Abstract
To facilitate the management of multidrug-resistant (MDR) tuberculosis, two nucleic acid sequence-based methods, the GenoType MTBDRplus test and DNA sequencing, were assessed for the rapid detection of drug-resistant Mycobacterium tuberculosis for the first time in the Asia-Pacific region. The performances of these two assays in detecting the presence of rifampin (rifampicin) (RIF) and isoniazid (INH) resistance-associated mutations in the rpoB, katG, inhA regulatory region, inhA, and oxyR-ahpC genes were compared to that of a conventional agar proportion drug susceptibility test. A total of 242 MDR and 30 pansusceptible M. tuberculosis isolates were evaluated in this study. The sensitivities obtained for RIF-resistant detection by the GenoType MTBDRplus test and by resistance gene sequencing were 95.5% and 97.9%, respectively. The sensitivities for INH resistance detection by the GenoType MTBDRplus test and by resistance gene sequencing were 81.8% and 93.4%, respectively. Together, the sensitivity for MDR tuberculosis detection was 78.5% with the GenoType MTBDRplus test and 91.3% by resistance gene sequencing. The specificity for RIF resistance, INH resistance, and MDR detection was 100% by both methods. The GenoType MTBDRplus test has the advantage of a short turnaround time for drug-resistant M. tuberculosis detection. Overall, the two assays performed equally well in detecting RIF resistance (P = 0.13). However, DNA sequencing demonstrated superior performance in detecting INH resistance (P < 0.001) and MDR tuberculosis (P < 0.001). We suggest that new alleles of INH resistance genes should be evaluated to improve the sensitivity of the GenoType MTBDRplus test, especially for different geographic areas with genetically diverse M. tuberculosis strains.
The emergence of multidrug-resistant tuberculosis (MDR-TB), defined as infection with a Mycobacterium tuberculosis complex isolate resistant to at least isoniazid (INH) and rifampin (rifampicin) (RIF), is a public health concern and threatens global TB control programs (22). In Taiwan, approximately 15,000 new TB cases are diagnosed annually, of which an estimated 4% are MDR-TB (12). Therefore, the Taiwan Centers for Disease Control (CDC) not only has strengthened directly observed treatment in the management of TB as of 2006, to prevent MDR generation, but also has implemented a DOTS-Plus (directly observed treatment, short-course) strategy for the management of MDR-TB patients as of 2007 (8). However, this program can be hampered by delayed laboratory diagnosis. The completion of diagnosis by conventional methods and drug susceptibility testing (DST) of M. tuberculosis normally take months.
The World Health Organization and partners have endorsed the use of the molecular test GenoType MTBDRplus (Hain Lifescience GmbH, Nehren, Germany) for rapid detection of high-risk MDR-TB cases, even directly from certain clinical specimens (1, 4, 6, 10, 15, 21). The GenoType MTBDRplus test is a PCR-based amplification and reverse blotting assay that employs specific probes hybridized to nitrocellulose strips to detect RIF and INH resistance. The assay detects mutations in the rpoB gene for RIF resistance, in the katG gene for high-level INH resistance, and in the inhA regulatory region gene for low-level INH resistance. To evaluate the reliability of the assay, DNA sequencing analyses of rpoB for RIF and katG, the inhA regulatory region gene, inhA, or oxyR-ahpC for INH were conducted in parallel.
Our previous study demonstrated the genetic diversity of MDR M. tuberculosis isolates with novel alleles in the rpoB gene in Taiwan (11). Likewise, the distribution of M. tuberculosis isolates differs in different geographic regions (5, 11). The GenoType MTBDRplus test has been assessed in Europe (6, 10, 15, 21), South Africa (4), and the Caribbean (1), but not in the Asia-Pacific region, where there is a high prevalence of Beijing family M. tuberculosis isolates. Here we report the performance of the revised GenoType MTBDRplus test compared to that of DNA sequencing using a culture-based phenotypic DST, which is considered the gold standard for routine clinical practice.
MATERIALS AND METHODS
Mycobacterium tuberculosis isolates.
One bacteriologically (cultured in Löwenstein-Jensen or MGIT medium) and biochemically (nitrate and niacin tests) identified M. tuberculosis complex isolate was collected from each of 272 TB cases in hospitals throughout Taiwan between May 2007 and April 2008. Of the 272 isolates, 242 isolates were MDR and 30 were fully susceptible, as determined by conventional DST. No INH-monoresistant isolate was used in this study. Of the 242 MDR isolates, 133 (55%) belonged to the Beijing family and 109 (45%) were non-Beijing isolates.
DST. (i) Conventional methods.
Test methods, media, and drug concentrations were used as previously described (7). Most laboratories in Taiwan have adopted the agar proportion method using either Middlebrook 7H10 or 7H11 medium (Creative Microbiologicals or Sancordon, Taipei, Taiwan). A few laboratories have employed BACTEC MGIT 960 SIRE kits (Becton Dickinson Diagnostic Systems, Sparks, MD) with a liquid culture system (2). Growth on a control medium was compared to growth on a drug-containing medium in order to determine susceptibility or resistance. The DST results were classified as resistant or susceptible. Tests were validated by the susceptibility of M. tuberculosis H37Ra, included in the same test. Although conventional DST was performed by clinical laboratories using either an agar proportion method or an MGIT method, all MDR-TB isolates used in this study were retested by both methods at the Reference Laboratory of Mycobacteriology in the Taiwan CDC.
(ii) Molecular methods.
The two genotypic assays were evaluated blindly by two medical technologists in parallel. Cell lysates, prepared according to the protocol provided by the manufacturer of the GenoType MTBDRplus kit, were also used for sequencing.
GenoType MTBDRplus test.
The GenoType MTBDRplus assay was performed according to the instructions provided by the manufacturer (Hain Lifescience GmbH, Nehren, Germany). Briefly, the amplification mixture contained 35 μl of the primer nucleotide mix, 5 μl of 10× polymerase incubation buffer, 5 μl of 25 mM MgCl2, 1 μl of AmpliTaq Gold polymerase (5 U/μl; Applied Biosystems), and 5 μl of the supernatant of the cell lysate, for a final volume of 50 μl. The amplification protocol consisted of 5 min of denaturation at 95°C, followed by 10 cycles comprising 30 s at 95°C and 2 min at 58°C, an additional 20 cycles comprising 25 s at 95°C, 40 s at 53°C, and 40 s at 70°C, and then a final extension at 70°C for 8 min. Hybridization and detection were performed with a TwinCubator (Hain Lifescience GmbH, Nehren, Germany). The hybridization procedure included the following steps: chemical denaturation of the amplification products at room temperature for 5 min, hybridization of the single-stranded biotin-labeled amplicons to membrane-bound probes at 45°C for 30 min, stringent washes, addition of a streptavidin-alkaline phosphatase (AP) conjugate at room temperature for 30 min, and an AP staining reaction to detect colorimetric bands. To detect RIF resistance, eight wild-type (WT) rpoB probes encoding amino acids 505 to 533 and four probes for common mutations were utilized. Probes used for INH resistance detection were designed to recognize a WT S315 region, with two mutant probes for the highly resistant katG gene and two probes specific for WT regions, as well as four mutant probes for the inhA gene, which demonstrates low-level resistance. When all WT probes showed positive staining for an isolate and mutant probes demonstrated no staining, the isolate was considered susceptible. In contrast, the isolate was considered resistant when either any one of the WT probes was absent or any one of the mutant probes was present.
DNA sequencing of the katG, inhA, inhA locus, oxyR-ahpC, and rpoB genes.
Mutations in katG, inhA, the inhA locus (inhA regulatory region), the oxyR-ahpC intergenic region (ahpC), and rpoB were analyzed by PCR amplification, and then were sequenced with the respective oligonucleotide primers: katG-F (5′-GTC ACA CTT TCG GTA AGA C-3′) and katG-R (5′-TTG TCG CTA CCA CGG AAC G-3′); inhA 1713-F (5′-CCG AGG ATG CGA GCT ATA TC-3′) and inhA 1713-R (5′-GGC TCG GGT CGA AGT CCA TG-3′); inhA 2194-F (5′-AGG CGC TGC TGC CGA TCA TG-3′) and inhA 2194-R (5′-CCG AAC GAC AGC AGC AGG AC-3′); inhA locus-F (5′-AAT TGC GCG GTC AGT TCC AC-3′) and inhA locus-R (5′-GTC GGT GAC GTC ACA TTC GA-3′); ahpC-F (5′-GCT TGA TGT CGG AGA GCA TCG-3′) and ahpC-R (5′-GGT CGC GTA GGC AGT GCC CC-3′); rpoB-F (5′-TCG GCG AGC CCA TCACGT CG-3′) and rpoB-R (5′-GCG TAC ACC GAC AGC GAG CC-3′). PCRs were performed as follows: 33 cycles at 96°C for 1 min; annealing for 1 min at 64°C for rpoB, 55°C for katG, and 60°C for inhA 1713, inhA 2194, the inhA regulatory region, and ahpC; and elongation at 72°C for 1 min. Thereafter, the PCR products were analyzed with an ABI 3730 automated sequencer (Applied Biosystems), and the sequence data were assembled and edited using Sequencing Analysis software (version 5.2.0; Applied Biosystems).
Data analysis.
The performances of the GenoType MTBDRplus test and DNA sequencing were compared to that of a conventional DST for the detection of RIF and INH resistance. The sensitivity, specificity, and accuracy of the molecular assays were calculated. Data were analyzed using Epi Info 6 (Centers for Disease Control and Prevention, Atlanta, GA). Comparisons were performed using chi-square analysis and Fisher's exact test. Statistical significance was defined as a P value of <0.05.
RESULTS
All 30 fully drug susceptible M. tuberculosis isolates demonstrated no mutations, as determined using both molecular assays.
GenoType MTBDRplus test and sequencing results conferring RIF resistance.
Of the 242 MDR M. tuberculosis isolates, 231 (95.4%) and 237 (97.9%) isolates revealed mutations conferring RIF resistance by the GenoType MTBDRplus test and DNA sequencing, respectively (Table 1). The frequencies of gene mutations in RIF-resistant isolates identified by the GenoType MTBDRplus test are shown in Table 2. Predominant mutations identified as RIF resistance mutations were rpoB S531L (63.2%), a missing rpoB WT pattern (20.8%), and rpoB H526Y (10%). Among the 48 RIF-resistant isolates with at least one missing rpoB WT pattern, 9 displayed a sole H526R mutation and 5 had a single L533P mutation. Both H526R and L533P were not included in the GenoType MTBDRplus test. Nevertheless, of the 11 (4.5%) isolates classified by the GenoType MTBDRplus test as RIF susceptible, 8 showed resistant mutations at L533P based on sequencing. Overall, the L533P mutation was identified in 13 (5.4%) isolates among 242 MDR isolates. In contrast, two MDR isolates having all eight positive WT stains and possessing additionally either the S531L (MUT3) or the H526Y (MUT2A) mutation, as identified by the GenoType MTBDRplus test, displayed the rpoB WT pattern by DNA sequencing (Table 1). These two isolates were judged to be resistant by the GenoType MTBDRplus test. The coexistence of a major WT peak and a minor mutation peak was found by reading the raw sequencing data visually for each sample. However, after data were analyzed using the Sequencing software, the two samples were judged drug susceptible. Finally, the results were later confirmed as drug resistant using conventional DST methods.
TABLE 1.
Genotype MTBDRplus assay and sequencing results for 242 MDR Mycobacterium tuberculosis isolates
| Result by the GenoType MTBDRplus test | Result by sequencing | No. (%) with the following result by conventional DST:
|
||
|---|---|---|---|---|
| RIF resistant | INH resistant | MDR | ||
| Resistant | Resistant | 229 (94.6) | 198 (81.8) | 188 (77.7) |
| Resistant | Susceptible | 2 (0.8) | 0 (0) | 2 (0.8) |
| Susceptible | Resistant | 8 (3.3) | 28 (11.6) | 33 (13.6) |
| Susceptible | Susceptible | 3 (1.2) | 16 (6.6) | 19 (7.9) |
TABLE 2.
Gene mutations in 231 RIF-resistant and 198 INH-resistant Mycobacterium tuberculosis isolates using the GenoType MTBDRplus test
| Gene(s) and mutation probe(s) | Mutation(s) or codon(s) analyzed | No. (%) of isolates |
|---|---|---|
| rpoB | ||
| MUT1 | D516V | 6 (2.6) |
| MUT2A | H526Y | 23 (10) |
| MUT2B | H526D | 8 (3.5) |
| MUT3 | S531L | 146 (63.2) |
| Missing WT | 505-533 | 48 (20.8) |
| katG | ||
| MUT1 | S315T1 | 96 (48.5) |
| MUT1,2 | S315T1, S315T2 | 1 (0.5) |
| MUT2 | S315T2 | 0 (0) |
| Missing WT | 315 | 10 (5.1) |
| inhA | ||
| MUT1 | C15T | 60 (30.3) |
| MUT2 | A16G | 0 (0) |
| MUT3A | T8C | 5 (2.5) |
| MUT3B | T8A | 0 (0) |
| katG and inhA | ||
| katG MUT1, inhA MUT1 | S315T1, C15T | 19 (9.6) |
| katG MUT1, inhA MUT3A | S315T1, T8C | 2 (1.0) |
| katG MUT1, inhA MUT3B | S315T1, T8A | 3 (1.5) |
| katG MUT1, missing inhA WT | S315T1, (−15/−16/−8) | 1 (0.5) |
| Missing katG WT, inhA MUT1 | 315, C15T | 1 (0.5) |
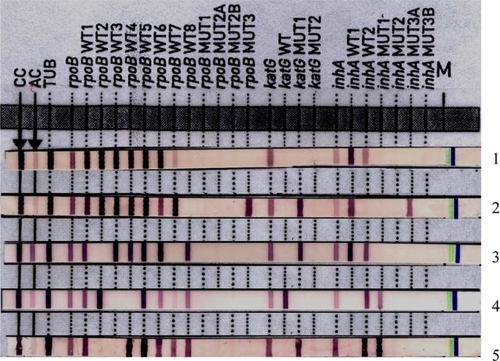
Sequencing data revealed that five MDR isolates each had two mutated codons, as shown in Fig. 1. Of the mutated rpoB codons identified, D444V and M558K were not within the hot spot region of the rpoB gene. In addition, two MDR isolates were identified by the GenoType MTBDRplus test. Those two isolates with discordant results were identified by sequencing as harboring either an rpoB H526N (Fig. 1, lane 1) or an rpoB T525I (lane 4) mutation; they stained WT7 positive by the GenoType MTBDRplus test (Fig. 1). Three MDR Beijing genotype isolates confirmed by conventional DST could not be identified as RIF resistant by either genotypic assay.
FIG. 1.
Representative patterns obtained by the GenoType MTBDR-plus test for isolates with dual mutations in the rpoB gene based on sequencing. Lane 1, ΔWT8 (sequenced mutations in rpoB, H526N and L533P); lane 2, ΔWT8 and MUT3 (rpoB D444V and S531L); lane 3, ΔWT2 and ΔWT7 (rpoB S511P and H526N); lane 4, ΔWT3 and ΔWT4 (rpoB D516Y and T525I); lane 5, ΔWT2 and ΔWT3 (rpoB Q513E and M558K).
GenoType MTBDRplus test and sequencing results for mutations conferring INH resistance.
Of the 242 MDR M. tuberculosis isolates, 198 (81.8%) and 226 (93.4%) isolates were detected by the GenoType MTBDRplus test and DNA sequencing, respectively, as INH resistant (Table 1). By the GenoType MTBDRplus test, 54.0% (107/198) demonstrated mutations in katG alone, 32.8% (65/198) showed mutations in inhA alone, and 13.1% (26/198) displayed mutations in both katG and inhA. The predominant mutation patterns identified as conferring INH resistance included katG S315T1 (48.5% of isolates [96/198]), inhA C15T (30.3% [60/198]), and the katG S315T1 inhA C15T double mutation (9.6% [19/198]). None of the single katG MUT2, inhA MUT2, or inhA MUT3B mutations were identified in our study population (Table 2).
Discordant results by sequencing compared to the GenoType MTBDRplus test were observed for 28 MDR isolates (Table 3). Based on DNA sequencing, 7.4% (18/242) of the MDR isolates had a mutation in the ahpC gene. Of those 18 isolates, 15 harbored a sole ahpC mutation. Overall, 16 (6.6%) MDR isolates still showed WT patterns in the katG, inhA, inhA regulatory region, and ahpC genes sequenced (Table 1). Of the 16 MDR isolates, 12 had Beijing family genotypes.
TABLE 3.
Discordance between results obtained by the GenoType MTBDRplus test and those obtained by DNA sequencing in the detection of INH resistance for 28 MDRa M. tuberculosis isolates
| INH resultb of Genotype MTBDRplus test for isolates with the WT katG/inhA regulatory region pattern | DNA sequencing
|
No. of isolates | |
|---|---|---|---|
| Mutated codon(s) (katG, inhA, ahpC) | INH result | ||
| S | ahpC C−10T | R | 6 |
| S | ahpC G−66A | R | 2 |
| S | ahpC G−9A | R | 2 |
| S | katG Y337C | R | 2 |
| S | katG D419H | R | 1 |
| S | katG Y413H | R | 1 |
| S | katG del401C | R | 1 |
| S | katG A379V | R | 1 |
| S | katG L378P, inhA E217D | R | 1 |
| S | katG del364T, ahpC C−12T | R | 1 |
| S | katG A362D | R | 1 |
| S | katG del310A, ahpC C−10T | R | 1 |
| S | katG G299A, ahpC C−10T | R | 1 |
| S | inhA E217D | R | 1 |
| S | inhA R202G | R | 1 |
| S | ahpC C−39T | R | 1 |
| S | ahpC −4/−5 insert A | R | 1 |
| S | ahpC G−5A, ahpC G−6A | R | 1 |
| S | ahpC C−15T | R | 1 |
| S | ahpC C−32T | R | 1 |
Resistance was confirmed by conventional DST.
S, susceptible; R, resistant.
Performances of the GenotypeMTBDRplus test and DNA sequencing.
For the detection of RIF resistance, the sensitivity, specificity, and accuracy of the GenoType MTBDRplus test were 95.4%, 100%, and 96.0%, respectively, and the corresponding values for DNA sequencing were 97.9%, 100%, and 98.2%, respectively. In contrast, for the detection of INH resistance, the sensitivity, specificity, and accuracy of the GenoType MTBDRplus test were 81.8%, 100%, and 83.8%, respectively, and the corresponding values for DNA sequencing were 93.4%, 100%, and 94.1%, respectively. Furthermore, for MDR-TB detection, the sensitivity, specificity, and accuracy of the GenoType MTBDRplus test were 78.5%, 100%, and 80.9%, respectively, and the corresponding values for resistance gene sequencing were 91.3%, 100%, and 92.3%, respectively. Overall, the two assays performed equally well in detecting RIF resistance (P = 0.13). However, DNA sequencing demonstrated superior performance for the detection of INH resistance (P < 0.001) and MDR-TB (P < 0.001).
DISCUSSION
We evaluated a revised version of the GenoType MTBDRplus test, including three extra WT rpoB probes for RIF, two extra WT inhA regulatory region probes, and four extra mutated probes for INH, enhancing the accuracy of the detection of resistance to RIF and INH. This test is useful due to its accuracy and specificity, as well as its short turnaround time and ease of use. Previous studies have demonstrated that the GenoType MTBDRplus test had outstanding performance: 91.7% to 100% accuracy in detecting RIF resistance, 34.6% to 94.6% accuracy in detecting INH resistance, and 92% to 100% accuracy in detecting MDR (1, 4, 6, 10, 15, 21). In comparison, the GenoType MTBDRplus test identified 95.4% of RIF-resistant isolates, 81.8% of INH-resistant isolates, and 78.5% of MDR isolates in our study. The overall performance for MDR detection was acceptable in our setting. However, resistance gene sequencing had to be employed as a supplement.
Based on DNA sequencing, all RIF-resistant isolates showed mutations within the hot spot region between codon 507 and codon 533. The frequency of the major mutation S531L (63.2%) is consistent with those found in other studies (50% and 86%) (1, 4, 6, 10, 15, 21). Our results were consistent despite reports that the D516V mutation was commonly found (38%) in Hungarian strains (5), while it was not common in our MDR isolates (2%) (data not shown). In addition, rare mutations in either D444V or M558K were considered associated with low-level RIF resistance (15), while dual mutations might correlate with high-level RIF resistance (23). Of the five isolates shown in Fig. 1, four were of the Beijing family genotype. Our previous study showed that the majority of our MDR isolates (60%) were of the Beijing family genotype (11). In addition, some RIF-resistant M. tuberculosis strains isolated either in Cape Town, South Africa (6%) (20), or in Hungary (10%) (5) did not possess an rpoB mutation, while 1.2% did not have the rpoB mutation in our study. This finding may be due to rare mutations in other regions of the rpoB gene, such as the V146F mutation, reported for a Hungarian population (5), or the V176F mutation, reported for a German population (9).
The frequency of INH-resistant isolates harboring a single C15T mutation in the inhA regulatory region gene in our study (30.3%) was higher than that found in a German population (3%) (10) and comparable to that found in a South African study (27%) (4). The high genetic diversity of INH resistance in our MDR isolates is shown in Table 2. A previous study reported a silent mutation that might result in the absence of one of the WT probes in the GenoType MTBDRplus test (1); however, no silent mutations were identified in our MDR isolates. Since 6.6% of our INH-resistant isolates demonstrated WT patterns, they might potentially have mutations in other genomic regions, such as the kasA, iniA, iniB, iniC, efpA, furA, and ndh genes (17, 24).
The association between mutations in codon 533 of the rpoB gene and RIF resistance remains controversial (10). Of the 13 (5.4%) MDR isolates carrying the L533P mutation, both resistant and susceptible results were obtained by the GenoType MTBDRplus test. Eight of these isolates were determined to be susceptible (the WT8 position showed weakly positive staining) by the GenoType MTBDRplus test. The coexistence of a major mutation peak and a minor WT peak was found by reading the raw sequencing data visually. However, after analysis of data using the Sequencing software, all eight samples were judged drug resistant. According to the manufacturer's instructions, conventional DST results must be obtained before a final report can be provided. Therefore, we suggest the inclusion of an L533P mutant probe to improve the accuracy and turnaround time of detection of RIF resistance. In addition, although mutations in the oxyR-ahpC intergenic region have been shown to be associated with INH resistance (3, 13, 14, 16, 18, 19, 25), detection of this region was not generally included in molecular methods. Of the 18 MDR isolates possessing mutations in the ahpC gene, 8 harbored the ahpC C−10T mutation, which has been described relative to the ahpC mRNA initiation site (14). Concurrent mutations in the ahpC and katG genes were identified in 3 of our MDR isolates and in 10 (12.6%) isolates in the study of Kiepiela et al. (14). Sixteen (88.9%) of our 18 isolates carried mutations in the ahpC promoter region between −45 and −4 relative to the ahpC mRNA initiation site (26). Since a single ahpC gene mutation plays an important role in detecting MDR isolates, it might be useful to include an ahpC mutation codon to improve the detection of INH-resistance.
In conclusion, the present study demonstrated that the GenoType MTBDRplus test is applicable for the rapid detection of RIF and INH resistance. The accuracy of the GenoType MTBDRplus test for the detection of RIF resistance was found to be comparable to that of DST using conventional culture-based methods, but the GenoType MTBDRplus test was less accurate for the detection of INH resistance. Although molecular techniques could be used for rapid DST, we were unable to replace conventional DST. Furthermore, the GenoType MTBDRplus test may need to include new alleles of RIF- and INH-resistant genes in order to improve sensitivity for genetically diverse M. tuberculosis isolates recovered from different geographic areas.
Acknowledgments
This work was supported by DOH97-DC-2502, a grant from the Centers for Disease Control, Department of Health, Taiwan.
We thank the clinical mycobacteriology laboratories for providing Mycobacterium tuberculosis isolates and Yen-Jung Lu and Tung-Ching Lin for technical support.
Footnotes
Published ahead of print on 3 June 2009.
REFERENCES
- 1.Akpaka, P. E., S. Baboolal, D. Clarke, L. Francis, and N. Rastogi. 2008. Evaluation of methods for rapid detection of resistance to isoniazid and rifampin in Mycobacterium tuberculosis isolates collected in the Caribbean. J. Clin. Microbiol. 463426-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardito, F., B. Posteraro, M. Sanguinetti, S. Zanetti, and G. Fadda. 2001. Evaluation of Bactec Mycobacteria Growth Indicator Tube (MGIT 960) automated system for drug susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 394440-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, L. V., T. J. Brown, O. Maxwell, A. L. Gibson, Z. Fang, M. D. Yates, and F. A. Drobniewski. 2005. Molecular analysis of isoniazid-resistant Mycobacterium tuberculosis isolates from England and Wales reveals the phylogenetic significance of the ahpC −46A polymorphism. Antimicrob. Agents Chemother. 491455-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnard, M., H. Albert, G. Coetzee, R. O'Brien, and M. E. Bosman. 2008. Rapid molecular screening for multidrug resistant tuberculosis in a high-volume public health laboratory in South Africa. Am. J. Respir. Crit. Care Med. 177787-792. [DOI] [PubMed] [Google Scholar]
- 5.Bártfai, Z., A. Somoskövi, C. Ködmön, N. Szabó, E. Puskás, L. Kosztolányi, E. Faragó, J. Mester, L. M. Parsons, and M. Salfinger. 2001. Molecular characterization of rifampin-resistant isolates of Mycobacterium tuberculosis from Hungary by DNA sequencing and the line probe assay. J. Clin. Microbiol. 393736-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Causse, M., P. Ruiz, J. B. Gutierrez, J. Zerolo, and M. Casal. 2008. Evaluation of new GenoType MTBDRplus for detection of resistance in cultures and direct specimens of Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 121456-1460. [PubMed] [Google Scholar]
- 7.Centers for Disease Control, Department of Health, Executive Yuan, Taiwan. 2004. A manual of mycobacteriology, 2nd ed. Centers for Disease Control, Taipei, Taiwan.
- 8.Centers for Disease Control, Department of Health, Executive Yuan, Taiwan. 2008. Annual report. http://www.cdc.gov.tw/public/data/8871524971.pdf.
- 9.Heep, M., U. Rieger, D. Beck, and N. Lehn. 2000. Mutations in the beginning of the rpoB gene can induce resistance to rifamycins in both Helicobacter pylori and Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 441075-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hillemann, D., S. Rüsch-Gerdes, and E. Richter. 2007. Evaluation of the GenoType MTBDRplus assay for rifampin and isoniazid susceptibility testing of Mycobacterium tuberculosis strains and clinical specimens. J. Clin. Microbiol. 452635-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jou, R., H. Y. Chen, C. Y. Chiang, M. C. Yu, and I. J. Su. 2005. Genetic diversity of multidrug-resistant Mycobacterium tuberculosis isolates and identification of 11 novel rpoB alleles in Taiwan. J. Clin. Microbiol. 431390-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jou, R., P. C. Chung, Y. S. Wu, J. J. Yang, and K. T. Luh. 2006. Drug-resistant Mycobacterium tuberculosis, Taiwan. Emerg. Infect. Dis. 12871-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley, C. L., D. A. Rouse, and S. L. Morris. 1997. Analysis of ahpC gene mutations in isoniazid-resistant clinical isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 412057-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiepiela, P., K. S. Bishop, A. N. Smith, L. Roux, and D. F. York. 2000. Genomic mutations in the katG, inhA and aphC genes are useful for the prediction of isoniazid resistance in Mycobacterium tuberculosis isolates from Kwazulu Natal, South Africa. Tuber. Lung Dis. 8047-56. [DOI] [PubMed] [Google Scholar]
- 15.Lacoma, A., N. Garcia-Sierra, C. Prat, J. Ruiz-Manzano, L. Haba, S. Rosés, J. Maldonado, and J. Domínguez. 2008. GenoType MTBDRplus assay for molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis strains and clinical samples. J. Clin. Microbiol. 463660-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramaswamy, S., and J. M. Musser. 1998. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber. Lung Dis. 793-29. [DOI] [PubMed] [Google Scholar]
- 17.Ramaswamy, S. V., R. Reich, S. J. Dou, L. Jasperse, X. Pan, A. Wanger, T. Quitugua, and E. A. Graviss. 2003. Single nucleotide polymorphisms in genes associated with isoniazid resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 471241-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sreevatsan, S., X. Pan, Y. Zhang, V. Deretic, and J. M. Musser. 1997. Analysis of the oxyR-ahpC region in isoniazid-resistant and -susceptible Mycobacterium tuberculosis complex organisms recovered from diseased humans and animals in diverse localities. Antimicrob. Agents Chemother. 41600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Telenti, A., N. Honore, C. Bernasconi, J. March, A. Ortega, B. Heym, H. E. Takiff, and S. T. Cole. 1997. Genotypic assessment of isoniazid and rifampin resistance in Mycobacterium tuberculosis: a blind study at reference laboratory level. J. Clin. Microbiol. 35719-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Rie, A., R. Warren, I. Mshanga, A. M. Jordaan, G. D. van der Spuy, M. Richardson, J. Simpson, R. P. Gie, D. A. Enarson, N. Beyers, P. D. van Helden, and T. C. Victor. 2001. Analysis for a limited number of gene codons can predict drug resistance of Mycobacterium tuberculosis in a high-incidence community. J. Clin. Microbiol. 39636-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vijdea, R., M. Stegger, A. Sosnovskaja, Å. B. Andersen, V. Ø. Thomsen, and D. Bang. 2008. Multidrug-resistant tuberculosis: rapid detection of resistance to rifampin and high or low levels of isoniazid in clinical specimens and isolates. Eur. J. Clin. Microbiol. Infect. Dis. 271079-1086. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. 2008. Anti-tuberculosis drug resistance in the world. Report WHO/HTM/TB/2008.394. World Health Organization, Geneva, Switzerland.
- 23.Zakerbostanabad, S. 2008. Multiple mutations in rpoB gene of M. tuberculosis correlate with high level of resistance to rifampicin, abstr. S-51. Abstr. 39th World Conf. on Lung Health of the International Union Against Tuberculosis and Lung Disease.
- 24.Zhang, M., J. Yue, Y. P. Yang, H. M. Zhang, J. Q. Lei, R. L. Jin, X. L. Zhang, and H. H. Wang. 2005. Detection of mutations associated with isoniazid resistance in Mycobacterium tuberculosis isolates from China. J. Clin. Microbiol. 435477-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, S. L., J. G. Shen, P. H. Xu, D. X. Li, Z. Q. Sun, L. Li, Z. R. Yang, and Q. Sun. 2007. A novel genotypic test for rapid detection of multidrug-resistant Mycobacterium tuberculosis isolates by a multiplex probe array. J. Appl. Microbiol. 1031262-1271. [DOI] [PubMed] [Google Scholar]
- 26.Zhang, Y., S. Dhandayuthapani, and V. Deretic. 1996. Molecular basis for the exquisite sensitivity of Mycobacterium tuberculosis to isoniazid. Proc. Natl. Acad. Sci. USA 9313212-13216. [DOI] [PMC free article] [PubMed] [Google Scholar]