Abstract
We designed a study to investigate the molecular epidemiology of group A streptococcal (GAS) and group C and G streptococcal (GCS and GGS) disease in Fiji, a country which is known to have a high burden of streptococcal disease. Molecular typing of the N-terminal portion (emm typing) of the M protein was performed with 817 isolates (535 GAS and 282 GCS/GGS). We also performed genotyping of the C-repeat region in 769 of these isolates to identify J14 sequence types. The profile of emm types for Fiji was very different from that found for the United States and Europe. There were no dominant emm types and a large number of overlapping types among clinical disease states. Commonly found GAS emm types in industrialized countries, including emm1, emm12, and emm28, were not found among GAS isolates from Fiji. Over 93% of GAS isolates and over 99% of GCS/GGS isolates that underwent J14 sequence typing contained either J14.0 or J14.1. Our data have implications for GAS vaccine development in developing countries and suggest that a vaccine based upon the conserved region of the M protein may be a feasible option for Fiji and potentially for other tropical developing countries.
The group A streptococcus (GAS) is an important cause of morbidity and mortality globally, with variation in disease burden between populations (9). A greater burden of GAS disease occurs in developing countries, particularly those located in the tropics, than in industrialized nations (9). The spectrums of GAS disease also differ between developed and developing countries. In many developing countries, GAS impetigo is often endemic, with resultant high rates of acute poststreptococcal glomerulonephritis, acute rheumatic fever, and rheumatic heart disease leading to at least 200,000 deaths annually, and the burden of invasive disease has probably been underestimated (9, 43). In industrialized countries, a massive number of cases of GAS pharyngitis leads to significant economic impact (27) and invasive disease leads to a significant number of deaths (25, 26).
The molecular epidemiologies of GAS disease appear to differ between industrialized and developing nations, although there is a paucity of data from developing nations (5, 9). There are a number of different methods used to characterize GAS, with sequence typing of the 5′ N-terminal end of the M protein gene (emm) the most widely used (3, 17, 18). There have been recent large epidemiologic studies that have used emm sequence typing to investigate the molecular epidemiology of GAS pharyngitis and invasive GAS disease in industrialized nations, most notably in the United States, Canada, and Europe (16, 26, 37) (R. R. Tanz, S. T. Shulman, W. Kabat, E. Kabat, E. Cederlund, D. Patel, Z. Li, V. Sakota, J. B. Dale, and B. Beall, presented at the XVIth Lancefield International Symposium on Streptococci and Streptococcal Diseases, Palm Cove, Queensland, Australia, 2005). Far fewer studies have been conducted in developing nations. The limited available data suggest that numerous new emm types and subtypes have been discovered that have not previously been observed in industrialized countries (40, 45), that the diversity of emm types in developing countries is greater than that in industrialized countries (23, 32-34, 40, 45), and that the majority of isolates are of emm types traditionally associated with impetigo, irrespective of the clinical site of recovery of the isolates (5, 6, 23, 33, 40).
Molecular epidemiologic data have implications for vaccine design. Although a number of antigens have shown promise as potential vaccine candidates, only one vaccine, a 26-valent M-protein-based vaccine, has reached clinical trials in recent times (15, 24). Serotypes for this vaccine were chosen if they were known to be common causes of invasive GAS disease or uncomplicated pharyngitis in the United States or if they were associated with rheumatic fever in classical studies from the United States in the mid-20th century (14). While this vaccine is likely to be efficacious in the United States, concerns have been raised about the transferability of this vaccine to developing-country settings (8).
Alternative approaches to a multivalent vaccine strategy include the development of a conserved-epitope vaccine. A number of conserved epitopes have been identified and are under investigation, including some within the portion of the M protein closest to the cell wall (the C-repeat region) which appear to be relatively conserved (2, 11, 31, 36). An example is the J8 peptide, a B-cell epitope, contained within the larger sequence J14 (named J14.0 in this article for clarity) (28, 29). Following the discovery of J14.0, a number of J14 sequence types have been identified (47). To date, across all C-repeat regions, there have been 55 different J14 sequence types described, which have been named in the order that they have been discovered (J14.0 to J14.54) (47). Typing of the C-repeat region by the J14 sequence type of GAS has been employed previously (47). J14 sequence type is relevant to J8 because antibodies raised against J8 in mice provide cross-protective immunity against GAS isolates containing J14.0 and J14.1 (Michael Batzloff, Queensland Institute of Medical Research, unpublished data). Antibodies raised against the J14.0 peptide in mice have been shown to opsonize GAS strains belonging to a variety of emm subtypes that contain J14 sequences other than the J14.0 sequence type, including J14.2 (47).
Group C streptococci (GCS) and group G streptococci (GGS) are emerging infectious agents, particularly as a cause of invasive disease and of epidemic pharyngitis (19, 35, 48). We have observed higher than expected rates of invasive GCS/GGS in Fiji, as well as high pharyngeal carriage among school children (43). There is also some evidence to suggest that these organisms may play a role in the pathogenesis of acute rheumatic fever and poststreptococcal glomerulonephritis (13, 30). There are very few data regarding emm sequence typing of GCS/GGS and no available data regarding sequence typing of the C-repeat region of these organisms (22).
We designed a study to investigate the molecular epidemiology of GAS and GCS/GGS disease in a tropical setting known to have a high burden of invasive, pharyngeal, and impetiginous streptococcal disease (42-44). Because of the implications for vaccine development, we included molecular typing of both the 5′ end of the M protein (emm sequence typing) and the C-repeat region of the M protein (J14 sequence typing).
MATERIALS AND METHODS
Source of isolates.
Eight hundred and seventeen beta-hemolytic streptococcal isolates collected in a series of population-based studies underwent emm sequence typing, of which 535 were GAS and 282 were GCS/GGS (Table 1). We analyzed GCS and GGS isolates as a single subspecies, Streptococcus dysgalactiae subsp. equisimilis, because all of the GCS and GGS isolates shared the same large colony morphology and because 16S rRNA gene sequencing was used to avoid misclassification where necessary (23, 46). Sterile site isolates were collected in an all-ages prospective surveillance study of invasive streptococcal disease conducted in the Colonial War Memorial Hospital in Suva, Fiji, over a 2-year period (December 2005 to November 2007) (43). Pharyngeal isolates were collected in a 10-month prospective cohort survey of school children aged 5 to 15 years in four schools in 2006 (42). Swabs of the pharynx were taken from asymptomatic children (carriage) and from children who complained of a sore throat. Over the same time period, 455 children also had a swab taken of any crusted or purulent impetigo lesions noted at repeated skin examinations every 2 months (total of six visits).
TABLE 1.
Numbers of isolates that were emm typed by clinical disease type and by beta-hemolytic streptococcal groupa
| Disease type | BHS group | No. of swabs | No. of isolates available | No. of isolates emm typed |
|---|---|---|---|---|
| Invasive disease | GAS | NA | 64 | 55 |
| GCS/GGS | NA | 18 | 12 | |
| Throat carriage | GAS | 665 | 40 | 40 |
| GCS/GGS | 126 | 120 | ||
| Culture-positive sore throat | GAS | 678 | 61 | 61 |
| GCS/GGS | 120 | 113 | ||
| Impetigo | GAS | 563 | 449 | 439b |
| GCS/GGS | 39 | 37b |
BHS, beta-hemolytic streptococcal; NA, not applicable.
There were 108 children who had more than one impetigo swab taken at each visit, and only one of two isolates was counted when the same emm subtype was found in an individual at the same visit, meaning that there were 379 GAS isolates and 37 GCS/GGS isolates included in the final analysis.
Laboratory methods.
Swabs were collected, transported, and cultured by conventional methods, as previously described (21, 41). All beta-hemolytic isolates were transported to the Queensland Institute of Medical Research for genotyping of the emm region and the C-repeat region of the M protein. emm sequence typing was performed using the standard methodology developed by the U.S. Centers for Disease Control and Prevention (CDC) (http://www.cdc.gov/ncidod/biotech/strep/strepindex.htm), and novel emm nucleotide sequences were submitted to the Streptococcal Reference Laboratory at the CDC, where they were assigned a sequence type or subtype by the laboratory moderator. Sequences were generated for the C-repeat region using the same method used for emm typing and were translated into a single-letter amino acid code using the ExPASy online translation tool (http://ca.expasy.org/tools/dna.html). J14 sequence types were assigned to each bacterial strain based on the amino acid sequence corresponding to the conserved region of the M protein. Novel sequences were checked by resequencing of the region. C-repeat regions were named by convention (C3 being closest to the cell membrane), although we added C1-1 and C1-2 for isolates that contained more than three C-repeat regions.
Statistical analysis.
emm sequence type and emm subtype distribution were analyzed by Lancefield group, by specimen type, by clinical disease profile, and by acquisition over time. We measured the variation of the number of emm sequence subtypes using Simpson's index of diversity; the higher the index, the greater the probability that any two isolates will be of different sequence types (12, 38). When comparing proportions of isolates belonging to individual GAS emm subtypes with site of specimen and severity of impetigo, we calculated relative risks using standard methods. We assessed the number of isolates in our study that belonged to emm types that are included in the 26-valent M protein-based GAS vaccine currently under clinical investigation (24). We combined the J14 sequence types in the C3, C2, and C1 regions to create “overall” J14 C-repeat types for each isolate (for example, an isolate with J14.1 in C3, J14.2 in C2, and J14.4 in C1 had an overall J14 C-repeat type of J14.1-J14.2-J14.4). The statistical package STATA version 10.0 (Stata Corporation, College Station, TX) was used to analyze the data.
Ethical approval and consent.
Ethical approval was obtained from the Fiji National Research Ethics Review Committee, the Fiji National Health Research Committee, the University of Melbourne Human Research Ethics Committee, and the Queensland Institute of Medical Research Human Research Ethics Committee. Children were enrolled only if written consent from a parent or guardian was obtained. Children aged 10 years or older were enrolled only if written assent by the child was also obtained.
RESULTS
Of the 817 beta-hemolytic streptococcal isolates included in the study, there were 105 different emm sequence types and 122 emm sequence subtypes. There were no emm sequence subtypes that were shared between GAS and GCS/GGS isolates.
GAS emm sequence typing.
Among the 535 GAS isolates, there were 67 emm types and 74 emm subtypes. There was high diversity of GAS emm subtypes overall (Simpson's index of diversity, 97.1; 95% confidence interval [CI], 96.8 to 97.5), with the greatest diversity occurring in invasive isolates (index, 98.2; 95% CI, 97.2 to 99.3), followed by pharyngeal isolates (index, 97.9; 95% CI, 97.2 to 98.5) and then impetigo isolates (index, 96.2; 95% CI, 95.6 to 96.8). There was one entirely new emm sequence type (st465) discovered in a throat carriage swab, and four new emm subtypes (emm39.4 and emm60.7 from pharyngeal swabs and emm89.11 and emm93.4 from impetigo lesions).
Among the 101 pharyngeal GAS isolates that underwent emm sequence typing, there were 45 emm types and 46 emm subtypes. There were no apparent dominant emm subtypes among the pharyngeal isolates (Fig. 1). There were 14 emm subtypes (30%) that were common to both throat carriage and sore throat (n = 47, 46.5%), and of these, none was significantly associated with carriage or sore throat.
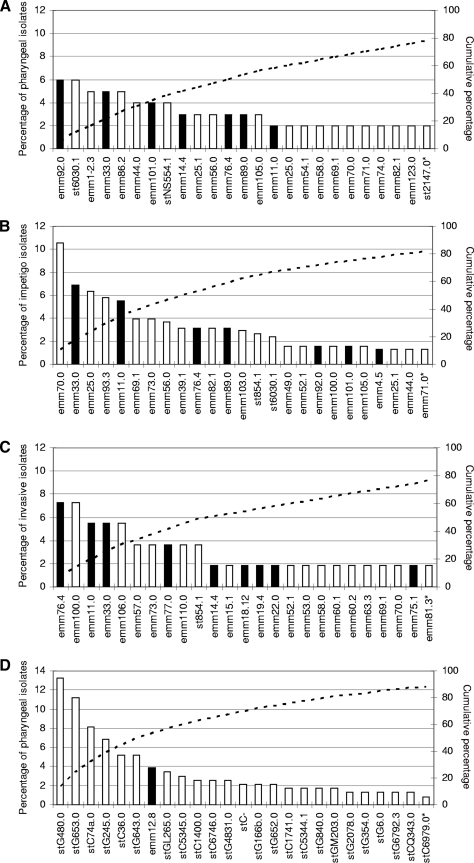
FIG. 1.
The 25 most common emm subtypes as proportions of total isolates causing GAS and GCS/GGS disease, grouped by GAS isolates found in the pharynx (A), GAS isolates causing impetigo (B), GAS isolates causing invasive disease (C), and GCS and GGS isolates found in the pharynx (D). (Black bars represent emm subtypes that are included in the current experimental multivalent GAS vaccine, while white bars represent those emm subtypes that are not included; the dotted line represents the cumulative percentage of all isolates). The 25 most common emm subtypes contributing to pharyngeal isolates are shown in panel A (there was a further emm subtype, st854.1, that was ranked 25th equally); these 26 emm subtypes accounted for 78.2% of all pharyngeal isolates, with 21 other emm subtypes contributing to the remaining 21.8%. The 25 most common emm subtypes contributing to impetigo isolates are shown in panel B (there were a further three emm subtypes, emm74.0, emm123.0 and st2147.0, that were ranked 25th equally); these 28 emm subtypes accounted for 86% of all impetigo isolates, with 28 other emm subtypes contributing to the remaining 14%. The 25 most common emm subtypes contributing to invasive isolates are shown in panel C (there were a further 13 emm subtypes, emm82.1, emm86.2, emm87.0, emm101.0, emm104.0, emm105.0, emm113.0, emm116.1, emm123.0, st2037.0, st2147.0, st6030.1.0, and stD631.0, that were ranked 25th equally); these 38 isolates accounted for 100% of all isolates. The 25 most common emm subtypes contributing to GCS and GGS pharyngeal isolates are shown in panel D (there were a further six emm subtypes, st839.0, stC922.0, stG2574.0, stG5420.0, stG652.1, and stGM220.0, that were ranked 25th equally); these 31 emm subtypes accounted for 93.6% of all pharyngeal isolates, with 15 other emm subtypes contributing to the remaining 6.4%.
There were 52 emm types and 56 emm subtypes recovered from 379 GAS impetigo lesions (Fig. 1). Of all GAS impetigo cases, 137 (36.1%) were classified as being moderate or severe impetigo (defined as the presence of more than five vesiculopustular impetigo lesions). Of the 56 emm subtypes, there were two emm subtypes that were statistically significantly associated with moderate or severe impetigo: emm25.0 (relative risk [RR], 2.5; 95% CI, 1.2 to 5.1) and emm74.0 (RR, 10.1; 95% CI 1.9 to 53.8).
When impetigo and pharyngeal GAS isolates were pooled, there were 60 emm types and 66 emm subtypes among 480 isolates (Tables 2, 3, and 4). Of these, there were 36 emm subtypes (55%) that were common to both sites (n = 383, 79.8%), 20 (30%) that were found only in isolates from impetigo lesions (n = 81, 16.9%), and 10 (12%) that were found only in isolates from the pharynx (n = 16, 3.3%). Therefore, 84.2% of all isolates found in isolates from the pharynx belonged to emm subtypes that were also found in isolates from impetigo lesions. Four emm subtypes were statistically significantly associated with isolation from the pharynx (emm86.2, emm92.0, st6030.1, and stNS554.1), and one was significantly associated with isolation from impetigo lesions (emm70.0) (Table 2).
TABLE 2.
emm subtypes recovered from GAS from both the pharynx and impetigo lesions
| emm subtypea | Pharynx (n = 85)
|
Impetigo (n = 298)
|
Total (n = 383)
|
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| emm11.0 | 2 | 2.4 | 21 | 7.0 | 23 | 6.0 |
| emm25.0 | 2 | 2.4 | 24 | 8.1 | 26 | 6.8 |
| emm25.1 | 3 | 3.5 | 5 | 1.7 | 8 | 2.1 |
| emm33.0 | 5 | 5.9 | 26 | 8.7 | 31 | 8.1 |
| emm42.0 | 1 | 1.2 | 2 | 0.7 | 3 | 0.8 |
| emm44.0 | 4 | 4.7 | 5 | 1.7 | 9 | 2.3 |
| emm49.0 | 1 | 1.2 | 6 | 2.0 | 7 | 1.8 |
| emm54.1 | 2 | 2.4 | 3 | 1.0 | 5 | 1.3 |
| emm56.0 | 3 | 3.5 | 14 | 4.7 | 17 | 4.4 |
| emm58.0 | 2 | 2.4 | 1 | 0.3 | 3 | 0.8 |
| emm69.1 | 2 | 2.4 | 15 | 5.0 | 17 | 4.4 |
| emm70.0** | 2 | 2.4 | 40 | 13.4 | 42 | 11.0 |
| emm71.0 | 2 | 2.4 | 5 | 1.7 | 7 | 1.8 |
| emm74.0 | 2 | 2.4 | 5 | 1.7 | 7 | 1.8 |
| emm75.1 | 1 | 1.2 | 1 | 0.3 | 2 | 0.5 |
| emm76.4 | 3 | 3.5 | 12 | 4.0 | 15 | 3.9 |
| emm77.0 | 1 | 1.2 | 2 | 0.7 | 3 | 0.8 |
| emm82.1 | 2 | 2.4 | 12 | 4.0 | 14 | 3.7 |
| emm86.2* | 5 | 5.9 | 3 | 1.0 | 8 | 2.1 |
| emm89.0 | 3 | 3.5 | 12 | 4.0 | 15 | 3.9 |
| emm92.0* | 6 | 7.1 | 6 | 2.0 | 12 | 3.1 |
| emm93.3 | 1 | 1.2 | 22 | 7.4 | 23 | 6.0 |
| emm101.0 | 4 | 4.7 | 6 | 2.0 | 10 | 2.6 |
| emm105.0 | 3 | 3.5 | 6 | 2.0 | 9 | 2.3 |
| emm109.1 | 1 | 1.2 | 1 | 0.3 | 2 | 0.5 |
| emm112.2 | 1 | 1.2 | 2 | 0.7 | 3 | 0.8 |
| emm116.1 | 1 | 1.2 | 2 | 0.7 | 3 | 0.8 |
| emm122.2 | 1 | 1.2 | 1 | 0.3 | 2 | 0.5 |
| emm123.0 | 2 | 2.4 | 5 | 1.7 | 7 | 1.8 |
| st2147.0 | 2 | 2.4 | 5 | 1.7 | 7 | 1.8 |
| st6030.1* | 6 | 7.1 | 9 | 3.0 | 15 | 3.9 |
| st854.1 | 2 | 2.4 | 10 | 3.4 | 12 | 3.1 |
| stCK401.0 | 1 | 1.2 | 1 | 0.3 | 2 | 0.5 |
| stD631.0 | 1 | 1.2 | 1 | 0.3 | 2 | 0.5 |
| stNS1033.0 | 1 | 1.2 | 4 | 1.3 | 5 | 1.3 |
| stNS554.1* | 4 | 4.7 | 3 | 1.0 | 7 | 1.8 |
*, subtypes that were significantly associated with recovery from the pharynx were emm86.2 (RR, 6.7, 95% CI, 1.6 to 27.4), emm92.0 (RR, 4; 95% CI, 1.3 to 12.1), st6030.1 (RR, 2.7; 95% CI 1.0 to 7.3), and stNS554.1 (RR, 5.3; 95% CI, 1.2 to 23.5); **, subtype that was associated with impetigo was emm70.0 (RR, 5.1; 95% CI, 1.3 to 20.8).
TABLE 3.
emm subtypes recovered from GAS from the pharynx only
| emm subtypea | No. recovered (n = 16) | % Recovered |
|---|---|---|
| emm1-2.3* | 5 | 31.3 |
| emm14.4 | 3 | 18.8 |
| emm39.4** | 1 | 6.3 |
| emm60.7** | 1 | 6.3 |
| emm63.3 | 1 | 6.3 |
| emm87.0 | 1 | 6.3 |
| emm110.0 | 1 | 6.3 |
| st465.0*** | 1 | 6.3 |
| st5282.0 | 1 | 6.3 |
| st11014.0 | 1 | 6.3 |
*, emm1-2.3 is sufficiently divergent from emm1 to be considered a distinct emm type; **, new subtype; ***, new type.
TABLE 4.
emm subtypes recovered from GAS from impetigo lesions only
| emm subtypea | No. recovered (n = 81) | % Recovered |
|---|---|---|
| emm4.5 | 5 | 6.2 |
| emm11.1 | 1 | 1.2 |
| emm19.4 | 3 | 3.7 |
| emm39.1 | 12 | 14.8 |
| emm52.1 | 6 | 7.4 |
| emm53.0 | 2 | 2.5 |
| emm55.0 | 1 | 1.2 |
| emm57.0 | 2 | 2.5 |
| emm60.1 | 1 | 1.2 |
| emm73.0 | 15 | 18.5 |
| emm85.0 | 4 | 4.9 |
| emm93.4* | 1 | 1.2 |
| emm89.11* | 1 | 1.2 |
| emm97.1 | 2 | 2.5 |
| emm98.1 | 3 | 3.7 |
| emm100.0 | 6 | 7.4 |
| emm103.0 | 11 | 13.6 |
| emm104.0 | 3 | 3.7 |
| st4119.0 | 1 | 1.2 |
| st9505.0 | 1 | 1.2 |
*, new subtype.
Of the 55 GAS isolates from sterile sites, there were 37 emm types and 38 emm subtypes. There were no clearly dominant emm subtypes causing invasive disease (Fig. 1). There were seven emm subtypes causing invasive disease that were not found in skin or pharyngeal isolates (n = 9, 16%). Forty-three of the emm subtypes causing invasive disease (78% of isolates) were also found in impetigo lesion isolates.
Overall, 12 of the 74 emm subtypes recovered in our study are included in the 26-valent GAS vaccine currently undergoing clinical trials, representing 137 isolates overall (25.6%; 95% CI, 22.0 to 29.5). The highest proportion of isolates included in the vaccine was found in invasive GAS isolates (32.7%; 95% CI, 20.7 to 46.7), followed by carriage (30%; 95% CI, 16.6 to 46.5), sore throat (26.2%; 95% CI, 15.8 to 39.1), and impetigo (24%; 95% CI, 19.8 to 28.6) isolates.
GCS and GGS emm sequence typing.
There were 38 emm types and 48 emm subtypes found among 282 GCS/GGS isolates. There were eight emm subtypes that were common to both GCS and GGS, 19 that were found in GCS isolates only, and 21 that were found in GGS only. There were six new emm types that accounted for 21 isolates: stCQ343, stCN204, stGL265, stG228, stGM203, and stGM220. There were five new emm subtypes, all individual isolates: emm12.45, stG245.1b, stC5345.3, stC749.0, and stC839.3.
Of the 270 isolates from pharyngeal or impetigo swabs, all but two emm subtypes out of 48 emm subtypes (stG643.1 and stG5240.0, one isolate each) were found in isolates from the pharynx, with 20 emm subtypes shared between isolates from the pharynx and skin (n = 173, 64.1%) and 26 emm subtypes found only in isolate from the pharynx (n = 95, 35.2%). Therefore, of the 37 GCS/GGS isolates from impetigo lesions, 35 (95%) were of emm subtypes also found in isolates from the pharynx. Of the 12 isolates from sterile sites, there were nine emm subtypes, and all of these subtypes were also found in isolates from both the pharynx and impetigo lesions.
Pharyngeal isolates accounted for 233 of all GCS/GGS isolates recovered on the study, and of these there were 46 emm subtypes. There were six dominant GCS/GGS emm subtypes, accounting for 49.8% of all GCS/GGS pharyngeal isolates (Fig. 1). There were 20 emm subtypes (43%) that were found in both sore throat cases and carriage swabs (n = 189, 81.1%).
C-repeat region sequence typing.
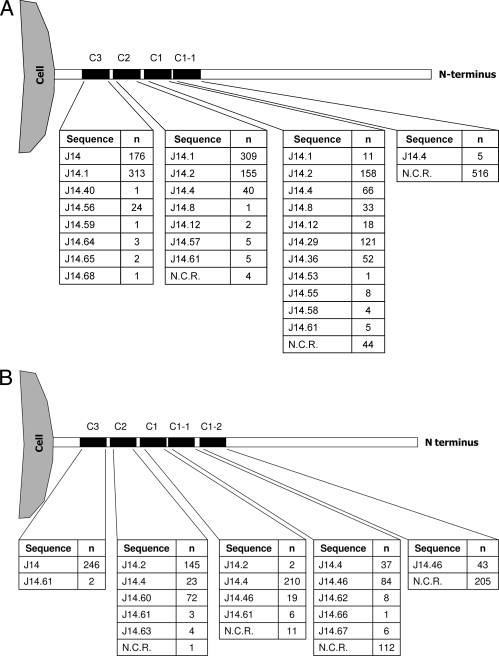
Of the 817 isolates in the study, 769 (94.1%) underwent successful sequencing of the C-repeat region for J14 sequence types (521 of 535 GAS isolates, 97.4%; and 248 of 282 GCS/GGS isolates, 87.9%). The majority of GAS isolates contained three C repeats (n = 477, 91.6%), with 40 GAS isolates containing two C repeats and 4 isolates containing only one C repeat (Fig. 2). More than half of the GCS/GGS isolates contained four C repeats (n = 136, 54.8%), with 57 isolates containing three C repeats (23%), 43 isolates containing five C repeats (17.3%), 11 isolates containing two C repeats (4.4%), and 1 isolate containing one C repeat (pharyngeal isolate, stG653).
FIG. 2.
J14 sequences in GAS, GCS, and GGS isolates recovered from Fiji. N.C.R., no C repeat. (A) J14 sequence types in GAS isolates (n = 521). (B) J14 sequence types in GCS and GGS isolates (n = 248).
There were 25 different J14 sequence types identified. Four J14 sequence types were common to both GAS and GCS/GGS (J14.0, J14.2, J14.4, and J14.61), 15 were unique to GAS (including J14.1), and 6 were unique to GCS/GGS (J14.46, J14.60, J14.62, J14.63, J14.66, and J14.67). Of the 25 J14 sequence types, 14 have not been described previously (J14.55 to J14.68, inclusive). When the J14 sequence types at C3, C2, and C1 were combined to create “overall” J14 C-repeat types, there were 29 overall J14 types among the 521 GAS isolates, and 12 overall J14 types among the 248 GCS/GGS isolates.
The most common J14 sequence type among the GAS isolates was J14.1 (n = 313 isolates, 60.1%), occurring in the C3 region alone in 4 GAS isolates, in both the C3 and C2 regions in 298 GAS isolates, and in all three C-repeat regions in 11 GAS isolates. The second most common sequence type was J14.0, which was found only in the C3 region (n = 176, 33.8%). Overall, J14.0 and J14.1 were recovered from 93.8% of GAS isolates. With GCS/GGS isolates, the J14.0 was found in the C3 region in all but two of the isolates (both stG354 isolates). There was a clear relationship between emm subtype and J14 sequence type in the C3 region. Forty-three emm subtypes contained J14.1 at C3, and 22 contained J14.0 at C3; no emm subtype had both J14.0 and J14.1 types.
DISCUSSION
This study is the most comprehensive investigation of the molecular epidemiology of beta-hemolytic streptococci in a developing country reported to date. It is also the largest report of J14 sequence typing. The emm profile of our isolates was unique. A comparison of the 45 GAS emm types among the pharyngeal isolates in our study with 51 emm types recovered in a large surveillance study of pharyngitis in the United States between 2000 and 2005 revealed that there were only 17 shared emm types, accounting for only 37% of pharyngeal isolates in our study (37) (S. T. Shulman, R. R. Tanz, W. Kabat, K. Kabat, J. Rippe, B. Beall, and J. B. Dale, presented at the XVII Lancefield Symposium on Streptococci and Streptococcal Diseases, Porto Heli, Greece, 2008). In contrast, 29 of the 37 emm subtypes recovered from invasive isolates in our study were also found among 89 emm types recovered from a large surveillance study of invasive isolates in the United States between 2000 and 2004. However, of the 10 most common invasive emm types in the U.S. study, only two emm types were also seen in Fiji (68.3% of all isolates versus 6%, respectively; P < 0.001).
The emm profile for Fiji was similar to that found for other tropical countries, in that there was a much flatter distribution of emm types, with less evidence of dominant types, compared to profiles in industrialized nations. However, the emm profile for Fiji was by no means identical to that for other tropical countries. For example, when comparing our data to those from a similar study in Ethiopia, only 24 out of 76 emm types recovered from pharyngeal and impetigo swabs in Ethiopia were also found in Fiji, accounting for 40.9% of Fiji isolates (45). A similar study conducted with the Aboriginal population of the Northern Territory of Australia found that 26 out of 60 emm types recovered from pharyngeal and impetigo swabs in the Northern Territory were also found in isolates from Fiji, accounting for 45.7% of Fiji isolates (23). Of note, 5 out of the 10 most common emm types recovered from pharyngeal swabs from the Northern Territory and 7 of the 10 most common emm types recovered from impetigo swabs were also seen among the Fiji isolates, accounting for 8% and 20% of Fiji isolates, respectively.
There was a great deal of sharing of emm types between pharyngeal and impetigo sites, with 55% of emm subtypes being found both in isolates from the pharynx and impetigo lesions and with emm subtypes associated with impetigo accounting for 84.2% of all pharyngeal isolates. There was even evidence of sharing of emm subtypes between the pharynx and the skin in individual children. Classical reports of GAS epidemiology from the United States described a division among serotypes such that some serotypes had the potential to infect mainly the pharynx, while others the potential to infect mainly the skin (1, 7). However, these reports also described a significant number of serotypes that appeared to have the potential to equally infect both sites. These classical reports were followed by the classification of GAS isolates into class I types that are serum opacity factor-negative and associated with pharyngeal infection and class II types that are serum opacity factor-positive and associated with impetigo infection (4). The molecular basis of this division is derived from sequencing of the C-repeat region (4) and corresponds to J14.0 and J14.1 sequence types, respectively (47). We did not find a statistically significant association between clinical site tropism and J14.0 and J14.1 sequence types in the C3-repeat region in GAS isolates, suggesting that many of the isolates in our study are able to move freely from skin to pharynx. This is in concert with findings by other authors in tropical settings where impetigo GAS strains predominate and where these skin strains account for the majority of pharyngeal GAS isolates (23, 40). However, the phenomenon is not isolated to tropical settings; the sequence of the spread of GAS from normal skin to impetigo to the throat has previously been described for temperate zones (10).
The profile of J14 sequence types in our study showed some similarities to the profile found in a study of 37 GAS isolates from northern India (47). In the study from India, investigators observed that J14.0 and J14.1 were the most common J14 sequence types recovered and that J14.0 was found only in the C3 region. However, there were also differences between the two studies, including a greater number of J14 types in the C3 region in our study (nine versus two).
Compared to a study of emm sequence typing of GCS/GGS pharyngeal isolates in the Northern Territory of Australia in which there were 24 emm subtypes identified among 257 isolates, there was a high diversity among the pharyngeal isolates in our study (46 emm subtypes among 233 isolates) (22). The high degree of throat tropism of GCS/GGS isolates in our study, which was also found in the Australian study, was striking. The finding of emm12 among GCS/GGS pharyngeal and impetigo isolates was of particular interest. While it is not uncommon to identify GAS isolates with emm types normally associated with GCS/GGS (22), it is rare to find emm types associated with GAS among GCS/GGS isolates. An exception to this is emm12; GGS isolates with type M12 antigen from both impetigo and throat cultures were first described for strains isolated in Trinidad in the 1960s (20). The explanation for this phenomenon appears to be that there is horizontal transfer of the emm12 gene between GAS and GCS/GGS (39). Interestingly, there were no emm12 GAS isolates in our study.
All of these findings have significant implications for GAS vaccine design and development. When comparing the emm profile of isolates in our study to that of the 26-valent vaccine currently in clinical trials, only 26% of isolates would be potentially covered in the current formulation of the vaccine. Moreover, the lack of dominant types in our study suggests that it would be difficult to target common epitopes. Further, GCS and GGS isolates were also highly prevalent in our study, and of the emm types found in our study among GCS and GGS isolates, only one emm type, emm12, is included in the vaccine.
In our study 93.8% of GAS isolates and 99.3% of GCS/GGS isolates that underwent J14 sequence typing contained either J14.0 or J14.1. These are encouraging data for a J8 vaccine, because it is known that antibodies against the J8 peptide provide cross-protection against GAS strains containing J14.0 and J14.1. All of the remaining isolates contained J14.2; based upon amino acid sequence, it is likely that cross-protection by a J8 vaccine will be afforded to isolates containing the J14.2 sequence, as there is only a one-amino-acid difference between J14.1 and J14.2 and a two-amino-acid difference between J14.0 and J14.2. In addition, antibodies raised against the J14.0 peptide in mice have been shown to opsonize GAS strains that contain J14.2 (47). An additional advantage of a J8 vaccine may be potential cross-protection against GCS/GGS isolates; all except two of the GCS/GGS isolates in our study contained J14.0.
Our data confirm that the molecular epidemiology of GAS is very different in this tropical setting from that found in temperate industrialized countries. These findings suggest that there may be some difficulties with a multiple emm-specific GAS vaccine approach and that a conserved epitope strategy, such as the J8 vaccine, may be more appropriate.
Acknowledgments
This study was funded by a grant from the National Institutes of Allergy and Infectious Diseases grant number U01AI60579.
There is no conflict of interest for any author.
We thank all of the patients, children, parents, schools, and communities for participating in this study and the Fiji Ministry of Health for their close collaboration in the project. We acknowledge the able assistance of the Fiji Group A Streptococcal Project research team, including Roselyn Ritika, Loraine Kelpie, Laisiana Matatolu, Frances Matanatabu, Jyotishna Mani, and Maureen Ah-Kee.
Footnotes
Published ahead of print on 10 June 2009.
REFERENCES
- 1.Anthony, B. F., E. L. Kaplan, L. W. Wannamaker, and S. S. Chapman. 1976. The dynamics of streptococcal infections in a defined population of children: serotypes associated with skin and respiratory infections. Am. J. Epidemiol. 104652-666. [DOI] [PubMed] [Google Scholar]
- 2.Batzloff, M. R., W. A. Hayman, M. R. Davies, M. Zeng, S. Pruksakorn, and E. R. Brandt. 2003. Protection against group A streptococcus by immunization with J8-diptheria toxoid: contribution of J8- and diptheria toxoid-specific antibodies to protection. J. Infect. Dis. 1871598-1608. [DOI] [PubMed] [Google Scholar]
- 3.Beall, B., R. Facklam, and T. Thompson. 1996. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J. Clin. Microbiol. 34953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bessen, D., K. F. Jones, and V. A. Fischetti. 1989. Evidence for two distinct classes of streptococcal M protein and their relationship to rheumatic fever. J. Exp. Med. 169269-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessen, D. E., J. R. Carapetis, B. Beall, R. Katz, M. Hibble, B. J. Currie, T. Collingridge, M. W. Izzo, D. A. Scaramuzzino, and K. S. Sriprakash. 2000. Contrasting molecular epidemiology of group A streptococci causing tropical and nontropical infections of the skin and throat. J. Infect. Dis. 1821109-1116. [DOI] [PubMed] [Google Scholar]
- 6.Bessen, D. E., T. R. Fiorentino, and S. K. Hollingshead. 1997. Molecular markers for throat and skin isolates of group A streptococci. Adv. Exp. Med. Biol. 418537-543. [DOI] [PubMed] [Google Scholar]
- 7.Bisno, A. L., I. A. Pearce, H. P. Wall, M. D. Moody, and G. H. Stollerman. 1970. Contrasting epidemiology of acute rheumatic fever and acute glomerulonephritis. N. Engl. J. Med. 283561-565. [DOI] [PubMed] [Google Scholar]
- 8.Carapetis, J. R., A. C. Steer, and E. K. Mulholland. 2005. The current evidence for the burden of group A streptococcal diseases. Department of Child and Adolescent Health and Development, World Health Organization, Geneva, Switzerland.
- 9.Carapetis, J. R., A. C. Steer, E. K. Mulholland, and M. Weber. 2005. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5685-694. [DOI] [PubMed] [Google Scholar]
- 10.Ferrieri, P., A. S. Dajani, L. W. Wannamaker, and S. S. Chapman. 1972. Natural history of impetigo. I. Site sequence of acquisition and familial patterns of spread of cutaneous streptococci. J. Clin. Investig. 512851-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischetti, V. A. 1989. Streptococcal M protein: molecular design and biological behavior. Clin. Microbiol. Rev. 2285-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundmann, H., S. Hori, and G. Tanner. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J. Clin. Microbiol. 394190-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haidan, A., S. R. Talay, M. Rohde, K. S. Sriprakash, B. J. Currie, and G. S. Chhatwal. 2000. Pharyngeal carriage of group C and group G streptococci and acute rheumatic fever in an Aboriginal population. Lancet 3561167-1169. [DOI] [PubMed] [Google Scholar]
- 14.Hu, M. C., M. A. Walls, S. D. Stroop, M. A. Reddish, B. Beall, and J. B. Dale. 2002. Immunogenicity of a 26-valent group A streptococcal vaccine. Infect. Immun. 702171-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotloff, K. L., and J. B. Dale. 2004. Progress in group A streptococcal vaccine development. Pediatr. Infect. Dis. J. 23765-766. [DOI] [PubMed] [Google Scholar]
- 16.Lamagni, T. L., A. Efstratiou, J. Vuopio-Varkila, A. Jasir, and C. Schalen. 2005. The epidemiology of severe Streptococcus pyogenes associated disease in Europe. Eurosurveillance 10179-184. [PubMed] [Google Scholar]
- 17.Lancefield, R. C. 1928. The antigenic complex of Streptococcus hemolyticus. I. Demonstration of a type-specific substance in extracts of Streptococcus hemolyticus. J. Exp. Med. 479-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lancefield, R. C. 1962. Current knowledge of type-specific M antigens of group A streptococci. J. Immunol. 89307-313. [PubMed] [Google Scholar]
- 19.Martin, N. J., E. L. Kaplan, M. A. Gerber, M. A. Menegus, M. Randolph, K. Bell, and P. P. Cleary. 1990. Comparison of epidemic and endemic group G streptococci by restriction enzyme analysis. J. Clin. Microbiol. 281881-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maxted, W. R., and E. V. Potter. 1967. The presence of type 12 M-protein antigen in group G streptococci. J. Gen. Microbiol. 49119-125. [DOI] [PubMed] [Google Scholar]
- 21.McDonald, M., R. Towers, P. Fagan, M. McKinnon, N. Benger, R. Andrews, B. J. Currie, and J. R. Carapetis. 2006. Recovering streptococci from the throat in remote tropical communities: a practical alternative to direct plating. J. Clin. Microbiol. 44547-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald, M., R. J. Towers, R. M. Andrews, J. R. Carapetis, and B. J. Currie. 2007. Epidemiology of Streptococcus dysgalactiae subsp. equisimilis in tropical communities, northern Australia. Emerg. Infect. Dis. 131694-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald, M. I., R. J. Towers, P. Fagan, J. R. Carapetis, and B. J. Currie. 2007. Molecular typing of Streptococcus pyogenes from remote Aboriginal communities where rheumatic fever is common and pyoderma is the predominant streptococcal infection. Epidemiol. Infect. 1381398-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNeil, S. A., S. A. Halperin, J. M. Langley, B. Smith, A. Warren, G. P. Sharratt, D. M. Baxendale, M. A. Reddish, M. C. Hu, S. D. Stroop, J. Linden, L. F. Fries, P. E. Vink, and J. B. Dale. 2005. Safety and immunogenicity of 26-valent group A streptococcus vaccine in healthy adult volunteers. Clin. Infect. Dis. 411114-1122. [DOI] [PubMed] [Google Scholar]
- 25.O'Grady, K.-A., L. Kelpie, R. A. Andrews, N. Curtis, T. M. Nolan, G. Selvaraj, J. W. Passmore, F. Oppedisano, J. Carnie, and J. R. Carapetis. 2007. The epidemiology of invasive group A streptococcal disease in Victoria, Australia. Med. J. Aust. 186565-569. [DOI] [PubMed] [Google Scholar]
- 26.O'Loughlin, R. E., A. Roberson, P. R. Cieslak, R. Lynfield, K. Gershman, A. Craig, B. A. Albanese, M. M. Farley, N. L. Barrett, B. Beall, L. H. Harrison, A. Reingold, and C. Van Beneden. 2007. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin. Infect. Dis. 45853-862. [DOI] [PubMed] [Google Scholar]
- 27.Pfoh, E., M. R. Wessels, D. Goldmann, and G. M. Lee. 2008. Burden and economic cost of group A streptococcal pharyngitis. Pediatrics 121229-234. [DOI] [PubMed] [Google Scholar]
- 28.Pruksakorn, S., B. Currie, E. Brandt, D. Martin, A. Galbraith, C. Phornphutkul, S. Hunsakunachai, A. Manmontri, and M. F. Good. 1994. Towards a vaccine for rheumatic fever: identification of a conserved target epitope on M protein of group A streptococci. Lancet 344639-642. [DOI] [PubMed] [Google Scholar]
- 29.Pruksakorn, S., A. Galbraith, R. A. Houghten, and M. F. Good. 1992. Conserved T and B cell epitopes on the M protein of group A streptococci. Induction of bactericidal antibodies. J. Immunol. 1492729-2735. [PubMed] [Google Scholar]
- 30.Reid, H. F., D. C. Bassett, T. Poon-King, J. B. Zabriskie, and S. E. Read. 1985. Group G streptococci in healthy school-children and in patients with glomerulonephritis in Trinidad. J. Hyg. (London) 9461-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabharwal, H., F. Michon, D. Nelson, W. Dong, K. Fuchs, R. C. Manjarrez, A. Sarkar, C. Uitz, A. Viteri-Jackson, R. S. Suarez, M. Blake, and J. B. Zabriskie. 2006. Group A streptococcus (GAS) carbohydrate as an immunogen for protection against GAS infection. J. Infect. Dis. 193129-135. [DOI] [PubMed] [Google Scholar]
- 32.Sagar, V., D. K. Bakshi, S. Nandi, N. K. Ganguly, R. Kumar, and A. Chakraborti. 2004. Molecular heterogeneity among north Indian isolates of group A Streptococcus. Lett. Appl. Microbiol. 3984-88. [DOI] [PubMed] [Google Scholar]
- 33.Sagar, V., R. Kumar, N. K. Ganguly, and A. Chakraborti. 2008. Comparative analysis of emm type pattern of group A streptococcus throat and skin isolates from India and their association with closely related SIC, a streptococcal virulence factor. BMC Microbiol. 8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakota, V., A. M. Fry, T. M. Lietman, R. Facklam, Z. Li, and B. Beall. 2006. Genetically diverse group A streptococci from children in far-Western Nepal share high genetic relatedness with isolates from other countries. J. Clin. Microbiol. 442160-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma, M., R. Khatib, and M. Fakih. 2002. Clinical characteristics of necrotizing fasciitis caused by group G streptococcus: case report and review of the literature. Scand. J. Infect. Dis. 34468-471. [DOI] [PubMed] [Google Scholar]
- 36.Shet, A., E. L. Kaplan, D. R. Johnson, and P. P. Cleary. 2003. Immune response to group A streptococcal C5a peptidase in children: implications for vaccine development. J. Infect. Dis. 188809-817. [DOI] [PubMed] [Google Scholar]
- 37.Shulman, S. T., R. R. Tanz, W. Kabat, K. Kabat, E. Cederlund, D. Patel, Z. Li, V. Sakota, J. B. Dale, and B. Beall. 2004. Group A streptococcal pharyngitis serotype surveillance in North America, 2000-2002. Clin. Infect. Dis. 39325-332. [DOI] [PubMed] [Google Scholar]
- 38.Simpson, E. 1949. Measurement of diversity. Nature 163688. [Google Scholar]
- 39.Simpson, W. J., J. M. Musser, and P. P. Cleary. 1992. Evidence consistent with horizontal transfer of the gene (emm12) encoding serotype M12 protein between group A and group G pathogenic streptococci. Infect. Immun. 601890-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smeesters, P. R., A. Vergison, D. Campos, E. DeAguiar, V. Y. M. Deyi, and L. V. Van Melderen. 2006. Differences between Belgian and Brazilian group A streptococcus epidemiologic landscape. PLoS One 1e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steer, A. C., A. J. Jenney, F. Oppedisano, M. R. Batzloff, J. Hartas, J. Passmore, F. M. Russell, J. H. Kado, and J. R. Carapetis. 2008. High burden of invasive beta-haemolytic streptococcal infections in Fiji. Epidemiol. Infect. 136621-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steer, A. C., A. W. J. Jenney, J. Kado, M. F. Good, M. Batzloff, G. Magor, R. Ritika, E. K. Mulholland, and J. R. Carapetis. 2009. Prospective surveillance of streptococcal sore throat in a tropical country. Pediatr. Infect. Dis. J. 28477-482. [DOI] [PubMed] [Google Scholar]
- 43.Steer, A. C., A. W. J. Jenney, J. Kado, M. F. Good, M. Batzloff, L. Waqatakirewa, E. K. Mulholland, and J. R. Carapetis. 2009. Invasive group A streptococcal disease in Fiji: prospective surveillance 2005-2007. Emerg. Infect. Dis. 15216-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steer, A. C., L. V. Tikoduadua, E. M. Manalac, S. Colquhoun, J. R. Carapetis, and C. Maclennan. 2009. Validation of an integrated management of childhood illness algorithm for the management of common skin conditions in Fiji. Bull. W. H. O. 67173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tewodros, W., and G. Kronvall. 2005. M protein gene (emm-type) analysis of group A beta-hemolytic streptococci from Ethiopia reveals unique patterns. J. Clin. Microbiol. 434369-4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vance, D. W., Jr. 1992. Group C streptococci: “Streptococcus equisimilis” or Streptococcus anginosus? Clin. Infect. Dis. 14616-617. [DOI] [PubMed] [Google Scholar]
- 47.Vohra, H., N. Dey, S. Gupta, A. K. Sharma, R. Kumar, D. McMillan, and M. F. Good. 2005. M protein conserved region antibodies opsonise multiple strains of Streptococcus pyogenes with sequence variations in C-repeats. Res. Microbiol. 156575-582. [DOI] [PubMed] [Google Scholar]
- 48.Williams, G. S. 2003. Group C and G streptococci infections: emerging challenges. Clin. Lab. Sci. 16209-213. [PubMed] [Google Scholar]