Abstract
A collection of 1,308 clinical Mycobacterium tuberculosis isolates from Ontario, Canada, was genotyped by IS6110 restriction fragment length polymorphism (RFLP) and mycobacterial interspersed repetitive-unit-variable-number tandem-repeat (MIRU-VNTR) analysis. RFLP or >12 MIRU-VNTR loci were necessary for resolution of Indo-Oceanic strains. The low clustering rate and high strain diversity indicate that, in Ontario, most tuberculosis results from reactivation of latent infections.
Tuberculosis (TB), which is caused by pathogens of the Mycobacterium tuberculosis complex (MTBC), remains a global scourge (19). In Canada, the average incidence rate is 5.0 cases/100,000 people, but the burden of disease varies across the country. In 2007, the four Atlantic provinces accounted for only ∼1% of TB cases, whereas ∼42% of new cases occurred in the province of Ontario (11). The Public Health Laboratories (PHL) of the Ontario Agency for Health Protection and Promotion provide diagnostic testing for TB in Ontario. The TB and Mycobacteriology Laboratory at PHL-Toronto is the largest facility of its kind in Canada, processing 50,000 patient samples plus 2,000 referred acid-fast positive cultures, with 600 to 650 new cases of TB detected annually (8, 11). Historically, the PHL has employed IS6110 restriction fragment length polymorphism (RFLP) for genotyping. Despite its utility, IS6110 RFLP is labor-intensive and only performed upon request. The current turnaround time of 21 days also makes the method incompatible with the PHL goal of universal, real-time MTBC genotyping. More recently, mycobacterial interspersed repetitive-unit-variable-number tandem-repeat (MIRU-VNTR) typing has been introduced (6, 7, 9, 15). The current PHL strategy relies upon agarose gel electrophoresis for comparison of PCR products. This method is low throughput, and gel-to-gel variability confounds comparison of samples processed at different times. Here, we describe implementation and validation of an improved MIRU-VNTR strategy and its utility for analysis of a large clinical strain collection.
The semiautomated MIRU-VNTR strategy was derived from the 12-loci method of Cowan et al. (6, 7). Briefly, multiplex PCR was performed with dye-labeled primers in 96-well plates. For each reaction, 5 ng of template DNA was combined with 11 μl of a master mix (Red Taq; Sigma-Aldrich, Oakville, Canada) containing three primer pairs. PCR conditions were 95°C for 10 min and 34 cycles of 94°C for 30 s, 62°C for 30 s, and 72°C for 60 s, with a final extension at 72°C for 7 min. PCR amplification was confirmed by electrophoresis on 1% Tris-borate-EDTA agarose gels. Samples were diluted 1:20 in sequence loading solution (Beckman Coulter, Mississauga, Canada) containing a 600-bp sequencing standard (Beckman Coulter) and then subjected to fragment analysis on a CEQ8800 genetic analysis system (Beckman Coulter). To reduce the manual labor and potential for human error associated with extensive pipetting, a Biomek NX Span-8 automation workstation (Beckman Coulter) was programmed to set up the initial PCRs and dilute PCR products for fragment analysis.
To validate the method, a blinded set of 99 DNA samples was provided by the Public Health Research Institute (NJ). Strain identities and MIRU-VNTR patterns were unblinded only after complete 12-digit patterns were generated for all 99 DNA samples. Concordance between PHL and Public Health Research Institute results was 100%.
To evaluate the utility of MIRU-VNTR in Ontario, typing was performed on 1,308 clinical samples from the PHL strain collection for which IS6110 RFLP profiles were also available. Strains were identified as MTBC by using DNA probes (AccuProbe; Gen-Probe, San Diego, CA) and were originally isolated during 1999 to 2001. Strains identified as Mycobacterium bovis or M. bovis BCG and samples containing multiple Mycobacterium species were excluded from analysis. For cases with multiple cultures, only the first isolate was used. Genomic DNA was extracted according to standard protocols (17) in a dedicated biosafety containment facility.
MIRU-VNTR and IS6110 RFLP data were analyzed with BioNumerics 5.0 (Applied Maths, St-Martin Latem, Belgium). RFLP patterns were compared using band-based Dice statistics with 1% position tolerance such that clustered strains exhibited bands of identical number and position. Strains clustered by MIRU-VNTR were identical at all 12 loci. Analysis by MIRU-VNTR plus IS6110 RFLP employed the unweighted-pair group method with arithmetic mean. MTBC lineages were assigned by comparison to the MIRU-VNTR reference strain database (1).
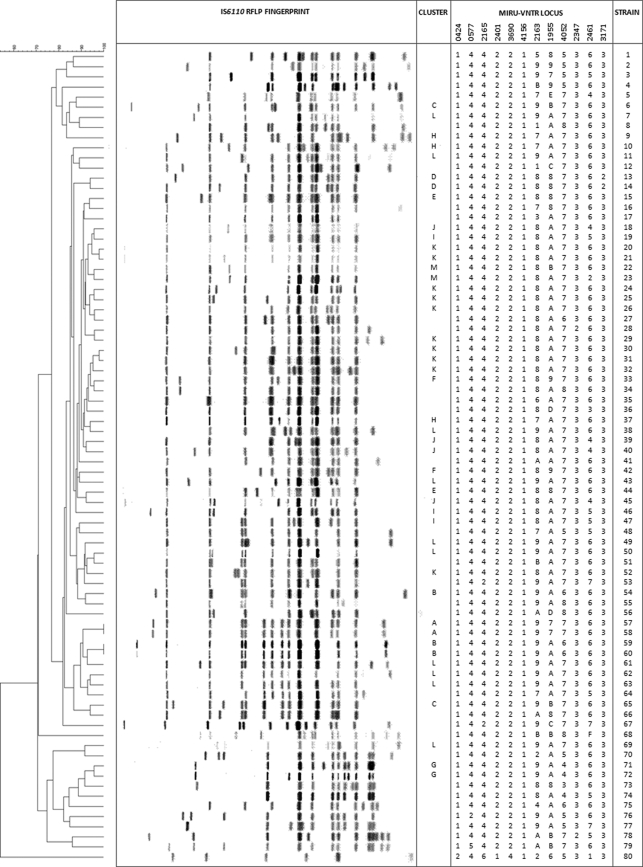
Independently, both methods revealed a large number of unique profiles and some clustered isolates (Table 1). Maximum strain resolution was achieved when both methods were combined. In general, RFLP was superior for multiband IS6110 strains, whereas resolution of single-band IS6110 isolates required MIRU-VNTR. Initial typing at 12 MIRU-VNTR loci generated two large “pseudoclusters.” One contained East Asian lineage strains (pattern 223325173533; n = 110), which are known to be poorly resolved by these 12 loci (12, 16). The second was comprised of strains from the Indo-Oceanic lineage (pattern 254326223432; n = 80). This group was typed at 12 additional loci (15). Even though four of the new loci were invariant, extended typing generated 47 new patterns (Fig. 1). Thirty-five Indo-Oceanic strains had unique profiles, whereas the remaining isolates formed 12 MIRU-VNTR-defined clusters (6 clusters with 2 isolates in each cluster, 3 with 3 each, 1 with 4, and 2 with 10 each). However, 11 of these could be resolved further by RFLP.
TABLE 1.
Clustering results from MIRU-VNTR and IS6110 RFLP genotyping
| Method | Total no. of patterns | No. of unique patterns | No. of clusters | No. of isolates in largest cluster | Clustering ratea |
|---|---|---|---|---|---|
| MIRU-VNTR only | 653 | 493 | 160 | 110 | 0.623 |
| IS6110 RFLP only | 1,067 | 985 | 82 | 65 | 0.247 |
| MIRU-VNTR + RFLP | 1,185 | 1,108 | 77 | 9 | 0.153 |
The clustering rate is based on the number of isolates in all clusters divided by the total number of isolates.
FIG. 1.
Improved typing of the Indo-Oceanic pseudocluster. Whereas all strains (n = 80) shared the same, original 12-loci pattern (254326223432), typing at 12 additional loci produced 47 distinct patterns, including 35 unique profiles. The remaining strains formed 12 clusters (6× n = 2; 3× n = 3; 1× n = 4; 2× n = 10), most of which could be further resolved by RFLP. However, one pair (cluster A) was identical by both methods, and two (clusters D and G) exhibited single band shifts. Conversely, one RFLP-defined pair (cluster M) exhibited differences at two loci upon extended MIRU-VNTR typing. For each strain, IS6110 RFLP profiles and repeat values at the extended MIRU-VNTR loci are shown. Relationships between strains are indicated by the phylogenetic (unweighted-pair group method with arithmetic mean) tree, and strains in MIRU-VNTR-defined clusters are labeled (clusters A to L).
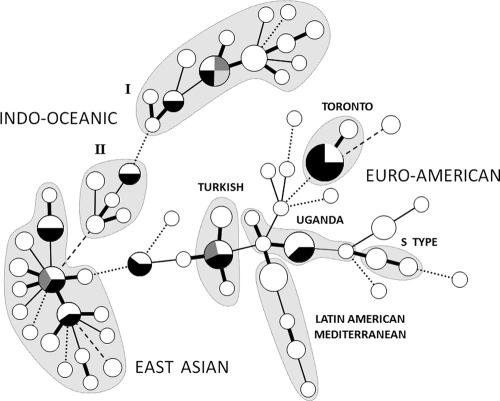
Previous genotyping studies have suggested that there is extensive local transmission of TB in Canada. A single MTBC strain is responsible for ∼25% of all cases in the province of Manitoba (2, 3). In Quebec, a pyrazinamide-resistant strain and its drug-sensitive ancestor are endemic (10). In contrast, 84.7% (1,108/1,308) of strains in the present study displayed unique genotypes. The remaining 200 strains formed 77 clusters (Fig. 2). The largest cluster, 12 strains associated with an outbreak in a homeless shelter in the urban metropolis of Toronto, accounts for <1% of all provincial cases (20). Although identical by MIRU-VNTR, RFLP distinguished two groups within this “Toronto” cluster: nine strains of one pattern and three with an extra IS6110 band.
FIG. 2.
Minimum spanning tree of 77 strain clusters. Each circle represents a cluster of strains (2 to 12 isolates) identical by 12-loci MIRU-VNTR typing. Circle sizes are proportional to the number of isolates. Divisions within circles represent sets of clusters that have identical MIRU-VNTR patterns but different IS6110 RFLP profiles. Connected clusters differ at one (thick line), two (thin line), three (dotted line), or four (dashed line) MIRU-VNTR loci. Lineage names were assigned by comparison of MIRU-VNTR patterns to the MIRU-VNTRplus reference strain database (1). The Toronto cluster is endemic to our population.
The genotypic diversity of MTBC strains found in this study is likely due to the ethnic diversity of the provincial population. Many Ontarians (3.4 million/12.1 million people) are foreign-born (14). Since 1996, ∼687,000 immigrants have arrived from the 22 nations identified as high-burden countries by the World Health Organization (13, 19). Reactivation disease is common among recent immigrants to both the United States and Canada (4, 18). The proportion of total TB cases (∼85%) attributed to foreign-born Ontarians is similar to trends in Minnesota (85.3%) and New York (71.1%) but much higher than levels in other Great Lakes states (e.g., Illinois, 58.9%; Wisconsin, 54.3%; Pennsylvania, 51.4; Ohio, 45.6%; Indiana, 43.0; Michigan, 37.6%) (5, 11).
This study, the first to evaluate the utility of MIRU-VNTR in Ontario, Canada, indicates that the method is an effective first-line genotyping tool. However, the 12-loci strategy can generate pseudoclusters. Resolution of some strains, especially those from the East Asian and Indo-Oceanic lineages, require second-line testing with IS6110 RFLP, additional loci, or spoligotyping. Genotyping revealed MTBC isolates from diverse global lineages, which is consistent with the multicultural origins of Ontario's population. Despite the predominance of reactivation disease, 77 clusters, comprising 200 isolates, were identified. Rapid detection of such clusters, especially those involving unrelated individuals, is essential for effective TB control. Due to its speed and high throughput, MIRU-VNTR will be an important component of the universal, real-time genotyping strategy in Ontario, Canada.
Acknowledgments
We acknowledge the efforts of the staff from the TB and Mycobacteriology Lab (PHL-Toronto) responsible for the initial isolation and cultivation of the clinical isolates used in the study.
This work was funded in part by an Innovations Fund grant awarded to F.J. by the Ontario Ministry of Government Services.
Footnotes
Published ahead of print on 3 June 2009.
REFERENCES
- 1.Allix-Béguec, C., D. Harmsen, T. Weniger, P. Supply, and S. Niemann. 2008. Evaluation and strategy for use of MIRU-VNTRplus, a multifunctional database for online analysis of genotyping data and phylogenetic identification of Mycobacterium tuberculosis complex isolates. J. Clin. Microbiol. 462692-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackwood, K. S., A. Al Azem, L. J. Elliott, E. S. Hershfield, and A. M. Kabani. 2003. Conventional and molecular epidemiology of tuberculosis in Manitoba. BMC Infect. Dis. 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackwood, K. S., J. N. Wolfe, and A. M. Kabani. 2004. Application of mycobacterial interspersed repetitive unit typing to Manitoba tuberculosis cases: can restriction fragment length polymorphism be forgotten? J. Clin. Microbiol. 425001-5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cain, K. P., S. R. Benoit, C. A. Winston, and W. R. Mac Kenzie. 2008. Tuberculosis among foreign-born persons in the United States. JAMA 300405-412. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2008. Reported tuberculosis in the United States, 2007. Centers for Disease Control and Prevention, Department of Tuberculosis Elimination, Atlanta, GA.
- 6.Cowan, L. S., L. Diem, T. Monson, P. Wand, D. Temporado, T. V. Oemig, and J. T. Crawford. 2005. Evaluation of a two-step approach for large-scale, prospective genotyping of Mycobacterium tuberculosis isolates in the United States. J. Clin. Microbiol. 43688-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowan, L. S., L. Mosher, L. Diem, J. P. Massey, and J. T. Crawford. 2002. Variable-number tandem repeat typing of Mycobacterium tuberculosis isolates with low copy numbers of IS6110 by using mycobacterial interspersed repetitive units. J. Clin. Microbiol. 401592-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marras, T. K., P. Chedore, A. M. Ying, and F. Jamieson. 2007. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario, 1997-2003. Thorax 62661-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazars, E., S. Lesjean, A. L. Banuls, M. Gilbert, V. Vincent, B. Gicquel, M. Tibayrenc, C. Locht, and P. Supply. 2001. High-resolution minisatellite-based typing as a portable approach to global analysis of Mycobacterium tuberculosis molecular epidemiology. Proc. Natl. Acad. Sci. USA 981901-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen, D., P. Brassard, D. Menzies, L. Thibert, R. Warren, S. Mostowy, and M. Behr. 2004. Genomic characterization of an endemic Mycobacterium tuberculosis strain: evolutionary and epidemiologic implications. J. Clin. Microbiol. 422573-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Public Health Agency of Canada. 2008. Tuberculosis in Canada 2007 (pre-release). Catalogue no. HP37-5/2007-1E-PDF. Public Health Agency of Canada, Ottawa, Ontario, Canada.
- 12.Sola, C., I. Filliol, E. Legrand, S. Lesjean, C. Locht, P. Supply, and N. Rastogi. 2003. Genotyping of the Mycobacterium tuberculosis complex using MIRUs: association with VNTR and spoligotyping for molecular epidemiology and evolutionary genetics. Infect. Genet. Evol. 3125-133. [DOI] [PubMed] [Google Scholar]
- 13.Statistics Canada. 2007. Place of birth for the immigrant population by period of immigration, 2006 counts and percentage distribution, for Canada, provinces and territories, 20% sample data. Statistics Canada, Ottawa, Ontario, Canada.
- 14.Statistics Canada. 2007. Population by immigrant status and period of immigration, 2006 counts, for Canada, provinces and territories, and census metropolitan areas and census agglomerations, 20% sample data. Statistics Canada, Ottawa, Ontario, Canada.
- 15.Supply, P., C. Allix, S. Lesjean, M. Cardoso-Oelemann, S. Rusch-Gerdes, E. Willery, E. Savine, P. de Haas, H. van Deutekom, S. Roring, P. Bifani, N. Kurepina, B. Kreiswirth, C. Sola, N. Rastogi, V. Vatin, M. C. Gutierrez, M. Fauville, S. Niemann, R. Skuce, K. Kremer, C. Locht, and D. van Soolingen. 2006. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J. Clin. Microbiol. 444498-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surikova, O. V., D. S. Voitech, G. Kuzmicheva, S. I. Tatkov, I. V. Mokrousov, O. V. Narvskaya, M. A. Rot, D. van Soolingen, and M. L. Filipenko. 2005. Efficient differentiation of Mycobacterium tuberculosis strains of the W-Beijing family from Russia using highly polymorphic VNTR loci. Eur. J. Epidemiol. 20963-974. [DOI] [PubMed] [Google Scholar]
- 17.van Soolingen, D., P. E. de Haas, P. W. Hermans, and J. D. van Embden. 1994. DNA fingerprinting of Mycobacterium tuberculosis. Methods Enzymol. 235196-205. [DOI] [PubMed] [Google Scholar]
- 18.Wobeser, W. L., L. Yuan, M. Naus, P. Corey, J. Edelson, N. Heywood, and D. L. Holness. 2000. Expanding the epidemiologic profile: risk factors for active tuberculosis in people immigrating to Ontario. CMAJ 163823-828. [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. 2008. Global tuberculosis control 2008: surveillance, planning, financing. World Health Organization, Geneva, Switzerland.
- 20.Yuan, L., A. E. Simor, L. P. S. Louie, R. Gould, and F. Jamieson. 1997. Tuberculosis clusters among the homeless in Toronto, Canada, abstr. K-173. Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., Toronto, Canada. American Society for Microbiology, Washington, DC.