Abstract
Human respiratory syncytial virus (HRSV) is a common etiological agent of acute lower respiratory tract disease in infants. We report the molecular epidemiology of HRSV in Niigata, Japan, over six successive seasons (from 2001 to 2007) and the emerging genotypes of HRSV subgroup A (HRSV-A) strains. A total of 488 HRSV samples were obtained from 1,103 screened cases in a pediatric clinic in Niigata. According to the phylogenetic analysis, among the PCR-positive samples, 338 HRSV-A strains clustered into the previously reported genotypes GA5 and GA7 and two novel genotypes, NA1 and NA2, which were genetically close to GA2 strains. One hundred fifty HRSV-B strains clustered into three genotypes, namely, GB3, SAB3, and BA, which has a 60-nucleotide insertion in the second hypervariable region of the G protein. The NA1 strains emerged first, in the 2004-2005 season, and subsequently, the NA2 strain emerged in the 2005-2006 season. Both strains caused large epidemics in the 2005-2006 and 2006-2007 seasons. The average age of children who were infected with NA2 strains was significantly higher than that of those infected with GA5 and the frequency of reinfection by NA2 was the highest among all genotypes, suggesting that this genotype possessed new antigenicity for evading past host immunity. This is the first paper to show a possible correlation between an emerging genotype, NA2, and large outbreaks of HRSV in Japan. Continuing studies to follow up the genetic changes and to clarify the mechanism of reinfection in HRSV are important steps to understand HRSV infections.
Human respiratory syncytial virus (HRSV) is the most common cause of serious acute lower respiratory tract disease among infants and young children, and is found mainly in late fall, winter, and spring in temperate zones of the world (6). Some 50% to 70% of infants experience infection in the first year of life, and virtually all are infected by 2 years of age (3). In a population-based birth cohort study, 1.1% of the cohort were admitted to the hospital within 12 months of birth with HRSV-induced bronchiolitis (14). The consequences of HRSV infection in children with underlying conditions, such as prematurity, cardiac and pulmonary disease, or immunosuppression, may include prolonged substantial illness and even death (18, 38). Reinfections are very common throughout life (7, 10, 11, 13).
HRSV belongs to the family Paramyxoviridae and has a nonsegmented, negative-sense RNA genome of approximately 15,200 nucleotides (3). HRSV has been classified into antigenic subgroups A and B (HRSV-A and HRSV-B, respectively), initially on the basis of the reactivity of the virus with monoclonal antibodies directed against the attachment glycoprotein (G protein) (1, 5, 15, 21) and now by genetic analyses (9, 19, 31-33).
The G protein is the most variable HRSV protein, with two hypervariable regions. Its C-terminal region (the second hypervariable region) accounts for strain-specific epitopes (2-4, 9, 16, 27, 29, 31). The molecular epidemiology and evolutionary patterns of the G protein have provided important information about the epidemiological features of HRSV. Some studies showed that several different genotypes cocirculated and some predominated in a community every year (23, 27). However, the relationship between strain diversity and the clinical and epidemiological features of HRSV has yet to be elucidated in detail.
The subgroups have been subdivided further, into genotypes, by genetic analyses. HRSV-A is divided into seven genotypes (GA1 to -7) and HRSV-B into four genotypes (GB1 to -4) (3, 8). An additional HRSV-A genotype, SAA1, has been proposed, as well as the new HRSV-B genotypes SAB1 to -3 (37). Another HRSV-B genotype includes the Buenos Aires (BA) type strain, which has a 60-nucleotide insertion in the second hypervariable region of the G protein and has been reported in Buenos Aires in 1999, as well as in other areas of the world (31, 35, 40). BA strains are further subdivided into six clusters (BA-I to BA-VI) (36).
National surveillance of HRSV infection based on weekly reports from sentinel pediatric clinics throughout Japan began in November 2003 under the Infectious Disease Control Law. The surveillance indicated that the number of HRSV-infected patients increased dramatically for unknown reasons in the 2005-2006 and 2006-2007 seasons. This study reports large HRSV-A epidemics in the 2005-2006 and 2006-2007 seasons in Niigata, Japan, and provides a possible link to high-level circulation in the same seasons nationwide. It also discusses the relationship between antigenic variation and clinical features of HRSV in regard to molecular epidemiology and the evolutionary patterns of the G protein.
MATERIALS AND METHODS
HRSV surveillance system in Japan.
National surveillance of HRSV infection under the Infectious Disease Control Law began in November, 2003 in Japan (http://idsc.nih.go.jp/iasr/20/230/de2309.html; http://idsc.nih.go.jp/iasr/25/287/tpc287.html). Each HRSV case is defined on the basis of clinical respiratory symptoms, such as rhinorrhea, wheeze, and cough, and laboratory diagnosis, such as virus isolation, serologic test, or rapid antigen test, is carried out at the outpatient clinic or on admission to the hospital. The number of patients diagnosed with HRSV infection is reported on a weekly basis from approximately 3,000 sentinel pediatric clinics/hospitals throughout Japan. These sentinel sites forward clinical data to approximately 60 prefectural or municipal public health institutes, and the data generated are electronically reported to the Infectious Disease Surveillance Center in the National Institute of Infectious Diseases (Tokyo). The weekly total numbers of HRSV cases in Japan that were released on the web site (http://idsc.nih.go.jp/idwr/index.html) was compiled into monthly data to compare with our clinic survey.
Study population and clinical samples.
This study was conducted for six successive seasons, from November 2001 through June 2007, at one pediatric outpatient clinic in Niigata City, Japan. Niigata City is the prefectural capital of Niigata Prefecture, and its total population is approximately 0.8 million. Children under 5 years of age with acute lower respiratory tract illness, such as wheezing, cough, rhinorrhea, and fever, were enrolled in the study. We obtained written informed consent from their guardians and nasopharyngeal aspirates or nasal swabs from the patients. Basic clinical data, such as sex, age, date of clinic visit, and date of onset, were recorded by a pediatrician at the clinic. This study was approved by the Medical Faculty Ethics Committee of Niigata University.
Clinical specimens were kept at 4°C in the clinic; were transported to the Department of Public Health, Graduate School of Medical and Dental Sciences, Niigata University, within 5 days of sampling; and were then kept frozen at −80°C for further examination.
RT-PCR and nucleotide sequencing.
Briefly, for reverse transcription (RT)-PCR and nucleotide sequencing, viral RNA was extracted from 100-μl samples of the nasopharyngeal aspirates or swabs by using an Extragen II kit (Kainos, Tokyo, Japan) according to the manufacturer's instructions. RT to create cDNA was performed using random primers and Moloney murine leukemia virus reverse transcriptase (Invitrogen Corp. Carlsbad, CA) (31).
In accordance with a method previously published (31), first and heminested PCRs targeting a 270-nucleotide segment of the G protein gene's second hypervariable region were performed. Amplified PCR products were purified with a MicroSpin S-300 HR column PCR purification kit (Amersham Bioscience, Buckinghamshire, United Kingdom), labeled with a BigDye terminator (version 3.1) cycle sequencing kit (Applied Biosystems, Foster, CA) according to the manufacturer's instructions, and then analyzed on an ABI 3100 automatic DNA sequencer.
Phylogenetic analysis.
Multiple sequence alignments of G genes for HRSV-A and -B strains were complied with BioEdit, version 7.0.9.0 (12). Phylogenetic trees of the G protein gene's second hypervariable region were generated by using the neighbor-joining method with MEGA, version 4 (34). Bootstrap probabilities for 1,000 iterations were calculated to evaluate confidence estimates. All sequences for both subgroup A and B viruses were included in the phylogenetic analysis. In the phylogeny, strains from Rochester, NY; Winnipeg, Manitoba, Canada; Houston, TX.; St. Louis, MO; Soweto, South Africa; Birmingham, AL; West Virginia; and Buenos Aires, Argentina, were included for comparison. Pairwise nucleotide distances (p distances), that is, the number of pairwise nucleotide differences divided by the total number of nucleotides in the sequenced segment, between representative paired sequences from each genotype of HRSV-A were calculated using MEGA, version 4 (34).
Patient characteristics by genotypes.
Clinical data on patients, such as sex and average age, were compared among seasons and infecting-strain genotypes. Statistical analysis for comparison of mean values in more than three groups was performed by using Scheffe's test. Comparison of the proportions was accomplished with 2-by-multiple tables. Statistical significance was concluded if the P value was less than 0.05.
Deduced amino acids and N-glycosylation site analysis.
The deduced amino acid sequences at the second hypervariable region of HRSV-A and HRSV-B strains were compared to those of the prototype A2 strain, spanning 270 nucleotides, and the BA type strain (BA4128/99B), with 330 nucleotides, respectively (16, 35). Potential N-glycosylation sites were predicted if the encoded amino acids were NXT, where X is not a proline (16, 25, 28).
Nucleotide sequence accession numbers.
The GenBank accession numbers of the nucleotide sequences obtained in the present study are AB470478 to AB470482.
RESULTS
National surveillance of HRSV cases in Japan.
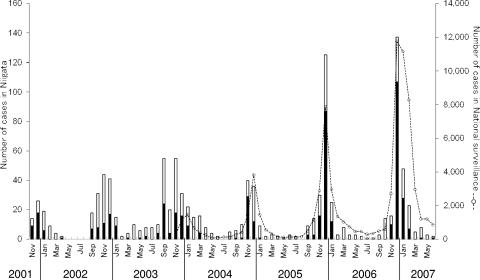
National surveillance for HRSV in Japan started in November 2003, and the disease was most prevalent and clearly active during autumn and early winter (Fig. 1). Large outbreaks of HRSV occurred in the 2005-2006 and 2006-2007 seasons, and the size of the outbreak was bigger in the latter season. In general, the seasonality and the size of the epidemic in our survey matched the national surveillance data.
FIG. 1.
Comparison of monthly number of HRSV cases between a hospital-based survey in Niigata and national surveillance in Japan. The monthly distribution of numbers of screened cases in hospital-based surveillance of HRSV in Niigata City is shown by the stacked bar graph; closed boxes indicate numbers of PCR-positive samples, and open boxes denote PCR-negative samples. The numbers of cases in national surveillance of HRSV in Japan are shown by the line graph.
Study population and demographic details.
Among 1,103 screened cases, a total of 488 HRSV strains were positive by PCR for the six successive seasons from November 2001 through June 2007 in Niigata City, Japan (Table 1). All HRSV-positive samples were sequenced and further analyzed for genotype. The yearly distribution of positive cases varied, depending on the season, from 33 to 154, with the highest positivity rate being observed in the 2006-2007 season (Table 1). The average age of patients in the 2006-2007 season (1.8 ± 1.1 [average ± standard deviation] years old) was significantly higher than in the 2001-2002 (0.8 ± 0.6 years old, P < 0.001), 2002-2003 (1.1 ± 0.8 years old, P < 0.005), and 2003-2004 (1.2 ± 0.8 years old, P < 0.05) seasons, respectively. On the other hand, the average ages of HRSV-negative infants were not significantly different during the study period.
TABLE 1.
Demographic characteristics of the study population divided into HRSV-positive or -negative cases
| Season | Total no. of cases screened | HRSV-positive cases
|
HRSV-negative cases
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases | Avg age (yr) ± SDa | Age distribution [no. (%) of patients]b
|
No. (%) of males | No. of cases | Avg age (yr) ± SDc | Age (yr) distribution [no. (%) of patients]b
|
No. (%) of males | ||||||
| 0-1 yr | 1-2 yr | >2 yr | 0-1 yr | 1-2 yr | >2 yr | ||||||||
| 2001-2002 | 74 | 33 | 0.8 ± 0.6 | 24 (72.7) | 7 (21.2) | 2 (6.1) | 17 (51.5) | 41 | 0.9 ± 0.6 | 24 (58.5) | 14 (34.1) | 3 (7.3) | 25 (61.0) |
| 2002-2003 | 197 | 60 | 1.1 ± 0.8 | 33 (55.0) | 18 (30.0) | 9 (15.0) | 35 (58.3) | 137 | 1.0 ± 0.8 | 84 (61.3) | 34 (24.8) | 19 (13.9) | 85 (62.0) |
| 2003-2004 | 233 | 75 | 1.2 ± 0.8 | 39 (52.0) | 19 (25.3) | 17 (22.7) | 43 (57.3) | 158 | 1.1 ± 0.8 | 83 (52.5) | 55 (34.8) | 20 (12.7) | 90 (57.0) |
| 2004-2005 | 114 | 45 | 1.4 ± 0.8 | 16 (35.6) | 19 (42.2) | 10 (22.2) | 22 (48.9) | 69 | 1.0 ± 0.7 | 35 (50.7) | 25 (36.2) | 9 (13.0) | 40 (58.0) |
| 2005-2006 | 225 | 121 | 1.3 ± 0.9 | 44 (36.4) | 51 (42.1) | 26 (21.5) | 65 (53.7) | 104 | 1.3 ± 1.0 | 48 (46.2) | 30 (28.8) | 26 (25.0) | 61 (58.7) |
| 2006-2007 | 262 | 154 | 1.8 ± 1.1 | 41 (26.6) | 52 (33.8) | 62 (40.3) | 89 (57.8) | 106 | 1.4 ± 1.1 | 47 (44.3) | 32 (30.2) | 26 (24.5) | 64 (60.4) |
| All seasons | 1103 | 488 | 1.4 ± 1.0 | 197 (40.4) | 166 (34.0) | 126 (25.8) | 271 (55.5) | 615 | 1.1 ± 0.9 | 321 (52.2) | 190 (30.9) | 103 (16.7) | 365 (59.3) |
Statistically significant differences in average age of HRSV-positive patients were shown between seasons 2001-2002 and 2006-2007 (P = 0.000), 2002-2003 and 2006-2007 (P = 0.002), 2003-2004 and 2006-2007 (P = 0.013), and 2001-2002 and 2005-2006 (P = 0.046).
No statistically significant differences in age distribution were shown between seasons in HRSV-positive or -negative patients.
No statistically significant differences in average ages of HRSV-negative patients were shown between seasons.
Molecular epidemiology and emergence of new genotype of HRSV in Niigata City.
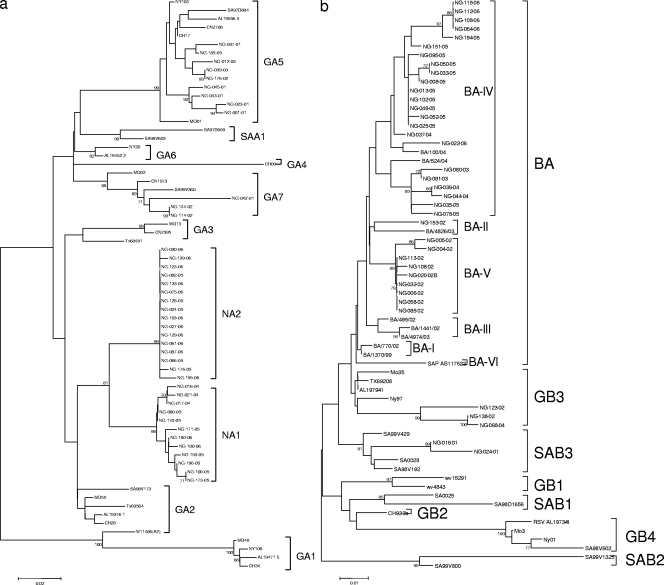
Phylogenetic analysis revealed that 338 samples were HRSV-A and 150 were HRSV-B (Table 2). The HRSV-A strains clustered into four genotypes, namely, GA5, GA7, and the two new genotypes NA1 and NA2, with bootstrap values of 70% to 100% (Fig. 2a). The NA1 and the NA2 strains were genetically close to the GA2 strains. The p distance between each NA1 strain and the representative GA2 strain, which was prototype A2 (M11486), ranged from 0.119 to 0.137, and the p distance between each NA2 strain and the representative GA2 strain ranged from 0.115 to 0.119. Furthermore, the NA1 strains were genetically very close to the NA2 strains, with the p distances between the two groups being 0.041 to 0.070. For reference purposes, the p distances between the HRSV-A and HRSV-B strains in our study were 0.548 to 0.989.
TABLE 2.
Number of cases divided by genotypes
| Season | No. of HRSV-A-positive cases/total no. (%)
|
No. of HRSV-B-positive cases/total no. (%)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Totala | GA5 | GA7 | NA1 | NA2 | Totalb | GB3 | SAB3 | BA | |
| 2001-2002 | 31/33 (93.9) | 30/33 (90.9) | 1/33 (3.0) | 2/33 (6.1) | 2/33 (6.1) | ||||
| 2002-2003 | 13/60 (21.7) | 9/60 (15.0) | 4/60 (6.7) | 47/60 (78.3) | 4/60 (6.7) | 43/60 (71.7) | |||
| 2003-2004 | 67/75 (89.3) | 67/75 (89.3) | 8/75 (10.7) | 8/75 (10.7) | |||||
| 2004-2005 | 36/45 (80.0) | 7/45 (15.6) | 29/45 (64.4) | 9/45 (20.0) | 1/45 (2.2) | 8/45 (17.8) | |||
| 2005-2006 | 85/121 (70.2) | 8/121 (6.6) | 37/121 (30.6) | 40/121 (33.1) | 36/121 (29.8) | 36/121 (29.8) | |||
| 2006-2007 | 106/154 (68.8) | 6/154 (3.9) | 100/154 (64.9) | 48/154 (31.2) | 48/154 (31.2) | ||||
| All seasons | 338/488 (69.3) | 121/488 (24.8) | 5/488 (1.0) | 72/488 (14.8) | 140/488 (28.7) | 150/488 (30.7) | 5/488 (1.0) | 2/488 (0.4) | 143/488 (29.3) |
All genotypes of HRSV-A-positive cases.
All genotypes of HRSV-B-positive cases.
FIG. 2.
Phylogenetic trees for HRSV-A (a) and HRSV-B (b) nucleotide sequences based on the second variable region of the G protein (270 or 330 bp) using the neighbor-joining method with MEGA, version 4. Genotypes were assigned by Peret et al. (genotypes GA1 to GA7 and GB1 to GB4) (27) and Venter et al. (genotypes SAA1 and SAB1 to SAB3) (37). The new type, named BA virus, comprises strains with a 60-nucleotide insertion. Reference GenBank sequences of strains from throughout the world were compared with strains from Niigata (NG); the comparison strains were from Rochester, NY (CH) (27); Winnipeg, Manitoba, Canada (CN) (26); Houston, TX (TX) (26); Rochester, NY (NY) (26); St. Louis, MO (MO) (26); Soweto, South Africa (SA) (37); Birmingham, AL (AL) (26); West Virginia (WV) (32); Sapporo, Japan (SAP) (22); and Buenos Aires, Argentina (BA) (35). The scale bars show the proportions of nucleotide substitutions, and the numbers at the branches are bootstrap values determined for 1,000 iterations. Only bootstrap values of greater than 70% significance are shown.
On the other hand, the HRSV-B strains clustered into three genotypes, namely, GB3, SAB3, and BA, with bootstrap values of 70% to 100% (Fig. 2b). The BA strains, first reported in Buenos Aires in 1999, have a 60-nucleotide insertion in the second hypervariable region of the G protein (35).
The predominant genotype changed during the study period. In HRSV-A strains, genotype GA5 circulated intermittently from the 2001-2002 season through the 2003-2004 season, shifting to NA1 and NA2 from the 2004-2005 season to the 2006-2007 season (Table 2). Among the HRSV-B strains, BA viruses were predominant from the 2002-2003 to the 2006-2007 season. BA viruses in this study were further classified into three groups, namely, BA-II, BA-IV, and BA-V (36). In the 2002-2003 season, 1 BA-II and 40 BA-V strains were detected, and in the subsequent seasons, from the 2003-2004 through the 2006-2007 season, only the BA-IV genotype was found, with the numbers of isolates being 3, 5, 33, and 16 in the 2003-2004, 2004-2005, 2005-2006, and 2006-2007 seasons, respectively.
Age distribution of children with HRSV infections by genotype.
A significant difference in average ages was observed between patients infected with genotypes GA5 and NA2 (1.0 ± 0.8 years old versus 1.7 ± 1.1 years old, P < 0.001) (Table 3) in HRSV-A infection cases. On the other hand, the average age of patients with each genotype among HRSV-B infection cases and between patients infected with HRSV-B and HRSV-A strains as a whole were not statistically different.
TABLE 3.
Age comparison among groups divided by genotypes
| Virus and genotype | No. of cases | Avg age (yr) ± SD | Age distribution [no. (%) of patients]
|
||
|---|---|---|---|---|---|
| 0-1 yr | 1-2 yr | >2 yr | |||
| HRSV-Aa | |||||
| NA1 | 71 | 1.4 ± 0.9 | 26 (36.6) | 30 (42.3) | 16 (22.5) |
| NA2 | 141 | 1.7 ± 1.1 | 35 (24.8) | 53 (37.6) | 53 (37.6) |
| GA5 | 121 | 1.0 ± 0.8 | 71 (58.7) | 31 (25.6) | 19 (15.7) |
| GA7 | 5 | 1.3 ± 1.2 | 3 (60.0) | 0 (0.0) | 2 (40.0) |
| HRSV-Bb | |||||
| BA | 143 | 1.4 ± 1.0 | 58 (40.6) | 51 (35.7) | 34 (23.8) |
| GB3 | 5 | 1.4 ± 1.1 | 3 (60.0) | 0 (0.0) | 2 (40.0) |
| SAB3 | 2 | 0.7 ± 0.3 | 1 (50.0) | 1 (50.0) | 0 (0.0) |
For HRSV-A strains, a statistically significant difference in average age was shown between patients with genotypes NA2 and GA5 (P < 0.001), and a statistically significant higher proportion of patients with genotype NA2 than with GA5 that were >2 years old (P < 0.001) was shown.
For HRSV-B strains, no statistically significant age differences were found between patients with different genotypes.
The proportion of children more than 2 years old infected with NA2 was significantly higher than the proportion infected with GA5 (37.6% versus 15.7%, P < 0.001). Among patients infected with other genotypes, the proportions were not statistically different (Table 3).
Amino acid analysis and N-glycosylation sites.
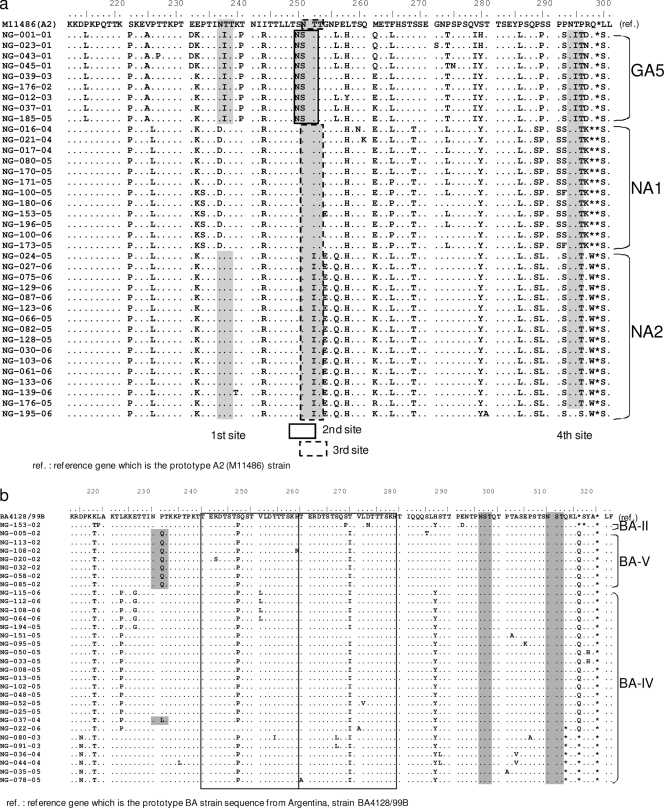
Four putative N-glycosylation sites were identified on the second variable region of the G protein among the HRSV-A strains. The first site, amino acid (aa) position 237, was conserved among the GA5 and NA2 strains but disappeared in the NA1 strain. The second site, aa 250, was present in the genotype GA5 strain only; the third site, aa 251, was identified in the prototype A2 and the NA1 strains as having amino acid coding NTT, and the NA2 strain as having NIT. The fourth site, aa 294, was conserved among all the three genotypes. Other amino acid mutations in comparison to the prototype were observed at residues 226, 256, 262, 274, 290, and 297 (Fig. 3a).
FIG. 3.
Deduced amino acid alignments of the second variable region of the G protein gene from HRSV-A (a) and HRSV-B (b) Niigata strains. Alignments are shown relative to the sequences of prototype strain A2 (GenBank accession number M11486) (16) and genotype BA strain BA4128/99B (GenBank accession number AY333364) (35). The amino acid numbering corresponds to strain A2 G protein positions 220 to 298 for the HRSV-A viruses and to strain BA4128/99B G protein positions 219 to 315 for the HRSV-B viruses. Identical residues are indicated by dots. Stop codons are indicated by asterisks. Potential N-glycosylation sites (NXT, where X is not a proline) are indicated by gray shading. In panel a, second N-glycosylation sites (aa 250) are indicated by rectangles and third sites (aa 251) by dotted rectangles. In panel b, the two copies of the duplicated 20-amino-acid region in HRSV-B viruses are indicated by rectangles.
Three N-glycosylation sites were identified among the group B strains (Fig. 3b). The first site was identified at aa 230 in the BA-V strains only, while the other two sites, at aa 296 and aa 310, were conserved among almost all the strains at the C-terminal end of the G protein gene. Other amino acid mutations in comparison to the prototype were observed at residues 218, 247, 270, and 287 (Fig. 3b).
Reinfection.
Reinfection was defined as an infection that occurred after a month-long period from the first infection without any HRSV clinical manifestation of acute respiratory infection. Over the study period, 39 (8.0%) of 488 patients were documented with HRSV reinfections (Table 4). Among them, eight (20.5%) were homologous reinfections, four (10.3%) being GA5, three (7.7%) NA2, and one (2.6%) BA. In our study, 20 of 39 reinfections were caused by NA2, the highest frequency of all the genotypes. Of these, the first infection was due to homologous NA2 in three cases, to NA1 and BA strains in eight cases each, and to a GA5 strain in one case.
TABLE 4.
Genotypes among children who were infected with HRSV twice during the 2001-2002 through 2006-2007 seasons
| 1st infection genotype | No. of children infected with indicated genotype at 2nd infection
|
||||
|---|---|---|---|---|---|
| GA5 | NA1 | NA2 | BA | Total | |
| GA5 | 4 | 1 | 1 | 4 | 10 |
| NA1 | 0 | 0 | 8 | 1 | 9 |
| NA2 | 0 | 0 | 3 | 1 | 4 |
| BA | 4 | 3 | 8 | 1 | 16 |
| Total | 8 | 4 | 20 | 7 | 39 |
DISCUSSION
Our epidemiological study indicates that large epidemics of HRSV occurred in Niigata in the 2005-2006 and 2006-2007 seasons, which coincided with the Japanese national surveillance data. It is well known that large influenza outbreaks occurred due to the emergence of new serotypes or subtypes of the influenza virus (30, 39), but this phenomenon has not been reported for HRSV. In Niigata, the BA strain emerged as a predominant genotype in the 2002-2003 season and continued to circulate during six successive seasons until the 2006-2007 season but did not cause large outbreaks thereafter, although a variety of genotypes were detected within the BA strains (31).
In addition, two novel genotypes, NA1 and NA2, which were GA2 variants of HRSV-A, were detected. The two genotypes were very close to each other but distinguished from GA2 in the phylogenetic tree and pairwise nucleotide distances. Both genotypes caused large epidemics in the 2005-2006 season, with NA1 and NA2 cocirculating, and in the 2006-2007 season, with NA2 predominating. In recent years, GA5 and GA2 were the most common genotypes among HRSV-A (17, 25, 41), but the new GA2 variants NA1 and NA2 have not been reported in Japan before. Thus, it was speculated that the large epidemics occurred due to the emergence of NA1 and NA2, because most of the children did not have immunity. We also detected NA2 strains from pediatric samples in Lebanon and Vietnam in 2008 (unpublished data), suggesting that this strain might be circulating globally. However, there are no reports of large outbreaks caused by the GA2 variants in other areas, so further studies are warranted to confirm our findings.
In general, most individuals are infected with HRSV in the first year of life and few escape infection by this virus during infancy or early childhood. That is why the age of children infected with HRSV tended to be younger. However, the results of our study indicated that the average age of the HRSV cases during the large outbreak in the 2006-2007 season was significantly older than in the other seasons. Of note, the ages of NA2-infected children were significantly older than the ages of children infected with other genotypes. This could be due to a low level of past immunity against the newly emerged NA2 among most infants and children.
Antigenic variation between and within the subgroups may contribute to reinfections by evading the host immune responses. Almost all homologous HRSV reinfections observed in this study were caused by HRSV-A viruses. This is in line with the findings of previous studies (20, 41) which suggested that HRSV-A may not elicit a complete and/or long-lasting subgroup-specific immune response compared to that elicited by HRSV-B. Interestingly, reinfection caused by NA2 occurred more frequently than reinfections by other genotypes, although it only circulated during two seasons. Thus, our observations led us to surmise that a weaker neutralizing immune response against NA2 was a cause for a higher rate of reinfection with NA2 strains.
However, it was unclear why NA1 was replaced by NA2 and why it did not become the dominant genotype in the following epidemic. Genetically, NA1 strains were very close to NA2 strains, as the p distance between the NA1 and NA2 strains was 0.041 to 0.070. However, several amino acid changes and different N-glycosylation sites in the second hypervariable region on the G protein might have contributed to a change in antigenicity between the genotypes (3, 24). Yet, this remains to be confirmed by serological studies.
After the appearance of the BA virus, which has a duplication of 60 nucleotides, in the 2002-2003 season (31), BA viruses were in circulation Niigata consistently thereafter. Trento et al. analyzed HRSV-B collected in Buenos Aires and classified the isolates into clusters BA-I to BA-IV by analyzing the entire G gene sequence (36). Also, they compared a worldwide collection of BA strains by partial sequence analysis of the last 330 nucleotides and reported six clusters, BA-I to BA-VI. In our analysis, we found that three clusters, BA-II, -IV, and -V, circulated during the study period. In particular, BA-V was unique to Niigata, and it circulated only in the 2002-2003 season. A specific amino acid change of P231Q in the second hypervariable region was observed, which can lead to aa position 230 being a potential N-glycosylation site. After the 2002-2003 season, BA-V disappeared and BA-IV became dominant. It eventually became the most diverse genetic cluster, as can be seen from the results of this study and previous reports from other countries (36). The reason for the successful circulation of this subgenotype for years remains to be determined.
In conclusion, we found novel emerging genotypes of HRSV-A, namely, NA1 and NA2, in Niigata, Japan. The analysis of both clinical and molecular biological information showed a possible correlation between the emergence of genotype NA2 and higher chances for reinfection, which may eventually lead to large outbreaks of HRSV in Japan. Furthermore, we would like to emphasize the importance of long-term molecular epidemiological surveys for early detection of newly emerging genotypes and the benefit of studies to elucidate the genetic and antigenic mechanisms underlying reinfection for better understanding of HRSV infections.
Acknowledgments
We thank the staff at Sano Pediatric Clinic who assisted with collection of the clinical information and samples; Naohito Tanabe and Asami Sasaki and the staff in the Department of Public Health, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan, for help with the study design and statistical analysis; Akemi Watanabe for her excellent technical assistance; Akinori Miyashita and Ryozo Kuwano in the Center for Bioresources, Brain Research Institute, Niigata University, for utilization of the DNA sequencer; and Miwako Saikusa in Yokohama Municipal Laboratory for valuable advice and providing positive controls to initiate this study.
Footnotes
Published ahead of print on 24 June 2009.
REFERENCES
- 1.Anderson, L. J., J. C. Hierholzer, C. Tsou, R. M. Hendry, B. F. Fernie, Y. Stone, and K. McIntosh. 1985. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J. Infect. Dis. 151626-633. [DOI] [PubMed] [Google Scholar]
- 2.Cane, P. A. 1997. Analysis of linear epitopes recognised by the primary human antibody response to a variable region of the attachment (G) protein of respiratory syncytial virus. J. Med. Virol. 51297-304. [DOI] [PubMed] [Google Scholar]
- 3.Cane, P. A. 2001. Molecular epidemiology of respiratory syncytial virus. Rev. Med. Virol. 11103-116. [DOI] [PubMed] [Google Scholar]
- 4.Cane, P. A., and C. R. Pringle. 1995. Evolution of subgroup A respiratory syncytial virus: evidence for progressive accumulation of amino acid changes in the attachment protein. J. Virol. 692918-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coates, H. V., D. W. Alling, and R. M. Chanock. 1966. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am. J. Epidemiol. 83299-313. [DOI] [PubMed] [Google Scholar]
- 6.Collins, P. L., and J. E. Crowe, Jr. 2006. Respiratory syncytial virus, p. 1601-1646. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields Virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 7.Cox, M. J., R. S. Azevedo, P. A. Cane, E. Massad, and G. F. Medley. 1998. Seroepidemiological study of respiratory syncytial virus in Sao Paulo state, Brazil. J. Med. Virol. 55234-239. [DOI] [PubMed] [Google Scholar]
- 8.Galiano, M. C., C. Palomo, C. M. Videla, J. Arbiza, J. A. Melero, and G. Carballal. 2005. Genetic and antigenic variability of human respiratory syncytial virus (groups A and B) isolated over seven consecutive seasons in Argentina (1995 to 2001). J. Clin. Microbiol. 432266-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García, O., M. Martín, J. Dopazo, J. Arbiza, S. Frabasile, J. Russi, M. Hortal, P. Perez-Breña, I. Martínez, and B. García-Barreno. 1994. Evolutionary pattern of human respiratory syncytial virus (subgroup A): cocirculating lineages and correlation of genetic and antigenic changes in the G glycoprotein. J. Virol. 685448-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glezen, W. P., L. H. Taber, A. L. Frank, and J. A. Kasel. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 140543-546. [DOI] [PubMed] [Google Scholar]
- 11.Hall, C. B., J. M. Geiman, R. Biggar, D. I. Kotok, P. M. Hogan, and G. R. Douglas, Jr. 1976. Respiratory syncytial virus infections within families. N. Engl. J. Med. 294414-419. [DOI] [PubMed] [Google Scholar]
- 12.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 4195-98. [Google Scholar]
- 13.Henderson, F. W., A. M. Collier, W. A. Clyde, Jr., and F. W. Denny. 1979. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N. Engl. J. Med. 300530-534. [DOI] [PubMed] [Google Scholar]
- 14.Henderson, J., T. N. Hilliard, A. Sherriff, D. Stalker, N. Al Shammari, and H. M. Thomas. 2005. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr. Allergy Immunol. 16386-392. [DOI] [PubMed] [Google Scholar]
- 15.Hendry, R. M., A. L. Talis, E. Godfrey, L. J. Anderson, B. F. Fernie, and K. McIntosh. 1986. Concurrent circulation of antigenically distinct strains of respiratory syncytial virus during community outbreaks. J. Infect. Dis. 153291-297. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, P. R., M. K. Spriggs, R. A. Olmsted, and P. L. Collins. 1987. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc. Natl. Acad. Sci. USA 845625-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuroiwa, Y., K. Nagai, L. Okita, I. Yui, T. Kase, T. Nakayama, and H. Tsutsumi. 2005. A phylogenetic study of human respiratory syncytial viruses group A and B strains isolated in two cities in Japan from 1980-2002. J. Med. Virol. 76241-247. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald, N. E., C. B. Hall, S. C. Suffin, C. Alexson, P. J. Harris, and J. A. Manning. 1982. Respiratory syncytial viral infection in infants with congenital heart disease. N. Engl. J. Med. 307397-400. [DOI] [PubMed] [Google Scholar]
- 19.Melero, J. A., B. Garcia-Barreno, I. Martinez, C. R. Pringle, and P. A. Cane. 1997. Antigenic structure, evolution and immunobiology of human respiratory syncytial virus attachment (G) protein. J. Gen. Virol. 78(pt 10)2411-2418. [DOI] [PubMed] [Google Scholar]
- 20.Mufson, M. A., R. B. Belshe, C. Orvell, and E. Norrby. 1987. Subgroup characteristics of respiratory syncytial virus strains recovered from children with two consecutive infections. J. Clin. Microbiol. 251535-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mufson, M. A., C. Orvell, B. Rafnar, and E. Norrby. 1985. Two distinct subtypes of human respiratory syncytial virus. J. Gen. Virol. 66(pt 10)2111-2124. [DOI] [PubMed] [Google Scholar]
- 22.Nagai, K., H. Kamasaki, Y. Kuroiwa, L. Okita, and H. Tsutsumi. 2004. Nosocomial outbreak of respiratory syncytial virus subgroup B variants with the 60 nucleotides-duplicated G protein gene. J. Med. Virol. 74161-165. [DOI] [PubMed] [Google Scholar]
- 23.Ostlund, M. R., A. T. Lindell, S. Stenler, H. M. Riedel, B. Z. Wirgart, and L. Grillner. 2008. Molecular epidemiology and genetic variability of respiratory syncytial virus (RSV) in Stockholm, 2002-2003. J. Med. Virol. 80159-167. [DOI] [PubMed] [Google Scholar]
- 24.Palomo, C., P. A. Cane, and J. A. Melero. 2000. Evaluation of the antibody specificities of human convalescent-phase sera against the attachment (G) protein of human respiratory syncytial virus: influence of strain variation and carbohydrate side chains. J. Med. Virol. 60468-474. [PubMed] [Google Scholar]
- 25.Parveen, S., W. M. Sullender, K. Fowler, E. J. Lefkowitz, S. K. Kapoor, and S. Broor. 2006. Genetic variability in the G protein gene of group A and B respiratory syncytial viruses from India. J. Clin. Microbiol. 443055-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peret, T. C., C. B. Hall, G. W. Hammond, P. A. Piedra, G. A. Storch, W. M. Sullender, C. Tsou, and L. J. Anderson. 2000. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J. Infect. Dis. 1811891-1896. [DOI] [PubMed] [Google Scholar]
- 27.Peret, T. C., C. B. Hall, K. C. Schnabel, J. A. Golub, and L. J. Anderson. 1998. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J. Gen. Virol. 79(pt 9)2221-2229. [DOI] [PubMed] [Google Scholar]
- 28.Roca, A., M. P. Loscertales, L. Quinto, P. Perez-Brena, N. Vaz, P. L. Alonso, and J. C. Saiz. 2001. Genetic variability among group A and B respiratory syncytial viruses in Mozambique: identification of a new cluster of group B isolates. J. Gen. Virol. 82103-111. [DOI] [PubMed] [Google Scholar]
- 29.Rueda, P., T. Delgado, A. Portela, J. A. Melero, and B. Garcia-Barreno. 1991. Premature stop codons in the G glycoprotein of human respiratory syncytial viruses resistant to neutralization by monoclonal antibodies. J. Virol. 653374-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakai, T., H. Suzuki, A. Sasaki, R. Saito, N. Tanabe, and K. Taniguchi. 2004. Geographic and temporal trends in influenzalike illness, Japan, 1992-1999. Emerg. Infect. Dis. 101822-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato, M., R. Saito, T. Sakai, Y. Sano, M. Nishikawa, A. Sasaki, Y. Shobugawa, F. Gejyo, and H. Suzuki. 2005. Molecular epidemiology of respiratory syncytial virus infections among children with acute respiratory symptoms in a community over three seasons. J. Clin. Microbiol. 4336-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullender, W. M., M. A. Mufson, L. J. Anderson, and G. W. Wertz. 1991. Genetic diversity of the attachment protein of subgroup B respiratory syncytial viruses. J. Virol. 655425-5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullender, W. M., L. Sun, and L. J. Anderson. 1993. Analysis of respiratory syncytial virus genetic variability with amplified cDNAs. J. Clin. Microbiol. 311224-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 241596-1599. [DOI] [PubMed] [Google Scholar]
- 35.Trento, A., M. Galiano, C. Videla, G. Carballal, B. Garcia-Barreno, J. A. Melero, and C. Palomo. 2003. Major changes in the G protein of human respiratory syncytial virus isolates introduced by a duplication of 60 nucleotides. J. Gen. Virol. 843115-3120. [DOI] [PubMed] [Google Scholar]
- 36.Trento, A., M. Viegas, M. Galiano, C. Videla, G. Carballal, A. S. Mistchenko, and J. A. Melero. 2006. Natural history of human respiratory syncytial virus inferred from phylogenetic analysis of the attachment (G) glycoprotein with a 60-nucleotide duplication. J. Virol. 80975-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venter, M., S. A. Madhi, C. T. Tiemessen, and B. D. Schoub. 2001. Genetic diversity and molecular epidemiology of respiratory syncytial virus over four consecutive seasons in South Africa: identification of new subgroup A and B genotypes. J. Gen. Virol. 822117-2124. [DOI] [PubMed] [Google Scholar]
- 38.Welliver, R. C. 2003. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J. Pediatr. 143S112-S117. [DOI] [PubMed] [Google Scholar]
- 39.Zaraket, H., R. Saito, N. Tanabe, K. Taniguchi, and H. Suzuki. 2008. Association of early annual peak influenza activity with El Nino southern oscillation in Japan. Influenza Other Respir. Viruses 2127-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zlateva, K. T., P. Lemey, E. Moes, A. M. Vandamme, and M. Van Ranst. 2005. Genetic variability and molecular evolution of the human respiratory syncytial virus subgroup B attachment G protein. J. Virol. 799157-9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zlateva, K. T., L. Vijgen, N. Dekeersmaeker, C. Naranjo, and M. Van Ranst. 2007. Subgroup prevalence and genotype circulation patterns of human respiratory syncytial virus in Belgium during ten successive epidemic seasons. J. Clin. Microbiol. 453022-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]