Abstract
Sonication of implants has been shown to be a promising method for diagnosis of prosthetic infections due to its improved sensitivity, simplicity, and low cost. The aim of the present study was to evaluate the effects of ultrasound performed under different conditions regarding temperature, duration, and composition of sonication tubes on bacterial species often associated with prosthetic infections. We found that ultrasound had an inhibitory effect on bacteria, of which gram-negative bacteria, in particular Escherichia coli, were almost eradicated after 5 min of sonication at 35°C. Gram-positive bacteria were found to be resistant to the effect of ultrasound. Four factors were important for the inhibitory effect of sonication: the type of microorganism, the temperature of the sonication buffer, the duration of exposure to ultrasound (minutes), and the material and composition of the sonication tube in which sonication is performed. On the basis of the results from the present study, we propose a protocol for sonication and recovery of bacteria associated with biofilm on infected implants prior to conventional culture. From the present protocol, we recommend sonication for 7 min at 22°C at the maximum effect which permits survival of gram-negative bacteria.
In the United States, 250,000 hip and 400,000 knee replacements are performed annually, and the numbers are expected to double within the next 2 decades due to an increasing number of elderly persons (9). Prosthetic joint infection is second to aseptic failure as a cause of joint replacement and is associated with a substantial morbidity rate and cost (20). The levels of risk for infection within 2 years after insertion are less than 1% and 2% for hip and knee implants, respectively, compared with 5 to 40% after revision surgery (20). The main sources of infection are contamination from the skin flora of patients or hospital staff (12).
Diagnosis of prosthetic infections is still a challenge, as clinical signs and a laboratory investigation, including microbiological findings, do not always distinguish aseptic loosening from loosening due to infection (6, 8, 27). Microbiological investigation of implants is complicated due to the sizes of the prosthetic components and problems associated with conventional culture, i.e., presence of intracellular bacteria, microorganisms in biofilm, and small-colony variants of bacteria (1, 5, 9, 14), the last of which may be induced after treatment with gentamicin (26).
Due to its simplicity and low cost, sonication appears to be the most promising method among the newer techniques for diagnosis of infected implants (2, 4, 10, 15, 17, 19, 21, 23). Several studies have shown that sonication of implants improves sensitivity compared with that obtained with periprosthetic-tissue culture (2, 8, 19, 24).
In medicine, ultrasound transducers are used for diagnosis of abnormalities and fetal, abdominal, and heart diseases. Ultrasound has been applied in microbiology for sonication and inactivation of bacteria in food processing (13). However, the effect of ultrasound has mainly been studied for food- and water-associated bacteria, and data are lacking for clinically important species, such as staphylococci, enterococci, Haemophilus influenzae, and Pseudomonas aeruginosa (3, 13).
The aim of the present study was to evaluate the effects of temperature, duration, composition of the sonication buffer, and material in the sonication tube during sonication of bacteria often associated with prosthetic infections. On the basis of the results from these experiments, we propose a protocol for sonication of biofilm on extracted implants prior to conventional culture.
MATERIALS AND METHODS
Definitions.
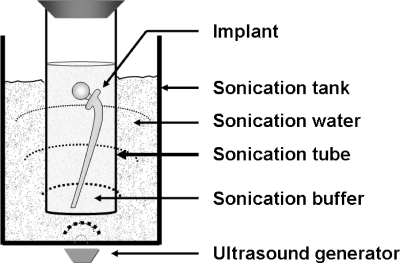
The terms used in this article are defined as follows: ultrasound, sound waves with frequencies of ≥20 kHz (13); sonication apparatus, a generator of ultrasound (Fig. 1); sonication tank, a container with water through which the ultrasound is transduced; sonication buffer, a sterilized buffer in which bacteria are suspended or released during sonication prior to conventional culture (Fig. 1); sonicate, sonication buffer after sonication; and sonication tube, a space where sonication of bacteria or implants is performed (Fig. 1).
FIG. 1.
Sonication system.
Bacterial isolates and sonication tubes.
The bacterial species and the culture conditions tested are presented in Table 1.
TABLE 1.
Bacterial species and culture conditions
| Bacterial species | CCUGa no. | Agar | Culture conditions |
|---|---|---|---|
| Escherichia coli | CCUG 17620 | Horse blood | Aerobic, 35°C, 24 h |
| Haemophilus influenzae | CCUG 23946 | McLeod | 5% CO2, 35°C, 24 h |
| Pseudomonas aeruginosa | CCUG 17619 | Horse blood | Aerobic, 35°C, 24 h |
| Enterococcus faecalis | CCUG 9997 | Horse blood | Aerobic, 35°C, 24 h |
| Staphylococcus aureus | CCUG 1800 | Horse blood | Aerobic, 35°C, 24 h |
| Staphylococcus epidermidis | CCUG 1621 | Horse blood | Aerobic, 35°C, 24 h |
CCUG, Culture Collection University of Göteborg, Sweden.
Influence of sonication temperature and time on the outcome of sonication.
Bacteria were grown overnight on agar plates under the different conditions described in Table 1.
Overnight colonies were suspended in sonication buffer (0.1 M phosphate-buffered saline [PBS] or 0.9% [wt/vol] sodium chloride [saline]) and diluted to a final concentration of 1 × 103 CFU/ml. A volume of 5 ml was added to a sonication tube of soda glass (10.9 by 160 by 1.0 mm) (catalogue no. 005-1400-16110; Bergman Labora, Sweden) with a screw cap and a volume of 10 ml. Alternatively, a 100-ml bacterial suspension was added to a 600-ml beaker (JenaerGlas, Rasotherm, Germany) or a 500-ml measure cylinder of glass (Witeg, Diffico, Germany). A Transonic Digital S (type, T490 DH; 40 kHz, 350 W; Elma, Singen, Germany) at 100% effect was used for sonication. Sonication for 60 min was performed with intermittent sonication at fixed temperatures (22°C and 35°C) or with continuous sonication at increasing temperatures from 22°C to 42°C after 60 min. Sonication at fixed temperatures (22°C and 35°C) was accomplished by adding a small amount of ice into the sonication tank after each episode of 5 min of uninterrupted sonication until a total of 60 min of sonication was completed (referred to as intermittent sonication). Samples (100 μl) from the inoculated sonication buffer were collected for culture at the start (0 min) and after 5, 10, 15, 20, 25, 30, 45, and 60 min of sonication. Escherichia coli, Pseudomonas aeruginosa, Enterococcus faecalis, Staphylococcus aureus, and Staphylococcus epidermidis were inoculated on horse blood (5%) agar, and Haemophilus influenzae was inoculated on McLeod agar. For growth conditions, see Table 1. All experiments were performed at least twice.
Influence of sonication tubes' exposure to ultrasound on outcome of sonication.
A 5-ml bacterial suspension of Staphylococcus aureus or Escherichia coli in sonication tubes of glass (10 ml) were submerged from partial to full (1/4 to 1/2 to 1/1) contact with water in the sonication tank. The inoculated sonication tubes were sonicated for 5 to 60 min at 22°C before plating.
Influence of composition of sonication tubes on outcome of sonication.
The effect of sonication on bacteria was evaluated with and without use of sonication tubes. A bacterial suspension of 100 ml was added into a measure cylinder (500 ml [thick-walled glass]) and a beaker (600 ml [thin-walled glass]) and sonicated. Sonication without sonication tubes was accomplished with substitution of the water within the sonication tank with a bacterial suspension. The bacterial suspensions, without or within the beaker/measure cylinders/sonication tube, were continuously sonicated for 60 min.
RESULTS
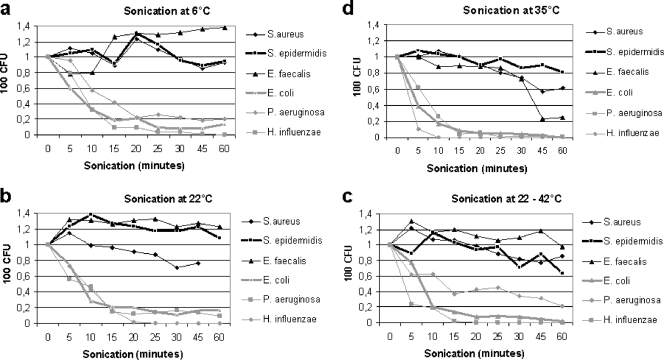
Data for recovery (mean values) of bacteria after sonication at different temperatures are presented in Fig. 2a to d. The effects of ultrasound varied among bacteria. Gram-positive bacteria were in general more resistant to the effect of ultrasound than were gram-negative bacteria (Fig. 2a to d). For gram positives, in particular Staphylococcus epidermidis as well as Enterococcus faecalis, the concentrations of bacteria increased from 1 ×103 to 1.3 ×103 CFU/ml during the first 10 min of sonication at 22°C and remained above the initial concentration (Fig. 2b) during the 60 min of sonication.
FIG. 2.
Sonication of bacteria at different temperatures. Results are shown for sonication (100% effect) of bacteria at different temperatures (a to d) at 6, 22, 35, and 22 to 42°C. “CFU” denotes numbers of CFU on agar plates after culture of 100 μl of a bacterial solution (1,000 CFU/ml in test tubes) exposed to sonication of ultrasound. “Sonication (minutes)” denotes the duration of exposure to ultrasound in minutes.
Among the tested gram-negative bacteria, H. influenzae was the most sensitive species and was almost eradicated after 5 min of sonication at 35°C (Fig. 2b).
S. aureus was intermediately resistant to the effect of ultrasound, with an approximately 40% reduction of bacteria after 60 min of sonication at 22°C (Fig. 2).
Sonication at 22°C yielded a higher rate of recovery of bacteria than sonication at increased temperatures (Fig. 2a to d). Sonication at 35°C was the temperature with the lowest recovery rates, in particular for gram-negative bacteria, which were almost eradicated after sonication for 15 min (Fig. 2d). The result for continuous sonication at 22 to 42°C was similar to the results for sonication at 22°C (Fig. 2b and c).
Two buffers, PBS and sodium chloride, were tested on E. coli and H. influenzae. The effects of ultrasound revealed only minor differences between these buffers (data not shown).
Influence of composition of sonication tubes.
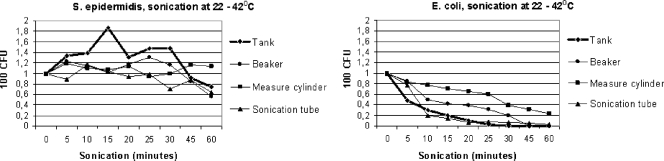
The effect of sonication was more pronounced in sonication tubes of glass than in those of plastic, especially of softer material (data not shown). Sonication in thin-walled glass (beaker) gave a lower recovery rate than sonication in the thick-walled sonication tube (measure cylinder), and this effect was more pronounced for gram-negative bacteria (Fig. 3). The reduced effect of ultrasounds passing through glass was visualized by comparing the results from sonication of bacteria with and without sonication tubes (i.e., substitution of water with bacterial suspension in the sonication tank). After 10 and 60 min of continuous sonication in thick-walled sonication tubes, 77% and 23% of E. coli bacteria, respectively, were recovered, compared with 30% and <2%, respectively, without sonication tubes (Fig. 3).
FIG. 3.
Evaluation of continuous sonication of bacteria without and with different sonication tubes. “CFU” denotes numbers of CFU on agar plates after culture of 100 μl of a bacterial solution (1,000 CFU/ml in test tubes) exposed to sonication of ultrasound. “Sonication (minutes)” denotes the duration of exposure to ultrasound in minutes. “Tank,” “beaker,” “measure cylinder,” and “sonication tube” denote the environments in which sonication of bacteria was performed.
Exposure of sonication tubes.
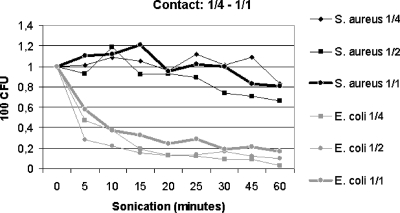
Sonication tubes with S. aureus or E. coli submerged to 1/4 to 1/1 contact with the water within the sonication tank revealed only minor differences in bacterial recovery after sonication at 22°C (Fig. 4). The results were similar to those observed for sonication at 22°C, as shown in Fig. 2.
FIG. 4.
Contact study using inoculated sonication tubes exposed to 1/4 to 1/1 contact with the water in the sonication tank. Results are shown for sonication (100% effect) at 22°C with 100% effect with partial (1/4 to 1/2) to full (1/1) contact between the sonication tube (10 ml) and the water in the sonication tank. “CFU” denotes numbers of CFU on agar plates after culture of 100 μl of a bacterial solution (1,000 CFU/ml in test tubes) exposed to sonication of ultrasound. “Sonication (minutes)” denotes the duration of exposure to ultrasound in minutes.
DISCUSSION
Conventional tissue culture is at present the gold standard for diagnosis of prosthetic infections (28). Ultrasound sonication of extracted implants prior to culture is a new and promising technique for improved diagnosis of implant infections associated with biofilm.
In the present study, we evaluated the in vitro effects of ultrasound under different conditions (temperature, composition of sonication fluid, and material in the sonication tube) on common pathogens associated with implant infections. From the in vitro results, we propose a protocol for sonication of biofilm from extracted implants prior to conventional culture.
From the in vitro studies, we identified four specific factors that influenced the bacteria. First, gram-negative bacteria were more susceptible than gram positives to the effect of ultrasound. This difference is probably related to the fact that gram positives possess a thicker and more robust cell wall due to cross-linking of peptidoglycan and teichoic acid (25), which make these bacteria less susceptible to ultrasound. For staphylococci and streptococci, we observed increased concentrations of bacteria (CFU/ml) during the sonication, probably due to a split of aggregates and chains of bacteria. A similar effect was not seen among gram-negative bacteria, which normally do not form aggregates or chains during culture.
Second, a longer duration of exposure to ultrasound was associated with decreased recovery. This effect was most pronounced for E. coli and H. influenzae, which were almost eradicated after 15 min of exposure at 22°C. Similar results have also been reported for other species (13, 16).
Third, the temperature of the sonication buffer was important for bacterial recovery, in particular for gram-negative bacterial species, such as H. influenzae. Room temperature (22°C) was associated with improved recovery compared with that obtained with sonication at 35°C. Sonication at 6°C only slightly improves bacterial recovery. In clinical practice, sonication at 22°C is preferred since no equipment for regulation of temperature is required.
Fourth, the material and composition of the sonication tube influence the outcome of bacterial recovery. Sonication was most efficient without the use of sonication tubes (i.e., bacterial solution in a sonication tank). Sonication in the thin-walled glass tube was more efficient, probably due to less absorption of energy than for thick-walled glass or soft plastic material (data not shown). For these reasons and for practicality, we recommend sonication in thin-walled sonication tubes, preferably of glass. Moreover, glass is impermeable, inert, and easily sterilized by heat according to good laboratory practice.
In agreement with other studies, we found that the composition of the sonication buffer was of minor importance (3, 13). We found no conclusive difference in the survival rates of E. coli upon comparing the effects of PBS to those of saline. PBS may be preferred due to a slightly higher efficacy for most of the species.
Prosthetic components are often large, and an important question is whether sonication tubes with partial contact with the water in the sonication tank are associated with loss of efficacy. We found only minor differences in bacterial recovery between tubes exposed to 1/4 and 1/1 water contact. These results indicate that ultrasound is effectively transduced even into sonication tubes that are in partial contact with the tank water. In clinical practice, we propose that large implants can be sonicated in a vertical position with good efficacy even when there is only a partial contact between the sonication tube and the surrounding tank water (Fig. 1).
In a previously described method reported by Trampuz et al., a fixed volume (400 ml) was used for sonication (19). In the present method, the required volume of sonication buffer varies with the sizes of implant and the sonication tube. We recommend the use of sonication tubes as small as possible, still allowing the buffer to cover the entire implant. This will reduce the number of centrifugations required for sedimentation of the sonicate. However, it is important that implants are completely soaked in buffer during sonication (Fig. 1).
The cutoff for significant numbers of bacteria per implant associated with infection may be difficult to define. In the method reported by Trampuz et al. (19), the cutoff and the detection limit for significant numbers of CFU/implant were set to ≥4.000 CFU/implant and ≥800 CFU/implant, respectively, compared with an estimated detection limit below 100 and a detection limit of ≥1 CFU/implant in the present method. The cutoff set by Trampuz et al. is probably too high, resulting in low sensitivity, mainly due to the inherent limitations of that protocol (19). Moreover, laboratory contamination, usually coagulase-negative staphylococci, is difficult to eliminate but usually generates low bacterial counts compared with the number of bacteria recovered from infected implants with the proposed protocol.
Extraction and sonication of prosthetic components are always associated with risk for contamination, and contamination of plastic bags due to penetration of sharp bone or cement fragments and/or effects of irradiation has previously been reported (18). An important conclusion from that study was that sonication should be done in impermeable sonication tubes, preferably of glass.
There are some limitations of the present study. First, only a limited number of bacterial species were analyzed. However, the included species represents a variety of gram-negative and gram-positive bacteria, of which some often are associated with prosthetic infections (11, 22). Other species, such as E. coli and H. influenzae, were included due to their suspected susceptibility to ultrasound. Despite the limited number of species analyzed, we found similar trends in outcome for gram-positive and gram-negative bacteria. Second, our results are based only on in vitro studies. However, in vivo studies of colonized implants may be very difficult to standardize and to carry out.
The interexperiment variation in outcome among gram-positive bacteria complicated to some extent the reproducibility and interpretation of the results. This interexperiment variation may also to some extent be explained by the nature of sound waves as the effect of interference between sound waves. Hence, the position of the test tubes in the sonication tank may influence the outcome. Despite our efforts to standardize the sonication procedure, we were not able to eliminate the interexperiment variation in outcome. We have from our recent experiments also observed a considerable variation in outcome between different sonication apparatus. For this reason, we recommend that users calibrate the sonication method prior to examination of clinical samples in order to optimize the recovery of bacteria. Since gram-negative bacteria are most susceptible to the effect of ultrasound, we suggest calibration against Escherichia coli CCUG 17620. The time of exposure to ultrasound that reduces the number of viable bacteria from 100 to 30 CFU/ml (approximately 70% reduction) at 22°C (Fig. 2b) is the maximum time of exposure that does not adventure culture of viable bacteria, whether this time of exposure is less or more than the 7 min in the present method.
The amount of bacteria released from biofilm associated with implants probably depends on the time of exposure to ultrasound. However, if infection due to gram-positive bacteria is suspected, a repeated sonication for another 30 min may increase the outcome. We do recommend that the 7-min sonicate be cultured prior to repeated sonication.
From our experiment, we propose sonication for 7 min, which seems reasonable with respect to the susceptibility of gram-negative bacteria to ultrasound versus the resistance of gram-positive bacteria (Fig. 2). Further studies are needed to evaluate if 7 min of sonication is the optimal time associated with a maximum release of bacteria from real extracted implants versus the lethal effect of ultrasound.
Suggested protocol for sonication of biofilm associated to implants.
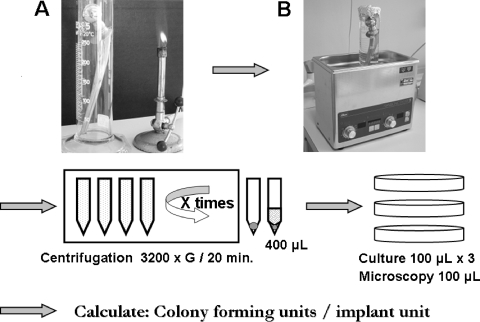
From the results of the present study, we propose the following protocol for sonication of implants in clinical practice (Fig. 5). (i) Extracted implants should be soaked in sterilized buffer and immediately transported to the laboratory. (ii) At the laboratory, the implant is then transferred to a new and sterilized sonication tube, preferably of glass, and totally soaked in a sterilized sonicate solution (PBS or saline). (iii) The sonication tube is sealed (to prevent contamination) and sonicated for 7 min at room temperature. (iv) After sonication, the sonicate solution is sedimented by centrifugation (3,200 × g for 20 min). (v) The sediment(s), preferably in one centrifuge tube after the final centrifugation, is resuspended in sterilized saline to give a total volume of 400 μl. The suspended pellet is divided into four portions of 100 μl each for direct microscopy and culture on appropriate agar media (blood, hematin, and anaerobic agar plates) (7). (vi) The number of CFU per implant is calculated from the agar plate with the highest number of CFU by multiplying this number by 4 (thus compensating for the 1:4 split of the bacterial suspension prior to culture). (vii) Finally, bacteria are identified and tested for antimicrobial susceptibility according to standard laboratory practice.
FIG. 5.
Protocol for sonication of prosthetic units. (A) Sonication tube (glass cylinder) with the implant. (B) Sonication apparatus with the sonication tube and implant within the sonication tank.
The benefits of the present protocol are that the risk for contamination is low and that the recovered number of bacteria is independent of the volume of sonication buffer used, especially as the sizes of implants differ considerably. Moreover, the method allows calculation of the number of CFU per implant.
In summary, we found that recovery of bacteria after sonication is dependent on the type of microorganism tested, the temperature of the sonication buffer, the time of exposure to ultrasound, and the material and composition of the sonication tube. From our studies, we propose a protocol for sonication of biofilm from extracted implants prior to conventional culture.
Acknowledgments
This work was financed by an ALF grant from Västerbotten Läns Landsting and by Northern County Councils project number 25/2007.
Thanks to Johan Wiström for the prereview and comments on the article.
There are no conflicts of interest related to this article.
Footnotes
Published ahead of print on 17 June 2009.
REFERENCES
- 1.Brown, W. J. 1994. Microbiology of the infected total joint arthroplasty. Semin. Arthroplasty 5107-113. [PubMed] [Google Scholar]
- 2.Dobbins, J. J., D. Seligson, and M. J. Raff. 1988. Bacterial colonization of orthopedic fixation devices in the absence of clinical infection. J. Infect. Dis. 158203-205. [DOI] [PubMed] [Google Scholar]
- 3.Duckhouse, H., T. J. Mason, S. S. Phull, and J. P. Lorimer. 2004. The effect of sonication on microbial disinfection using hypochlorite. Ultrason. Sonochem. 11173-176. [DOI] [PubMed] [Google Scholar]
- 4.Esteban, J., E. Gomez-Barrena, J. Cordero, N. Z. Martin-de-Hijas, T. J. Kinnari, and R. Fernandez-Roblas. 2008. Evaluation of quantitative analysis of cultures from sonicated retrieved orthopedic implants in diagnosis of orthopedic infection. J. Clin. Microbiol. 46488-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain, M., M. H. Wilcox, and P. J. White. 1993. The slime of coagulase-negative staphylococci: biochemistry and relation to adherence. FEMS Microbiol. Rev. 10191-207. [DOI] [PubMed] [Google Scholar]
- 6.Ince, A., J. Rupp, L. Frommelt, A. Katzer, J. Gille, and J. F. Lohr. 2004. Is “aseptic” loosening of the prosthetic cup after total hip replacement due to nonculturable bacterial pathogens in patients with low-grade infection? Clin. Infect. Dis. 391599-1603. [DOI] [PubMed] [Google Scholar]
- 7.Monsen, T., C. Olofsson, M. Rönnmark, and J. Wiström. 2000. Clonal spread of staphylococci among patients with peritonitis associated with continuous ambulatory peritoneal dialysis. Kidney Int. 57613-618. [DOI] [PubMed] [Google Scholar]
- 8.Nelson, C. L., A. C. McLaren, S. G. McLaren, J. W. Johnson, and M. S. Smeltzer. 2005. Is aseptic loosening truly aseptic? Clin. Orthop. Relat. Res. 43725-30. [DOI] [PubMed] [Google Scholar]
- 9.Neut, D., H. C. van der Mei, S. K. Bulstra, and H. J. Busscher. 2007. The role of small-colony variants in failure to diagnose and treat biofilm infections in orthopedics. Acta Orthop. 78299-308. [DOI] [PubMed] [Google Scholar]
- 10.Neut, D., J. R. van Horn, T. G. van Kooten, H. C. van der Mei, and H. J. Busscher. 2003. Detection of biomaterial-associated infections in orthopaedic joint implants. Clin. Orthop. Relat. Res. 413261-268. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen, L. L., C. L. Nelson, M. Saccente, M. S. Smeltzer, D. L. Wassell, and S. G. McLaren. 2002. Detecting bacterial colonization of implanted orthopaedic devices by ultrasonication. Clin. Orthop. Relat. Res. 40329-37. [DOI] [PubMed] [Google Scholar]
- 12.Pittet, D., and G. Ducel. 1994. Infectious risk factors related to operating rooms. Infect. Control Hosp. Epidemiol. 15456-462. [DOI] [PubMed] [Google Scholar]
- 13.Piyasena, P., E. Mohareb, and R. C. McKellar. 2003. Inactivation of microbes using ultrasound: a review. Int. J. Food Microbiol. 87207-216. [DOI] [PubMed] [Google Scholar]
- 14.Proctor, R. A., and G. Peters. 1998. Small colony variants in staphylococcal infections: diagnostic and therapeutic implications. Clin. Infect. Dis. 27419-422. [DOI] [PubMed] [Google Scholar]
- 15.Schindler, O. S., R. F. Spencer, and M. D. Smith. 2006. Should we use a separate knife for the skin? J. Bone Joint Surg. Br. 88382-385. [DOI] [PubMed] [Google Scholar]
- 16.Stanley, K. D., D. A. Golden, R. C. Williams, and J. Weiss. 2004. Inactivation of Escherichia coli O157:H7 by high-intensity ultrasonication in the presence of salts. Foodborne Pathog Dis. 1267-280. [DOI] [PubMed] [Google Scholar]
- 17.Tollefson, D. F., D. F. Bandyk, H. W. Kaebnick, G. R. Seabrook, and J. B. Towne. 1987. Surface biofilm disruption. Enhanced recovery of microorganisms from vascular prostheses. Arch. Surg. 12238-43. [DOI] [PubMed] [Google Scholar]
- 18.Trampuz, A., K. E. Piper, A. D. Hanssen, D. R. Osmon, F. R. Cockerill, J. M. Steckelberg, and R. Patel. 2006. Sonication of explanted prosthetic components in bags for diagnosis of prosthetic joint infection is associated with risk of contamination. J. Clin. Microbiol. 44628-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trampuz, A., K. E. Piper, M. J. Jacobson, A. D. Hanssen, K. K. Unni, D. R. Osmon, J. N. Mandrekar, F. R. Cockerill, J. M. Steckelberg, J. F. Greenleaf, and R. Patel. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. N. Engl. J. Med. 357654-663. [DOI] [PubMed] [Google Scholar]
- 20.Trampuz, A., and A. F. Widmer. 2006. Infections associated with orthopedic implants. Curr. Opin. Infect. Dis. 19349-356. [DOI] [PubMed] [Google Scholar]
- 21.Trampuz, A., and W. Zimmerli. 2006. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury 37(Suppl. 2)S59-S66. [DOI] [PubMed] [Google Scholar]
- 22.Trampuz, A., and W. Zimmerli. 2005. Prosthetic joint infections: update in diagnosis and treatment. Swiss Med. Wkly. 135243-251. [DOI] [PubMed] [Google Scholar]
- 23.Tunney, M. M., S. Patrick, M. D. Curran, G. Ramage, D. Hanna, J. R. Nixon, S. P. Gorman, R. I. Davis, and N. Anderson. 1999. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J. Clin. Microbiol. 373281-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tunney, M. M., S. Patrick, S. P. Gorman, J. R. Nixon, N. Anderson, R. I. Davis, D. Hanna, and G. Ramage. 1998. Improved detection of infection in hip replacements. A currently underestimated problem. J. Bone Joint Surg. Br. 80568-572. [DOI] [PubMed] [Google Scholar]
- 25.Vandamme, P. A. R. 2007. Taxonomy and classification of bacteria, p. 275-290. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed., vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 26.von Eiff, C., D. Bettin, R. A. Proctor, B. Rolauffs, N. Lindner, W. Winkelmann, and G. Peters. 1997. Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin. Infect. Dis. 251250-1251. [DOI] [PubMed] [Google Scholar]
- 27.Zimmerli, W., and P. E. Ochsner. 2003. Management of infection associated with prosthetic joints. Infection 3199-108. [DOI] [PubMed] [Google Scholar]
- 28.Zimmerli, W., A. Trampuz, and P. E. Ochsner. 2004. Prosthetic-joint infections. N. Engl. J. Med. 3511645-1654. [DOI] [PubMed] [Google Scholar]