Abstract
Isolates from Campylobacter jejuni-infected patients were collected and fresh poultry meat from retail sources was sampled during the same time period and within the same geographical area. The patients were interviewed about exposure to known risk factors, and a significant correlation between the presence of a poultry subtype in patients and the consumption of fresh poultry meat was observed.
Thermophilic Campylobacter spp. are the most common cause of reported bacterial gastroenteritis in Sweden and many other countries worldwide. In Sweden, domestically acquired Campylobacter infections are caused almost exclusively by C. jejuni (27). Case control studies performed for the identification of infection sources have demonstrated that the consumption of poultry, unpasteurized milk, and untreated water and contact with pets such as dogs and cats and with food-producing animals such as cattle and poultry are common risk factors (7, 12, 13, 28, 31). However, contradictory results have been reported by different case control studies. For example, eating chicken at home is usually cited as a risk factor, but in two studies, this behavior was found to be protective (1, 7). Furthermore, the consumption of undercooked beef was identified as a risk factor in a recent French study, but the consumption of chicken was not (9).
Comparing the subtypes of isolates from patients and suspected sources is a more direct way than the case control approach for determining whether a specific source constitutes an important transmission vehicle or not. Many studies have concluded that there is a substantial overlap of subtypes between patients and chickens but that the overlap is smaller for potential sources such as water, wild birds (black-headed gulls), wild mammals, sandy beaches, and cattle (4, 6, 14, 18, 24, 25). However, making a direct correlation between the overlap of subtypes and the source of infection assumes that all subtypes are equal in terms of virulence and survival until the time of ingestion. It also excludes the possibility of a third, unidentified source transmitting Campylobacter to both the suspected source and humans.
In this study, called Campy-SET, we enhanced the surveillance of campylobacteriosis from 1 August to 31 October 2003 in three Swedish counties, Gävleborg, Örebro, and Halland. Patients from the hospital in Borås, in the county of Västra Götaland, were also included. These four counties are evenly distributed along a diagonal cross-section of southern Sweden, from the northeast to the southwest. Confirmed Campylobacter isolates recovered from stool samples at the participating hospitals were sent to the Swedish Institute for Infectious Disease Control. On arrival, the isolates were classified as C. jejuni or C. coli by PCR assays as described by Linton et al. (19). Patients with domestically acquired Campylobacter infections were interviewed by telephone within a 2-week period after the onset of illness by using a standard questionnaire divided into the following sections: (i) illness and patient characteristics (4 questions), (ii) consumption and handling of poultry meat (14 questions), and (iii) contact with other potential infection sources (14 questions). A few patients received the questionnaire by post.
Participating hospitals reported 114 cases of campylobacteriosis to the national surveillance program SmiNet during the 3-month study period. Ninety-three (82%) of these cases were also reported to the Campy-SET study. The number of patients was evenly distributed among the four counties, with between 20 and 30% per county. A completed questionnaire and an associated isolate were obtained from 79 patients.
The gender distribution among patients was even (39 females and 40 males), and the median age was 39 years (range, 1 to 90 years). Patients aged 31 to 40 years constituted the largest group (n = 15), and the next largest groups consisted of patients aged 51 to 60 years (n = 14) and those aged 41 to 50 years (n = 13). Five patients were children aged 1 to 10 years. The duration of clinical symptoms was up to 10 days for 48 patients. Fifteen patients had symptoms lasting between 11 and 20 days, and 13 patients were ill for more than 20 days. Four patients with symptoms lasting between 11 and 20 days were still ill when the questionnaire was filled out, and three patients did not state the number of days with symptoms. In Sweden, Campylobacter infection is a reportable communicable disease, and cross comparison of data revealed that the gender and age group distributions recorded in the Campy-SET study were similar to those reported in the mandatory system.
The food standards agencies of the main municipal authorities in the participating counties sampled fresh poultry products from retail sources every week between 27 July and 25 October 2003. The sampling staff was instructed to avoid collecting samples that could be suspected to originate from the same slaughter group (those stamped with the same branches and dates). Identical analytical methods were applied to all samples, but the analyses were carried out in different commercial laboratories employed by the different municipal authorities. In brief, the whole meat sample was transferred into a sterile plastic bag, and a volume of peptone water corresponding to half the weight of the sample was added. After the plastic bag was manually shaken three times for 30 s each time, 1 ml of the rinse fluid was spread onto a large (14-cm-diameter) modified charcoal cefoperazone deoxycholate agar plate and 0.1 ml of the fluid and a serial dilution thereof were spread onto normal-sized (9-cm-diameter) modified charcoal cefoperazone deoxycholate agar plates. The plates were incubated in a microaerophilic atmosphere created by gas packs at 42°C for 48 h. Colonies with a Campylobacter-like appearance and with cells displaying the typical corkscrew-like movement under the microscope were sent to the National Food Administration. On arrival, the isolates were classified as thermophilic Campylobacter and C. jejuni by PCR assays (8, 23).
In total, 458 fresh poultry products were collected from retail stores during the 3 months of sampling. Campylobacter spp. were detected in 101 (25%) of 397 chicken products. The spreading of 1 ml of chicken rinse fluid onto a large plate increases the sensitivity to the level obtained by enrichment (16). The observed prevalence is in agreement with the results of a Swedish surveillance study conducted in 2000, in which the prevalence in fresh chicken meat from retail sources sampled from June to October 2000 was found to be 22% (2). Approximately 50% of the chicken samples were whole carcasses, and the other 50% were evenly divided among breast fillets and other products. The majority (85%) of the chicken products were from the three largest slaughterhouses in Sweden. The prevalence of Campylobacter spp. varied significantly (chi-square test; P < 0.05) among these three slaughterhouses, at 33, 20, and 14%. Similar differences among these slaughterhouses have been observed previously within the national Campylobacter surveillance program in Sweden, in which almost all slaughter groups are tested upon arrival at slaughterhouses. Thus, differences in contamination levels were probably due mainly to differences in prevalence among slaughter groups rather than differences in slaughter hygiene. This finding is also in agreement with the results of another Swedish study in which the prevalence in slaughter groups was found to be related to the prevalence on carcasses postchilling (15). No significant difference (chi-square test; P > 0.05) in prevalence among the following categories of chicken products from the slaughterhouse with the most positive samples was observed: fillets (n = 65), whole carcasses (n = 34), drumsticks (n = 32), and spiced/marinated products (n = 18). The 11 most contaminated products carried concentrations between 101 and 1,000 CFU/g and were not clustered according to a specific slaughterhouse or product category. Half the positive samples had concentrations equal to or less than 10 CFU/g.
Only 1 (1.6%) of 61 samples of turkey meat tested was culture positive for Campylobacter spp., a result similar to those of an earlier Swedish study in which 4 of 111 turkey samples from retail sources were positive. A Danish study from 1997 found the percentages of Campylobacter-contaminated turkey and chicken products at retail stores to be similar, 29 and 25%, respectively (22). Studies in the United States and Ireland revealed that the prevalence of Campylobacter spp. in turkey products was lower than that in chicken, although the prevalences in turkey products in both studies were over 35% (20, 30). Recently, a Canadian study reported a higher prevalence in turkey flocks (46%) than in broiler flocks (37%) (3). It is still unclear whether a low infection rate during breeding or good hygiene practices during slaughter are the main reason for the low prevalence on turkey meat in Sweden.
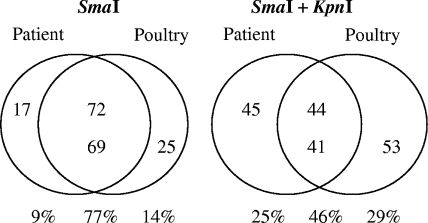
In total, 89 patient isolates and 94 poultry isolates were typed by SmaI digestion and pulsed-field gel electrophoresis (PFGE) as described in the Campynet protocol (http://campynet.vetinst.dk). The PFGE analysis generated 51 pulsotypes, of which 13 (groups A to N) included isolates from both patients and poultry. These 13 pulsotypes comprised the majority of the isolates from both patients and poultry (Fig. 1A). The number of patient isolates with a pulsotype matching that of a poultry isolate was reduced from 72 to 44 after the use of a second restriction enzyme (KpnI) as described by Lindmark et al. (18) (Fig. 1B). Similarly, KpnI cleavage reduced the number of poultry isolates with a pulsotype matching that of a patient isolate from 69 to 41. This degree of overlap between pulsotypes of human and chicken isolates is similar to previously reported values (14, 18).
FIG. 1.
Number of C. jejuni isolates displaying a subtype present among only one source or present in both patients and poultry. The results of PFGE with SmaI and with SmaI and KpnI are shown.
The vast majority of isolates within some groups, e.g., C and D, were also indistinguishable after cleavage with KpnI, whereas many isolates in other groups, e.g., A and G, displayed different pulsotypes after cleavage with KpnI. The indistinguishable isolates in groups C and D, comprising 16 and 18 isolates, respectively, were evenly distributed among the four participating counties. Chicken and patient isolates of both pulsotypes C and D were found in the four counties, with the exception of Örebro, where no chicken isolate of pulsotype C was found. Isolates from both groups C and D were present in chickens slaughtered during each of the three months of sampling. Similarly, onsets of disease in patients carrying isolates of these two groups occurred during each of these three months.
The usefulness of genetic subtyping methods such as PFGE has been questioned due to the low degree of clonality within the species. Using antibiotic resistance genes, de Boer et al. (5) showed that DNA is easily exchanged between both homologous and heterologous C. jejuni strains. However, PFGE profiles can be very stable during a large number of passages in vitro as well as in vivo (21). There are also reports showing that most strains display a stable PFGE profile along the food chain but that in rare cases frequent recombination occurs, giving rise to numerous PFGE profiles (10, 17, 29). The generation of new PFGE profiles seems to be favored when the host is infected with more than one strain, and thus, interrecombination rather than intrarecombination seems to occur (5, 17). The evidence for genetic stability is also supported by the results of a Finnish study, which found the same PFGE profiles in strains collected in three different years (11). Similarly, the major PFGE groups in the present study were also found in an earlier Swedish study that analyzed 177 isolates collected in 2000 from retail meat products (mostly chicken), patients, and water (18). In fact, the five major PFGE groups from that study were all among the six largest groups in the Campy-SET study. Thus, the relative proportions of different PFGE groups seem to be fairly stable over time, at least in chickens and humans. It can be speculated that C. jejuni frequently recombines under certain conditions but that most of the new genotypes have reduced fitness and consequently disappear. Overall, it seems likely that PFGE profiles in most cases remain stable from the time of introduction into a broiler flock to the point of recovery from a patient, especially in regions where broiler flocks are rarely infected with more than one strain. We previously tested whether identical pulsotypes obtained after cleavage with SmaI and KpnI reflect true genetic relatedness (26).
Indistinguishable human and chicken isolates were compared by using microarray-based comparative genomic hybridizations. One human and one chicken isolate from each of groups B, C, D, and H and two human and two chicken isolates from group A were tested. The microarray data clustered all but one isolate according to the five groups determined by PFGE. The exception was one of the human isolates in group A, which according to the comparative genomic hybridization analysis was more closely related to the isolates in group C than to the other three isolates in group A. Multilocus sequence typing revealed that the isolates in both groups were of the same sequence type (ST21). For group A, the production dates for the two chicken isolates matched and, of the two human isolates, the isolate more closely related to the chicken isolates was from a patient who became ill 14 days after the production date. The onset of disease in the second patient occurred 2½ months after the production date, indicating that the source of infection was not the present broiler flock. Overall, it seems that PFGE results reflect true genetic relatedness but that the discriminatory power is sometimes limited to perfectly clustered isolates that are closely related.
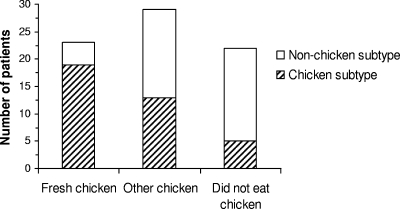
According to the questionnaires, 52 patients had consumed chicken during the 2 weeks before the onset of disease and 23 of these patients had consumed a dish cooked using fresh chicken (raw or marinated). Twenty-two patients had not eaten chicken, and five could not recall their consumption histories. Figure 2 shows the proportions of patients within three consumption categories (consumption of fresh chicken, consumption of other chicken [frozen, barbequed before retail sale, or not specified], and no consumption of chicken) carrying an isolate matching a poultry isolate. The proportion of patients in the fresh-chicken consumption category with isolate pulsotypes matching those of chicken isolates was significantly higher than the proportion in the no-chicken consumption category (chi-2 test; P < 0.05). This correlation was not seen with data generated only with SmaI. PFGE group H consisted of isolates from four of the five patients infected with a chicken subtype but reporting no contact with poultry (through cooking or eating), isolates from two patients reporting consumption of chicken, and two chicken isolates. The two chicken isolates were obtained from chicken products produced the same day at the same slaughterhouse. The production date for the chicken samples and the dates of the onset of disease in the patients infected with group H isolates indicated that the immediate slaughter group was not the infection source. The patients were from three different counties, and the dates of disease onset were not clustered but spread over 2½ months. In addition, a separate group of five indistinguishable human isolates from PFGE group A was formed after digestion with KpnI. The corresponding patients were from three different counties, and the dates of disease onset in four of the patients were similar. Only one of these patients reported the consumption of fresh chicken in the fortnight before the onset of illness. Thus, it seems likely that the patients with isolates in groups A and H were infected through a transmission route other than chicken. The questionnaire data did not indicate any common plausible infection source, e.g., pets, unpasteurized milk, or untreated water, etc., for the patients.
FIG. 2.
Distributions of chicken and nonchicken subtypes within the patient groups, those who had consumed fresh chicken, those who had consumed other chicken, and those who had consumed no chicken.
The proportion of patients infected with nonchicken subtypes was higher for patients with any of the risk factors other than chicken consumption included in the questionnaire, but the difference was not statistically significant (Table 1). Daily contact with cattle or pigs was recorded for four patients, and they all carried a nonchicken subtype. Three of these patients also lived on a farm. Tentatively, using controls and subtracting the number of patients infected with a poultry subtype may enhance the possibility of identifying infection sources other than chicken. Patients infected with isolates of PFGE group A had significantly longer illnesses than patients infected with isolates of PFGE group G (t test; P < 0.5).
TABLE 1.
Distribution of nonchicken subtypes among patients with risk factors
| Risk factor | No. with nonchicken subtype/total no. with risk factor (%) | No. with nonchicken subtype/total no. without risk factor (%) |
|---|---|---|
| Living on a farm | 3/4 (75) | 36/74 (49) |
| Daily contact with dog | 12/21 (57) | 22/43 (51) |
| Daily contact with cat | 11/18 (61) | 25/51 (49) |
| Consumption of unpasteurized milk | 3/4 (75) | 36/75 (48) |
| Consumption of untreated water | 6/11 (55) | 33/68 (49) |
There is sharp seasonal variation in Campylobacter incidence in both chickens and humans in Sweden. During winter, Swedish broiler flocks are almost free of Campylobacter spp. and the number of reported domestic cases of campylobacteriosis is approximately 50 per month. During July and August, 30 to 40% of broiler flocks are contaminated with Campylobacter spp. and the number of reported domestic campylobacteriosis cases is around 500 per month. By itself, this pattern may indicate that humans acquire Campylobacter spp. from eating fresh chicken meat or that both humans and broilers become infected from a third, hitherto unidentified source. The clear finding that the consumption of fresh chicken is correlated with infection by a subtype present in chicken meat points to chicken as an important infection source. This correlation opens up the possibility of a connection between some nonchicken subtypes and other risk factors. Such a connection was indicated by the fact that patients reporting these potential risk factors (having daily contact with a dog or cat, drinking untreated water or unpasteurized milk, and living on a farm) had a higher frequency of infection with nonchicken subtypes than patients who did not report these risk factors.
To our knowledge, this is the first study in which the subtyping of isolates collected from patients and a food item in a coordinated program has been combined with the use of a questionnaire. Although performed on a pilot scale here, we believe that this type of study can be fruitfully applied for the investigation of risk factors other than the consumption of chicken.
Acknowledgments
The staff at the county medical offices in the counties of Gävleborg, Örebro, Halland, and Västra Götaland are gratefully acknowledged for administering the questionnaires. We also thank the food and health offices of the participating municipalities for the retail sampling.
Footnotes
Published ahead of print on 3 June 2009.
REFERENCES
- 1.Adak, G. K., J. M. Cowden, S. Nicholas, and H. S. Evans. 1995. The Public Health Laboratory Service national case-control study of primary indigenous sporadic cases of campylobacter infection. Epidemiol. Infect. 11515-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 2000. Campylobacter in meat and water. Report 10. National Food Administration, Uppsala, Sweden. (In Swedish.)
- 3.Arsenault, J., A. Letellier, S. Quessy, V. Normand, and M. Boulianne. 2007. Prevalence and risk factors for Salmonella spp. and Campylobacter spp. caecal colonization in broiler chicken and turkey flocks slaughtered in Quebec, Canada. Prev. Vet. Med. 81250-264. [DOI] [PubMed] [Google Scholar]
- 4.Broman, T., H. Palmgren, S. Bergstrom, M. Sellin, J. Waldenstrom, M. L. Danielsson-Tham, and B. Olsen. 2002. Campylobacter jejuni in black-headed gulls (Larus ridibundus): prevalence, genotypes, and influence on C. jejuni epidemiology. J. Clin. Microbiol. 404594-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer, P., J. A. Wagenaar, R. P. Achterberg, J. P. van Putten, L. M. Schouls, and B. Duim. 2002. Generation of Campylobacter jejuni genetic diversity in vivo. Mol. Microbiol. 44351-359. [DOI] [PubMed] [Google Scholar]
- 6.Dingle, K. E., F. M. Colles, R. Ure, J. A. Wagenaar, B. Duim, F. J. Bolton, A. J. Fox, D. R. Wareing, and M. C. Maiden. 2002. Molecular characterization of Campylobacter jejuni clones: a basis for epidemiologic investigation. Emerg. Infect. Dis. 8949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eberhart-Phillips, J., N. Walker, N. Garrett, D. Bell, D. Sinclair, W. Rainger, and M. Bates. 1997. Campylobacteriosis in New Zealand: results of a case-control study. J. Epidemiol. Community Health 51686-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fermer, C., and E. Olsson Engvall. 1999. Specific PCR identification and differentiation of the thermophilic campylobacters, Campylobacter jejuni, C. coli, C. lari, and C. upsaliensis. J. Clin. Microbiol. 373370-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallay, A., V. Bousquet, V. Siret, V. Prouzet-Mauleon, H. Valk, V. Vaillant, F. Simon, Y. Le Strat, F. Megraud, and J. C. Desenclos. 2008. Risk factors for acquiring sporadic Campylobacter infection in France: results from a national case-control study. J. Infect. Dis. 1971477-1484. [DOI] [PubMed] [Google Scholar]
- 10.Hanninen, M. L., M. Hakkinen, and H. Rautelin. 1999. Stability of related human and chicken Campylobacter jejuni genotypes after passage through chick intestine studied by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 652272-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanninen, M. L., P. Perko-Makela, A. Pitkala, and H. Rautelin. 2000. A three-year study of Campylobacter jejuni genotypes in humans with domestically acquired infections and in chicken samples from the Helsinki area. J. Clin. Microbiol. 381998-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapperud, G., G. Espeland, E. Wahl, A. Walde, H. Herikstad, S. Gustavsen, I. Tveit, O. Natas, L. Bevanger, and A. Digranes. 2003. Factors associated with increased and decreased risk of Campylobacter infection: a prospective case-control study in Norway. Am. J. Epidemiol. 158234-242. [DOI] [PubMed] [Google Scholar]
- 13.Kapperud, G., E. Skjerve, N. H. Bean, S. M. Ostroff, and J. Lassen. 1992. Risk factors for sporadic Campylobacter infections: results of a case-control study in southeastern Norway. J. Clin. Microbiol. 303117-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karenlampi, R., H. Rautelin, and M. L. Hanninen. 2007. Evaluation of genetic markers and molecular typing methods for prediction of sources of Campylobacter jejuni and C. coli infections. Appl. Environ. Microbiol. 731683-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindblad, M., I. Hansson, I. Vågsholm, and R. Lindqvist. 2006. Postchill campylobacter prevalence on broiler carcasses in relation to slaughter group colonization level and chilling system. J. Food Prot. 69495-499. [DOI] [PubMed] [Google Scholar]
- 16.Lindblad, M., H. Lindmark, S. T. Lambertz, and R. Lindqvist. 2006. Microbiological baseline study of broiler chickens at Swedish slaughterhouses. J. Food Prot. 692875-2882. [DOI] [PubMed] [Google Scholar]
- 17.Lindmark, H., I. C. Diedrich, L. Andersson, R. Lindqvist, and E. O. Engvall. 2006. Distribution of Campylobacter genotypes on broilers during slaughter. J. Food Prot. 692902-2907. [DOI] [PubMed] [Google Scholar]
- 18.Lindmark, H., B. Harbom, L. Thebo, L. Andersson, G. Hedin, B. Osterman, T. Lindberg, Y. Andersson, A. Westöö, and E. Olsson Engvall. 2004. Genetic characterization and antibiotic resistance of Campylobacter jejuni isolated from meats, water, and humans in Sweden. J. Clin. Microbiol. 42700-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linton, D., A. J. Lawson, R. J. Owen, and J. Stanley. 1997. PCR detection, identification to species level, and fingerprinting of Campylobacter jejuni and Campylobacter coli direct from diarrheic samples. J. Clin. Microbiol. 352568-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logue, C. M., J. S. Sherwood, L. M. Elijah, P. A. Olah, and M. R. Dockter. 2003. The incidence of Campylobacter spp. on processed turkey from processing plants in the midwestern United States. J. Appl. Microbiol. 95234-241. [DOI] [PubMed] [Google Scholar]
- 21.Manning, G., B. Duim, T. Wassenaar, J. A. Wagenaar, A. Ridley, and D. G. Newell. 2001. Evidence for a genetically stable strain of Campylobacter jejuni. Appl. Environ. Microbiol. 671185-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen, N. 1998. Campylobacter i fødevarer fra detailhandlen. Alimenta 2110-11. [Google Scholar]
- 23.Nogva, H. K., A. Bergh, A. Holck, and K. Rudi. 2000. Application of the 5′-nuclease PCR assay in evaluation and development of methods for quantitative detection of Campylobacter jejuni. Appl. Environ. Microbiol. 664029-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen, L., E. M. Nielsen, J. Engberg, S. L. On, and H. H. Dietz. 2001. Comparison of genotypes and serotypes of Campylobacter jejuni isolated from Danish wild mammals and birds and from broiler flocks and humans. Appl. Environ. Microbiol. 673115-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ragimbeau, C., F. Schneider, S. Losch, J. Even, and J. Mossong. 2008. Multilocus sequence typing, pulsed-field gel electrophoresis, and fla short variable region typing of clonal complexes of Campylobacter jejuni strains of human, bovine, and poultry origins in Luxembourg. Appl. Environ. Microbiol. 747715-7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodin, S., A. F. Andersson, V. Wirta, L. Eriksson, M. Ljungström, B. Björkholm, H. Lindmark, and L. Engstrand. 2008. Performance of a 70-mer oligonucleotide microarray for genotyping of Campylobacter jejuni. BMC Microbiol. 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ronner, A. C., E. O. Engvall, L. Andersson, and B. Kaijser. 2004. Species identification by genotyping and determination of antibiotic resistance in Campylobacter jejuni and Campylobacter coli from humans and chickens in Sweden. Int. J. Food Microbiol. 96173-179. [DOI] [PubMed] [Google Scholar]
- 28.Studahl, A., and Y. Andersson. 2000. Risk factors for indigenous Campylobacter infection: a Swedish case-control study. Epidemiol. Infect. 125269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wassenaar, T. M., B. Geilhausen, and D. G. Newell. 1998. Evidence of genomic instability in Campylobacter jejuni isolated from poultry. Appl. Environ. Microbiol. 641816-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whyte, P., K. McGill, D. Cowley, R. H. Madden, L. Moran, P. Scates, C. Carroll, A. O'Leary, S. Fanning, J. D. Collins, E. McNamara, J. E. Moore, and M. Cormican. 2004. Occurrence of Campylobacter in retail foods in Ireland. Int. J. Food Microbiol. 95111-118. [DOI] [PubMed] [Google Scholar]
- 31.Wingstrand, A., J. Neimann, J. Engberg, E. M. Nielsen, P. Gerner-Smidt, H. C. Wegener, and K. Molbak. 2006. Fresh chicken as main risk factor for campylobacteriosis, Denmark. Emerg. Infect. Dis. 12280-285. [DOI] [PMC free article] [PubMed] [Google Scholar]