Abstract
Lpf (stands for long polar fimbriae) is one of the few adhesive factors of enterohemorrhagic Escherichia coli O157:H7 associated with colonization of the intestine. E. coli O157:H7 strains possess two lpf loci encoding highly regulated fimbrial structures. Database analysis of the genes encoding the major fimbrial subunits demonstrated that they are present in commensal as well as pathogenic (both intestinal and extraintestinal) E. coli strains and in Salmonella strains and that the lpfA1 and lpfA2 genes are highly prevalent among LEE (locus of enterocyte effacement)-positive E. coli strains associated with severe and/or epidemic disease. Further DNA sequence analysis of the lpfA1 and lpfA2 genes from different attaching-and-effacing E. coli strains has led us to the identification of several polymorphisms and the classification of the major fimbrial subunits into distinct variants. Using collections of pathogenic E. coli isolates from Europe and Latin America, we demonstrated that the different lpfA types are associated with the presence of specific intimin (eae) adhesin variants and, most importantly, that they are found in specific E. coli pathotypes. Our results showed that the use of these fimbrial genes as markers, in combination with the different intimin types, resulted in a specific test for the identification of E. coli O157:H7, distinguishing it from other pathogenic E. coli strains.
During the infection process, enterohemorrhagic Escherichia coli (EHEC) O157:H7 adheres to the intestinal epithelium, where it produces Shiga toxins responsible for the hemorrhagic symptoms associated with bloody diarrhea or during development of hemolytic-uremic syndrome (HUS). The adhesion of E. coli O157:H7 to enterocytes induces the formation of the attaching and effacing (A/E) lesion (reviewed in references 14 and 39). The A/E phenotype is conferred mainly by the locus of enterocyte effacement (LEE), a pathogenicity island containing genes encoding structural components of a type III secretion apparatus, translocator and secreted effector proteins, an adhesin (intimin), and the intimin receptor, Tir (reviewed in reference 35). The association of intimin with Tir triggers a host cell response leading to pedestal formation, and although this phenotype is best characterized in vitro, its expression correlates with the ability of the A/E organisms to colonize the intestine and cause disease in human and other animal hosts (reviewed in reference 20). Interestingly, it has been postulated that different intimin types (differences in the amino acid sequence of the intimin proteins) influence the pattern of colonization and tissue tropism in the host (10, 24). Therefore, initial experimental approaches provided evidence for the existence of at least four distinct types, known as intimin α, β, γ, and δ (1, 2). Subsequent studies have proposed that additional intimin types exist, and based on differences at the nucleotide level, they have been classified as intimins ζ, η, θ, ι, and κ, etc. (3, 4, 13, 18, 29, 41).
While the correlation between the expression of some of the intimin types and the tissue tropism of different E. coli strains has been demonstrated experimentally using in vitro human intestinal organ cultures (6, 10, 11, 25), very little is known about other E. coli O157:H7 colonization factors, including those controlling the expression of fimbriae. EHEC O157:H7 contains two nonidentical lpf loci homologous to the long polar fimbriae (LPF) of Salmonella enterica serovar Typhimurium (33, 34). Expression of E. coli O157:H7 lpf operon 1 (lpf1) in E. coli K-12 has been associated with increased adherence to tissue-cultured cells and with the appearance of long fimbriae (33, 38). The lpf2 operon has also been linked to adherence to epithelial cells (34), and its expression in other pathogenic E. coli strains is believed to be important for the development of severe diarrhea (8, 23). E. coli O157:H7 strains harboring mutations in one or both of the lpf loci have diminished colonization abilities in animal models (swine and sheep) (12) and also display an altered human intestinal tissue tropism (9). Furthermore, the role of LPF as a colonization factor associated with persistence in the intestine was elucidated using a lamb model of infection (37). Recently, we established the connection between regulatory proteins and expression of the lpf1 loci in response to environmental cues, and we found that these fimbriae are regulated by H-NS, a protein that binds to the regulatory sequence of lpfA1 and “silences” transcription, while the LEE-encoded Ler regulator binds to the regulatory sequence and inhibits the action of H-NS (36). Further, we found that deregulation of the lpf1 operon produced constitutive expression of the fimbriae, a phenotype associated with adherence and hemagglutination phenotypes in E. coli O157:H7 (38).
Because our data indicated that LPF constitute an important colonization factor of EHEC O157:H7 strains and because cumulative evidence indicates that homologues to the lpf genes are found in other pathogenic E. coli and Salmonella strains (7, 8, 28, 31, 34), in the current study, we identified several polymorphisms within the lpfA genes, which were used to classify the major fimbrial subunit genes into distinct variants. Further, we showed that lpf genes, in combination with the different intimin types, are reliable markers for the differentiation of E. coli O157:H7 and other pathogenic E. coli strains.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
Diarrheagenic and extraintestinal pathogenic E. coli (ExPEC) strains from reference laboratories in Spain, Chile, and Brazil were employed in this study (4, 18, 19, 22, 40). The Spanish collection comprised 100 strains including 18 Shiga toxin-producing E. coli (STEC), 30 enteropathogenic E. coli (EPEC), and 52 atypical EPEC (aEPEC) strains. The Chilean collection comprised 125 strains, including 64 STEC, 39 EPEC, and 22 aEPEC strains. Finally, the collection from Brazil comprised 4 EPEC and 33 aEPEC strains. For the PCR tests, EHEC strain EDL933 and E. coli K-12 MG1655 were used as positive and negative controls, respectively. Strains were maintained at −80°C, and when needed, they were grown in Luria-Bertani broth (17) at 37°C.
Recombinant DNA techniques.
Standard methods were used to perform genomic DNA isolation, PCR, and gel electrophoresis (27). Recombinant Taq polymerase enzyme (1 U) was used in combination with 2 mM MgCl2 and 1 μM oligonucleotide primer in each reaction. All amplifications began with a 5-min hot start at 94°C, followed by 35 cycles of denaturation at 94°C for 30 s, annealing for 30 s in a range of 52°C to 72°C (depending on the lpfA variant amplified), and extension at 72°C for 30 s. In some cases, PCRs were performed with boiled bacterial colonies. On the basis of multiple sequence alignments, the polymorphic regions in the lpfA genes were chosen (see below), and PCR primers were derived from those regions with the help of OLIGO primer analysis software. All oligonucleotide primers are listed in Table 1.
TABLE 1.
PCR primer pairs for the amplification of the different lpfA types
| Gene type (predominant serotype) and primer | Sequence (5′-3′) | Position | Amplicon length (bp) |
|---|---|---|---|
| lpfA1 | |||
| 1 (O127:H6) | |||
| LPFA1-AF | AGTTGGTGATAAATCACCAT | 186-205 | 222 |
| LPFA1-AR | GTGCTGGATTCACCACTATTCATCG | 383-407 | |
| 2 (O26:H11) | |||
| LPFA1-B1F | AAGTCTGTATTTACTGCTATG | 169-189 | 273 |
| LPFA1-B1R | GAAATACAGAACGGTCTGA | 423-441 | |
| 3 (O157:H7) | |||
| LPFA1-CF | GGTTGGTGACAAATCCCCG | 186-204 | 244 |
| LPFA1-CR1 | CGTCTGGCCTTTACTCAGA | 411-429 | |
| 4 (ONT:H10) | |||
| LPFA1-B2F | AAGTCTGTGTTTACCACTACT | 64-84 | 273 |
| LPFA1-B2R | AAAATACAGAACAGTCTGG | 318-336 | |
| 5 (ONT:H26) | |||
| LPFA1-CF | GGTTGGTGACAAATCCCCG | 81-99 | 250 |
| LPFA1-CR1 | GAGAACCGTCTGGCCTGTTT | 311-330 | |
| lpfA2 | |||
| 1 (O113:H21) | |||
| LPFA2-B1F | GGTAGTCTGGCGTCGCCACAGA | 130-151 | 207 |
| LPFA2-B1R | AATACGAATACCAACGCCG | 318-336 | |
| 2 (O157:H7) | |||
| LPFA2-CF | CTACAGGCGGCTGATGGAACA | 61-81 | 297 |
| LPFA2-CR | GCTAATACCAGCGGCAGCATCGT | 335-357 | |
| 3 (O44) | |||
| LPFA2-B2F | GGTAGTCTGGCGTCACCACAGC | 190-211 | 207 |
| LPFA2-B2R | AATACGAATACCGACACCC | 378-396 |
Phylogenetic analysis and gene accession numbers.
The E. coli and Salmonella lpfA gene sequences available from public databases were analyzed using the Discovery Studio gene program (version 1.5; Accelrys). Multiple sequence alignments were performed using ClustalW with open and extended gap penalties of 10.0 and 5.0, respectively. Bootstrap subsets (1,000 sets) and phylogenetic trees were generated with the neighbor-joining algorithm, and the distance model used was the Kimura two-parameter model (15).
LpfA1 protein NCBI GenBank accession numbers for E. coli serotype O157:H7 strains are as follows: for EDL933, AAG58695; for EC4115, ACI36002; for Sakai, BAB37854. For other E. coli serotypes (with strains given in parentheses), LpfA1 protein accession numbers are as follows: for O55:H7 (DEC5A), BAE48422; for ONT:H26 (ECOR42), BAE48423; for O119:NM (O119-53), BAE48424; for O127:H6 (E2348/69), CAS11346; for O8 (IAI1), CAR00508; for O26:H11, BAD69589; for O81 (ED1a), CAR10220; for O4:H43 (ECOR67), BAE48419; for O111:H21 (DEC15A), BAE48418; for O111:H8 (DEC8B), BAE48417; for O104:NM (ECOR28), BAE48416; for O86:H43 (ECOR23), BAE48415; for O128:H2 (DEC11A), BAE48420; for ONT:H10 (ECOR65), BAE48421; for rabbit EPEC (REPEC) O15:H− (83/39), AAO22843; for enteroaggregative E. coli (EAEC) (55989), CAV00478. For Salmonella enterica strains, LpfA1 protein accession numbers are as follows: for Salmonella enterica serovar Enteritidis P125109, CAR35040; for Salmonella enterica serovar Dublin CT02021853, ACH74212; for Salmonella enterica serovar Newport SL254, ACF63868; for Salmonella enterica serovar Heidelberg SL476, ACF70317; for Salmonella serovar Typhimurium LT2, AAL22500.
LpfA2 protein accession numbers for E. coli serotype O157:H7 strains are as follows: for EDL933, AAG58930; for EC4115, ACI39341; for Sakai, BAB38093. For other E. coli serotypes (with strains given in parentheses), LpfA2 protein accession numbers are as follows: for O55:H7 (DEC5A), BAE48400; for O119:NM (O119-53), BAE48402; for ONT:H26 (ECOR42), BAE48401; for O113:H21 (EH41), AAL18161; for O152:H28 (SE11), BAG79542; for O78 (789), AAY18076; for O78:H9 (chi7122), AAS99229; for O13:H21 (ECOR30), BAE48408; for O7:H21 (ECOR33), BAE48407; for O26:H11 (DEC10A), BAE48410; for O111:H8 (DEC8B), BAE48409; for O86:H43 (ECOR23), BAE48406; for O157:H43 (DEC7A), BAE48405; for O104::NM (ECOR28), BAE48404; for O85:HNT (ECOR7), BAE48403; for ONT:HNT (ECOR48), BAE48413; for O1:H6 (ECOR46), BAE48412; for O7:NM (ECOR40), BAE48411; for O79:H25 (ECOR36), BAE48414; for O8 (IAI1), CAR00706; for enterotoxigenic E. coli (ETEC) O139:H28 (E24377A), ABV19201; for verotoxigenic E. coli O15, AAT76975; for EAEC (55989), CAV00812; for O44 (O44-20), BAE48399.
Statistical analysis.
Analysis of variance and Pearson's chi-square test were used to test associations between the clinical courses of E. coli O157:H7 infections (acute diarrhea, bloody diarrhea, or HUS) and the presence of the lpfA genes.
RESULTS AND DISCUSSION
Phylogenetic trees based on lpfA1 and lpfA2 gene sequences of E. coli and Salmonella strains.
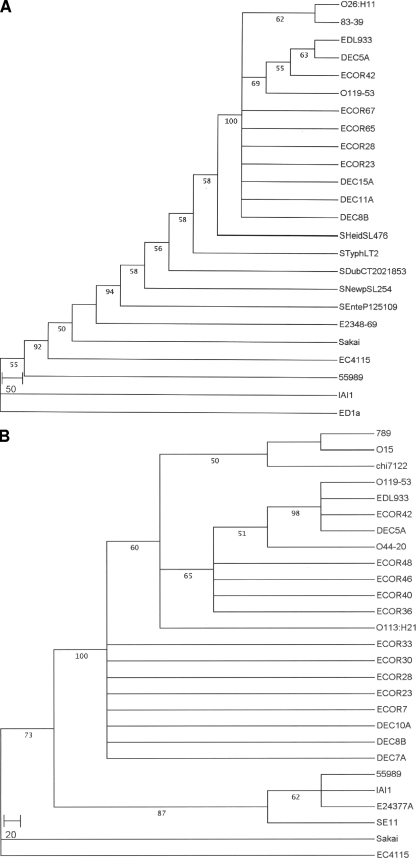
Previously, it was demonstrated that the lpfA1 and lpfA2 genes are highly prevalent among LEE-positive E. coli strains, including EHEC O157:H7 strains associated with severe and/or epidemic disease (28, 31, 34). Further, homologues of lpf genes have also been detected in non-O157:H7 LEE-positive E. coli strains, LEE-negative pathogenic E. coli strains, and REPEC strains (8, 21, 23, 32). Therefore, we performed BLAST analysis to identify the DNA sequences currently available in the database that display homology to the lpfA1 and lpfA2 genes of EHEC O157:H7 strain EDL933. Using phylogenetic analysis of DNA sequences, distinct clades could be distinguished corresponding to the diversity of lpfA1 and lpfA2 genes (Fig. 1). In the lpfA1 tree analysis, we found that genes with 69 to 99% identity to the EDL933 lpfA1 gene were present in a range of E. coli strains, including EPEC strains DEC11A, DEC5A, O119-53, and E2348/69, STEC strain DEC8B, EAEC strains DEC15A and 55989, EHEC strains Sakai, EC4115, and O26:H11, REPEC strain 83-39, E. coli Reference Collection (ECOR) strains ECOR67, ECOR65, ECOR28, ECOR23, and ECOR42, ExPEC strain IAI1, and commensal E. coli strain ED1a, as well as in Salmonella enterica serovars Dublin (CT02021853), Newport (SL254), Enteritidis (P125109), Heidelberg (SL476), and Typhimurium (LT2). As previously described, we now confirmed that the EDL933 lpfA1 gene is phylogenetically related to the lpfA genes found in EPEC DEC5A, O119-53, and ECOR42 (96 to 99% identity) and is less related (69% identity) to the lpfA1 genes found in the different serovars of Salmonella (Fig. 1A). To our surprise, the lpfA1 genes from O157:H7 strains Sakai and EC4115 were more phylogenetically related at the nucleotide level to EPEC O127:H6 strain E2348/69 than to O157:H7 strain EDL933. Our tree also showed that the lpfA1 gene is also present in several ECOR strains and that these genes shared close phylogenetic relationships to the genes found in the other ECOR strains and strains DEC15A, DEC11A, and DEC8B. Three new genome sequences recently included in the database indicated that the lpfA1 gene is also present in EAEC strain 55989, ExPEC strain IAI1, and commensal E. coli strain ED1a (Fig. 1A). Finally, the two strains of E. coli that carried the more distantly related (72 to 73% identity) lpfA1 genes are REPEC strain 83-39 and EHEC strain O26:H11.
FIG. 1.
Trees based on sequence data from the lpfA1 (A) and lpfA2 (B) genes. Shown are the phylogenetic positions of the 525-bp and 603-bp E. coli O157:H7 lpfA1 and lpA2 genes from strain EDL933, respectively, and the corresponding lpfA1 and lpfA2 DNA sequences from E. coli and Salmonella strains currently available in GenBank (for accession numbers, see Materials and Methods). The occurrence (percentage) of the branching order in 1,000 bootstrapped trees is given at each branch. E. coli strains (with serotypes given in parentheses) listed include EDL933, EC4115, and Sakai (O157:H7), DEC5A (O55:H7), DEC7A (O157:H43), DEC8B (O111:H8), DEC10A (O26:H11), DEC11A (O128:H2), DEC15A (O111:H21), ECOR7 (O85:HNT), ECOR23 (O86:H43), ECOR28 (O104:NM), ECOR30 (O13:H21), ECOR33 (O7:H21), ECOR36 (O79:H25), ECOR40 (O7:NM), ECOR42 (ONT:H26), ECOR46 (O1:H6), ECOR48 (ONT:HNT), ECOR65 (ONT:H10), ECOR67 (O4:H43), O119-53 (O119:NM), E2348/69 (O127:H6), EH41 (O113:H21), O44-20 (O44), IAI1 (O8), 83/39 (O15:H−), O26:H11 (O26:H11), ED1a (O81), 789 (O78), chi7122 (O78:H9), SE11 (O152:H28), O15 (verocytotoxigenic E. coli O15), E24377A (ETEC O139:H28), and 55989 (EAEC). Salmonella enterica strains listed (with serovars given in parentheses) include P125109 (S. Enteritidis), CT02021853 (S. Dublin), SL254 (S. Newport), SL476 (S. Heidelberg), and LT2 (S. Typhimurium).
The tree analysis of the lpfA2 genes revealed a totally distinct distribution of the genes and indicated that the EDL933 lpfA2 gene is also closely related at the nucleotide level (98 to 99% identity) to the genes found in EPEC strains DEC5A and O119-53 and strain ECOR42 (Fig. 1B). Genes with homology to EDL933 lpfA2 are also found in STEC strains O15, DEC10A, and DEC8B; EHEC strains Sakai, EC4115, and O113:H21; and other members of the ECOR reference collection (ECOR48, ECOR46, ECOR40, ECOR36, ECOR33, ECOR30, ECOR28, ECOR23, and ECOR7). As in the lpfA1 tree analysis, addition of new genome sequences to the database demonstrated that genes with homology to lpfA2 are also present in other categories of pathogenic E. coli, such as EAEC strains O44-20 and 55989, ETEC strains E24377A and DEC7A, avian-pathogenic E. coli strain chi7122, ExPEC strains IAI1 and 789, and commensal E. coli strains ED1a and SE11 (Fig. 1B). Our database search and analysis also revealed that genes with homology to lpfA2 were also present in Shigella sonnei, Shigella flexneri, and Shigella boydii (data not shown).
We selected the lpfA genes for our analysis for several reasons. (i) The LPF are a novel determinant of EHEC O157:H7 tropism for the human intestinal tract (9). The expression of the LPF in LEE-negative strains of EHEC is believed to be important for the development of severe diarrhea and hence is potentially clinically relevant (8, 23). (ii) A large number of intestinal pathogenic E. coli strains associated with severe and/or epidemic disease possess the lpf genes, and it is postulated that they express the LPF (28, 31, 34). A study analyzing lpf genes in a collection of pathogenic E. coli strains of different categories isolated from the intestine found that the lpf genes are not specific to EHEC O157:H7; they are present in other diarrheagenic E. coli (DEC) strains and in the standard collection of ECOR strains (28, 31, 34). These findings suggest that there is a relationship between the lpfA gene variant and the phylogenetic group. (iii) Our current data confirmed that lpfA genes are present in intestinal pathogens, such as E. coli, Salmonella, and Shigella spp.; however, elucidation of genome sequences recently deposited in GenBank has now demonstrated that lpfA1 and lpfA2 homologues are also present in the genomes of ExPEC strains, in E. coli strains of other pathotypes causing infection in animals, and, unexpectedly, in some isolates from healthy humans that are considered to be commensal E. coli strains. The presence of these genes seems to be widespread in pathogenic E. coli strains of different origins, which justified their study as putative markers to identify outbreak strains of specific pathotypes that occur in specific locations around the world.
Prevalence of lpf1 and lpf2 genes in reference collections of pathogenic E. coli strains.
Because a large portion of lpfA DNA sequences available in the database belong to pathogenic E. coli strains producing A/E lesions (A/E E. coli [AEEC]), we hypothesized that the lpfA genes might contain conserved regions useful for classifying these genes into different types (variants) and that these variants might be present in specific virulent serotypes. We aligned all the available DNA sequences and found several conserved regions (see Fig. S1 in the supplemental material), allowing us to group the lpfA1 genes into at least five different types (we named them alleles 1, 2, 3, 4, and 5) and the lpfA2 genes into three distinct types (alleles 1, 2, and 3). Using these conserved regions, we designed pairs of oligonucleotides (Table 1) that specifically amplified these segments in the different lpfA types, and then we determined by PCR analysis whether these lpfA variants were present in all strains or only in specific subsets of AEEC strains as well as in E. coli strains in reference collections. As indicated in Table 2, by using the DEC reference collection (strains were kindly provided by the late Thomas Whittam [Michigan State University]) and ECOR, as well as other prototypic AEEC strains, we determined that the different lpfA types are present in a wide variety of serotypes, and we observed no apparent correlation between the type of lpfA1 and/or lpfA2 gene and the bacterial pathotype. Such observations have been reported previously by C. Toma and colleagues (31); however, their study also suggested the existence of a relationship between the lpfA type and the bacterial phylogenetic group. Because it has been determined previously that AEEC strains possess distinct variants of intimin and that some of the genes encoding these proteins are associated with specific pathotypes (26), we investigated whether there was an association between intimin (eae), the lpfA types, and the different pathotypes. As shown in Table 2, an interesting correlation emerged from this association; e.g., we found that the lpfA1-1 variant was present only in those E. coli strains carrying the intimin gene types α1, δ/κ, η1, η2, λ, μ, and π and that EPEC O127:H6 was the predominant serotype representative of that group. The lpfA1-2 gene is associated with E. coli strains carrying intimin types β1, γ2/θ1, ɛ1, and ɛ2. To our surprise, the lpfA1-3 gene was found only in AEEC strains belonging to serotype O157:H7 and in O55:H7 strains (both of these serotypes possess γ1 intimin, a type of intimin found only in EHEC O157 strains and in some of the phylogenetically related serotype O55:H7 and O145 strains). In contrast, no association with any intimin type was found for strains carrying the lpfA1-4 and lpfA1-5 gene types.
TABLE 2.
Different lpfA types and their associations with intimin types in reference collections of virulent E. coli strains
| lpfA type | Predominant serotype(s) (strain[s]) possessing the lpfA type | Specific intimin (eae) type(s) associated |
|---|---|---|
| lpfA1-1 | EPEC O127:H6 (E2348/69) | α1 (alpha), δ (delta), κ (kappa), η1 (eta), η2, λ (lambda), μ (mu), π (pi) |
| lpfA1-2 | REPEC O15:H− (83/39), EPEC2 O128:H2 (DEC11a), O86:H43 (ECOR23), EPEC O128:H21 (DEC15A), O4:H43 (ECOR67), EHEC2 O111:H8 (DEC8B), O104:NM (ECOR28), O26:H11 (AB161111) | β1 (beta), θ1 (theta), ɛ1 (epsilon), ɛ2 |
| lpfA1-3 | EHEC1 O157:H7 (EDL933), EPEC O55:H7 (DEC5A) | γ1 (gamma) |
| lpfA1-4 | ONT:H10 (ECOR65) | NAa |
| lpfA1-5 | ONT:H26 (ECOR42), O119:NM (O119-53) | NA |
| lpfA2-1 | EHEC O113:H21, O15 (O15), O78:K80:H9 (chi7122), O78 (789), O85:H− (ECOR07), O104:NM (ECOR28), ETEC O157:H43 (DEC7A), O86:H43 (ECOR23), O7:H21 (ECOR33), EHEC2 O111:H8 (DEC8B), EHEC2 O26:H11 (DEC10A) | β1, θ1, ɛ2, ζ1 (zeta), ι1B (iota), ι1C |
| lpfA2-2 | EHEC1 O157:H7 (EDL933), EPEC O55:H7 (DEC5A), ONT:H26 (ECOR42), O119:NM (O119-53) | γ1 |
| lpfA2-3 | O44 (O44-20), O1:H6 (ECOR46), ONT:HNT (ECOR48), O7:NM (ECOR40), O79:H25 (ECOR36) | NA |
NA, no association; these types of lpfA1 or lpfA2 genes were not found in any of the intimin-positive strains from the E. coli Reference Laboratory (Lugo, Spain) analyzed.
For the lpfA2 genes, the associations were not as defined as those observed for the lpfA1 genes. We found that the lpfA2-1 gene was associated with E. coli strains carrying intimin types β1, γ2/θ, ɛ2, ζ, and ι1 and that the lpfA2-2 gene was associated with E. coli strains carrying intimin type γ1 (EPEC O55:H7 and EHEC O157:H7) (Table 2). Interestingly, a combination of the lpfA1-3 and lpfA2-2 types was observed only for serotypes O55:H7 and O157:H7. In contrast, no association with intimin types was found for strains carrying the lpfA2-3 gene variant. Our data strongly suggest that a correlation exists between the intimin and lpfA gene variants carried by different pathogenic E. coli strains. In the case of EHEC O157:H7, because the O55:H7 clinical isolates are rarely found, the use of lpfA genes as probes in combination with the use of the intimin types could result in a specific test for the O157:H7 strains and for other pathogenic E. coli strains.
The idea of distinguishing pathogenic E. coli strains belonging to different pathotypes through sequence-based comparison of their virulence-associated genes has been demonstrated previously (30). In that study, 12 putative virulence genes from ExPEC strains were evaluated based on single-nucleotide polymorphisms. The investigators found that only polymorphisms in the fimH gene (which encodes a minor component of the type 1 fimbriae) were able to distinguish uropathogenic E. coli strains from other ExPEC organisms. With those concepts in mind, we performed a comprehensive analysis of a large collection of EPEC and STEC strains for the presence of the different intimin and lpfA gene variants.
Specific combinations of lpfA and intimin gene types are present in STEC and EPEC strains.
To determine whether the lpfA gene types could be used as a simple, inexpensive screening test for epidemiological studies of pathogenic E. coli strains, we analyzed collections of EPEC and STEC strains located in our reference laboratory in Spain, mainly representing isolates from Europe and Brazil (Table 3). The identification of the different intimin and lpfA gene variants in these strains produced the following results. The lpfA1-3 and lpfA2-2 alleles were present only in strains carrying the intimin γ1 gene (STEC O157:H7, EPEC O55:H7, and two rare aEPEC isolates of serotypes O33:H7 and O163:H7). These combinations of alleles are not present in other STEC strains and can be used as a discriminatory tool because, while other STEC strains from serotypes O26:H11, O111:H−, and O111:H8 possessed the lpfA1 and lpfA2 genes, they carried the lpfA1-2 and lpfA2-1 alleles in combination with intimin gene types β1 and θ1 (Table 3). Among the EPEC strains (this pathotype represents typical EPEC strains that possess the EPEC adherence factor [EAF] virulence plasmid and carry the bfp fimbrial genes), the majority possess only one of the two lpfA genes. The majority of the EPEC strains analyzed possess the lpfA1-1 allele in combination with eae type α1 (O55:H6, O127:H6), δ (O86:H34), κ (O86:H34), η1 (O125:H−), η2 (ONT:H45), or μ (O55:H− and O55:H51). In contrast, the majority of the aEPEC strains (which lack the EAF virulence plasmid and the bfp genes) possess the lpfA1-2 and lpfA2-1 genes in combination with eae types β1 and ɛ2 (Table 3). Overall, these results indicated that a strong correlation exists between the intimin types and the lpfA gene variants, and they also suggested that the presence of both lpfA1 and lpfA2 alleles is associated with pathogenic E. coli strains, particularly with those belonging to the STEC pathotype.
TABLE 3.
Correlation of lpfA types with STEC and EPEC serotypes in a collection of strains from the LRECa
| LREC no. | Pathotypeb | Serotypec | Country of origin | Intimin (eae) type | lpfA1 type | lpfA2 type |
|---|---|---|---|---|---|---|
| IH30873A-03 | aEPEC | O51:H41 | Spain | α1 | 1 | |
| IH52368A/03 | aEPEC | O51:H49 | Spain | α1 | 1 | |
| FV10087 | EPEC | O55:H6 | United Kingdom | α1 | 1 | |
| FV10088-2348III | EPEC | O127:H6 | Unknown | α1 | 1 | |
| EPEC-21 | EPEC | O127:H− | Unknown | α1 | 1 | |
| EPEC-23 | EPEC | O142:H6 | Unknown | α1 | 1 | |
| IH1658-A | EPEC | O157:H45 | Spain | α1 | 1 | |
| FV10089 | EPEC | O157:H45 | Switzerland | α1 | 1 | |
| IH26845A-05 | aEPEC | O5:H6 | Spain | α2 | ||
| IH9661A-04 | aEPEC | O20:H6 | Spain | α2 | ||
| FV10090 | aEPEC | O125:H6 | Spain | α2 | ||
| FV10091 | aEPEC | O63:H33 | Spain | α2 | ||
| FV10092 | aEPEC | O132:H34 | Spain | α2 | ||
| FV10094-IH27256-03-A | STEC | O26:H11 | Spain | β1 | 2 | 1 |
| O26-7 | STEC | O26:H11 | Spain | β1 | 2 | 1 |
| O26-22 | STEC | O26:H11 | Spain | β1 | 2 | 1 |
| VTH-62 | STEC | O118:H16 | Spain | β1 | 2 | 1 |
| FV10095 | EPEC | O111:H2 | Uruguay | β1 | ||
| EPEC-11 | EPEC | O111:H− | Unknown | β1 | ||
| IH22561A-04 | EPEC | O111:H− | Spain | β1 | ||
| FV10096 | aEPEC | O177:H11 | Spain | β1 | 2 | 1 |
| VTB-266 | STEC | O177:H11 | Spain | β1 | 2 | 1 |
| VTB-163 | STEC | O177:H11 | Spain | β1 | 2 | 1 |
| FV12055-IH40495-06-A | aEPEC | O103:H− | Spain | β1 | 2 | 1 |
| FV12056-IH46769-07-A | aEPEC | O103:H− | Spain | β1 | 2 | 1 |
| FV11582-IH11922A07 | aEPEC | O104:H2 | Spain | β1 | 2 | |
| FV5751 | aEPEC | O104:H2 | Brazil | β1 | 2 | |
| FV11697-T2932-2 | aEPEC | ONT:H7 | Brazil | β1 | 2 | 1 |
| IH51463A-04 | EPEC | O88:H6 | Spain | β2 | ||
| FV10097 | EPEC | O119:H6 | Uruguay | β2 | ||
| FV10098 | aEPEC | O113:H6 | Spain | β2 | ||
| FV4575 | aEPEC | O139:H14 | Brazil | β2 | ||
| IH2056A-03 | EPEC | O167:H6 | Spain | β2 | ||
| FV10099 | EPEC | O167:H6 | Brazil | β2 | ||
| FV10101-IH34136-03-C | aEPEC | O128:H7 | Spain | β3 | 1 | |
| FV10102-IH42584-03-A | aEPEC | O128:H− | Spain | β3 | ||
| FV5667 | aEPEC | O33:H7 | Brazil | γ1 | 3 | 2 |
| FV10105-IH28143-03-A | aEPEC | O55:H7 | Spain | γ1 | 3 | 2 |
| IH44336A-04 | aEPEC | O55:H7 | Spain | γ1 | 3 | 2 |
| IH5027/06A | aEPEC | O55:H7 | Spain | γ1 | 3 | 2 |
| FV5701 | aEPEC | O55:H7 | Brazil | γ1 | 3 | 2 |
| FV5665 | aEPEC | O55:H7 | Brazil | γ1 | 3 | 2 |
| O157-847 | STEC | O157:H7/SF− | Spain | γ1 | 3 | 2 |
| O157-881 | STEC | O157:H7/SF− | Spain | γ1 | 3 | 2 |
| FV10103 | STEC | O157:H7/SF− | Spain | γ1 | 3 | 2 |
| FV10104-EDL933 | STEC | O157:H7 | Canada | γ1 | 3 | 2 |
| FV10108-FV5570 | STEC | O157:H−/SF+ | Germany | γ1 | 3 | 2 |
| FV10107-FV5569 | STEC | O157:H−/SF+ | Germany | γ1 | 3 | 2 |
| FV5668 | aEPEC | O163:H7 | Brazil | γ1 | 3 | 2 |
| IH7548-05A | aEPEC | ONT:H− | Spain | γ1 | 5 | |
| IH15752-07A | aEPEC | O2 | Spain | γ1 | 5 | |
| FV10106 | aEPEC | O145:H28 | Spain | γ1 | 5 | |
| IH11218-04A | aEPEC | O145:H28 | Spain | γ1 | ||
| IH38354A/05 | STEC | O145:H− | Spain | γ1 | 5 | |
| IH34365-05A | aEPEC | O145:H− | Spain | γ1 | 3 | |
| IH10248A-05 | aEPEC | O2:H40 | Spain | θ1 | ||
| FV11585-IH8663A06 | aEPEC | O2:H49 | Spain | θ1 | ||
| FV10109 | STEC | O111:H− | Spain | θ1 | 2 | 1 |
| FV10110 | STEC | O111:H8 | Germany | θ1 | 2 | 1 |
| IH45218A-06 | aEPEC | O111:H25 | Spain | θ1 | ||
| FV10111 | EPEC | O127:H40 | Uruguay | θ1 | ||
| IH5098A-07 | EPEC | O131:H46 | Spain | θ1 | 1 | |
| FV11695-T1871-1 | aEPEC | O34:H− | Brazil | θ2 | 2 | 1 |
| IH25556A-03 | EPEC | O49:H10 | Spain | κ | ||
| FV10114 | EPEC | O86:H34 | Brazil | δ | 1 | |
| FV10112 | EPEC | O86:H34 | Unknown | κ | 1 | |
| IH48480A-06 | EPEC | O88:H− | Spain | κ | ||
| FV10113 | EPEC | O118:H5 | Germany | κ | ||
| FV5713 | aEPEC | O1:H45 | Brazil | ɛ1 | ||
| FV5718 | aEPEC | O21:H38 | Brazil | ɛ1 | 1 | |
| FV10116 | aEPEC | O26:H− | Brazil | ɛ1 | ||
| FV5715 | aEPEC | O80:H26 | Brazil | ɛ1 | ||
| FV10115 | STEC | O103:H2 | Spain | ɛ1 | 2 | |
| FV5676 | EPEC | O111:H40 | Brazil | ɛ1 | ||
| FV10117 | aEPEC | O157:H16 | Spain | ɛ1 | ||
| FV10118 | aEPEC | O6:H19 | Spain | ɛ2 | 2 | 1 |
| FV10119 | aEPEC | O103:H19 | Brazil | ɛ2 | ||
| FV10120 | aEPEC | O123:H19 | Spain | ɛ2 | 2 | 1 |
| FV12050-IH9456-07-A | EPEC | O88:H25 | Spain | ɛ2 | 2 | 1 |
| FV12051-IH37508-06-A | EPEC | O88:H25 | Spain | ɛ2 | 2 | 1 |
| FV11698-73382-9 | aEPEC | O109:H9 | Brazil | ɛ2 | 1 | |
| FV11680-T2332-7 | aEPEC | O123:H− | Brazil | ɛ2 | 1 | |
| FV11681-T1482-11 | aEPEC | O123:H− | Brazil | ɛ2 | 1 | |
| FV11678-T0811-4 | aEPEC | ONT:H− | Brazil | ɛ2 | 1 | |
| FV10121-IH31923-03-A | aEPEC | O181:H− | Spain | ɛ3 | ||
| FV10123-IH37159-03-A | EPEC | O109:H− | Spain | ɛ4 | ||
| FV10142 | STEC | O80:H− | Spain | ɛ5/ξ | ||
| FV10143 | aEPEC | O80:H2 | Slovakia | ɛ5/ξ | ||
| FV10144 | aEPEC | O157:H− | Spain | ɛ5/ξ | ||
| FV10124 | STEC | O156:H− | Spain | ζ1 | 1 | |
| FV10125 | aEPEC | O156:H− | Spain | ζ1 | 1 | |
| FV11212-E110019 | aEPEC | O111:H9 | Finland | ζ2 | 1 | |
| FV11584-IH46488A07 | EPEC | O111:H− | Spain | ζ2 | 1 | |
| FV11677-T2381-8 | aEPEC | O154:H9 | Brazil | ζ2 | 1 | |
| FV11682-T2232-6 | aEPEC | O49:H− | Brazil | ζ2 | 1 | |
| FV10126 | aEPEC | O85:H31 | Spain | ζ3 | ||
| FV10127 | EPEC | O125:H− | Burundi | η1 | 1 | |
| FV10128-IH53199-03-A | aEPEC | ONT:H45 | Spain | η2 | ||
| FV10129 | EPEC | ONT:H45 | Switzerland | η2 | 1 | |
| FV10130 | aEPEC | O145:H4 | Germany | ι1A | ||
| FV5750 | aEPEC | O145:H2 | Brazil | ι1A | - | |
| FV11583-IH47502A07 | aEPEC | O2:H49 | Spain | ι1A | ||
| FV10131 | EPEC | O153:H8 | Spain | ι1B | 1 | |
| FV11679-T3281-6 | aEPEC | O85:H− | Brazil | ι1B/C | 1 | |
| FV11868-IH40760A/06 | EPEC | O55:H8 | Spain | ι1C | 1 | |
| FV10132 | EPEC | O119:H8 | Switzerland | ι1C | 1 | |
| FV10133 | aEPEC | ONT:H45 | Spain | ι2 | ||
| FV11873-IH39717A/03 | aEPEC | O101:H− | Spain | ι2 | ||
| FV11874-LORENY217.2 | aEPEC | O101:H− | Brazil | ι2 | ||
| FV10134 | aEPEC | O34:H− | Brazil | λ | ||
| FV10135 | aEPEC | O33:HNT | Brazil | λ | 1 | |
| FV10136 | aEPEC | O101:H33 | Uruguay | λ | ||
| FV5737 | aEPEC | O101:H33 | Brazil | λ | ||
| FV5752 | aEPEC | O124:H11 | Brazil | λ | 1 | |
| FV10137 | EPEC | O55:H− | Uruguay | μ | 1 | |
| FV10138 | EPEC | O55:H51 | Uruguay | μ | 1 | |
| LR-88-1 | EPEC | O55:H51 | Brazil | μ | 1 | |
| FV10139 | EPEC | O55:H− | Uruguay | μ | 1 | |
| FV10140 | aEPEC | O10:H− | Spain | ν | ||
| FV10141 | aEPEC | ONT:H− | Spain | ν | ||
| FV10145 | aEPEC | O129:H− | Spain | o | ||
| FV10146 | aEPEC | O84:H− | Spain | o | ||
| FV12052-IH28343-03-A | aEPEC | O84:H− | Spain | o | ||
| FV12053-IH36299-04-A | aEPEC | O105:H4 | Spain | o | 2 | 1 |
| IH21822A | aEPEC | ONT:H− | Spain | o | ||
| FV10430-T1551-2 | aEPEC | ONT:H− | Brazil | o | ||
| FV10147 | aEPEC | O14:H5 | Brazil | π | 1 | |
| FV10148 | aEPEC | ONT | Brazil | π | 1 | |
| FV5724 | aEPEC | ONT:H5 | Brazil | π | 1 | |
| FV10149 | aEPEC | O149:H− | Spain | ρ | ||
| FV10150 | aEPEC | O149:H− | Spain | ρ | ||
| FV10151 | aEPEC | O180:H− | Spain | ρ | ||
| FV10152 | aEPEC | O86:H− | United Kingdom | σ | ||
| FV10153 | aEPEC | O86:H− | United Kingdom | σ | ||
| FV10425-T0621-6 | aEPEC | ONT:H− | Brazil | σ | ||
| FV11772A-T4281-7 | aEPEC | O104:H− | Brazil | τ | ||
| FV11696-T1632-7 | aEPEC | O26:H− | Brazil | υ |
LREC, E. coli Reference Laboratory, Lugo, Spain.
Pathotypes were established based on the presence of virulence factors: EPEC strains were positive for the bfpA gene and the presence of the EAF plasmid; aEPEC strains were negative for bfpA and EAF; and STEC strains carried the stx gene.
SF− and SF+, negative and positive for sorbitol fermentation, respectively.
We then independently analyzed a collection of strains isolated predominantly in Chile (collection located at the Center for Vaccine Development, Santiago, Chile), with no relationship to our other reference strains. Sixty-two STEC O157:H7 strains were evaluated for the presence of the lpfA and intimin genes. As shown in Table 4, we found a perfect correlation (100%) between the EHEC serotype O157:H7 strains and the intimin (eae) types and lpfA gene variants. All 62 strains possessed the intimin γ1 gene, and they all carried the lpfA1-3 and lpfA2-2 gene combination, strongly supporting the idea that these three genes are reliable markers for the identification of this highly virulent serotype. Then we determined whether a correlation exists between the presence of these genes and the pathotypes in a collection of EPEC strains. As shown in Table 4, different types of lpfA1 and lpfA2 genes were found in combination with intimin gene variants. However, some trends were evident. (i) The majority of lpfA genes belong to the lpfA1-2 and/or the lpfA2-1 variant. (ii) The strains possessing lpfA1-2 and lpfA2-1 type genes also carried intimin gene types β1, θ1, ɛ, ο, and γ1. (iii) The majority of the Chilean EPEC strains, whether they belonged to the typical or the atypical EPEC pathotype, either possessed both lpfA1 and lpfA2 genes or lacked both of these genes. Interestingly, novel trends also emerged from our analysis; for example, we identified strains of serotype O145:H8 carrying a unique combination of lpfA genes (lpfA1-5 with lpfA2-1).
TABLE 4.
Correlation of lpfA types with EHEC and EPEC serotypes in a collection of strains from the Center for Vaccine Development, Santiago, Chile
| Strain reference no. | Pathotype | Serotypea | Intimin (eae) typeb | lpfA1 type | lpfA2 type |
|---|---|---|---|---|---|
| O157:H7 isolates (n = 62) | STEC | O157:H7 | γ1 | 3 | 2 |
| Di-304 | aEPEC | O63:H6 | α2 | ||
| PH-78 | aEPEC | O125 | α2 | ||
| Di-12 | aEPEC | O15:H25 | β1 | 1 | |
| Di-126 | aEPEC | O20:H7 | β1 | 2 | 1 |
| Di-140 | aEPEC | O20:H7 | β1 | 2 | 1 |
| Di-91 | aEPEC | O26:H− | β1 | 2 | 1 |
| O26-187 | STEC | O26:H11 | β1 | 2 | 1 |
| O26-215 | STEC | O26:H11 | β1 | 2 | 1 |
| J-80 | aEPEC | O119:H− | β1 | 2 | 1 |
| FV10037 | aEPEC | O153:H7 | β1 | 2 | 1 |
| Di-358 | aEPEC | O137:H6 | β1 | 1 | |
| Di-178 | EPEC | ND | β1 | ||
| Di-210 | EPEC | O98:HNT | β1 | 2 | 1 |
| Di-282 | EPEC | O158 | β1 | 1 | |
| Di-305 | EPEC | O119 | β1 | 2 | |
| Di-333 | EPEC | ND | β1 | 2 | |
| J-11 | EPEC | O126 | β1 | 2 | 1 |
| J-145 | aEPEC | ND | β1 | 2 | |
| J-215 | EPEC | ND | β1 | ||
| J-275 | EPEC | ND | β1 | ||
| J-294 | EPEC | ND | β1 | 5 | |
| FV9988 | EPEC | O145:H8 | γ1 | 5 | |
| Di-141 | aEPEC | O145:H8 | γ1 | 5 | 1 |
| J-104 | EPEC | ND | γ1 | 2 | 1 |
| FV9965 | EPEC | O23:H8 | θ1 | 2 | 1 |
| FV9986 | aEPEC | O23:H8 | θ1 | 2 | 1 |
| Di-4 | aEPEC | O23:H8 | θ1 | 2 | 1 |
| J-9 | EPEC | O23:H8 | θ1 | 2 | 1 |
| FV9967 | aEPEC | O55:H40 | θ1 | ||
| FV10039 | EPEC | O111:H25 | θ1 | ||
| J-97 | aEPEC | ONT:H40 | θ1 | ||
| J-98 | aEPEC | O55 | θ1 | ||
| J-151 | EPEC | ND | θ1 | ||
| Di-174 | EPEC | ONT:H19 | ɛ | 2 | 1 |
| Di-219 | EPEC | ND | ɛ | 2 | 1 |
| J-168 | EPEC | ND | ɛ | ||
| PH-11 | EPEC | ND | ɛ | ||
| FV9968 | EPEC | O109:H− | ɛ4 | ||
| Di-45 | EPEC | ONT:H1,H12 | ζ1 | ||
| Di-224 | EPEC | ND | ζ1 | ||
| PH-92 | EPEC | O1:H1,H12 | ζ1 | 3 | |
| FV9970 | EPEC | O1:H1,H12 | ζ3 | ||
| FV9991 | EPEC | O1:H1,H12 | ζ3 | 3 | |
| Di-101 | EPEC | O86:H8 | ι1 | 1 | |
| Di-307 | aEPEC | O145:H34 | ι1 | 2 | |
| FV9969 | aEPEC | ONT:H4 | ο | 2 | 1 |
| J-222 | aEPEC | ONT:H4 | ο | 2 | 1 |
| FV9984 | aEPEC | O76:H2 | ρ | ||
| Di-124 | aEPEC | O76:H2 | ρ | ||
| Di-192 | aEPEC | O145:H34 | NT | ||
| J-70 | EPEC | ND | NT | ||
| J-206 | EPEC | ND | NT | 1 | |
| J-280 | EPEC | ND | NT | 5 | |
| J-303 | EPEC | ND | NT | ||
| J-349 | EPEC | ND | NT | ||
| J-351 | EPEC | ND | NT | ||
| J-94 | EPEC | ND | NT | 3 | |
| J-281 | EPEC | ND | NT | ||
| PH-32 | EPEC | ND | NT | 3 | |
| PH-74 | EPEC | ND | NT | 2 | 3 |
| PH-112 | EPEC | ND | NT | ||
| PH-120 | EPEC | ND | NT | 2 | 1 |
| S-28 | EPEC | ND | NT |
ND, not determined.
NT, not typeable.
Finally, we determined what the probability of association was between the presence of the lpfA gene and the type of disease produced by the different pathotypes isolated in Chile (acute diarrhea, bloody diarrhea, or HUS). Our statistical analysis indicated that a strong association existed between the lpfA gene and E. coli strains carrying the stx genes (STEC strains). Further, we found that the lpfA (P = 0.0058) and stx2 (P = 0.0014) genes were significantly associated with HUS. Neither lpfA nor stx was significantly associated with acute diarrhea or bloody diarrhea. Therefore, we can conclude that lpfA and stx2 are STEC virulence factors showing strong associations with the HUS pathology that can result from a STEC infection; however, additional research is needed to confirm this association with a larger collection of pathogenic E. coli strains.
Our own initial studies, combined with observations by other groups, suggested that the lpf genes are associated with particular serotypes and/or specific genotypes (16, 28, 32-34). However, further studies indicated that the EHEC O157:H7 lpfA genes are widely distributed among DEC strains (31). Our current study confirmed and expanded these observations, because we now demonstrated that certain variants of the lpfA1 and lpfA2 genes are restricted to strains carrying intimin type γ (mainly EHEC O157:H7 and EPEC O55:H7). The study by Toma and colleagues tried to understand the relationship between lpfA variants and phylogenetic groups (31); unfortunately, at the time of that study, the number of variants available in the database was limited, and the phylogenetic trees obtained were incongruent with the strain phylogeny. Although it is evident that the lpf gene clusters are widely distributed in different E. coli lineages, and the lpfA genes seem not to be specific to EHEC strains, the availability of additional sequences in the database and the incorporation of the different intimin types into our analysis led us to identify specific combinations of genes present only in AEEC strains that are associated with severe and/or epidemic disease. Overall, our results indicate that the combination of these three gene markers (eae, lpfA1, and lpfA2) could be sufficient for performing a quick identification of AEEC isolates, specifically for the quick identification of the highly virulent serotype O157:H7.
One additional task that we are currently undertaking is the complete elucidation of the evolutionary history of the lpf gene clusters in DEC strains, as well as in ExPEC, commensal E. coli, Shigella, and Salmonella strains. This analysis includes mapping of the chromosomal location of the lpf gene clusters (in EHEC O157:H7, lpfA1 is linked to O-island 141 [OI-141], while lpfA2 is located in OI-154) and characterization of the other open reading frames within the lpf operons, because studies with other fimbrial gene clusters have suggested that different regions within an operon have diverse evolutionary histories (5). But it is now evident that the acquisition of different lpf gene clusters in specific lineages of E. coli might be contributing to the emergence of highly virulent strains derived from commensal organisms, which also possess unique lpf variants.
Supplementary Material
Acknowledgments
We thank Julia María Pita of the Unidade de Microbioloxía at the Complexo Hospitalario Xeral-Calde of Lugo, Spain, for providing us with bacterial strains.
This work was supported in part by a John Sealy Memorial Endowment Fund Bridging Grant and NIH grant AI079154-01A2 to A.G.T. Work in the laboratories of T.A.T.G. was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo, the Conselho Nacional de Desenvolvimento Científico e Tecnológico, and the Programa de Apoio a Núcleos de Excelência (PRONEX MCT/CNPq/FAPERJ); R.V. was funded by FONDECYT project 1061088; and J.B. was funded by the Fondo de Investigación Sanitaria, by the Xunta de Galicia, and by the Comisión Interministerial de Ciencia y Tecnología.
Footnotes
Published ahead of print on 3 June 2009.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Adu-Bobie, J., G. Frankel, C. Bain, A. G. Goncalves, L. R. Trabulsi, G. Douce, S. Knutton, and G. Dougan. 1998. Detection of intimins alpha, beta, gamma, and delta, four intimin derivatives expressed by attaching and effacing microbial pathogens. J. Clin. Microbiol. 36662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agin, T. S., and M. K. Wolf. 1997. Identification of a family of intimins common to Escherichia coli causing attaching-effacing lesions in rabbits, humans, and swine. Infect. Immun. 65320-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco, M., J. E. Blanco, A. Mora, G. Dahbi, M. P. Alonso, E. A. González, M. I. Bernárdez, and J. Blanco. 2004. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from cattle in Spain and identification of a new intimin variant gene (eae-xi). J. Clin. Microbiol. 42645-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco, M., S. Schumacher, T. Tasara, C. Zweifel, J. E. Blanco, G. Dahbi, J. Blanco, and R. Stephan. 2005. Serotypes, intimin variants and other virulence factors of eae positive Escherichia coli strains isolated from healthy cattle in Switzerland. Identification of a new intimin variant gene (eae-η2). BMC Microbiol. 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd, E. F., and D. L. Hartl. 1999. Analysis of the type 1 pilin gene cluster fim in Salmonella: its distinct evolutionary histories in the 5′ and 3′ regions. J. Bacteriol. 1811301-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantey, J. R., and L. R. Inman. 1981. Diarrhea due to Escherichia coli strain RDEC-1 in the rabbit: the Peyer's patch as the initial site of attachment and colonization. J. Infect. Dis. 143440-446. [DOI] [PubMed] [Google Scholar]
- 7.Cergole-Novella, M. C., L. S. Nishimura, L. F. Dos Santos, K. Irino, T. M. Vaz, A. M. Bergamini, and B. E. Guth. 2007. Distribution of virulence profiles related to new toxins and putative adhesins in Shiga toxin-producing Escherichia coli isolated from diverse sources in Brazil. FEMS Microbiol. Lett. 274329-334. [DOI] [PubMed] [Google Scholar]
- 8.Doughty, S., J. Sloan, V. Bennett-Wood, M. Robertson, R. M. Robins-Browne, and E. L. Hartland. 2002. Identification of a novel fimbrial gene cluster related to long polar fimbriae in locus of enterocyte effacement-negative strains of enterohemorrhagic Escherichia coli. Infect. Immun. 706761-6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzhenry, R., S. Dahan, A. G. Torres, Y. Chong, R. Heuschkel, S. Murch, M. Thomson, J. B. Kaper, G. Frankel, and A. D. Phillips. 2006. Long polar fimbriae and tissue tropism in Escherichia coli O157:H7. Microbes Infect. 81741-1749. [DOI] [PubMed] [Google Scholar]
- 10.Fitzhenry, R. J., D. J. Pickard, E. L. Hartland, S. Reece, G. Dougan, A. D. Phillips, and G. Frankel. 2002. Intimin type influences the site of human intestinal mucosal colonisation by enterohaemorrhagic Escherichia coli O157:H7. Gut 50180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzhenry, R. J., M. P. Stevens, C. Jenkins, T. S. Wallis, R. Heuschkel, S. Murch, M. Thomson, G. Frankel, and A. D. Phillips. 2003. Human intestinal tissue tropism of intimin ɛ O103 Escherichia coli. FEMS Microbiol. Lett. 218311-316. [DOI] [PubMed] [Google Scholar]
- 12.Jordan, D. M., N. Cornick, A. G. Torres, E. A. Dean-Nystrom, J. B. Kaper, and H. W. Moon. 2004. Long polar fimbriae contribute to colonization by Escherichia coli O157:H7 in vivo. Infect. Immun. 726168-6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jores, J., K. Zehmke, J. Eichberg, L. Rumer, and L. H. Wieler. 2003. Description of a novel intimin variant (type ζ) in the bovine O84:NM verotoxin-producing Escherichia coli strain 537/89 and the diagnostic value of intimin typing. Exp. Biol. Med. (Maywood) 228370-376. [DOI] [PubMed] [Google Scholar]
- 14.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2123-140. [DOI] [PubMed] [Google Scholar]
- 15.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16111-120. [DOI] [PubMed] [Google Scholar]
- 16.Low, A. S., F. Dziva, A. G. Torres, J. L. Martinez, T. Rosser, S. Naylor, K. Spears, N. Holden, A. Mahajan, J. Findlay, J. Sales, D. G. Smith, J. C. Low, M. P. Stevens, and D. L. Gally. 2006. Cloning, expression, and characterization of fimbrial operon F9 from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 742233-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 18.Mora, A., M. Blanco, J. E. Blanco, G. Dahbi, C. López, P. Justel, M. P. Alonso, A. Echeita, M. I. Bernárdez, E. A. González, and J. Blanco. 2007. Serotypes, virulence genes and intimin types of Shiga toxin (verocytotoxin)-producing Escherichia coli isolates from minced beef in Lugo (Spain) from 1995 through 2003. BMC Microbiol. 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreira, F. C., M. A. Vieira, A. J. Ferreira, D. M. Girão, T. M. Vaz, A. C. Rosa, T. Knobl, K. Irino, E. Freymüller, and T. A. T. Gomes. 2008. Escherichia coli strains of serotype O51:H40 comprise typical and atypical enteropathogenic E. coli strains and are potentially diarrheagenic. J. Clin. Microbiol. 461462-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newton, H. J., J. Sloan, V. Bennett-Wood, L. M. Adams, R. M. Robins-Browne, and E. L. Hartland. 2004. Contribution of long polar fimbriae to the virulence of rabbit-specific enteropathogenic Escherichia coli. Infect. Immun. 721230-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira, M. G., J. R. Brito, T. A. T. Gomes, B. E. Guth, M. A. Vieira, Z. V. Naves, T. M. Vaz, and K. Irino. 2008. Diversity of virulence profiles of Shiga toxin-producing Escherichia coli serotypes in food-producing animals in Brazil. Int. J. Food Microbiol. 127139-146. [DOI] [PubMed] [Google Scholar]
- 23.Osek, J., M. Weiner, and E. L. Hartland. 2003. Prevalence of the lpfO113 gene cluster among Escherichia coli O157 isolates from different sources. Vet. Microbiol. 96259-266. [DOI] [PubMed] [Google Scholar]
- 24.Phillips, A. D., and G. Frankel. 2000. Intimin-mediated tissue specificity in enteropathogenic Escherichia coli interaction with human intestinal organ cultures. J. Infect. Dis. 1811496-1500. [DOI] [PubMed] [Google Scholar]
- 25.Phillips, A. D., S. Navabpour, S. Hicks, G. Dougan, T. Wallis, and G. Frankel. 2000. Enterohaemorrhagic Escherichia coli O157:H7 target Peyer's patches in humans and cause attaching/effacing lesions in both human and bovine intestine. Gut 47377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid, S. D., D. J. Betting, and T. S. Whittam. 1999. Molecular detection and identification of intimin alleles in pathogenic Escherichia coli by multiplex PCR. J. Clin. Microbiol. 372719-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 28.Szalo, I. M., F. Goffaux, V. Pirson, D. Piérard, H. Ball, and J. Mainil. 2002. Presence in bovine enteropathogenic (EPEC) and enterohaemorrhagic (EHEC) Escherichia coli of genes encoding for putative adhesins of human EHEC strains. Res. Microbiol. 153653-658. [DOI] [PubMed] [Google Scholar]
- 29.Tarr, C. L., and T. S. Whittam. 2002. Molecular evolution of the intimin gene O111 clones of pathogenic Escherichia coli. J. Bacteriol. 184479-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tartof, S. Y., O. D. Solberg, and L. W. Riley. 2007. Genotypic analyses of uropathogenic Escherichia coli based on fimH single nucleotide polymorphisms (SNPs). J. Med. Microbiol. 561363-1369. [DOI] [PubMed] [Google Scholar]
- 31.Toma, C., N. Higa, S. Iyoda, M. Rivas, and M. Iwanaga. 2006. The long polar fimbriae genes identified in Shiga toxin-producing Escherichia coli are present in other diarrheagenic E. coli and in the standard E. coli collection of reference (ECOR) strains. Res. Microbiol. 157153-161. [DOI] [PubMed] [Google Scholar]
- 32.Toma, C., E. Martínez Espinosa, T. Song, E. Miliwebsky, I. Chinen, S. Iyoda, M. Iwanaga, and M. Rivas. 2004. Distribution of putative adhesins in different seropathotypes of Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 424937-4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres, A. G., J. A. Giron, N. T. Perna, V. Burland, F. R. Blattner, F. Avelino-Flores, and J. B. Kaper. 2002. Identification and characterization of lpfABCC′DE, a fimbrial operon of enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 705416-5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres, A. G., K. J. Kanack, C. B. Tutt, V. Popov, and J. B. Kaper. 2004. Characterization of the second long polar (LP) fimbriae of Escherichia coli O157:H7 and distribution of LP fimbriae in other pathogenic E. coli strains. FEMS Microbiol. Lett. 238333-344. [DOI] [PubMed] [Google Scholar]
- 35.Torres, A. G., and J. B. Kaper. 2001. PAIs of intestinal E. coli, p. 31-48. In J. Hacker and J. B. Kaper (ed.), Pathogenicity islands and the evolution of pathogenic microbes, vol. 1. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 36.Torres, A. G., G. N. López-Sánchez, L. Milflores-Flores, S. D. Patel, M. Rojas-López, C. F. Martínez de la Peña, M. M. Arenas-Hernández, and Y. Martínez-Laguna. 2007. Ler and H-NS, regulators controlling expression of the long polar fimbriae of Escherichia coli O157:H7. J. Bacteriol. 1895916-5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres, A. G., L. Milflores-Flores, J. G. Garcia-Gallegos, S. D. Patel, A. Best, R. M. La Ragione, Y. Martinez-Laguna, and M. J. Woodward. 2007. Environmental regulation and colonization attributes of the long polar fimbriae of Escherichia coli O157:H7. Int. J. Med. Microbiol. 297177-185. [DOI] [PubMed] [Google Scholar]
- 38.Torres, A. G., T. M. Slater, S. D. Patel, V. L. Popov, and M. M. Arenas-Hernández. 2008. Contribution of the Ler- and H-NS-regulated long polar fimbriae of Escherichia coli O157:H7 during binding to tissue-cultured cells. Infect. Immun. 765062-5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres, A. G., X. Zhou, and J. B. Kaper. 2005. Adherence of diarrheagenic Escherichia coli strains to epithelial cells. Infect. Immun. 7318-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vidal, M., P. Escobar, V. Prado, J. C. Hormazabal, and R. Vidal. 2007. Distribution of putative adhesins in Shiga toxin-producing Escherichia coli (STEC) strains isolated from different sources in Chile. Epidemiol. Infect. 135688-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, W. L., B. Köhler, E. Oswald, L. Beutin, H. Karch, S. Morabito, A. Caprioli, S. Suerbaum, and H. Schmidt. 2002. Genetic diversity of intimin genes of attaching and effacing Escherichia coli strains. J. Clin. Microbiol. 404486-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.