Abstract
The objective of this study was to evaluate the performance of a low-cost method, the thin layer agar (TLA) method, for the diagnosis of smear-negative patients. This prospective study was performed in Homa Bay District Hospital in Kenya. Out of 1,584 smear-negative sputum samples, 212 (13.5%) were positive by culture in Löwenstein-Jensen medium (LJ) and 220 (14%) were positive by the TLA method. The sensitivities of LJ and TLA were 71% and 74%, respectively. TLA could become an affordable method for the diagnosis of smear-negative tuberculosis in resource-limited settings, with results available within 2 weeks.
The prevalence of smear-negative pulmonary tuberculosis (TB) has been increasing in countries with a high incidence of human immunodeficiency virus (HIV), especially in low-income countries where many patients are coinfected with HIV and TB and where culture of Mycobacterium tuberculosis is often not available (3, 4, 5, 6, 13, 15). The incidence of TB in Kenya is estimated at 384/100,000 inhabitants (23). Kenya ranks 13th on the World Health Organization list of 22 high-burden countries for TB worldwide, and HIV prevalence in new TB cases is estimated at 52% (23). Culture is more sensitive than smear microscopy (1, 3, 7), but cultivation in Löwenstein-Jensen (LJ) medium is very slow. The thin layer agar (TLA) method has been described as a simple, rapid, and inexpensive method (11, 12, 18) allowing initial identification of M. tuberculosis based on colony morphology, visualized microscopically, and by incorporation of para-nitrobenzoic acid (PNB) in the medium (10, 17, 19). We performed a prospective study to evaluate the performance of the TLA method for detection of M. tuberculosis in smear-negative samples compared to cultivation in LJ medium.
The study was conducted in Homa Bay District Hospital, Kenya. All smear-negative respiratory samples received between November 2007 and September 2008 from patients suspected of having TB were included. A total of 1,584 smear-negative samples were analyzed. Sputum was digested and decontaminated using the sodium hydroxide-N-acetyl-l-cysteine method (9). LJ cultures were examined twice weekly for up to 8 weeks (22). Cultures were considered positive for M. tuberculosis according to their morphological characteristics, positive acid-fast bacilli staining, and inhibition of growth by PNB on TLA (8). A culture was considered negative if no growth was observed after 8 weeks, and it was considered contaminated if there was growth but it was negative for acid-fast bacilli. TLA plates were prepared as previously described (18) with small modifications. One hundred microliters of decontaminated sample was inoculated on a biplate petri dish of 100 mm by 15 mm (Becton Dickinson, Sparks, MD) containing 20 ml of 7H11 agar supplemented with 10% oleic acid-albumin-dextrose-catalase (Becton Dickinson) plus piperacillin, trimethoprim, and amphotericin B (Sigma Aldrich) at 0.05 μg/ml, 0.02 μg/ml, and 0.02 μg/ml, respectively. PNB at 500 μg/ml was incorporated in one compartment. The plates were sealed with sterile parafilm, leaving a space of 1 to 2 cm, and incubated at 37°C in 5% CO2. Plates were checked after 24 h for contamination and examined twice weekly for up to 6 weeks using a standard microscope. A positive culture was identified by the characteristic cords of M. tuberculosis growth. Nontuberculous mycobacteria (NTM) were recognized by their lack of cording and growth on PNB. Fungal or bacterial contamination was recognized by rapid overgrowth of the plates.
The reference standard was considered when a sample was positive in any of the two culture media, TLA or LJ. Sensitivity was calculated as the number of positive cultures on LJ or TLA divided by the total number of positive cultures in any culture medium. The Wilcoxon test for nonnormal distribution was applied to compare differences in time to detection between the two culture media; paired comparisons were performed, excluding contaminated specimens. A P value of <0.05 was considered to be statistically significant. Analysis was performed with MedCalc version 9.6.4.0 program (MedCalc Software, Mariakerke, Belgium). The time to detection of growth and contamination rates were recorded for each culture medium.
Out of 1,584 smear-negative samples, 212 were positive by LJ (13.5%) and 220 were positive by TLA (14%). All positive cultures were presumptively identified as M. tuberculosis. Eight NTM isolates were identified by growth on PNB, morphology, and colony pigmentation, and molecular identification was performed at the Reference TB Laboratory in Antwerp, Belgium, for final confirmation. The NTM found were Mycobacterium gordonae, Mycobacterium flavescens, and Mycobacterium kubicae. Out of the 1,584 samples, 1,099 (69.5%) were culture negative by LJ and 951 (60%) by TLA. Two-hundred five (17%) samples inoculated on LJ and 325 (26%) on TLA were contaminated. The sensitivity of LJ was 71% and for TLA it was 74%. The median time to growth was 23 days for LJ and 14 days for TLA (P < 0.0001).
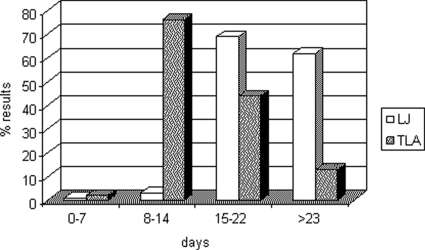
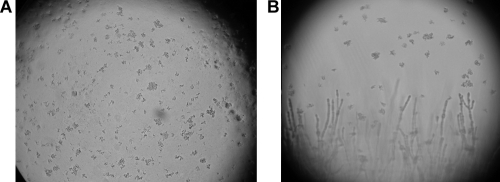
Among the positive cultures, the combination of both media increased the positivity. One hundred thirty-five positive cultures on LJ were also found positive on TLA. Forty-seven cultures found positive on LJ were negative on TLA, and 30 positive cultures on LJ were found contaminated on TLA. For TLA, 47 positive cultures were found negative on LJ and another 38 positive cultures were found contaminated on LJ. Figure 1 shows the average times to obtain a positive culture on LJ and TLA. More than 70% of cultures were found positive between 8 and 14 days on TLA, while the majority of cultures were found positive on LJ between 15 and 22 days and after 23 days. Figure 2 shows a typical cord formation characteristic for M. tuberculosis on TLA (Fig. 2A) and cords together with contaminants (Fig. 2B).
FIG. 1.
Time to detection (days) of M. tuberculosis culture-positive results in TLA and in LJ medium.
FIG. 2.
Microcolonies of M. tuberculosis in TLA seen under a microscope (10× objective). (A) Characteristic cord formation of M. tuberculosis. (B) Cord and contamination (filament) together.
This is the first operational study performed in a low-income country, in a rural setting, that has demonstrated that the TLA method performs as well as the LJ for the diagnosis of smear-negative samples, with a higher speed for results than culture on LJ. All reagents were available from local suppliers. The TLA method was able to differentiate presumptively between M. tuberculosis complex and NTM, which is an advantage compared to LJ. The support of other facilities for complete species identification is still required. Another method called the microscopic observation drug susceptibility assay (14) is similar to the TLA method, but disadvantages of this other assay are the use of liquid medium and the need for an inverted microscope. Contamination was a serious problem in our study and caused the loss of several samples. The rate of contamination was 17% for LJ and 26% for TLA. We have to point out that contamination was high in both media, not only in TLA. The concentration of NaOH used in the decontamination was 2% but could be increased up to 4% to attempt to reduce contamination. However, care should be taken to avoid toxicity to mycobacteria, which might cause negative culture results (21). Another important point could be the delay between sputum collection and processing, but in this study, it was only 2 to 3 days and samples were kept at 4°C. Concerning antibiotic mixtures, they are frequently used to inhibit the growth of contaminants, and the concentrations of piperacillin, trimethoprim, and amphotericin B used in this study could be increased, since they were rather low. Preliminary results using a concentration of 4.0 μg/ml of the three antibiotics showed that the contamination rate in TLA decreased to less than 10% (A. Martin et al., unpublished data). Piperacillin is active against many gram-positive and -negative bacteria, trimethoprim is a bacteriostatic agent, and amphotericin B is an antifungal drug. The most common contaminants were Candida albicans, coagulase-negative Staphylococcus spp., Staphylococcus aureus, Pseudomonas spp., and Aspergillus spp. Since some contamination was due to S. aureus, one possibility is the addition of vancomycin to TLA to reduce this contamination. We noticed that the contamination rate decreased slowly during the study. Such improvement suggests the need to set up a training period during which technicians can become familiar with culture method in order to easily recognize cording specific for M. tuberculosis. In the few published studies using TLA, the contamination rate was reported as 5 to 16% (2, 11, 12, 18). However, those studies were carried out in reference laboratories in middle-income countries with smear-positive results, contrary to the present study, which was performed in a rural laboratory in a low-income country on smear-negative samples. Concerning the workload, in Homa Bay, an average of 10 samples per day is inoculated into both media in duplicate. At least two technicians should be dedicated full time for preparation of the media, registration of data, decontamination, inoculation, and reading of TLA plates and LJ tubes. A cost-effectiveness study is underway to evaluate the impact of the introduction of the TLA method for diagnosis of TB in smear-negative patients. Despite the high contamination rate found in both media, we believe that TLA could be implemented in TB/HIV settings to increase TB case detection in smear-negative samples and patients. TLA is faster than conventional culture, practical and inexpensive, and allows the presumptive identification of M. tuberculosis complex. Smooth variants of M. tuberculosis have been described and named Mycobacterium canettii (20). These variants are, however, infrequent and have been found until now only in the Horn of Africa (16). TLA provides an alternative method to implement when more sophisticated techniques are not available or affordable.
Acknowledgments
We thank all of the MSF-F team in Homa Bay and Nairobi, including local and expat personnel that gave support to this study. Thanks to Juan Carlos Palomino for critically reviewing the manuscript.
Footnotes
Published ahead of print on 3 June 2009.
This work is dedicated to the memory of Peter Munga Waweru.
REFERENCES
- 1.Aber, V. R., B. W. Allen, D. A Mitchison, P. Ayuma, E. A. Edwards, and A. B. Keyes. 1980. Quality control in tuberculosis bacteriology. 1. Laboratory studies on isolated positive cultures and the efficiency of direct smear examination. Tubercle 61123-133. [DOI] [PubMed] [Google Scholar]
- 2.Almeida da Silva, P. E., F. Wiesel, M. M. Santos Boffo, A. Von Groll, I. Gomes de Mattos, G. Mejia, and J. Robledo. 2007. Microcolony detection in thin layer culture as an alternative method for rapid detection of Mycobacterium tuberculosis in clinical samples. Braz. J. Microbiol. 38421-423. [Google Scholar]
- 3.Colebunders, R., and I. Bastian. 2000. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 497-107. [PubMed] [Google Scholar]
- 4.Foulds, J., and R. O'Brien. 1998. New tools for the diagnosis of tuberculosis: the perspective of developing countries. Int. J. Tuberc. Lung Dis. 2778-783. [PubMed] [Google Scholar]
- 5.Hargreaves, N. J., O. Kadzakumanja, C. J. Whitty, F. M. Salaniponi, A. D. Harries, and S. B. Squire. 2001. ‘Smear-negative’ pulmonary tuberculosis in a DOTS programme: poor outcomes in an area of high HIV seroprevalence. Int. J. Tuberc. Lung Dis. 5847-854. [PubMed] [Google Scholar]
- 6.Harries, A. D., D. Maher, and P. Nunn. 1998. An approach to the problems of diagnosing and treating adult smear-negative pulmonary tuberculosis in high-HIV-prevalence settings in sub-Saharan Africa. Bull. W. H. O. 76651-662. [PMC free article] [PubMed] [Google Scholar]
- 7.Ipuge, Y. A. I., H. L. Rieder, and D. A. Enarson. 1996. The yield of acid-fast bacilli from serial smears in routine microscopy laboratories. Trans. R. Soc. Trop. Med. Hyg. 90258-261. [DOI] [PubMed] [Google Scholar]
- 8.International Union Against Tuberculosis and Lung Disease. 2007. Priorities for tuberculosis bacteriology services in low-income countries, 2nd ed. International Union against Tuberculosis and Lung Disease, Paris, France.
- 9.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology: a guide for the level III laboratory. Centers for Disease Control and Prevention, Atlanta, GA.
- 10.Laszlo, A., and S. H. Siddiqi. 1984. Evaluation of a rapid radiometric differentiation test for the Mycobacterium tuberculosis complex by selective inhibition with p-nitro-acetylamino-hydroxypropriophenone. J. Clin. Microbiol. 19694-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mejia, G. I., L. Castrillon, H. Trujillo, and J. A. Robledo. 1999. Microcolony detection in 7H11 thin layer culture is an alternative for rapid diagnosis of Mycobacterium tuberculosis infection. Int. J. Tuberc. Lung Dis. 3138-142. [PubMed] [Google Scholar]
- 12.Mejía, G. I., A. Guzmán, C. A. Agudelo, H. Trujillo, and J. Robledo. 2004. Five year experience with thin layer agar medium for rapid diagnosis of tuberculosis. Biomedica 152-59. [PubMed] [Google Scholar]
- 13.Mendelson, M. 2007. Diagnosing tuberculosis in HIV-infected patients: challenges and future prospects. Br. Med. Bull. 82149-165. [DOI] [PubMed] [Google Scholar]
- 14.Moore, D. A., C. A. Evans, R. H. Gilman, L. Caviedes, J. Coronel, A. Vivar, E. Sanchez, Y. Piñedo, J. C. Saravia, C. Salazar, R. Oberhelman, M. G. Hollm-Delgado, D. LaChira, A. R. Escombe, and J. S. Friedland. 2006. Microscopic observation drug susceptibility assay for the diagnosis of TB. N. Engl. J. Med. 151539-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunn, P. P., A. M. Elliott, and K. P. W. J. McAdam. 1994. Impact of human immunodeficiency virus on tuberculosis in developing countries. Thorax 49511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfyffer, G. E., R. Auckenthaler, J. D. Van Embden, and D. Van Soolingen. 1998. Mycobacterium canettii, the smooth variant of M. tuberculosis, isolated from a Swiss patient exposed in Africa. Emerg. Infect. Dis. 4631-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rastogi, N., K. S. Goh, and H. I. David. 1989. Selective inhibition of the Mycobacterium tuberculosis complex by p-nitro-acetylamino-beta-hydroxypropriophenone (NAP) and p-nitrobenzoic acid (PNB) used in 7H11 agar medium. Res. Microbiol. 140419-423. [DOI] [PubMed] [Google Scholar]
- 18.Robledo, J. A., G. I. Mejía, N. Morcillo, L. Chacón, M. Camacho, J. Luna, J. Zurita, A. Bodon, M. Velasco, J. C. Palomino, A. Martin, and F. Portaels. 2006. Evaluation of a rapid culture method for tuberculosis diagnosis: a Latin American multi-center study. Int. J. Tuberc. Lung Dis. 10613-619. [PubMed] [Google Scholar]
- 19.Tsukamura, M., and S. Tsukamura. 1964. Differentiation of Mycobacterium tuberculosis and Mycobacterium bovis by p-nitrobenzoic acid susceptibility. Tubercle 4564-65. [DOI] [PubMed] [Google Scholar]
- 20.Van Soolingen, D., T. Hoogenboezem, P. E. de Haas, P. W. Hermans, M. A. Koedam, K. S. Teppema, P. J. Brennan, G. S Besra, F. Portaels, J. Top, L. M. Schouls, and J. D. Van Embden. 1997. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa. Int. J. Syst. Bacteriol. 471236-1245. [DOI] [PubMed] [Google Scholar]
- 21.Whyte, T., M. Cormican, B. Hanahoe, G. Doran, T. Collins, and G. Corbett-Feeney. 2000. Comparison of BACTEC MGIT 960 and BACTEC 460 for culture of mycobacteria. Diagn. Microbiol. Infect. Dis. 38123-126. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. 1998. Laboratory services in tuberculosis control. Part II. Microscopy. World Health Organization, Geneva, Switzerland.
- 23.World Health Organization. 2008. Global tuberculosis control: surveillance, planning, financing. WHO/HTM/TB/2008.393. World Health Organization, Geneva, Switzerland.