Abstract
Retrospective testing of neonatal Guthrie card blood spots for specific immunoglobulin M (IgM) can distinguish congenital toxoplasmosis from acquired toxoplasmosis. We determined whether storage temperature reduced IgM detection, using filter paper blood samples “spiked” with anti-Toxoplasma IgM. After 300 days, IgM detection deteriorated with storage at room temperature but not at temperatures of 4°C or lower.
Guthrie card filter paper blood spots (GCs) are taken within 8 days of birth for neonatal screening for phenylketonuria (PKU) and other conditions. In the United Kingdom, samples are stored for at least 5 years and therefore provide a valuable biological sample for retrospective clinical testing and research (7). GC samples have previously been used for retrospective immunoglobulin G (IgG) testing for a range of infections, but we know of no published reports evaluating the feasibility or performance of IgM testing of stored neonatal GCs. Retrospective Toxoplasma-specific IgM testing of neonatal GCs could be useful to distinguish between congenital and postnatal acquired toxoplasmosis in children presenting with symptoms later in childhood (2, 3).
In an experimental study, we created “spiked” Toxoplasma-specific IgM-positive filter paper blood samples and assessed the detection of IgM after storage at different temperatures. The “spiked” blood samples were made from washed red blood cells from a single Toxoplasma gondii-seronegative donor and were mixed with serum from 40 IgM-seropositive samples at a ratio of 1:1 to produce a hematocrit similar to that of a neonatal sample. The “spiked” filter paper samples were dried, placed in separate plastic bags, and stored at room temperature (21°C), 4°C, −20°C, or −80°C (40 samples stored at each temperature). Aliquots of the 40 serum samples were stored at the same temperatures. Toxoplasma-specific IgM antibodies were tested in filter paper and serum samples after 1, 92, 201, 306, and 400 days of storage using the fully automated AutoDelfia immunofluorometric assay (PerkinElmer, Turku, Finland) (5, 8).
In a parallel clinical study, we sought consent to retrieve and test stored neonatal GC samples from children with symptoms of congenital or postnatal acquired toxoplasmosis who were reported by pediatricians, ophthalmologists or laboratories to a national, active surveillance study between 2002 and 2004 (details reported elsewhere [2, 9]). Clinicians forwarded information to the parents, including a request for consent to retrieve and test stored neonatal GC samples. On initial testing of retrieved GC samples at the Statens Serum Institut in Copenhagen, Denmark, using the AutoDelfia assay (8), samples failed to elute with phosphate-buffered saline. Testing was repeated at the Laboratorio Nobel and Centro de Triagem Neonatal in Porto Alegre, Brazil. Again, elution with phosphate-buffered saline failed, but partial elution was achieved after crushing the filter paper disc inside a microplate well coated with streptavidin-anti-human IgM antibody, shaking the microplate, and incubating overnight. The eluate was analyzed for Toxoplasma-specific IgM antibodies by using a fluorometric enzyme immunoassay (Ani Labsytems, Helsinki, Finland) (1, 5, 6).
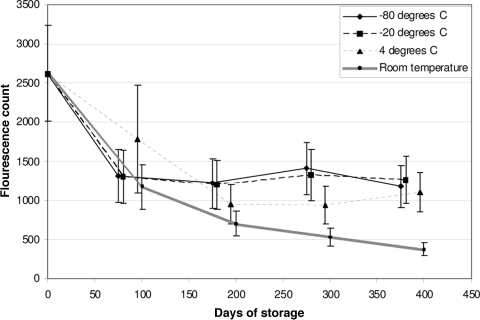
All of the GC samples “spiked” with anti-Toxoplasma IgM were successfully eluted and tested up to 400 days. Counts declined during the first 100 days at all temperatures. Similar declines were seen in the serum samples. At 300 and 400 days, the mean counts for the samples stored at room temperature were significantly lower than those for samples stored at 4°C or below (P < 0.003) (Fig. 1). After 400 days of storage, at least 83% of samples stored at 4°C or below were IgM positive, compared with 18% of samples stored at room temperature (P < 0.0005).
FIG. 1.
Change in IgM assay fluorescence counts in “spiked” filter paper blood spots, according to storage temperature and duration (n = 40 samples stored at each temperature).
Thirty-eight cases were identified in the national surveillance study, and 27 United Kingdom-born children were eligible for blood spot retrieval. Stored blood spots were retrieved and tested from only one-third of these children (n = 9), mainly because clinicians did not forward information and consent forms (n = 12) or because the laboratory failed to respond (n = 2) or failed to retrieve the GC (n = 2). No parent refused testing. Seven of the nine blood spots retrieved were for children 6 years of age or older, the oldest being 16 years old (Table 1). Of the six children classified with congenital toxoplasmosis, five had a positive result (sensitivity, 83%; 95% confidence interval, 36% to 99%) (Table 1). Three children were classified with postnatal acquired infection, and all had negative IgM results (specificity, 100%; lower 95% confidence interval, 33%).
TABLE 1.
Toxoplasma-specific IgM results for children identified with toxoplasmosis in a national surveillance study
| Toxoplasmosis type | Age (yr)a | Immunofluorometric assay countb | Interpretation |
|---|---|---|---|
| Congenital | 0.0 | 455 | Positive |
| 0.4 | 606 | Positive | |
| 3.7 | 700 | Positive | |
| 5.5 | 620 | Positive | |
| 0.7 | 680 | Positive | |
| 10.6 | 280 | Negative | |
| Postnatally acquired | 10.0 | 312** | Negative |
| 14.5 | 410 | Negative | |
| 15.9 | 334** | Negative |
Age when infection was first suspected.
Cutoff values for fluorescence counts of control samples: negative and borderline, <450; positive, ≥450 counts. **, poor quality sample.
Detection of Toxoplasma-specific IgM was significantly reduced in filter paper samples stored for more than 300 days at room temperature compared with those stored at 4°C or below. Despite this deterioration in IgM detection, the surveillance study showed that it is possible to detect Toxoplasma-specific IgM in neonatal GC samples from children with symptoms of congenital toxoplasmosis after nearly 6 years of storage at room temperature. These findings demonstrate the feasibility of retrospective testing in clinical practice, but because of the small sample, further studies are needed to evaluate the accuracy of retrospective testing under different storage temperatures. Further research is also needed to determine whether the elution of samples could be improved by storing GC samples in a plastic bag or an envelope (4, 11).
Diagnosis of congenital toxoplasmosis is problematic because visual or neurological abnormalities associated with congenital toxoplasmosis may not present until the preschool years or later, when ocular signs due to congenital or postnatal acquired toxoplasmosis are indistinguishable and Toxoplasma-specific IgM is usually undetectable (3). Stored neonatal GC samples can provide critically important samples that allow retrospective testing for toxoplasma IgM to distinguish between congenital and postnatal acquired toxoplasmosis. This information is important for counseling parents about associated neurological symptoms and prognosis and to inform policy about the burden of congenital toxoplasmosis (6, 10).
Long-term storage of neonatal GC samples is also important for retrospective testing for other conditions apart from congenital toxoplasmosis (6, 14). In the United Kingdom and in most of Europe (11), neonatal GC samples are stored at room temperature, except in Denmark, where storage is at −20°C (8). The results from our experimental study suggest that GC samples should be stored at 4°C or below in order to optimize retrospective IgM antibody testing after prolonged storage. Further clinical studies are needed to evaluate the clinical and cost effectiveness of cold storage for the range of potential uses for retrospective testing of neonatal GC samples.
Acknowledgments
This work was partly supported by grants from the British Council for Prevention of Blindness and the British Eye Research Foundation.
Miles Stanford contributed to the study design and ophthalmic assessments, and Pia Hardelid made helpful comments on the manuscript.
Footnotes
Published ahead of print on 3 June 2009.
REFERENCES
- 1.Eaton, R. B., E. Petersen, H. Seppanen, and T. Tuuminen. 1996. Multicenter evaluation of a fluorometric enzyme immunocapture assay to detect toxoplasma-specific immunoglobulin M in dried blood filter paper specimens from newborns. J. Clin. Microbiol. 343147-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert, R. E., H. K. Tan, S. Cliffe, E. Guy, and M. R. Stanford. 2005. Symptomatic toxoplasma infection due to congenital and postnatally acquired infection. Arch. Dis. Child. 91495-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert, R. E., L. Thalib, H. K. Tan, M. Paul, M. Wallon, and E. Petersen. 2007. Screening for congenital toxoplasmosis: accuracy of immunoglobulin M and immunoglobulin A tests after birth. J. Med. Screen. 148-13. [DOI] [PubMed] [Google Scholar]
- 4.Mei, J. V., J. R. Alexander, B. W. Adam, and W. H. Hannon.2001. Use of filter paper for the collection and analysis of human whole blood specimens. J. Nutr. 1311631S-1636S. [DOI] [PubMed] [Google Scholar]
- 5.Neto, E. C., E. Anele, R. Rubim, A. Brites, J. Schulte, D. Becker, and T. Tuuminen. 2000. High prevalence of congenital toxoplasmosis in Brazil estimated in a 3-year prospective neonatal screening study. Int. J. Epidemiol. 29941-947. [DOI] [PubMed] [Google Scholar]
- 6.Neto, E. C., R. Rubin, J. Schulte, and R. Giugliani. 2004. Newborn screening for congenital infectious diseases. Emerg. Infect. Dis. 101068-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollitt, R. J. 2006. International perspectives on newborn screening. J. Inherit. Metab. Dis. 29390-396. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen, T., J. Spenter, I. Jaliashvili, M. Christiansen, B. Norgaard-Pedersen, and E. Petersen. 2002. Automated time-resolved immunofluorometric assay for Toxoplasma gondii-specific IgM and IgA antibodies: study of more than 130,000 filter-paper blood-spot samples from newborns. Clin. Chem. 481981-1986. [PubMed] [Google Scholar]
- 9.Stanford, M. R., H. K. Tan, and R. E. Gilbert. 2006. Toxoplasmic retinochoroiditis presenting in childhood: clinical findings in a UK survey. Br. J. Ophthalmol. 901464-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan, H. K., D. Schmidt, M. Stanford, K. Tear-Fahnehjelm, N. Ferret, A. Salt, and R. Gilbert. 2007. Risk of visual impairment in children with congenital toxoplasmic retinochoroiditis. Am. J. Ophthalmol. 144648-653. [DOI] [PubMed] [Google Scholar]
- 11.UK Newborn Screening Programme Centre. 2005. Newborn blood spot screening in the UK. Policies and standards. UK Newborn Screening Programme Centre, London, United Kingdom.