Abstract
This study assesses human papillomavirus (HPV) detection and genotyping in self-sampled genital smears applied to an indicating FTA elute cartridge (FTA cartridge). The study group consisted of 96 women, divided into two sample sets. All samples were analyzed by the HPV SPF10-Line Blot 25. Set 1 consisted of 45 women attending the gynecologist; all obtained a self-sampled cervicovaginal smear, which was applied to an FTA cartridge. HPV results were compared to a cervical smear (liquid based) taken by a trained physician. Set 2 consisted of 51 women who obtained a self-sampled cervicovaginal smear at home, which was applied to an FTA cartridge and to a liquid-based medium. DNA was obtained from the FTA cartridges by simple elution as well as extraction. Of all self-obtained samples of set 1, 62.2% tested HPV positive. The overall agreement between self- and physician-obtained samples was 93.3%, in favor of the self-obtained samples. In sample set 2, 25.5% tested HPV positive. The overall agreement for high-risk HPV presence between the FTA cartridge and liquid-based medium and between DNA elution and extraction was 100%. This study shows that HPV detection and genotyping in self-obtained cervicovaginal samples applied to an FTA cartridge is highly reliable. It shows a high level of overall agreement with HPV detection and genotyping in physician-obtained cervical smears and liquid-based self-samples. DNA can be obtained by simple elution and is therefore easy, cheap, and fast. Furthermore, the FTA cartridge is a convenient medium for collection and safe transport at ambient temperatures. Therefore, this method may contribute to a new way of cervical cancer screening.
Infection with human papillomavirus (HPV) is a necessary event in the multistep process of cervical carcinogenesis (17, 27). As a result, the clinical value of HPV testing has been well established (1, 3, 5, 6, 10, 11). In the United States, the Food and Drug Administration has authorized a high-risk HPV (HR-HPV) assessment for primary screening in women aged 30 and older. This is in addition to regular cytological screening as well as for the triage of smears of atypical cells of undetermined significance. In The Netherlands, additional HR-HPV testing has been approved and recommended in all follow-up smears after the detection of a first-time borderline or mild dysplasia smear. The beneficial effect of HPV testing will most likely increase in case HR-HPV assessment replaces cytology as primary screening tool (1, 3, 6, 11).
Regarding (HR-)HPV testing, material from vaginal lavages or self-sampling brushes has proven to be highly representative for the cervical (HR-)HPV status (8, 12, 13, 18, 22, 29). In addition, cervicovaginal self-obtained samples have repetitively been proven to be as reliable as physician-taken samples (19, 20). Subsequently, several studies have shown that self-sampling for HPV testing was highly acceptable to women, although some women were concerned about performing the test properly (13, 28). HR-HPV testing on self-sampled materials might be a promising opportunity to increase the efficacy of population-based screening programs worldwide (8, 12, 18, 24). Cervicovaginal self-sampling may be an easy, accessible, user-friendly, and time-saving alternative for the physician-based collection of cervicovaginal material (13, 28).
In the Dutch cervical screening program, approximately 70% of the women who are invited actually take part. Tragically, half of the cervical carcinomas are diagnosed in the remaining group of nonresponders (7, 18, 21). Cervical cancer incidence would decrease significantly if these nonresponders could be reached (5, 6). Several studies have shown that nonresponders actually do take part in self-sampling studies (4, 8, 18). Self-sampling is a less-costly and less-invasive collection method (20). Self-sampled material could be more easily obtained in populations that are difficult to reach and in settings with limited resources, facilitating the introduction of organized HPV-based cervical screening programs in developing countries as well.
However, the vast majority of studies assessing self-sampling have used liquid-based storage and transport media (4, 8, 18, 22). Since these solutions can be inflammable, hazardous, and potentially infectious, careful handling is required and regular mailing may even not be allowed. This severely hampers the introduction of cervicovaginal self-sampling methods. Dried fluid spots or solid carriers have already been used for decades in the postnatal screening of certain congenital disorders and diseases. Solid carriers have also been successfully used in studies detecting and genetically characterizing measles virus strains, as well as in studies assessing viral load and genotypic resistance for human immunodeficiency virus (HIV) (2, 14, 23). As dried material on a solid carrier is neither hazardous nor inflammable, applying genital self-samples on these solid carriers (like FTA cartridges) can solve storage and transportation problems.
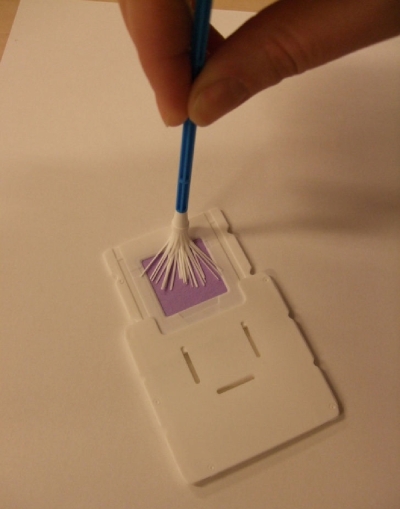
In this study, we have assessed the use of self-sampled cervicovaginal smears applied to a new FTA cartridge, i.e., the Whatman indicating FTA elute cartridge (Fig. 1 and 2) which allows easy storage and transport, as the virus is denaturized upon application. Additionally, the cartridge overcomes the uncertainty of women about performing the procedure properly, as it has an indicating dye which changes from purple to white when a (genital) sample is applied. Furthermore, we assessed the novel method of direct HPV DNA elution without requiring further purification.
FIG. 1.
Whatman indicating FTA elute cartridge.
FIG. 2.
Whatman indicating FTA elute cartridge upon application.
MATERIALS AND METHODS
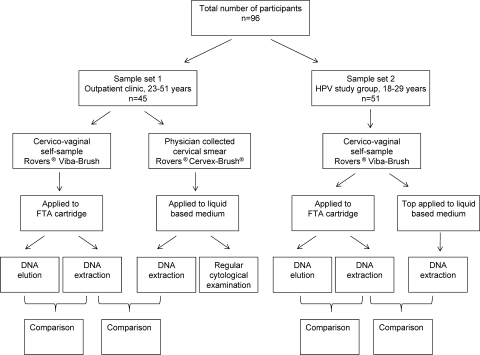
The study group consisted of 96 women, divided into two sample sets (Fig. 3).
FIG. 3.
Study design.
Sample set 1.
Between September and October 2008, 45 women were recruited at the Department of Obstetrics and Gynecology of the Radboud University Nijmegen Medical Centre, The Netherlands. These participants visited the gynecologist for follow-up after diethylstilbestrol exposition in utero, treatment of cervical dysplasia, or follow-up after two borderline or mild dysplasia smears. The median age was 38 years (standard deviation, 6.85 years; range, 23 to 51 years).
All women were asked to self-collect a cervicovaginal sample after having received instructions on how to perform the self-sample (verbally, written, and in cartoon). In brief, participants were instructed to wash their hands before opening the brush cover (Rovers Viba-Brush; Rovers Medical Devices B.V., Oss, The Netherlands), to hold the brush by the end of the handle, to insert the brush approximately 7 cm into the vagina (similar to inserting a tampon), and to gently turn the brush five times. Subsequently, the brush was applied to the FTA cartridge (Whatman indicating FTA elute cartridge; catalog number WB 659223; GE Healthcare, United Kingdom) (Fig. 1 and 2). The FTA cartridge was air dried. After self-sampling, a vaginal speculum was inserted and a physician obtained a regular cervical smear using a Rovers Cervex-Brush (Rovers Medical Devices B.V., Oss, The Netherlands) that was rinsed in a ThinPrep vial (Cytyc Corp., Boxborough, MA). Regular liquid-based cytological (LBC) examination was performed, and 0.5 ml of LBC homogenized medium was used for HPV assessment.
In order to assess the samples anonymously, all self-obtained samples and cervical LBC samples were provided with an unique patient code before they were sent to the laboratory.
Sample set 2.
Sample set 2 consisted of 51 healthy participants who were randomly recruited from a prospective self-sampling study of HPV prevalence, incidence, and clearance among 2,065 unscreened women between 18 and 29 years of age (15). All women were asked to self-collect a cervicovaginal sample in the privacy of their own home. Women received an explanatory letter, an informed consent form, and a self-sample kit by mail. The self-sample kit was provided with an anonymous code to ensure privacy. The self-sample kit contained a collection device (a small brush packaged in an individual sterile cover; Rovers Viba-Brush; Rovers Medical Devices B.V., Oss, The Netherlands), an FTA cartridge (Whatman indicating FTA elute cartridge; catalog number WB 659223; GE Healthcare, United Kingdom) (Fig. 1 and 2), a collection tube containing medium (SurePath; Tripath Imaging, Inc., Burlington, NC), instructions how to perform the cervicovaginal self-sample (written and in cartoon), and a return package consisting of a leakproof seal bag, absorption sheet, and a reclosable plastic return envelope (Easyslider; Transposafe Systems Holland B.V., Sassenheim, The Netherlands). Except for the fact that they used an additional liquid-based medium, the instructions of how to perform the self-sample were similar to the instructions described above. In brief, participants were instructed to first apply the self-sample on the FTA cartridge and to subsequently place the top of the brush in the collection tube. The collection tube was closed and enclosed in the seal bag. Finally, the collection tube was placed in the return envelope together with the dried FTA cartridge and sent to the Department of Obstetrics and Gynecology for further processing and HPV assessment at the Department of Medical Microbiology. The samples were stored at room temperature. In the original study of the 2,065 women, a control for sample sufficiency, i.e., detection of human beta-globin, was performed and showed less than 1% false negative samples (15).
The self-sampling material on the FTA cartridge was compared to the self-sampling material stored in the liquid-based medium (Fig. 3).
Specimen preparation LBC.
For isolation of DNA from cervical scrapes in LBC medium, the MagNAPure LC isolation station (Roche Diagnostics GmbH, Roche Applied Science, Mannheim, Germany) was used; 500 μl of material was isolated using the MagNAPure LC total nucleic acid isolation kit (Roche Diagnostics GmbH, Roche Molecular Biochemicals, Mannheim, Germany), as described by the manufacturer. With each set of 28 cervical scrape samples, four negative controls were included. Nucleic acid was resuspended in a final volume of 50 μl; 10 μl was used for PCR analysis (25).
Specimen preparation of the indicating FTA elute cartridge.
The indicating FTA elute matrix contains an indicating dye that changes from purple to white upon application of a colorless sample such as a cervicovaginal swab. The FTA cartridges were punched using a sterilized perforator specifically designed for the FTA cartridges (3-mm Harris Uni-Core device; Whatman). The sample amount varied between samples, and to optimize the number of punches to cover this variation, pilots were performed using a different number of punches. For this study, only three punches were considered to compare DNA elution and extraction, as well as individual genotypes.
The FTA elute matrix is chemically treated with proprietary reagents that lyses cells upon contact, causing the release of nucleic acids. DNA was recovered from the FTA elute matrix through a simplified elution process using heat and water. Inhibitory components, such as hemoglobin, are retained on the FTA elute matrix.
Elution.
The three punches were transferred into a 1.5-ml Microfuge tube and 1,500 μl of sterile water was added to the punches and immediately pulse vortexed three times for a total of 5 s. The water was removed with a sterile fine-tip pipette. Fifty microliters of sterile water was added to the punches, and the tube was transferred to a heating block at 95°C for 30 min. At the end of the incubation period, the sample was removed from the heating block and pulse vortexed approximately 60 times. It was additionally centrifuged for 30 s, and the eluted DNA was placed into a new microcentrifuge tube with a pipette. The eluted DNA was stored at −80°C.
Finally, 10 μl of the eluate was used for PCR analysis.
Isolation.
For additional comparison, DNA was extracted from three other punches using the Qiagen DNeasy tissue kit (Qiagen Inc., Valencia, CA), as described by the manufacturer.
Subsequently, HPV DNA assessment was performed identically as for the LBC specimens, as described below. All HPV tests were performed by laboratory assistants unaware of the cytological status and the results from the comparative HPV detection tests.
HPV detection and genotyping.
Broad-spectrum HPV DNA amplification was performed using a short-PCR-fragment assay (HPV SPF10-Line Blot 25; Labo Bio-medical Products B.V., Rijswijk, The Netherlands). This assay amplifies a 65-bp fragment of the L1 open reading frame and allows detection of a broad range of HR, low-risk (LR), and possible HR-HPV genotypes (16).
Twenty-eight oligonucleotide probes which recognize 25 different types were tailed with poly(dT) and immobilized as parallel lines to membrane strips (Labo Bio-medical Products B.V., Rijswijk, The Netherlands). The HPV genotypes detectable are HR-HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 59, and 68/73 and two probable HR-HPV types (53 and 66). Samples that tested positive using the DNA enzyme immunoassay but showed no results on the LiPA strip were considered to be HPV “X” type, i.e., genotypes not available on the LiPA strip. LR-HPV types were defined as HPV type 6, 11, 34, 40, 42, 43, 44, 54, 55, 58, 70, 74, and X. The HPV genotyping assay was performed as described previously (26). The LiPA strips were visually inspected and interpreted using the provided reference guide.
Study design.
All samples were assessed for HPV genotyping using the HPV SPF10-Line Blot 25 assay.
For the first sample set, the self-sampled material on the FTA cartridge was compared to a liquid-based cervical smear obtained for diagnostic purposes by a trained physician in the outpatient clinic. Additionally, as HPV DNA elution is a novel method to obtain HPV DNA from an FTA elute cartridge, results of DNA elution were compared to results from HPV DNA extraction (Fig. 3).
In the second sample set, the self-sampled material stored in liquid-based solution was compared to self-sampled material on the FTA cartridge. Again, results of DNA elution and extraction from the FTA cartridge were compared (Fig. 3). In the original study population of sample set 2 (n = 2,065), detection of beta-globin was used as a control for sample sufficiency and showed less than 1% false negatives.
Comparing the presence of HR-HPV between the two samples, results were termed concordant or discordant based on the following definitions. If analyses showed identical genotypes in both samples, the results were termed concordant. Genotyping results were termed discordant when no similarities in the genotypes existed.
This study was approved by the local medical ethics committee. All participants provided an informed consent.
Statistics.
All data were analyzed using SPSS version 16.0 for Windows (Chicago, IL). Agreement was measured by absolute agreement and Cohen's kappa statistics, a measure of the agreement between two methods that is in excess of that due to chance.
RESULTS
The study group consisted of 96 women between 18 and 51 years of age. The results of the two sample sets are described separately, since sample set 2 consisted of healthy unscreened women and sample set 1 consisted of women with a higher risk of an HPV infection than in the general population as they had initially been referred to the gynecologist for cervical follow-up for several reasons.
Sample set 1.
The median age of the 45 women in sample set 1 was 38 years (standard deviation, 6.85 years; range, 23 to 51 years).
Cervicovaginal self-obtained sample versus physician-obtained cervical smear.
Of the 45 self-collected cervicovaginal samples on the FTA cartridges, 62.2% (n = 28) tested positive for one or more HPV genotypes. This high prevalence was expected due to the nature of the follow-up. Of these 28 samples, 25 also tested positive for HPV in the cervical smear sample obtained by the physician.
Of the 28 HPV-positive samples, 19 samples showed similar types, 5 samples showed an additional genotype (sample no. 2, 5, 9, 16, and 25) (Table 1), and 4 samples showed a different genotype (sample no. 8, 10, 18, and 20) (Table 1). The overall agreement for HPV positivity between self-sampling and the cervical smear taken by the physician was 93.3% (kappa value, 0.86; 95% confidence interval [CI], 0.713 to 1.013).
TABLE 1.
HPV detection by SPF10-Line Blot 25 for corresponding genital self-obtained smears and physician-obtained cervical smears (sample set 1)a
| Sample no. | HPV detection using physician-obtained samples (LBC)
|
HPV detection using self-obtained sample (FTA cartridge)
|
HPV accordance
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| DNA extraction | HR | LR | DNA extraction | DNA elution | HR | LR | HR | LR | |
| 1 | 52 | + | − | 52 | 52 | + | − | c | c |
| 2 | 51 | + | − | 11, 31, 51 | 11, 31, 51 | + | + | c | d |
| 3 | 18, 31 | + | − | 18, 31 | 18, 31 | + | − | c | c |
| 4 | 16 | + | − | 16 | 16 | + | − | c | c |
| 5 | 53 | + | − | 6, 53 | 6, 53 | + | + | c | d |
| 6 | 16 | + | − | 16 | 16 | + | − | c | c |
| 7 | 6 | − | + | 6 | 6 | − | + | c | c |
| 8 | N | − | − | 52 | 52 | + | − | d | c |
| 9 | 16, 66 | + | − | 66 | 66 | + | − | c | c |
| 10 | N | − | − | 11 | 11 | − | + | c | d |
| 11 | 16 | + | − | 16 | 16 | + | − | c | c |
| 12 | 66 | + | − | 66 | 66 | + | − | c | c |
| 13 | X | − | + | X | X | − | + | c | c |
| 14 | 59 | + | − | 59 | 59 | + | − | c | c |
| 15 | 39 | + | − | 39 | 39 | + | − | c | c |
| 16 | 42 | − | + | 18, 42 | 18, 42 | + | + | d | c |
| 17 | 16 | + | − | 16 | 16 | + | − | c | c |
| 18 | N | − | − | 16 | 16 | + | − | d | c |
| 19 | 51 | + | − | 51 | 51 | + | − | c | c |
| 20 | 68, 70 | + | + | 52, 70 | 52, 70 | + | + | c | c |
| 21 | 51 | + | − | 51 | 51 | + | − | c | c |
| 22 | X | − | + | X | X | − | + | c | c |
| 23 | 6, 51, 58 | + | + | 6, 51, 58 | 6, 51, 58 | + | + | c | c |
| 24 | 58 | − | + | 58 | 58 | − | + | c | c |
| 25 | 31 | + | − | 31, 51 | 31, 51 | + | − | c | c |
| 26 | 70 | − | + | 70 | 70 | − | + | c | c |
| 27 | 6 | − | + | 6 | 6 | − | + | c | c |
| 28 | X | − | + | X | X | − | + | c | c |
| 29-45 | N | N | N | N | N | N | N | c | c |
HR-HPV types (HR) were 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 59, and 68/73; probable HR-HPV types were 53 and 66. LR-HPV types (LR) were 6, 11, 34, 40, 42, 43, 44, 54, 55, 58, 70, 74, and X. c, concordant results; d, discordant results; N, HPV negative; −, negative; +, positive.
Concordance and discordance of HR-HPV.
Table 1 shows a summary of the genotypes per sample set as well as the concordance and discordance for HR-HPV. Taking the samples of all 45 women into account, 42 samples (93.3%) were concordant and 3 samples (6.7%) were discordant for HR-HPV presence. In these three samples, the physician-obtained smear did not contain a HR-HPV type in contrast to the self-obtained sample (sample no. 8, 16, and 18) (Table 1).
Of the 42 concordant samples, 25 showed no HR-HPV DNA in both self- and physician-obtained samples. In 20 of the 45 (44.4%) self-obtained samples, one or more HR-HPV types were detected; 17 patients also tested HR-HPV positive in the cervical smears obtained by the physician. The overall agreement for HR-HPV positivity was 93.3% (kappa value, 0.86; 95% CI, 0.713 to 1.013).
Concordance and discordance of LR-HPV.
In 13 of the 45 (28.9%) self-obtained samples, one or more LR-HPV types were detected, 10 patients also tested LR-HPV positive in the cervical smears obtained by the physician. The overall agreement for LR-HPV positivity was 93.3% (kappa value, 0.83; 95% CI, 0.635 to 1.016).
Table 1 shows a summary of the genotypes per sample set as well as the concordance and discordance for LR-HPV. Taking the samples of all 45 women into account, 42 samples (93.3%) were concordant and 3 samples (6.7%) were discordant for LR-HPV presence. In these three samples, the physician-obtained smear did not contain a LR-HPV type in contrast to the self-obtained sample (sample no. 2, 5, and 10) (Table 1). Of the 42 concordant samples, 32 showed no LR-HPV DNA in both self- and physician-obtained samples.
DNA elution versus DNA extraction.
As DNA elution is a novel method of obtaining DNA from an FTA cartridge, HPV DNA from the self-sampled material on the FTA cartridge yielded by DNA elution was compared to HPV DNA yielded from the cartridge by DNA extraction. The results showed a perfect overall agreement of 100% (kappa value, 1; 95% CI, 1.0 to 1.0), indicating the reliability of this procedure (Table 1).
Sample set 2.
The median age of the women in sample set 2 was 21 years (range, 18 to 29 years).
Of the 51 self-collected cervicovaginal samples applied to a liquid-based medium, 13 (25.5%) tested positive for one or more HPV genotypes. All of these HPV-positive samples also tested positive for HPV on the FTA cartridge (Table 2). Moreover, the overall agreement for HPV positivity between the FTA cartridge and liquid-based medium was 100% (kappa value, 1.0; 95% CI, 1.0 to 1.0).
TABLE 2.
HPV detection by SPF10-Line Blot 25 for corresponding genital self-obtained smears with liquid-based medium versus FTA cartridge (sample set 2)a
| Sample no. | HPV detection using self-samples (LBC)
|
HPV detection using self-samples (FTA cartridge)
|
HPV accordance
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| DNA extraction | HR | LR | DNA extraction | DNA elution | HR | LR | HR | LR | |
| 1 | 18, 51, 54, 68 | + | + | 18, 51, 54, 68 | 18, 51, 54, 68 | + | + | c | c |
| 2 | 56 | + | − | 56 | 56 | + | − | c | c |
| 3 | X | − | + | X | X | − | + | c | c |
| 4 | X | − | + | X | X | − | + | c | c |
| 5 | 42, 51, 54 | + | + | 42, 51, 54 | 42, 51, 54 | + | + | c | c |
| 6 | 52 | + | − | 52 | 52 | + | − | c | c |
| 7 | X | − | + | X | X | − | + | c | c |
| 8 | 16 | + | − | 16 | 16 | + | − | c | c |
| 9 | 16, 45, 51 | + | − | 11, 16, 45, 51 | 11, 16, 45, 51 | + | + | c | d |
| 10 | 68 | + | − | 68 | 68 | + | − | c | c |
| 11 | 31 | + | − | 31 | 31 | + | − | c | c |
| 12 | 39, 54 | + | + | 39 | 39 | + | − | c | d |
| 13 | 54 | − | + | X | X | − | + | c | c |
| 14-51 | N | − | − | N | N | − | − | c | c |
HR-HPV types (HR) were 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 59, and 68/73; probable HR-HPV types were 53 and 66. LR-HPV types (LR) were 6, 11, 34, 40, 42, 43, 44, 54, 55, 58, 70, 74, and X. c, concordant; d, discordant; N, HPV negative; −, negative; +, positive.
Of the 13 HPV-positive samples, 10 samples showed similar types, 2 samples showed an additional genotype (sample no. 9 and 12) (Table 2), and 1 sample showed a different genotype (sample no. 13) (Table 2).
Concordance and discordance of HR-HPV.
Table 2 shows a summary of the genotypes per sample set as well as the concordance of HR-HPV between the liquid-based and filter-based samples. Taking all 51 samples into account, all samples were concordant for HR-HPV detection, of which 9 (17.6%) were HR-HPV positive. In these nine liquid-based stored self-obtained samples, one or more HR-HPV types were detected; all samples (100%) also tested HR-HPV positive on the FTA cartridges.
Additionally, the overall agreement for HR-HPV positivity between the FTA cartridge and liquid-based medium was 100% (kappa value, 1.0; 95% CI, 1.0 to 1.0).
Concordance and discordance of LR-HPV.
One or more LR-HPV types were detected in seven of the liquid-based stored self-obtained samples (13.7%) versus seven on the FTA cartridges (Table 2). However, not all samples showed similar types, resulting in only five concordant LR-HPV types. The overall agreement for LR-HPV positivity between FTA cartridge and liquid-based medium was 96.1% (kappa value, 0.83; 95% CI, 0.609 to 1.059).
DNA elution versus DNA extraction.
In sample set 2, HPV DNA yielded from the self-obtained material on the FTA cartridge by DNA elution was again compared to DNA yielded from the FTA cartridge by extraction. The results showed an overall agreement of 100% (kappa value, 1; 95% CI, 1.0).
DISCUSSION
HPV testing in cervical cancer screening has a beneficial effect in patient management and can increase the success rate of population-based screening programs in reducing cervical cancer incidence (1, 3, 6, 11). Regarding HR-HPV testing, cervicovaginal self-obtained samples have repetitively been proven to be as reliable as physician-obtained samples (19, 20). This present study underlines the reliability of using cervicovaginal self-samples for HR-HPV testing.
However, despite differences in self-sampling methods, many previous studies have used liquid-based sample storage and transport media. Use of these solutions may result in a delay or inability to implement at-home self-sampling of population-based screening nonresponders because of a number of reasons. For example, one reason is the high cost due to legislations for these potentially hazardous liquid-based techniques, which require difficult and therefore expensive logistics. An alternative for the transport of potentially hazardous solutions could be storage on filter papers, i.e., FTA cartridges. These FTA cartridges, for example, are less prone to contamination and are therefore easy to handle. For instance, filters have been used for decades in the postnatal screening of certain congenital disorders and diseases. The air-dried samples showed stability at room temperature for months, up to years (9). Furthermore, at-home collection for HIV testing on filter papers has been considered feasible and acceptable in a high-risk cohort. Additionally, also viral load and genotypic resistance assessments in applied whole blood and plasma of HIV-positive patients appear to be possible (2, 23).
To compare the transport media used in this study, it would be ideal if all conditions across the groups were equal. However, as we are particularly interested in whether the results of HPV detection in cervicovaginal self-obtained samples are comparable to the results of HPV detection in physician-taken cervical smears, the “golden standard,” despite or precisely because the conditions differ, we think it is important to compare the self-sampling method with the “regular” physician-taken smear as a proof of principle. This study shows a high level of overall agreement of HPV detection and genotyping between physician-obtained cervical smears which are applied to a liquid-based medium and self-obtained cervicovaginal samples that are subsequently applied to an FTA cartridge. Additionally, all HR-HPV-positive physician-obtained smears were HR-HPV positive in the cervicovaginal self-samples as well. Besides the reliability of the FTA cartridge regarding HR-HPV testing, its unique properties make it easy to handle. For instance, the air-dried FTA cartridges showed stability at room temperature for months. Furthermore, the uncertainty about performing the self-sampling procedure properly will be overcome since the indicating FTA cartridge has an indicating dye which changes upon application of the sample. Furthermore, the contamination risk is reduced as the virus is denatured upon application, making it biohazard free and safe for transport by mail. This allows cervical or self-obtained genital samples to be added to this FTA cartridge and sent to designated central laboratories for analysis. Even more important, by using the FTA cartridge, processing costs will be low as DNA is eluted by a simple method using only water and heat, without requiring expensive DNA extraction. Besides the use in existing screening programs, usage of the FTA cartridge could simplify the introduction of organized HPV-based cervical screening programs in developing countries as well.
It has been shown that self-sampling methods are unsuitable for cytological analysis (8, 18). To complete the diagnosis for the individual HR-HPV-positive patient, a subsequent physician-obtained smear ought to be performed. Preferably, this is done solely in women who are persistently HR-HPV positive. Whether the self-sampling women are willing to have an additional cytology smear taken in case of HR-HPV persistence has not yet been studied.
Since cervicovaginal self-sampling could be an easily accessible and user-friendly method, women not participating in the screening program due to fear or other reasons might be interested to actually participate since this technique could be applicable to at-home self-sampling. Therefore, the introduction of cervicovaginal self-sampling will probably increase the participation rate (8, 12, 18, 24). Recently, Bais et al. showed that the active response to self-sampling in population-based-screening nonresponders was significantly higher than the active response to an extra recall for conventional cytology (4).
For HPV detection and genotyping, we used the HPV SPF10-Line Blot 25. This assay has previously shown high concordance with various other systems (25, 26). This indicates the suitability of the FTA cartridge for various other HPV detection and genotyping systems like the Roche Amplicor and linear array assays. Preliminary studies indeed showed an excellent concordance (data not shown). However, further study may be needed to assess genital self-sampled FTA cartridges using other commercially available HPV detection tests with lower analytical sensitivity (e.g., Hybrid Capture II). Additionally, since this was a pilot study and sample sizes were small, further research should be conducted. Furthermore, since not all samples were checked for specimen sufficiency (e.g., beta-globin), future research should include a measure for sample sufficiency.
In conclusion, the results of HPV detection and genotyping on self-sampled cervicovaginal samples using a Rovers Viba-Brush and the Whatman indicating FTA elute cartridge are highly representative for the cervical HPV status.
Furthermore and equally important, this study shows that elution of DNA from the Whatman indicating FTA elute cartridge, without the necessity of DNA extraction procedures, is a fast, cheap, and reliable method. The Whatman indicating FTA elute cartridge technique is a convenient medium for collection, as the color of the FTA cartridge changes after application of the self-sampled material, confirming proper use. Additionally, the FTA cartridges can be stored at ambient temperatures for months, and since the method is nonhazardous, the samples are allowed to be sent by regular mail. This suggests that this method might be applicable to at-home self-sampling in population-based screening nonresponders, as well as for the introduction of primary HPV-based cervical cancer prevention and for establishing cervical cancer screening programs in developing countries.
Acknowledgments
This study was partially supported by GlaxoSmithKline.
The funder of this study had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Concerning this paper, no conflict of interest exists.
We thank Nel H. T. H. Struijk-van der Zanden for the logistical support critical to this study. Furthermore, the Delft Diagnostic Laboratory kindly provided the HPV SPF10-Line Blot 25 genotyping test and detection reagents, Rovers Medical Devices B.V. supplied the Rovers Cervex-Brush and Rovers Viba-Brush, and GE Healthcare provided the Whatman indicating FTA elute cartridges.
Footnotes
Published ahead of print on 24 June 2009.
REFERENCES
- 1.Arbyn, M., E. Paraskevaidis, P. Martin-Hirsch, W. Prendiville, and J. Dillner. 2005. Clinical utility of HPV-DNA detection: triage of minor cervical lesions, follow-up of women treated for high-grade CIN: an update of pooled evidence. Gynecol. Oncol. 99S7-S11. [DOI] [PubMed] [Google Scholar]
- 2.Ayele, W., R. Schuurman, T. Messele, W. Dorigo-Zetsma, Y. Mengistu, J. Goudsmit, W. A. Paxton, M. P. de Baar, and G. Pollakis. 2007. Use of dried spots of whole blood, plasma, and mother's milk collected on filter paper for measurement of human immunodeficiency virus type 1 burden. J. Clin. Microbiol. 45891-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bais, A. G., M. Rebolj, P. J. Snijders, F. A. de Schipper, D. A. van der Meulen, R. H. Verheijen, F. Voorhorst, M. van Ballegooijen, C. J. Meijer, and T. J. Helmerhorst. 2005. Triage using HPV-testing in persistent borderline and mildly dyskaryotic smears: proposal for new guidelines. Int. J. Cancer 116122-129. [DOI] [PubMed] [Google Scholar]
- 4.Bais, A. G., F. J. van Kemenade, J. Berkhof, R. H. Verheijen, P. J. Snijders, F. Voorhorst, M. Babovic, M. van Ballegooijen, T. J. Helmerhorst, and C. J. Meijer. 2007. Human papillomavirus testing on self-sampled cervicovaginal brushes: an effective alternative to protect nonresponders in cervical screening programs. Int. J. Cancer 1201505-1510. [DOI] [PubMed] [Google Scholar]
- 5.Bekkers, R. L., L. F. Massuger, J. Bulten, and W. J. Melchers. 2004. Epidemiological and clinical aspects of human papillomavirus detection in the prevention of cervical cancer. Rev. Med. Virol. 1495-105. [DOI] [PubMed] [Google Scholar]
- 6.Bekkers, R. L., C. J. Meijer, L. F. Massuger, P. J. Snijders, and W. J. Melchers. 2006. Effects of HPV detection in population-based screening programmes for cervical cancer: a Dutch moment. Gynecol. Oncol. 100451-454. [DOI] [PubMed] [Google Scholar]
- 7.Bos, A. B., M. Rebolj, J. D. Habbema, and M. van Ballegooijen. 2006. Nonattendance is still the main limitation for the effectiveness of screening for cervical cancer in The Netherlands. Int. J. Cancer 1192372-2375. [DOI] [PubMed] [Google Scholar]
- 8.Brink, A. A., C. J. Meijer, M. A. Wiegerinck, T. E. Nieboer, R. F. Kruitwagen, F. van Kemenade, D. N. Fransen, A. T. Hesselink, J. Berkhof, and P. J. Snijders. 2006. High concordance of results of testing for human papillomavirus in cervicovaginal samples collected by two methods, with comparison of a novel self-sampling device to a conventional endocervical brush. J. Clin. Microbiol. 442518-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chibo, D., M. A. Riddell, M. G. Catton, and C. J. Birch. 2005. Applicability of oral fluid collected onto filter paper for detection and genetic characterization of measles virus strains. J. Clin. Microbiol. 433145-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuzick, J., E. Beverley, L. Ho, G. Terry, H. Sapper, I. Mielzynska, A. Lorincz, W. K. Chan, T. Krausz, and P. Soutter. 1999. HPV testing in primary screening of older women. Br. J. Cancer 81554-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuzick, J., A. Szarewski, H. Cubie, G. Hulman, H. Kitchener, D. Luesley, E. McGoogan, U. Menon, G. Terry, R. Edwards, C. Brooks, M. Desai, C. Gie, L. Ho, I. Jacobs, C. Pickles, and P. Sasieni. 2003. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet 3621871-1876. [DOI] [PubMed] [Google Scholar]
- 12.De Alba, I., H. Anton-Culver, F. A. Hubbell, A. Ziogas, J. R. Hess, A. Bracho, C. Arias, and A. Manetta. 2008. Self-sampling for human papillomavirus in a community setting: feasibility in Hispanic women. Cancer Epidemiol. Biomarkers Prev. 172163-2168. [DOI] [PubMed] [Google Scholar]
- 13.Harper, D. M., W. W. Noll, D. R. Belloni, and B. F. Cole. 2002. Randomized clinical trial of PCR-determined human papillomavirus detection methods: self-sampling versus clinician-directed—biologic concordance and women's preferences. Am. J. Obstet. Gynecol. 186365-373. [DOI] [PubMed] [Google Scholar]
- 14.Kerr, R. J., G. Player, S. A. Fiscus, and J. A. Nelson. 2009. Qualitative human immunodeficiency virus RNA analysis of dried blood spots for diagnosis of infections in infants. J. Clin. Microbiol. 47220-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenselink, C. H., W. J. Melchers, W. G. Quint, A. M. Hoebers, J. C. Hendriks, L. F. Massuger, and R. L. Bekkers. 2008. Sexual behaviour and HPV infections in 18 to 29 year old women in the pre-vaccine era in The Netherlands. PLoS ONE 3e3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melchers, W. J., J. M. Bakkers, J. Wang, P. C. de Wilde, H. Boonstra, W. G. Quint, and A. G. Hanselaar. 1999. Short fragment polymerase chain reaction reverse hybridization line probe assay to detect and genotype a broad spectrum of human papillomavirus types. Clinical evaluation and follow-up. Am. J. Pathol. 1551473-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muñoz, N., F. X. Bosch, S. de Sanjose, R. Herrero, X. Castellsague, K. V. Shah, P. J. Snijders, and C. J. Meijer. 2003. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 348518-527. [DOI] [PubMed] [Google Scholar]
- 18.Nobbenhuis, M. A., T. J. Helmerhorst, A. J. van den Brule, L. Rozendaal, L. H. Jaspars, F. J. Voorhorst, R. H. Verheijen, and C. J. Meijer. 2002. Primary screening for high risk HPV by home obtained cervicovaginal lavage is an alternative screening tool for unscreened women. J. Clin. Pathol. 55435-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogilvie, G. S., D. M. Patrick, M. Schulzer, J. W. Sellors, M. Petric, K. Chambers, R. White, and J. M. FitzGerald. 2005. Diagnostic accuracy of self collected vaginal specimens for human papillomavirus compared to clinician collected human papillomavirus specimens: a meta-analysis. Sex. Transm. Infect. 81207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petignat, P., D. L. Faltin, I. Bruchim, M. R. Tramer, E. L. Franco, and F. Coutlee. 2007. Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis. Gynecol. Oncol. 105530-535. [DOI] [PubMed] [Google Scholar]
- 21.Sasieni, P. D., J. Cuzick, E. Lynch-Farmery, and the National Co-ordinating Network for Cervical Screening Working Group. 1996. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. Br. J. Cancer 731001-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sellors, J. W., A. T. Lorincz, J. B. Mahony, I. Mielzynska, A. Lytwyn, P. Roth, M. Howard, S. Chong, D. Daya, W. Chapman, and M. Chernesky. 2000. Comparison of self-collected vaginal, vulvar and urine samples with physician-collected cervical samples for human papillomavirus testing to detect high-grade squamous intraepithelial lesions. CMAJ 163513-518. [PMC free article] [PubMed] [Google Scholar]
- 23.Spielberg, F., C. Critchlow, E. Vittinghoff, A. S. Coletti, H. Sheppard, K. H. Mayer, D. Metzgerg, F. N. Judson, S. Buchbinder, M. Chesney, M. Gross, and the HIV Early Detection Study Group. 2000. Home collection for frequent HIV testing: acceptability of oral fluids, dried blood spots and telephone results. AIDS 141819-1828. [DOI] [PubMed] [Google Scholar]
- 24.Szarewski, A., L. Cadman, S. Mallett, J. Austin, P. Londesborough, J. Waller, J. Wardle, D. G. Altman, and J. Cuzick. 2007. Human papillomavirus testing by self-sampling: assessment of accuracy in an unsupervised clinical setting. J. Med. Screen. 1434-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Ham, M. A., J. M. Bakkers, G. K. Harbers, W. G. Quint, L. F. Massuger, and W. J. Melchers. 2005. Comparison of two commercial assays for detection of human papillomavirus (HPV) in cervical scrape specimens: validation of the Roche AMPLICOR HPV test as a means to screen for HPV genotypes associated with a higher risk of cervical disorders. J. Clin. Microbiol. 432662-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Hamont, D., M. A. van Ham, J. M. Bakkers, L. F. Massuger, and W. J. Melchers. 2006. Evaluation of the SPF10-INNO LiPA human papillomavirus (HPV) genotyping test and the Roche linear array HPV genotyping test. J. Clin. Microbiol. 443122-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walboomers, J. M., M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. Snijders, J. Peto, C. J. Meijer, and N. Munoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 18912-19. [DOI] [PubMed] [Google Scholar]
- 28.Waller, J., K. McCaffery, S. Forrest, A. Szarewski, L. Cadman, J. Austin, and J. Wardle. 2006. Acceptability of unsupervised HPV self-sampling using written instructions. J. Med. Screen. 13208-213. [DOI] [PubMed] [Google Scholar]
- 29.Winer, R. L., Q. Feng, J. P. Hughes, M. Yu, N. B. Kiviat, S. O'Reilly, and L. A. Koutsky. 2007. Concordance of self-collected and clinician-collected swab samples for detecting human papillomavirus DNA in women 18 to 32 years of age. Sex. Transm. Dis. 34371-377. [DOI] [PubMed] [Google Scholar]