Abstract
A total of 242 isolates were recovered from 76 patients with invasive diseases, 89 with scarlet fever, and 77 with pharyngitis. The most frequent emm types were types 12 (43.4%), 4 (18.2%), and 1 (16.9%). emm12 reemerged in 2005 and peaked in 2007. emm11 was recovered only from patients with invasive disease.
Streptococcus pyogenes, a group A Streptococcus (GAS) species, produces many diseases, ranging from impetigo, pharyngitis, and scarlet fever to life-threatening sepsis, necrotizing fasciitis, and streptococcal toxic shock syndrome (STSS) (8). M protein that is encoded by the emm gene is a major virulence factor of GAS. Different M protein types are epidemiologically related to particular clinical syndromes; e.g., emm28 was isolated more frequently than other types among women with puerperal sepsis (6, 10, 14). M1, M2, M3, M4, M6, M18, and M22 strains are associated with outbreaks of scarlet fever (1, 11, 15, 18, 20, 22-24), while M1 and M3 are highly associated with severe invasive disease (19, 25, 27) and M18 is associated with acute rheumatic fever (7).
The distribution of emm types in cases of invasive diseases tends to vary over time and within different geographic regions. In the United States, the most common emm types were 1, 28, 12, 3, and 11 during the period from 1995 to 1999 (19). emm types 1, 3, and 12 were predominant from 2000 to 2004 (21). In Europe, the distribution of the emm types differs among countries (16). For example, in Denmark, emm1 and emm28 were the most prevalent types from 2001 to 2004, whereas in Greece, emm1 and emm12 were predominant from 2003 to 2005. In Sweden, four emm types, 89, 81, 28, and 1, accounted for 56% of 746 patients with invasive GAS diseases during the period from 2002 to 2004. In Taiwan, emm1 associated with invasive GAS disease, while emm12 was more often associated with noninvasive GAS disease (13). The purpose of the current study was to investigate the changing epidemiology, genetic diversity, and epidemic virulence of GAS infections over a 10-year period in southern Taiwan, utilizing M genotyping and pulsed-field gel electrophoresis (PFGE) analysis.
Clinical isolates of S. pyogenes was obtained from patients seen at the National Cheng Kung University Hospital, Tainan City, Taiwan, from 1998 to 2007. They were divided into strains isolated from patients with invasive diseases or those from patients with noninvasive diseases. Invasive isolates were defined as those obtained from sterile body sites (blood, cerebrospinal, joint, pleural, peritoneal, or pericardial fluids) or from nonsterile sites (wounds associated with STSS or necrotizing fasciitis). Noninvasive strains were defined as GAS isolates obtained from patients with asymptomatic nasopharyngeal colonization, pharyngitis, scarlet fever, erysipelas, and impetigo.
A total of 242 nonduplicate GAS isolates recovered from normally sterile sites (165 from throat swabs, 30 from wounds, 25 from pus, 12 from blood, 10 from other materials) were collected. The mean age of patients with invasive diseases was significantly greater than that of those with noninvasive diseases (28.5 ± 27 years versus 8.7 ± 7.9 years [P < 0.0001]). Among the 76 patients with invasive diseases, 58 (76.3%) patients had skin and soft-tissue infections. Three had necrotizing fasciitis, eight had STSS, four had severe sepsis, and three had complicated infections including pneumonia or mastoiditis. Among the 166 patients with noninvasive diseases, 77 (46%) had pharyngitis (including one with lymphadenitis), and 89 (54%) had scarlet fever (including 59 patients with pharyngitis).
The distribution of emm types among patients with invasive and noninvasive diseases (subdivided into those with scarlet fever and those with pharyngitis) is shown in Table 1. GAS emm sequence typing was based on the 5′ end of the emm gene within the emm chromosomal region (2, 3). A unique emm type was defined as having ≥95% sequence identity to any other known emm type over 160 bp near the 5′ end of the gene, using a BLAST search (http://www.cdc.gov/ncidod/biotech/strep/strepblast.htm). Twenty emm sequence types were identified among the 242 GAS isolates, including types 1, 4, 6, 11, 12, 13, 22, 33, 49, 57, 77, 81, 82, 85, 87, 92, 94, 101, 102, and 123. The leading emm types were 12 (43.4%), 4 (18.2%), 1 (16.9%), 11 (4.5%), 6 (3.7%), and 22 (2.5%) (Table 1). All of the emm types were associated with both invasive and noninvasive diseases except for emm11, which was recovered only from patients with invasive disease. emm22 and other less frequent types were more likely to be associated with invasive diseases (P < 0.0001). The emm types 1, 4, 6, and 12 accounted for 82.2% of all patients with GAS infections, 95.2% of the patients with noninvasive diseases, and 53.9% of the patients with invasive disease. The most common type, emm12, was also significantly associated with noninvasive disease (85.7%) (P < 0.0001).
TABLE 1.
Distribution of Streptococcus pyogenes emm types isolated from 1998 to 2007 according to clinical characteristics
| emm type | No. (%) of indicated isolates
|
χ2c | P valueb | |||
|---|---|---|---|---|---|---|
| Invasive | Noninvasive
|
Total | ||||
| Scarlet fever | Pharyngitis | |||||
| 1 | 14 (34.1) | 14 (34.1) | 13 (31.7) | 41 (16.9) | 0.06 | 0.8 |
| 4 | 11 (25) | 21 (47.7) | 12 (27.3) | 44 (18.2) | 0.03 | 0.86 |
| 6 | 1 (11.1) | 3 (33.3) | 5 (55.6) | 9 (3.7) | 2.87 | 0.09 |
| 11 | 11 (100.0) | 0 (0.0) | 0 (0.0) | 11 (4.5) | 18.38 | <0.0001 |
| 12 | 15 (14.3) | 47 (44.8) | 43 (41.0) | 105 (43.4) | 20.22 | <0.0001 |
| 22 | 4 (66.7) | 2 (33.3) | 0 (0.0) | 6 (2.5) | 4.38 | 0.04 |
| Othera | 20 (76.9) | 2 (7.7) | 4 (15.4) | 26 (10.7) | ||
| Total | 76 (31.4) | 89 (36.8) | 77 (31.8) | 242 (100.0) | ||
The “Other” group includes ≤5 isolates of emm13, emm33, emm49, emm57, emm77, emm81, emm82, emm85, emm87, emm92, emm94, emm101, emm102, and emm123.
Variation in the proportion of each emm type, which is compared with the remaining emm types combined; determined by χ2 test. Two-sided P values of <0.05 are statistically significant.
The χ2 for trend statistic was used to assess differences in proportions and to test for departures from linear trends associated with clinical manifestations of GAS acquisition.
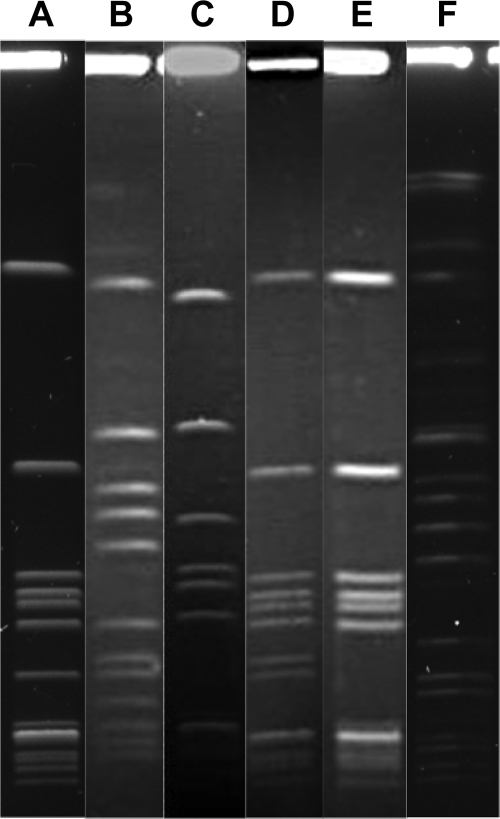
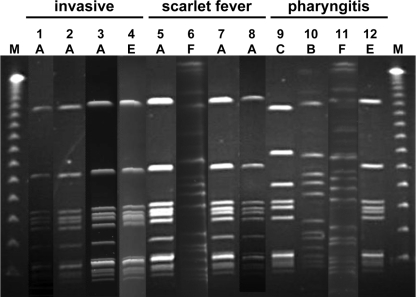
The most prevalent emm12 type was analyzed by PFGE after digestion of total DNA with SmaI or SgrAI for determining the clonal relatedness. The PFGE protocol for GAS was developed on the basis of Gautom's Escherichia coli rapid PFGE protocol, with minor modifications (9). Six PFGE patterns were observed among the isolates during the 10-year period from 1998 to 2007 (Fig. 1). Pattern A was shown as the most common pattern during the entire study period (Fig. 2). This clonal pattern has also been identified in northern and central Taiwan (4, 5). The reemergence of emm12 strains throughout Taiwan may explain the reported increase in cases of scarlet fever noted since 2005.
FIG. 1.
Illustration of the various PFGE patterns of SmaI (A to E)- or SgrAI (F)-digested chromosomal DNA of emm12 GAS isolates. Six major PFGE patterns are shown.
FIG. 2.
PFGE patterns of emm12 strains isolated from patients with GAS invasive diseases, scarlet fever, and pharyngitis. Chromosome DNA was digested with SmaI (lanes 1 to 5, 7 to 10, and 12) or SgrAI (lanes 6 and 11). The study periods were divided into the following: period I, 1998 to 1999; period II, 2000 to 2002; period III, 2003 to 2005; and period IV, 2006 to 2007. Lane 1, 5, and 9 represent period I. Lanes 2, 6, and 10 represent period II. Lanes 3, 7, and 11 represent period III. Lanes 4, 8, and 12 represent period IV. Lanes 1 to 4 are the patterns of emm12 strains isolated from patients with invasive diseases; lanes 5 to 8 are those from patients with scarlet fever; lanes 9 to 12 are those from patients with pharyngitis; and lane M shows the lambda DNA reference size markers (size range, 50 to 1,000 kb).
There is considerable controversy concerning the virulence of the various GAS emm types. There appear to be major differences in the frequency of purportedly disease-specific emm types in Taiwan (4, 5, 28). These apparent differences can be explained, in part, by the changes in the relative abundance of different emm types over time, herd immunity following epidemic peaks, epidemic virulence, age, sex, and location in the community. In the current study, emm type 12 accounted for 43.4% of all strains. Because of its relatively great abundance, it was also the most common invasive strain, accounting for 15 of 76 (19.7%) of all invasive strains. But only 14.3% of the emm type 12 strains were associated with invasive infections. Most were associated with noninvasive infections. emm types that were significantly associated with invasive infections were types 11 and 22 and several less-common types. These findings are in concert with a report of an outbreak of invasive infections in a nursing home in which emm11 was one of two distinct emm types identified in the outbreak (26). An emm11 strain expressing the ermA gene was identified in Spain (17). Nevertheless, 10 emm types (types 1, 6, 11, 12, 13, 22, 33, 77, 92, and 101) were covered by the 26-valent GAS vaccine (12). Therefore, this vaccine may provide antibodies to protect vulnerable groups from GAS infection.
In conclusion, emm12 was found to be prevalent throughout the past 10 years. All of the emm types were associated with both invasive and noninvasive diseases, except for type 11, which was associated only with invasive disease. The predominant emm type during this period was type 12, clonal pattern A. It was the major emm type associated with scarlet fever and pharyngitis. Types 12, 22, and a few less-common emm types appeared to have special epidemic virulence for invasive diseases. Long-term surveillance studies combined with emm sequence typing and clonal analysis are needed to understand the natural history and epidemic virulence of GAS infections.
Acknowledgments
We express our appreciation to Calvin M. Kunin for providing invaluable suggestions and a critical review of the manuscript. We thank Chao-Ping Liao for collection of clinical isolates.
This study was supported by grants from National Health Research Institutes, Taiwan (NHRI-EX95-9429 SP and NHRI-EX96-9429 SP).
Footnotes
Published ahead of print on 10 June 2009.
REFERENCES
- 1.Anthony, B. F., T. Yamauchi, J. S. Penso, I. Kamei, and S. S. Chapman. 1974. Classroom outbreak of scarlet fever and acute glomerulonephritis related to type 2 (M-2, T-2) group A Streptococcus. J. Infect. Dis. 129336-340. [DOI] [PubMed] [Google Scholar]
- 2.Bessen, D. E., J. R. Carapetis, B. Beall, R. Katz, M. Hibble, B. J. Currie, T. Collingridge, M. W. Izzo, D. A. Scaramuzzino, and K. S. Sriprakash. 2000. Contrasting molecular epidemiology of group A streptococci causing tropical and nontropical infections of the skin and throat. J. Infect. Dis. 1821109-1116. [DOI] [PubMed] [Google Scholar]
- 3.Bessen, D. E., M. W. Izzo, T. R. Fiorentino, R. M. Caringal, S. K. Hollingshead, and B. Beall. 1999. Genetic linkage of exotoxin alleles and emm gene markers for tissue tropism in group A streptococci. J. Infect. Dis. 179627-636. [DOI] [PubMed] [Google Scholar]
- 4.Chen, Y. Y., C. T. Huang, S. M. Yao, Y. C. Chang, P. W. Shen, C. Y. Chou, and S. Y. Li. 2007. Molecular epidemiology of group A Streptococcus causing scarlet fever in northern Taiwan, 2001-2002. Diagn. Microbiol. Infect. Dis. 58289-295. [DOI] [PubMed] [Google Scholar]
- 5.Chiou, C. S., T. L. Liao, T. H. Wang, H. L. Chang, J. C. Liao, and C. C. Li. 2004. Epidemiology and molecular characterization of Streptococcus pyogenes recovered from scarlet fever patients in central Taiwan from 1996 to 1999. J. Clin. Microbiol. 423998-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuang, I., C. Van Beneden, B. Beall, and A. Schuchat. 2002. Population-based surveillance for postpartum invasive group A streptococcus infections, 1995-2000. Clin. Infect. Dis. 35665-670. [DOI] [PubMed] [Google Scholar]
- 7.Colman, G., A. Tanna, A. Efstratiou, and E. T. Gaworzewska. 1993. The serotypes of Streptococcus pyogenes present in Britain during 1980-1990 and their association with disease. J. Med. Microbiol. 39165-178. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gautom, R. K. 1997. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 352977-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green, N. M., S. Zhang, S. F. Porcella, M. J. Nagiec, K. D. Barbian, S. B. Beres, R. B. LeFebvre, and J. M. Musser. 2005. Genome sequence of a serotype M28 strain of group A streptococcus: potential new insights into puerperal sepsis and bacterial disease specificity. J. Infect. Dis. 192760-770. [DOI] [PubMed] [Google Scholar]
- 11.Hsueh, P. R., L. J. Teng, P. I. Lee, P. C. Yang, L. M. Huang, S. C. Chang, C. Y. Lee, and K. T. Luh. 1997. Outbreak of scarlet fever at a hospital day care centre: analysis of strain relatedness with phenotypic and genotypic characteristics. J. Hosp. Infect. 36191-200. [DOI] [PubMed] [Google Scholar]
- 12.Hu, M. C., M. A. Walls, S. D. Stroop, M. A. Reddish, B. Beall, and J. B. Dale. 2002. Immunogenicity of a 26-valent group A streptococcal vaccine. Infect. Immun. 702171-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao, C. H., P. Y. Chen, F. L. Huang, C. W. Chen, C. S. Chi, Y. H. Lin, C. Y. Shih, B. S. Hu, C. R. Li, J. S. Ma, Y. J. Lau, K. C. Lu, and H. W. Yu. 2005. Clinical and genetic analysis of invasive and non-invasive group A streptococcal infections in central Taiwan. J. Microbiol. Immunol. Infect. 38105-111. [PubMed] [Google Scholar]
- 14.Kaplan, E. L., J. T. Wotton, and D. R. Johnson. 2001. Dynamic epidemiology of group A streptococcal serotypes associated with pharyngitis. Lancet 3581334-1337. [DOI] [PubMed] [Google Scholar]
- 15.Kohler, W., D. Gerlach, and H. Knoll. 1987. Streptococcal outbreaks and erythrogenic toxin type A. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 266104-115. [DOI] [PubMed] [Google Scholar]
- 16.Luca-Harari, B., J. Darenberg, S. Neal, T. Siljander, L. Strakova, A. Tanna, R. Creti, K. Ekelund, M. Koliou, P. T. Tassios, M. van der Linden, M. Straut, J. Vuopio-Varkila, A. Bouvet, A. Efstratiou, C. Schalen, B. Henriques-Normark, and A. Jasir. 2009. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J. Clin. Microbiol. 471155-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montes, M., B. Orden, E. Tamayo, J. I. Alos, and E. Perez-Trallero. 2006. Characterisation of the main clones of Streptococcus pyogenes carrying the ermA (subclass TR) gene in Spain. Int. J. Antimicrob. Agents 28408-412. [DOI] [PubMed] [Google Scholar]
- 18.Musser, J. M., K. Nelson, R. K. Selander, D. Gerlach, J. C. Huang, V. Kapur, and S. Kanjilal. 1993. Temporal variation in bacterial disease frequency: molecular population genetic analysis of scarlet fever epidemics in Ottawa and in eastern Germany. J. Infect. Dis. 167759-762. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien, K. L., B. Beall, N. L. Barrett, P. R. Cieslak, A. Reingold, M. M. Farley, R. Danila, E. R. Zell, R. Facklam, B. Schwartz, and A. Schuchat. 2002. Epidemiology of invasive group A streptococcus disease in the United States, 1995-1999. Clin. Infect. Dis. 35268-276. [DOI] [PubMed] [Google Scholar]
- 20.Ohga, S., K. Okada, K. Mitsui, T. Aoki, and K. Ueda. 1992. Outbreaks of group A beta-hemolytic streptococcal pharyngitis in children: correlation of serotype T4 with scarlet fever. Scand. J. Infect. Dis. 24599-605. [DOI] [PubMed] [Google Scholar]
- 21.O'Loughlin, R. E., A. Roberson, P. R. Cieslak, R. Lynfield, K. Gershman, A. Craig, B. A. Albanese, M. M. Farley, N. L. Barrett, N. L. Spina, B. Beall, L. H. Harrison, A. Reingold, and C. Van Beneden. 2007. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin. Infect. Dis. 45853-862. [DOI] [PubMed] [Google Scholar]
- 22.Parker, M. T. 1967. International survey of the distribution of serotypes of Streptococcus pyogenes (group A streptococci). Bull. W. H. O. 37513-527. [PMC free article] [PubMed] [Google Scholar]
- 23.Perea-Mejía, L. M., A. E. Inzunza-Montiel, and A. Cravioto. 2002. Molecular characterization of group A Streptococcus strains isolated during a scarlet fever outbreak. J. Clin. Microbiol. 40278-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perks, E. M., and R. T. Mayon-White. 1983. The incidence of scarlet fever. J. Hyg. (Lond.) 91203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharkawy, A., D. E. Low, R. Saginur, D. Gregson, B. Schwartz, P. Jessamine, K. Green, and A. McGeer. 2002. Severe group A streptococcal soft-tissue infections in Ontario: 1992-1996. Clin. Infect. Dis. 34454-460. [DOI] [PubMed] [Google Scholar]
- 26.Thigpen, M. C., D. M. Thomas, D. Gloss, S. Y. Park, A. J. Khan, V. L. Fogelman, B. Beall, C. A. Van Beneden, R. L. Todd, and C. M. Greene. 2007. Nursing home outbreak of invasive group A streptococcal infections caused by 2 distinct strains. Infect. Control Hosp. Epidemiol. 2868-74. [DOI] [PubMed] [Google Scholar]
- 27.Veasy, L. G., L. Y. Tani, J. A. Daly, K. Korgenski, L. Miner, J. Bale, E. L. Kaplan, J. M. Musser, and H. R. Hill. 2004. Temporal association of the appearance of mucoid strains of Streptococcus pyogenes with a continuing high incidence of rheumatic fever in Utah. Pediatrics 113e168-e172. [DOI] [PubMed] [Google Scholar]
- 28.Yan, J. J., C. C. Liu, W. C. Ko, S. Y. Hsu, H. M. Wu, Y. S. Lin, M. T. Lin, W. J. Chuang, and J. J. Wu. 2003. Molecular analysis of group A streptococcal isolates associated with scarlet fever in southern Taiwan between 1993 and 2002. J. Clin. Microbiol. 414858-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]