medium supplemented with 10 mM succinic acid and antibiotics. Strains were grown overnight, diluted 1/25, and incubated at 30°C with aeration, and the growth was monitored by measuring absorbance at 600 nm every hour.
Furthermore, we included a Flag tag containing two stop codons at different Hfq positions, respectively, by a modified method of a one-step gene inactivation epitope tagging of chromosomal genes (37) in the A. baylyi hfq gene. A mutant with the tags inside the Sm domain showed similar growth defects compared to the total hfq deletion, whereas an insertion of the tag before the hfq stop codon resulted in a very weak effect on growth compared to wild-type cells (data not shown). This indicates that hfq and especially the N-terminal protein part is necessary for normal growth in A. baylyi.
Verification of an unusually large Hfq in A. baylyi.
Given the observation that the unusual C-terminal extension of A. baylyi Hfq was not necessary for normal growth, we investigated whether the whole ORF was expressed. Therefore, all A. baylyi hfq strains were analyzed with regard to Hfq mRNA and protein expression (Fig. 6). Northern blot analysis with hfq-specific PCR probes revealed that in fact hfq deletion and complementation were successful on the mRNA level. In the wild type, a 525-base Hfq mRNA was expected, whereas deletion of the hfq ORF or of the glycine-rich domain should result in the absence of Hfq mRNA or in a 306-base mRNA, respectively. Indeed, the A. baylyi Δhfq strain expressed no hfq mRNA any longer (Fig. 6C). Chromosomal hfq complementation resulted in a smaller hfq mRNA due to the insertion of the hfq gene without its glycine-rich domain (Δ219 bp) compared to the wild-type mRNA. As expected, the hfq mRNA level of the wild type and the chromosomally complemented Δhfq strain appeared to be similar. In contrast, the in trans complementation by overexpression from the low-copy-number plasmid pRK415 led to a much higher amount of hfq mRNA (Fig. 6C). This observation strengthens the earlier assumption that Hfq is only effective at a certain concentration.
FIG. 6.
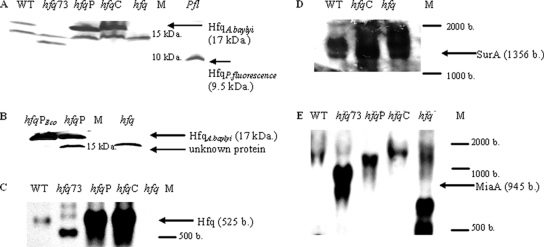
Analysis of hfq expression in A. baylyi hfq strains used in the present study by Western and Northern blot experiments. (A and B) Hfq Western blot analysis of 20 μg of total protein derived from all hfq mutant A. baylyi strains using a polyclonal antibody raised against P. putida Hfq. (C) Northern blot analysis of hfq mRNA detected with a specific DIG-labeled PCR probe (primer pair 232/234) in total RNA derived from all hfq mutant A. baylyi strains. (D) Detection of surA transcript with a specific DIG-labeled PCR probe (primer pair 243/267). (E) Detection of miaA transcript using a specific DIG-labeled PCR probe (primer pair 242/290). Abbreviations: WT, ADP1 wild type; hfq73, ADP1 HfqΔ73aa; hfqP, ADP1/pRK415 hfq; hfqC, ADP1 Δhfq/pRK415 hfq; hfq, ADP1 Δhfq; Pfl, P. fluorescence wild type; hfqPEco, E. coli/pRK415 hfqA. baylyi Plac; M, PageRulerProtein ladder (Fermentas, St. Leon-Roth, Germany) or ssRNA ladder (New England Biolabs, Frankfurt am Main, Germany).
Correspondingly, the Hfq protein expression was investigated in all hfq mutant A. baylyi strains by Western blot analysis with specific anti-Hfq antibodies raised against P. putida Hfq (Fig. 6). Both proteins share an identity of 83% in the N-terminal part (amino acid residues 1 to 70). A. baylyi Hfq could be detected as a 17-kDa band, confirming the existence of an unusually long Hfq in A. baylyi (Fig. 6A, lane WT). A. baylyi Hfq could also be detected as 17-kDa protein in the hfq complemented and the overexpressing strain (Fig. 6A, lanes hfqC and hfqP). According to the signal strength (plasmid versus chromosomally encoded hfq), overexpression of Hfq was successful, since the plasmid-containing strains caused a much stronger signal. In addition to the specific Hfq signal, we noticed a nonspecific binding of the antibody to a 15-kDa protein in all A. baylyi hfq strains. Western blot analysis of total protein from an E. coli strain expressing A. baylyi hfq did not reveal this band; it showed only one band according to Hfq of the same size as in A. baylyi wild type (Fig. 6B, lane hfqPEco). P. putida Hfq was detected at 10 kDa, a finding which agrees with the annotation of the protein (Fig. 6A, lane Pfl). The signal of the 15-kDa protein again was missing, indicating that it is derived from a nonspecific binding of the antibody with an A. baylyi protein.
A. baylyi hfq transcription starts within the miaA coding region or further upstream.
In contrast to the deviation from other bacteria in terms of Hfq C-terminal size and sequence conservation, hfq genomic localization is strictly conserved among gammaproteobacterial genomes. A. baylyi hfq, being no exception to this, is encoded within the mutL-miaA-hfq operon, whose transcription has been extensively studied in E. coli (34). This conservation does not apply to the genes downstream of hfq among gammaproteobacteria. In many cases, hfl genes encoding enzymes for bacteriophage λ lysogeny (4) follow hfq. Species of the family Moraxellaceae encode different genes downstream of hfq. In Moraxella catarrhalis it is kpsF encoding for arabinose-5-phosphate isomerase (1), and in A. baylyi it is surA encoding for a peptidyl-prolyl cis-trans isomerase. RT-PCR analysis was performed with RNA from A. baylyi to determine whether hfq transcription starts within the miaA coding region or further upstream. An RT step was carried out with primer 301 (Fig. 3) annealing from bp 119 till bp 97 downstream of the hfq 5′ end on the negative strand, whereas the second PCR primer 246 (Fig. 3) was located from bp 35 till bp 10 upstream of the miaA 3′ end, respectively. The RT-PCR yielded a ∼265-bp product that was missing in the control reaction without reverse transcriptase (Fig. 7), indicating that hfq transcription starts at least partly within the miaA ORF or further upstream.
FIG. 7.
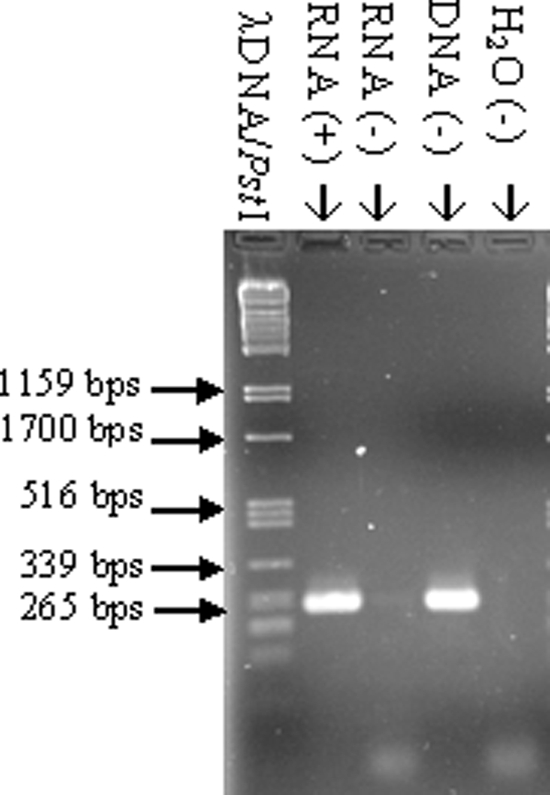
RT-PCR analysis of the A. baylyi miaA-hfq region. Lanes: RNA (+), RT-PCR with A. baylyi total RNA as a template; RNA (−), RT-PCR with A. baylyi total RNA as a template but without reverse transcriptase; DNA (−), RT-PCR with A. baylyi chromosomal DNA as a template; H2O (−), PCR with H2O instead of DNA as a template; λDNA/PstI, λDNA cut with PstI.
Influence of hfq on the expression of neighboring genes.
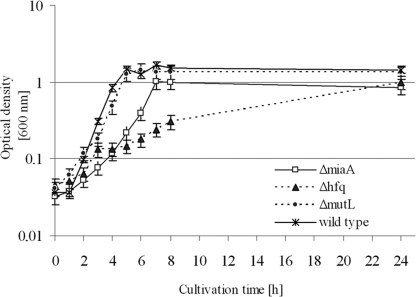
The A. baylyi hfq gene is located between the genes surA and miaA (Fig. 3). An hfq deletion could possibly affect surA or miaA expression, e.g., by destabilization of their transcripts, and subsequently the observed effect could be caused by miaA or surA rather than by hfq. Both neighbor genes are involved in important processes. miaA expression leads to tRNA modification by transfer of an isopentenyl moiety from dimethylallyl pyrophosphate to adenosine 37 of tRNAs (5, 28), and surA encodes a rotamase that is involved in protein folding (14). Therefore, possible effects toward transcript stability of both mRNAs were investigated. To address this issue, we studied the influence of hfq loss and insertion of the Ω-interposon in miaA and surA (Fig. 6D and E) via Northern blot analysis with specific DNA probes (miaA primer pair 290/242, surA primer pair 267/243). surA and miaA mRNA were detected in total RNA isolated from cells from the stationary growth phase. The deletion of hfq or the insertion of the Ω-interposon had no influence on the mRNA level of the downstream gene surA (Fig. 6D). miaA is encoded on the same DNA strand as hfq, and hfq expression starts within or upstream of the miaA ORF. Northern blot experiments revealed that (except for the A. baylyi hfq mutant) the miaA mRNA was detected in the size range between 1,500 and 2,000 bases. Given the lengths of the miaA gene (945 bp) and the hfq gene (525 bp), it is likely that there is cotranscription. In addition to the miaA mRNA, we saw two strong abundant RNAs with sizes between 500 and 1,000 bases appearing in the A. baylyi hfq mutant (Fig. 6E, lane hfq). It is known that Hfq specifically destabilizes its own mRNA at the posttranscriptional level by binding at two positions in the 5′-untranslated region of its mRNA, which results in inhibition of the translation initiation complex (39). Together with the fact that E. coli hfq transcription is regulated within the miaA gene by two promoters whose expression results in long untranslated transcripts (891 and 488 bases), a lack of Hfq could result in the accumulation of these 5′-untranslated Hfq mRNAs (34). Our hypothesis is supported by the fact that complementation of the hfq mutation in trans leads to the loss of both small abundant hfq transcripts (Fig. 6E, lane hfqC). In addition, also in case of the hfq wild type, C-terminal truncated, and Hfq overexpression situations, wherever Hfq was present, both small RNAs were also not detected (Fig. 6E, lane WT, hfq73 and hfqP). We checked whether insertion of the Ω-interposon is responsible for the miaA mRNA instability and would lead to the observed Δhfq growth defect. Therefore, we introduced the Ω-interposon into the miaA gene. We were concerned that a complete replacement of miaA with the interposon would lead to secondary effects due to hfq transcription failure. Along these lines was the finding that a complete deletion of miaA during the creation of single deletion mutants in A. baylyi was not possible (6). For both reasons, we created an artificial BamHI site by replacing TTGGC into CCTAG 55 bases downstream of the miaA start codon to create an incorporation site for the Ω-interposon. At this site the Ω-interposon was inserted to inactivate the miaA gene. A. baylyi mutL (preceding the miaA gene and kindly supplied by Valerie de Berardinis, Genoscope, France), miaA and hfq mutants were compared in growth experiments (Fig. 8) showing that deletion of hfq revealed the mutant with the biggest effect on growth. The effect of the miaA mutation was intermediate, whereas mutL mutants showed no apparent effect compared to wild-type cells.
FIG. 8.
Growth of A. baylyi strains manipulated in the mutL-miaA-hfq operon in minimal medium supplemented with 10 mM succinic acid and antibiotics. Strains were grown overnight, diluted 1/25, and incubated at 30°C with aeration, and the growth was monitored by measuring the absorbance at 600 nm every hour.
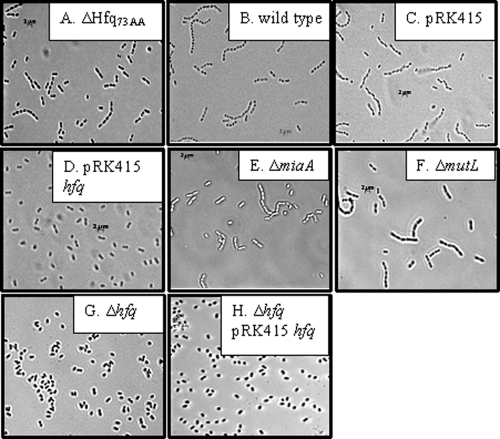
Given that A. baylyi Hfq is able to influence E. coli cell size (35), we checked the cells of all A. baylyi strains that are manipulated in the mutL-miaA-hfq operon (Fig. 9). Interestingly, hfq deletion (Fig. 9G), as well as overexpression (Fig. 9D), led to the inability of the cells to assemble in chains. Complementation of the deletion in trans (Fig. 9H) could not restore the original cell arrangement in contrast to chromosomal complementation (Fig. 9A), indicating that the amount of Hfq is critical for the cell phenotype. An effect of the cell phenotype due to the plasmid pRK415 (Fig. 9C) can be excluded. Neither mutL (Fig. 9F) nor miaA (Fig. 9E) mutants are affected in their cell phenotype compared to wild-type cells (Fig. 9B). Taken together, the described experiments provide evidence that the effects observed upon deletion of hfq are due to the absence of this gene and not to the disturbed expression of the neighboring genes.
FIG. 9.
Microscope comparison of different A. baylyi strains. The cells were grown in minimal medium supplemented with 10 mM succinic acid as carbon source and incubated at 30°C in flasks with aeration until an OD of ∼1 at 600 nm was reached.
DISCUSSION
Hfq is known to show a strong variation in its C terminus in gamma- and betaproteobacteria (40). Searching redundant protein databases showed that (except for a few betaproteobacteria especially Burkholderia species) the largest known Hfq proteins are annotated in members of the gammaproteobacterial family Moraxellaceae. The annotated Hfq lengths are between 168 and 174 amino acids for Acinetobacter species, 183 to 203 amino acids for Psychrobacter species, and up to 210 amino acids for M. catarrhalis (1, 38). Among the Moraxella spp., Hfq proteins differ in terms of the length of the C-terminal domain, its sequence, and the downstream genes (either surA in Acinetobacter species or kpsF encoded in other Moraxellaceae). In Burkholderia genomes such as in Burkholderia pseudomallei, small (79-amino-acid) and large (216-amino-acid) Hfq homologues have been annotated but not yet verified. Except for the abundance of glycine residues between the N and C termini, there is no obvious sequence conservation comparing both large Hfq homologues of Burkholderia and Acinetobacter spp. Surprisingly, the small and large Hfq Burkholderia proteins themselves share only low sequence conservation even in the conserved N terminus (53% between amino acids 9 and 79). In addition, the genomic locus is not conserved: neither the Burkholderia small Hfq protein nor the large Hfq protein is encoded within the mutL-miaA-hfq operon. Burkholderia small hfq precedes the hfl genes, which act as GTPases and are responsible for high frequency of bacteriophage lambda lysogenization in E. coli (23). This genetic localization also seems to be common for many small hfq genes in gammaproteobacteria. A. baylyi Hfq is twice as long as most gammaproteobacterial Hfq proteins (see references 7, 29, and 31). It is encoded upstream of surA, a peptidyl cis-trans isomerase. This enzyme class is involved in the correct folding of many eukaryotic and prokaryotic proteins (11, 15). In the present study, we could show by Northern blot analysis and RT-PCR that hfq transcription may start within the preceding miaA gene, as is the case in E. coli (34). All large Hfq homologues contain a glycine-rich domain between their N- and C-terminal ends, but only Acinetobacter species show a strictly repeated pattern within this domain. Searching the nonredundant protein databases showed that this pattern can be found in DNAJ-like chaperones (42). Until now, there have been no data available for the functionality of this protein region. For P. putida it was shown that its Hfq of 86 amino acids can complement hfq deletion effects in E. coli (30). We report here that the large gammaproteobacterial Hfq from A. baylyi (174 amino acids) shows the same ability to complement the hfq deletion effects on growth behavior and cell phenotype in E. coli. Furthermore, a complete hfq deletion in A. baylyi resulted in a drastic disruption of growth and change in the cell phenotype which could not completely be restored with the same expression plasmid used for E. coli hfq complementation, whereas chromosomal complementation resulted in complete restoration. The experiments reported here indicate that the amount of Hfq in the cell needs to be controlled: overexpression led to the loss of chain assembly; in trans hfq complementation resulted in failure to restore wild-type growth behavior. In contrast, the glycine-rich domain of A. baylyi Hfq is not important for normal growth and cell phenotype, as shown by the chromosomal deletion of the 73-amino-acid glycine-rich domain in A. baylyi. A deletion of hfq in A. baylyi has a strong effect on growth by causing an elongated lag phase but also a loss of the ability of exponential cell splitting. To exclude secondary effects on miaA transcript stability by the Ω-interposon, we investigated partial miaA deletion. Complete miaA disruption is not possible because it would interfere with hfq transcription, and we saw no effects on cell arrangement and only slight growth defects with exponentially growing cells in a strain where only 5% of the full-length of miaA is expressed (from the ATG to the start of the Ω-interposon).
In summary, A. baylyi Hfq is the second described member of large Hfq proteins encoded within the family Moraxellaceae. A. baylyi Hfq differs from M. catarrhalis in the amino acid composition of the large C-terminal domain and the genetic localization regarding hfq downstream genes. A. baylyi and M. catarrhalis share the same upstream gene (miaA) and the feature that hfq transcription starts within miaA or further upstream. Remarkable and unique for Acinetobacter is the Hfq glycine-rich domain, consisting of a strictly repeated amino acid pattern.
Acknowledgments
We thank Valerie de Berardinis, Genoscope, France, for providing the A. baylyi mutL mutant and Gerhardt Burchhardt, University of Greifswald, Greifswald, Germany, for the anti-Hfq antibodies. We also thank Thomas Böck, Hatice Öztürk, and Iris Steiner for technical support.
D.S. was supported by a grant from the state of Baden-Württemberg.
Footnotes
Published ahead of print on 26 June 2009.
REFERENCES
- 1.Attia, A. S., J. L. Sedillo, W. Wang, W. Liu, C. A. Brautigam, W. Winkler, and E. J. Hansen. 2008. Moraxella catarrhalis expresses an unusual Hfq protein. Infect. Immun. 762520-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbe, V., D. Vallenet, N. Fonknechten, A. Kreimeyer, S. Oztas, L. Labarre, S. Cruveiller, C. Robert, S. Duprat, P. Wincker, L. N. Ornston, J. Weissenbach, P. Marliere, G. N. Cohen, and C. Medigue. 2004. Unique features revealed by the genome sequence of Acinetobacter sp. ADP1, a versatile and naturally transformation competent bacterium. Nucleic Acids Res. 325766-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, L., and T. Elliott. 1996. Efficient translation of the RpoS sigma factor in Salmonella typhimurium requires host factor I, an RNA-binding protein encoded by the hfq gene. J. Bacteriol. 1783763-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng, H. H., P. J. Muhlrad, M. A. Hoyt, and H. Echols. 1988. Cleavage of the cII protein of phage lambda by purified HflA protease: control of the switch between lysis and lysogeny. Proc. Natl. Acad. Sci. USA 857882-7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connolly, D. M., and M. E. Winkler. 1991. Structure of Escherichia coli K-12 miaA and characterization of the mutator phenotype caused by miaA insertion mutations. J. Bacteriol. 1731711-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Berardinis, V., D. Vallenet, V. Castelli, M. Besnard, A. Pinet, C. Cruaud, S. Samair, C. Lechaplais, G. Gyapay, C. Richez, M. Durot, A. Kreimeyer, F. Le Fevre, V. Schachter, V. Pezo, V. Doring, C. Scarpelli, C. Medigue, G. N. Cohen, P. Marliere, M. Salanoubat, and J. Weissenbach. 2008. A complete collection of single-gene deletion mutants of Acinetobacter baylyi ADP1. Mol. Syst. Biol. 4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding, Y., B. M. Davis, and M. K. Waldor. 2004. Hfq is essential for Vibrio cholerae virulence and downregulates sigma expression. Mol. Microbiol. 53345-354. [DOI] [PubMed] [Google Scholar]
- 8.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 761648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folichon, M., V. Arluison, O. Pellegrini, E. Huntzinger, P. Regnier, and E. Hajnsdorf. 2003. The poly(A) binding protein Hfq protects RNA from RNase E and exoribonucleolytic degradation. Nucleic Acids Res. 317302-7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franze de Fernandez, M. T., L. Eoyang, and J. T. August. 1968. Factor fraction required for the synthesis of bacteriophage Qβ-RNA. Nature 219588-590. [DOI] [PubMed] [Google Scholar]
- 11.Gavini, N., S. Tungtur, and L. Pulakat. 2006. Peptidyl-prolyl cis/trans isomerase-independent functional NifH mutant of Azotobacter vinelandii. J. Bacteriol. 1886020-6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geissmann, T. A., and D. Touati. 2004. Hfq, a new chaperoning role: binding to messenger RNA determines access for small RNA regulator. EMBO J. 23396-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166557-580. [DOI] [PubMed] [Google Scholar]
- 14.Hermans, P. W., P. V. Adrian, C. Albert, S. Estevao, T. Hoogenboezem, I. H. Luijendijk, T. Kamphausen, and S. Hammerschmidt. 2006. The streptococcal lipoprotein rotamase A (SlrA) is a functional peptidyl-prolyl isomerase involved in pneumococcal colonization. J. Biol. Chem. 281968-976. [DOI] [PubMed] [Google Scholar]
- 15.Herrler, M., H. Bang, K. Brune, G. Fischer, and M. A. Marahiel. 1992. Peptidyl-prolyl cis-trans isomerase from Bacillus subtilis. A prokaryotic enzyme that is highly sensitive to cyclosporin A. FEBS Lett. 309231-234. [DOI] [PubMed] [Google Scholar]
- 16.Juni, E. 1978. Genetics and physiology of Acinetobacter. Annu. Rev. Microbiol. 32349-371. [DOI] [PubMed] [Google Scholar]
- 17.Kambach, C., S. Walke, R. Young, J. M. Avis, E. de la Fortelle, V. A. Raker, R. Luhrmann, J. Li, and K. Nagai. 1999. Crystal structures of two Sm protein complexes and their implications for the assembly of the spliceosomal snRNPs. Cell 96375-387. [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto, H., Y. Koide, T. Morita, and H. Aiba. 2006. Base-pairing requirement for RNA silencing by a bacterial small RNA and acceleration of duplex formation by Hfq. Mol. Microbiol. 611013-1022. [DOI] [PubMed] [Google Scholar]
- 19.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70191-197. [DOI] [PubMed] [Google Scholar]
- 20.Masse, E., F. E. Escorcia, and S. Gottesman. 2003. Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev. 172374-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moller, T., T. Franch, P. Hojrup, D. R. Keene, H. P. Bachinger, R. G. Brennan, and P. Valentin-Hansen. 2002. Hfq: a bacterial Sm-like protein that mediates RNA-RNA interaction. Mol. Cell 923-30. [DOI] [PubMed] [Google Scholar]
- 22.Muffler, A., D. Fischer, and R. Hengge-Aronis. 1996. The RNA-binding protein HF-I, known as a host factor for phage Qβ RNA replication, is essential for rpoS translation in Escherichia coli. Genes Dev. 101143-1151. [DOI] [PubMed] [Google Scholar]
- 23.Noble, J. A., M. A. Innis, E. V. Koonin, K. E. Rudd, F. Banuett, and I. Herskowitz. 1993. The Escherichia coli hflA locus encodes a putative GTP-binding protein and two membrane proteins, one of which contains a protease-like domain. Proc. Natl. Acad. Sci. USA 9010866-10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oelmüller, U., N. Krüger, A. Steinbüchel, and C. G. Freidrich. 1990. Isolation of prokaryotic RNA and detection of specific mRNA with biotinylated probes. J. Microbiol. Methods 1173-81. [Google Scholar]
- 25.Prentki, P., A. Binda, and A. Epstein. 1991. Plasmid vectors for selecting IS1-promoted deletions in cloned DNA: sequence analysis of the omega interposon. Gene 10317-23. [DOI] [PubMed] [Google Scholar]
- 26.Sauter, C., J. Basquin, and D. Suck. 2003. Sm-like proteins in Eubacteria: the crystal structure of the Hfq protein from Escherichia coli. Nucleic Acids Res. 314091-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schumacher, M. A., R. F. Pearson, T. Moller, P. Valentin-Hansen, and R. G. Brennan. 2002. Structures of the pleiotropic translational regulator Hfq and an Hfq-RNA complex: a bacterial Sm-like protein. EMBO J. 213546-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seif, E., and B. M. Hallberg. 2009. RNA-protein mutually induced fit: structure of Escherichia coli isopentenyl-tRNA transferase in complex with tRNAPhe. J. Biol. Chem. 2846600-6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sittka, A., V. Pfeiffer, K. Tedin, and J. Vogel. 2007. The RNA chaperone Hfq is essential for the virulence of Salmonella typhimurium. Mol. Microbiol. 63193-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonnleitner, E., I. Moll, and U. Blasi. 2002. Functional replacement of the Escherichia coli hfq gene by the homologue of Pseudomonas aeruginosa. Microbiology 148883-891. [DOI] [PubMed] [Google Scholar]
- 31.Sonnleitner, E., J. Napetschnig, T. Afonyushkin, K. Ecker, B. Vecerek, I. Moll, V. R. Kaberdin, and U. Blasi. 2004. Functional effects of variants of the RNA chaperone Hfq. Biochem. Biophys. Res. Commun. 3231017-1023. [DOI] [PubMed] [Google Scholar]
- 32.Sun, X., I. Zhulin, and R. M. Wartell. 2002. Predicted structure and phyletic distribution of the RNA-binding protein Hfq. Nucleic Acids Res. 303662-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trautwein, G., and U. Gerischer. 2001. Effects exerted by transcriptional regulator PcaU from Acinetobacter sp. strain ADP1. J. Bacteriol. 183873-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsui, H. C., G. Feng, and M. E. Winkler. 1996. Transcription of the mutL repair, miaA tRNA modification, hfq pleiotropic regulator, and hflA region protease genes of Escherichia coli K-12 from clustered Eσ32-specific promoters during heat shock. J. Bacteriol. 1785719-5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsui, H. C., H. C. Leung, and M. E. Winkler. 1994. Characterization of broadly pleiotropic phenotypes caused by an hfq insertion mutation in Escherichia coli K-12. Mol. Microbiol. 1335-49. [DOI] [PubMed] [Google Scholar]
- 36.Urlaub, H., V. A. Raker, S. Kostka, and R. Luhrmann. 2001. Sm protein-Sm site RNA interactions within the inner ring of the spliceosomal snRNP core structure. EMBO J. 20187-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uzzau, S., N. Figueroa-Bossi, S. Rubino, and L. Bossi. 2001. Epitope tagging of chromosomal genes in Salmonella. Proc. Natl. Acad. Sci. USA 9815264-15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaneechoutte, M., D. M. Young, L. N. Ornston, T. De Baere, A. Nemec, T. Van Der Reijden, E. Carr, I. Tjernberg, and L. Dijkshoorn. 2006. Naturally transformable Acinetobacter sp. strain ADP1 belongs to the newly described species Acinetobacter baylyi. Appl. Environ. Microbiol. 72932-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vecerek, B., I. Moll, and U. Blasi. 2005. Translational autocontrol of the Escherichia coli hfq RNA chaperone gene. RNA. 11976-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vecerek, B., L. Rajkowitsch, E. Sonnleitner, R. Schroeder, and U. Blasi. 2008. The C-terminal domain of Escherichia coli Hfq is required for regulation. Nucleic Acids Res. 36133-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vytvytska, O., I. Moll, V. R. Kaberdin, A. von Gabain, and U. Blasi. 2000. Hfq (HF1) stimulates ompA mRNA decay by interfering with ribosome binding. Genes Dev. 141109-1118. [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh, P., D. Bursac, Y. C. Law, D. Cyr, and T. Lithgow. 2004. The J-protein family: modulating protein assembly, disassembly and translocation. EMBO Rep. 5567-571. [DOI] [PMC free article] [PubMed] [Google Scholar]