Abstract
Typhoid fever is becoming an ever increasing threat in the developing countries. We have improved considerably upon the existing PCR-based diagnosis method by designing primers against a region that is unique to Salmonella enterica subsp. enterica serovar Typhi and Salmonella enterica subsp. enterica serovar Paratyphi A, corresponding to the STY0312 gene in S. Typhi and its homolog SPA2476 in S. Paratyphi A. An additional set of primers amplify another region in S. Typhi CT18 and S. Typhi Ty2 corresponding to the region between genes STY0313 to STY0316 but which is absent in S. Paratyphi A. The possibility of a false-negative result arising due to mutation in hypervariable genes has been reduced by targeting a gene unique to typhoidal Salmonella serovars as a diagnostic marker. The amplified region has been tested for genomic stability by amplifying the region from clinical isolates of patients from various geographical locations in India, thereby showing that this region is potentially stable. These set of primers can also differentiate between S. Typhi CT18, S. Typhi Ty2, and S. Paratyphi A, which have stable deletions in this specific locus. The PCR assay designed in this study has a sensitivity of 95% compared to the Widal test which has a sensitivity of only 63%. As observed, in certain cases, the PCR assay was more sensitive than the blood culture test was, as the PCR-based detection could also detect dead bacteria.
Salmonella enterica is an important enteric pathogen and is involved in causing both systemic and intestinal diseases in humans and a wide range of other hosts (7, 21). Serotypes within subspecies I (Salmonella enterica subsp. enterica) are responsible for the vast majority of salmonellosis in warm-blooded animals. S. enterica subsp. enterica serovar Typhi and S. enterica subsp. enterica serovar Paratyphi A cause typhoid fever strictly in humans mostly in developing countries, with no age exemption, but it is less common in children younger than 2 years old. According to one estimate, the worldwide incidence of typhoid fever is 16 million cases annually and the mortality rate is 600,000 individuals per year (23). According to a press release from the Press Information Bureau, Government of India, dated 22 February 2006, the morbidity due to typhoid fever varies from 102 to 2,219 per 100,000 population in different parts of India, and in some areas, typhoid fever is responsible for 2 to 5% of all deaths. The problem of typhoid fever has been exacerbated by the appearance of multiple-drug-resistant strains (25), the treatment of which would depend on newer and advanced antibiotics and early and precise diagnosis.
The existing modes of diagnosis are through the detection of antibodies against Salmonella bacteria by the Widal test and other serological tests like DOT enzyme immunoassay, dip stick assays, and semiquantitative tube agglutination test (22). Apart from this, the bacteremia observed in typhoid fever around day 6 to 9 enables it to be detected through the blood culture test (29) and PCR amplification of the bacterial DNA from blood. Of the commonly available diagnostic tests, Widal test and other serological diagnostic methods are limited because of the low specificity of the test. There are reports of a large number of false-positive cases especially in areas where typhoid fever is endemic and in patients exposed to typhoid fever earlier (6). The blood culture test has the major disadvantage of being a time-consuming test, which takes 2 to 3 days.
PCR-based diagnoses are superior to the classical serological method, Widal test, and blood culture test in terms of their specificity and sensitivity. The modification by Sanchez-Jimenez and Cardona-Castro (26) where the initial DNA purification step is omitted and the whole blood is used directly as the template for PCR has been used in our assay system with minor modifications.
The PCR-based systems currently use primers against flagellin genes (2, 4, 5, 12, 16, 17, 27), hilA (26), and invA and spvC genes (5). The different distributions of invA and spvC genes among Salmonella isolates from animals highlights the unsuitability of these two genes as PCR probes for Salmonella detection (20). Many genes carried on Salmonella pathogenicity islands have evolved differentially in typhoidal and nontyphoidal Salmonella serovars giving rise to different allelic variants of these genes (9). These genes are present in different Salmonella serovars, and their orthologs in other species of bacteria share various degrees of identity at the nucleotide levels (9). These differences, if minor, at certain PCR conditions can lead to promiscuous amplification, thereby leading to false-positive results. This problem can be overcome by choosing those regions that are unique to S. Typhi and S. Paratyphi A. Though certain pathogenicity islands are unique to S. Typhi and S. Paratyphi A, like Salmonella pathogenicity island 7 (SPI-7) and SPI-8, these islands are known to be unstable. These islands can be excised by the activation of certain recombinases as exemplified by isolation of the clinical variants lacking SPI-7 (19). Also, the presence of a gene encoding integrase on SPI-8 suggests that it is a mobile island (13). For the same reason, insertion sequences and bacteriophage genes are not good candidates for diagnostic purposes. However, a thorough examination of the whole-genome sequences of S. enterica serovar Typhi, S. Paratyphi A, and S. Typhimurium highlights the existence of genomic regions of unknown function with no homologous genes in related serovars and without the features of mobile DNA sequences. Using these criteria for the identification of a good diagnostic marker gene in S. Typhi CT18, we identified the genomic loci spanning the STY0312 gene, which is unique to S. Typhi CT18 and S. Paratyphi, the causative agents of typhoid fever. The adjoining locus spanning STY0313 to STY0316 was different in S. Typhi Ty2 and S. Paratyphi but otherwise conserved in most Salmonella strains. This region was found to be part of SPI-6, which is present in many Salmonella enterica subspecies I strains (10).
We hypothesized that these novel primers could differentiate between typhoidal and nontyphoidal serovars of Salmonella enterica. Hence, the aim of the present work was to amplify the genomic region using the unique set of primers and demonstrate whether this method can be useful for the early diagnosis of typhoid fever.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The different bacterial strains used in this study are given in Table 1. Routine culturing of bacteria was done in LB broth and on LB agar plates at 37°C. During blood culture diagnosis, the bacteria were plated on salmonella-shigella agar (Himedia), selective media for Salmonella species. S. enterica serovar Typhimurium LT2 strain is nalidixic acid resistant, and 50 μg/ml of nalidixic acid was added to the LB broth or agar during culture of this strain.
TABLE 1.
Bacterial species and strains used in this work
| Bacterial species | Strain | Sourcea |
|---|---|---|
| Staphylococcus aureus | ATCC 25923 | Diagnostic Lab, Institute of Clinical Microbiology, Immunology and Hygiene, Erlangen, Germany |
| Escherichia coli XL1-Blue | Laboratory stock | |
| E. coli K-12 | U. Varshney, MCB, IISc, Bangalore, India | |
| E. coli DH5α | Laboratory stock | |
| Uropathogenic E. coli | Clinical isolate; R.V. Diagnostic Center, Bangalore, India | |
| Shigella flexneri | S. Mahadevan, MRDG, IISc, India | |
| Yersinia enterocolitica | MTCC 840 | IMTECH, Chandigarh, India |
| Hafnia alvei | MTCC 1426 | IMTECH, Chandigarh, India |
| Vibrio cholerae | MTCC 3904 | IMTECH, Chandigarh, India |
| Citrobacter freundii | MTCC 6738 | IMTECH, Chandigarh, India |
| Proteus vulgaris | MTCC 426 | IMTECH, Chandigarh, India |
| Pseudomonas syringae | MTCC 673 | IMTECH, Chandigarh, India |
| Salmonella enterica subsp. arizonae | MTCC 660 | IMTECH, Chandigarh, India |
| S. enterica serovar Bovismorbificans | MTCC 1162 | IMTECH, Chandigarh, India |
| S. enterica serovar Virchow | MTCC 1166 | IMTECH, Chandigarh, India |
| S. enterica serovar Infantis | MTCC 1167 | IMTECH, Chandigarh, India |
| S. enterica serovar Brunei | MTCC 1168 | IMTECH, Chandigarh, India |
| S. enterica serovar Weltevreden | MTCC 1169 | IMTECH, Chandigarh, India |
| S. enterica serovar Enteritidis | MTCC 3219 | IMTECH, Chandigarh, India |
| S. enterica subsp. enterica serovar Nchauga | MTCC 3228 | IMTECH, Chandigarh, India |
| S. enterica subsp. enterica serovar Newport | MTCC 3229 | IMTECH, Chandigarh, India |
| S. enterica subsp. enterica serovar Schwarzengrund | MTCC 3230 | IMTECH, Chandigarh, India |
| S. enterica serovar Choleraesuis | MTCC 3858 | IMTECH, Chandigarh, India |
| S. enterica serovar Gallinarum | B-29-2 | CRI, Kasauli, India |
| S. enterica serovar Pullorum | B-28-193 | CRI, Kasauli, India |
| Salmonella bongori | M. Hensel, Germany | |
| S. enterica subsp. enterica serovar Typhimurium LT2 | PGIMER, Chandigarh, India | |
| S. enterica subsp. enterica serovar Typhi CT18 | M. Hensel, Germany | |
| S. enterica subsp. enterica serovar Typhi Ty2 | M. Hensel, Germany | |
| S. enterica subsp. enterica serovar paratyphi A SarB | NCTC 12023 | M. Hensel, Germany |
Abbreviations: MCB, Department of Microbiology and Cell Biology; IISc, Indian Institute of Science; MRDG, Department of Molecular Reproduction, Development and Genetics; IMTECH, Institute of Microbial Technology; CRI, Central Research Institute; PGIMER, Postgraduate Institute of Medical Education and Research.
Blood collection.
One-milliliter blood samples were collected from the healthy donors and the patients suspected to have typhoid fever. The blood samples were collected in tubes with EDTA. The samples were collected before antibiotic treatment. The samples were obtained from individuals at the Indian Institute of Science Health Center and R.V. Diagnostic Center, Bangalore, India, according to the institute's human ethics committee. A total of 50 patient samples were collected.
Processing of blood samples for PCR.
Artificially inoculated blood samples were made by adding various amounts of bacteria from serially diluted cultures to an optical density at 600 nm (OD600).The samples were then processed as discussed above, and PCR was performed with specific primers.
Templates for the PCR were prepared from both patient blood samples and healthy donor blood samples artificially inoculated with S. Typhi and S. paratyphi A. For clinical samples, 1 ml of blood was centrifuged at 10,000 × g for 5 min. One milliliter of 0.2% Triton X-100 was added to the pellet, vortexed, and incubated for 10 min at room temperature, followed by centrifugation at 10,000 × g for 10 min. The supernatant was decanted, and the pellet was washed with 0.2% Triton X-100 again. The final pellet was again washed with 1 ml of nuclease-free water. Finally, the pellet was resuspended in 30 μl of nuclease-free water.
Blood culture test.
Patient blood samples (2.5 ml) were drawn and added to brain heart infusion medium. This medium was then incubated at 37°C for 24 h and later streaked onto MacConkey agar plate. If the colony did not ferment lactose, further oxidase test and slide agglutination test were done. The colonies that gave a negative results by the oxidase test and a positive result by the slide agglutination test were further confirmed through biochemical tests where S. Typhi is indole negative, and urease negative and does not ferment mannitol.
Slide agglutination test.
On a slide, 2 or 3 drops of saline were added and the test colony picked from the growth plate was thoroughly mixed with the saline. To this mixture 1 drop of Widal test-positive indicator serum was added. The formation of clumps was an indication of the presence of Salmonella.
Widal test.
Rapid slide test for qualitative in vitro determination of antibodies in serum against Salmonella Typhi O and H antigens and/or Salmonella Paratyphi A(H) and B(H) antigens were done using TyDAL kit (Arsitha Diatech) following the manufacturer's instructions.
Primers.
The primers were designed after analyzing the complete genome of S. enterica serovar Typhimurium, S. Typhi CT18, S. Typhi Ty2, and S. Paratyphi A, available at GenBank NCBI. The sequences for these four strains were analyzed using wgVISTA; which is a set of programs for comparing the whole-genome sequences of two microbes which are less than 10 Mb along with annotation (http://genome.lbl.gov/cgi-bin/WGVistaInput) (11, 18). The program is implemented as an online server which provides access to the whole-genome alignment pipeline. Unique regions present in the systemic typhoid fever-causing organisms S. Typhi and S. Paratyphi A but absent in enteric disease-causing organism S. Typhimurium were identified. The regions of SPI-7 and SPI-8, though unique to S. Typhi and S. Paratyphi were omitted because of their unstable nature. The STY0312 gene from SPI-6 of S. Typhi was found to be present in S. Typhi and S. Paratyphi A and absent from S. Typhimurium though SPI-6 is present in S. Typhimurium as Salmonella genomic island. The STY0312, STY0313, STY0314, and STY0316 genes were subjected to homology searches through BLAST by comparison against all completely and partially sequenced microbial genomes. The STY0312 gene was found in the typhoidal Salmonella serovars S. Typhi CT18 and S. Paratyphi A. Salmonella enterica subsp. enterica serovar Heidelberg strain SL476, S. enterica subsp. enterica serovar Newport strain SL254, and S. enterica subsp. enterica serovar Typhi Ty2 have a partial gene sequence but will not be amplified using the given set of primers. The STY0313, STY0314, and STY0316 genes were found in many serovars of Salmonella enterica subspecies I. From this locus spanning genes STY0313 to STY0316, unique regions specific to S. Typhi CT18 and S. Typhi Ty2 were identified. This region was also present in the nontyphoidal strains S. enterica subsp. enterica serovar Heidelberg strain SL486 and S. enterica subsp. enterica serovar Newport strain SL254.
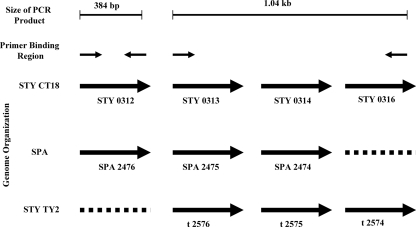
Using S. Typhi CT18 as the reference strain, two sets of primers were designed such that they would amplify two genomic loci spanning the STY0312 gene (SPA2476 present in S. Paratyphi A but absent in S. Typhi Ty2), which encodes a putative secreted protein, and gene STY0313 (SPA2475 in S. Paratyphi A, t2576 in S. Typhi Ty2) to gene STY0316 (not present in S. Paratyphi A, t2574 in S. Typhi Ty2) in a nonoverlapping way. The STY0312 gene is absent in S. Typhi Ty2, and the locus spanning STY0313 to STY0316 is absent in S. Paratyphi A, whereas the entire locus is absent in S. Typhimurium. PCR amplification using the two sets of primers with genomic loci of different Salmonella strains as the template would yield two bands of 1,043 and 384 bp for S. Typhi CT18, one band of 384 bp for S. Typhi Ty2, one band of 1,043 bp for S. Paratyphi A, and no band for S. Typhimurium (Fig. 1).
FIG. 1.
Schematic diagram of the genome organization of the loci used to design primers. The loci were from the three Salmonella strains S. Typhi CT18 (STY CT18), S. Paratyphi A (SPA), and S. Typhi Ty2 (STY Ty2). The expected size of the PCR product is shown at the top of the figure. Solid black arrows depict the position and direction of primer binding sites of the two sets of primers. The genome organizations of the three serovars are depicted as solid arrows, each representing a gene with its GenBank locus tag number given below. Broken lines represent genes that are absent in a particular serovar.
The complete genomes of Salmonella enterica subsp. enterica serovar Typhi CT18, (accession no. NC_003198), S. enterica subsp. enterica serovar Typhi Ty2 (accession no. NC_004631), S. enterica subsp. enterica serovar Paratyphi A ATCC 9150 (accession no. NC_006511), and Salmonella serovar Typhimurium LT2 (accession no. NC_003197) have been sequenced and deposited in the GenBank database.
Primer set 1 consists of STY0312/SPA2476 (forward) (5′-ATGTTCAGTAAAATAGTGTCATTGCTTTTG-3′) and STY0312/SPA2476 (reverse) (5′-TTGTAGCGCCGGAAATGATATTCT-3′). Primer set 2 consists of STY0313/SPA2475/t2576 (forward) (5′-CTTGACGTACCGGTAGAGAT ATACTGGCT-3′) and STY0316/t2574 (reverse) (5′-CTTTACATCTGTTCCGCCCCAGGCAAATAC-3′).
PCR conditions.
The same PCR protocol was used for clinical samples and artificially inoculated blood samples. The protocol used was a modification of the method used by Sanchez-Jimenez and Cardona-Castro (26). A volume of 200 to 300 μl of blood was taken and centrifuged at 12,000 × g for 2 min. To the pellet 1 ml of 0.2% Triton X-100 was added and kept for 5 min. The pellet containing the cell debris and bacteria were centrifuged, and the supernatant was discarded. The Triton X-100 wash was repeated, and the pellet was suspended in 20 μl of nuclease-free water. This pellet was boiled for 10 min at 99°C, and the entire volume was used as template for the PCR. The PCR mixture (50 μl) contained 67 mM Tris (pH 8.8), 16 mM (NH4)2SO4, 2.5 mM MgCl2, 0.1% Triton X-100, 200 μM (each) deoxynucleoside triphosphates, 25 picomoles of each primer, and two units of Taq DNA polymerase (Bioron GmbH, Germany). The parameters for amplification were as follows: initial denaturation at 94°C for 4 min, primer annealing at 54°C for 30 s, and extension at 72°C for 1.5 min. This step was repeated for 35 cycles in an automated DNA thermal cycler (Palm-Cycler Corbett Research, Australia). Final extension was done at 72°C for 10 min. The PCR products were run on 0.8 and 1.5% agarose gels, and the gels stained with ethidium bromide were visualized under the UV trans illuminator. Molecular size markers (1-kb DNA ladder; MBI Fermentas, Canada) were run concurrently. For colony PCR, the template was prepared by picking up isolated colonies of bacteria using a sterile toothpick and suspending them in 10 μl of nuclease-free water and boiling them at 99°C for 10 min in a PCR tube. The master mix containing other reagents of a PCR mixture was added later, and the amplification program discussed above was followed.
RESULTS
The PCR detection method is specific for Salmonella and differentiates between various typhoidal serovars and other common pathogenic and nonpathogenic bacteria.
To prove that the PCR-based detection method was specific for Salmonella, we carried out colony PCR with different Salmonella serovars and other common pathogenic and nonpathogenic bacteria listed in Table 1. PCR was performed as described in Materials and Methods, and the different-sized amplification products when run on an agarose gel was able to differentiate between the various serovars (the samples were loaded on the gel in the same order as in Table 1). PCR amplification in S. enterica serovar Typhi samples produced two bands of 384 bp and 1,043 bp, whereas S. enterica serovar Paratyphi A produced only the 384-bp band. All other bacteria were negative for both the products (Fig. 2). Megablast analysis of the open reading frames used for the assay revealed that Salmonella enterica serovars Heidelberg and Newport would produce the 1,043-bp band. Of the two serovars, serovar Newport was used for the assay, but it did not produce any bands. The reason for this remains unknown. The specificity was tested again with the same set of samples but with higher template concentrations by taking 20-μl portions of the stationary-phase cultures of all the bacteria, centrifuging, and resuspending the pellets in 10-μl volumes of water as the PCR template. When the annealing temperature was reduced to less than 50°C, nonspecific bands appeared. Hence, for all the further experiments, an annealing temperature of 54°C was used. The processing of whole blood for template DNA results in host genomic DNA contamination, which can serve as a template for nonspecific binding and therefore can give rise to spurious PCR products. To rule out this possibility, the PCR assay was repeated with dilutions done in blood instead of PBS. The results of this experiment were found to be similar with no spurious PCR products, though the intensity of the bands was less (data not shown). This may be because of the large amount of contaminating proteins, which may reduce the efficiency of the PCR.
FIG. 2.
The PCR detection method is specific for Salmonella and differentiates various typhoid-causing serovars. Colony PCR was performed for different serovars of Salmonella and other pathogenic and nonpathogenic bacteria. Salmonella enterica subsp. arizonae, many Salmonella enterica serovars, and Salmonella bonogori were used. The other pathogenic and nonpathogenic bacteria included Staphylococcus aureus, Escherichia coli, uropathogenic E. coli (UPEC), Shigella flexneri, Yersinia enterocolitica, Hafnia alvei, Vibrio cholerae, Citrobacter freundii, Proteus vulgaris, and Pseudomonas syringae. PCR amplification was performed with the two sets of primers for 30 cycles, and the product was run on a 1.5% agarose gel. The samples have been loaded on the gel in the same order as in Table 1. The 384-bp band is the amplification product of the STY0312 gene of S. Typhi CT18 and its homolog in S. Paratyphi A and is found in both the serovars. The 1,043-bp band is the amplification product of the locus spanning the genes STY0313 to STY0316 and their homologs in S. Typhi Ty2. This locus is absent in S. Paratyphi A. Both these bands are absent in all the other bacterial strains tested.
The genomic locus that is selected as a diagnostic marker is potentially stable.
The genomic locus that is used as a diagnostic marker should be genetically stable. An unstable region poses the possibility of it being deleted in certain isolates. This excision can take place through the prophage activation in the case of bacteriophage sequences and through specific recombinase in pathogenicity islands and by homologous recombination between paralogous genes in the same bacterium. The genetic stability of the amplified region was further tested by amplifying the region from the clinical isolates from various parts of India. Twenty samples were obtained from the Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry (southern India) and 12 samples from Nagpur (central India). The PCR assay conducted with pure cultures of these clinical isolates amplified the expected band from all the isolates (Fig. 3).
FIG. 3.
The genomic locus used as the diagnostic marker is potentially stable. Twenty clinical isolates from Pondicherry (southern India) and 12 clinical isolates from Nagpur (central India) were analyzed by colony PCR to show that the region is potentially stable. The amplified products were run on a 0.8% agarose gel. (A) The 20 clinical isolates (lanes 1 to 20) showed two bands, characteristic of S. Typhi CT18, which has also been confirmed through serotyping. (B) This representative gel shows 7 samples out of 12 samples obtained from patients in Nagpur, India. Among seven samples, three samples showed only the lower 384-bp band of S. Paratyphi A (lanes 2, 3, and 5), and four samples showed two bands of S. Typhi CT18 (lanes 1, 4, 6, and 7). Lanes 8, 9, and 10 contain controls with isolated colonies of S. Paratyphi A, S. Typhi CT18, and S. Typhi Ty2, respectively. M lanes contain molecular size markers.
Though the sample size of 32 is not sufficient to rule out the presence of allelic variants of the gene or mutations with complete deletion of the gene, further proof for stability of that particular genomic locus was gathered by bioinformatics analysis. In silico analysis was conducted to confirm that this region was not in any mobile pathogenicity islands or bacteriophage insertions by analyzing the GC percentage and subjecting the entire region to BLAST analysis to look for regions of homology to the bacteriophage genome (data not shown).
The PCR-based assay can detect as few as four bacteria per ml of blood.
After the specificity and stability of the chosen diagnostic marker gene was determined, the sensitivity of the assay was measured. The minimum number of bacteria that can be detected in one round of PCR amplification with 35 cycles was determined by using serial dilutions of a pure culture of S. Typhi as templates. The OD600 of the bacterial suspension was set at 0.3 which contains 1.5 × 108 bacteria/ml (precalculated through dilution plating), from which serial dilution were made. The PCR was repeated four times, and the lowest dilution that consistently produced a band was taken as the lowest detection limit. The dilutions were carried out in both blood and PBS, and in both the cases, as few as one bacterium in 300 μl of blood gave rise to the bands corresponding to the desired product size (Fig. 4). Detection limits can be increased by doing two rounds of PCR and by using the product of one round of amplification as the template for the next round, but this increases the time and cost. A major objective of this PCR diagnostic assay is to develop a reliable, easy, quick, and economical means of diagnosis of typhoid fever in the developing countries. To reduce the total time and cost of the assay, we tried to maximize the detection level in a single round of PCR amplification without compromising on the specificity. By taking 300 μl of blood as the template, we were able to detect four or less bacteria/ml of blood in a single round of PCR.
FIG. 4.
The PCR-based assay can detect as few as four bacteria/ml. The minimum number of bacteria that can be detected by this method was determined by diluting a culture which contains 1.5 × 108 bacteria and adjusting it to an OD600 of 0.3 with various dilutions in PBS and sterile blood. The diluted culture samples were then subjected to one round of PCR amplification for 35 cycles; the products were then visualized by electrophoresis on a 0.8% agarose gel. The corresponding number of bacteria/ml is given at the top of each lane. (A) Different numbers of S. Typhi CT18 diluted in PBS, showing that one bacterium/ml can produce a visible band. (B) Different numbers of S. Typhi CT18 diluted in blood, showing that the procedure can detect as few as four bacteria/ml of blood. (C) Different numbers of S. Paratyphi A diluted in PBS, showing a sensitivity of detection of four bacteria/ml. (D) Different numbers of S. Paratyphi A diluted in blood, showing a lower detection limit of one bacterium/ml of blood. M lanes contain molecular size markers.
The PCR assay is more sensitive than the Widal test is.
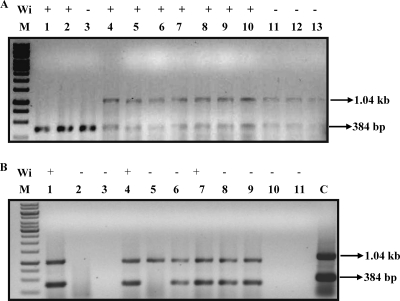
The conventional means of diagnosis of typhoid fever are the Widal test and blood culture test. The blood culture tests though time-consuming is considered the gold standard in typhoid fever diagnosis. The sensitivity of the PCR assay with the given primer set was next calculated and compared to the sensitivities of the Widal test and blood culture test, which are the conventional means of diagnosis for typhoid fever. Blood samples from patients suspected to have typhoid fever (e.g., exhibiting pyrexia symptoms) were collected before antibiotic treatment from the Indian Institute of Science Health Center and the R.V. Diagnostic Center, Bangalore, India. Of these samples, 58 samples were subjected to the Widal test, PCR assay, and blood culture test and 20 samples were subjected to PCR and Widal test (Table 2). A representative gel picture along with the Widal test results show that the PCR assay is more sensitive than the Widal test is (Fig. 5). Taking the blood culture test as the standard, the Widal test has a sensitivity of only 63%, whereas the PCR method has a sensitivity of 95% (among 58 samples with culture result). Using the sample set for which data are available for all three diagnostic methods, namely, PCR assay, Widal test, and blood culture, chi-square analysis using 2 × 2 contingency tables was done to compare the performance of the three methods. The PCR detection method compared with the blood culture method revealed no significant difference (P > 0.05). However, there was significant difference between the Widal test and blood culture (P < 0.05). Comparison between the Widal test and PCR analysis shows that the PCR test is significantly better than the Widal test is (P < 0.05). Among the 20 samples for which culture assay was not done, PCR assay detected 11 positive samples compared to only 5 positive samples detected by the Widal test.
TABLE 2.
Results of the Widal test, PCR assay, and blood culture on 78 clinical samples collected from two centers in Bangalore, India
| Total no. of samples | No. of samples with the following test result:
|
|||||
|---|---|---|---|---|---|---|
| Widal test
|
PCR assay of blood sample
|
Blood culture
|
||||
| Positive | Negative | Positive | Negative | Positive | Negative | |
| 58 | 32 | 26 | 48 | 10 | 51 | 7 |
| 20 | 5 | 15 | 11 | 9 | NDa | ND |
ND, not done.
FIG. 5.
The PCR assay is more sensitive than the Widal test is. Two representative gel pictures showing the PCR products amplified from blood samples from patients. The corresponding Widal test result (Wi) (+, positive; −, negative) is given above each lane. (A) Lanes 1, 2, and 3 contain samples positive for S. Paratyphi A. Lanes 4 to 13 contain samples positive for S. Typhi. (B) Lanes 1, 4, 6, 7, 8, and 9 contain samples positive for S. Typhi CT18. Lanes 2, 3, 10, and 11 contain PCR-negative samples. Lane 5 contains a sample positive for S. Typhi Ty2 by PCR. Lane C contains a positive control for S. Typhi CT18. The PCR assay is 40% more sensitive than the Widal test in this given partial sample data. M lanes contain molecular size markers.
DISCUSSION
The use of two novel sets of primers from two unique regions of S. enterica serovar Typhi is a remarkable improvement upon the existing methods of PCR-based diagnosis. The specificity of the PCR test is increased because of the simultaneous amplifications which give results similar to two rounds of PCR with nested primers. In the previous studies when a nested PCR was carried out for the diagnosis of Salmonella, one or two sets of primers were used in the second round of PCR (1, 16). Though the nested PCR increases detection limits by increasing the number of cycles of amplification, the increased sensitivity is equal to a single round of PCR with two sets of primers amplifying two different loci, which we have employed in our study. We have shown that the detection level of bacteria with a single round of amplification of 35 cycles in our study is very high (as few as 4 CFU was detected). Hence, our modified method is able to achieve the specificity similar to that of a nested PCR along with a high sensitivity in one round of amplification, saving both time and reagents. An estimated number of bacteria per ml of peripheral blood during the bacteremic phase in typhoid fever is 1 CFU/ml (28). Since our method is able to detect this range, it can be safely used for diagnosis without the fear of false-negative results. The primers used in this study are designed against a potentially stable genomic locus. Clinical isolates from different parts of India produced the expected band size. Most of the previous studies used primers designed against the hypervariable region of the flagellar genes, which are the regions susceptible to high mutation rates (14, 15). The use of a hypervariable gene as a diagnostic marker precludes the possibility of emergence of variants of the gene, which can give a dangerous false-negative result in PCR diagnosis using flagellar genes and serological tests involving detection of flagellar antibodies.
The use of whole-blood lysate as the PCR template is a modification over other previous studies involving the purification of DNA from blood, which is a costly and time-consuming process (1, 17, 27). In our modified method we have used whole-blood lysate instead of purified DNA. This step reduces the need to purify DNA from blood, which is an additional step and increases the cost and time. We have used 0.2% Triton X-100 for lysis without any other buffer or lysis agent, which allows us to use the lysate dissolved in this solution directly as template. The PCR yield did not change when different commercial PCR enzymes and their cognate buffers were used. This gives us the flexibility to use any commercially available PCR enzyme and buffer and eliminates the need for specialized buffer compositions.
This PCR test can also be used to determine the serovars of the Salmonella strain through a single PCR of colonies isolated from blood culture along with biochemical tests and slide agglutination tests. Serovar identification is crucial to predict the course of the disease and to determine the treatment regimen, as different serovars respond to different modes of treatment (3, 8). The practical value of PCR in the clinical diagnosis is the detection of Salmonella DNA in the blood samples from patients with suspected clinical symptoms but with negative cultures. The low level of bacteremia in typhoid fever patients can give rise to negative blood culture results, particularly if the patients have been treated with antibiotics before culture. Depending on the conventional diagnostic methods, these culture-negative cases cannot be confirmatively diagnosed as typhoid fever.
By using two pairs of primers in a single PCR instead of two reactions in nested PCR, the time taken for the diagnosis is considerably reduced. The whole procedure to differentiate the typhoidal Salmonella serovars in blood by agarose gel electrophoresis took only 5 h, demonstrating the PCR to be rapid, specific, and the most reliable method for early diagnosis of typhoid fever.
Typhoid fever is a very debilitating disease and hence demands very accurate and fast diagnosis. Pretreatment with antibiotics will lead to culture-negative condition but will not kill the bacteria residing in the gallbladder, which will lead to carrier condition and relapse of fever (24). Even with the antibiotic treatment, PCR diagnosis can detect dead bacteria in blood, thus helping to identify and monitor those patients who are susceptible for relapse of the disease or progress to a carrier state. To summarize, we have developed a novel method to detect blood-borne Salmonella serovars in a rapid and reliable manner. Studies in this line for identification of other pathogens will be a fruitful avenue of further research.
Acknowledgments
This work was supported by a grant, Provision (2A) Tenth Plan (191/MCB), from the Director of Indian Institute of Science, ICMR Center for Medical Microbiology, and DBT program support on Basic Biology of Microbial Pathogens. Grant support from the Department of Biotechnology, Government of India (DBT-197 and DBT-178) is greatly acknowledged. A.N. acknowledges CSIR, India, for the Senior Research fellowship.
Footnotes
Published ahead of print on 17 June 2009.
REFERENCES
- 1.Ambati, S. R., G. Nath, and B. K. Das. 2007. Diagnosis of typhoid fever by polymerase chain reaction. Indian J. Pediatr. 74909-913. [DOI] [PubMed] [Google Scholar]
- 2.Aziah, I., M. Ravichandran, and A. Ismail. 2007. Amplification of ST50 gene using dry-reagent-based polymerase chain reaction for the detection of Salmonella typhi. Diagn. Microbiol. Infect. Dis. 59373-377. [DOI] [PubMed] [Google Scholar]
- 3.Bhan, M. K., R. Bahl, and S. Bhatnagar. 2005. Typhoid and paratyphoid fever. Lancet 366749-762. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhry, R., B. V. Laxmi, N. Nisar, K. Ray, and D. Kumar. 1997. Standardisation of polymerase chain reaction for the detection of Salmonella typhi in typhoid fever. J. Clin. Pathol. 50437-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu, C. H., and J. T. Ou. 1996. Rapid identification of Salmonella serovars in feces by specific detection of virulence genes, invA and spvC, by an enrichment broth culture-multiplex PCR combination assay. J. Clin. Microbiol. 342619-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemens, J., S. Hoffman, B. Ivanoff, K. Klugman, M. M. Levine, M. Neira, and T. Pang. 1999. Typhoid fever vaccines. Vaccine 172476-2478. [DOI] [PubMed] [Google Scholar]
- 7.Coburn, B., G. A. Grassl, and B. B. Finlay. 2007. Salmonella, the host and disease: a brief review. Immunol. Cell Biol. 85112-118. [DOI] [PubMed] [Google Scholar]
- 8.Connor, B. A., and E. Schwartz. 2005. Typhoid and paratyphoid fever in travellers. Lancet Infect. Dis. 5623-628. [DOI] [PubMed] [Google Scholar]
- 9.Eswarappa, S. M., J. Janice, A. G. Nagarajan, S. V. Balasundaram, G. Karnam, N. M. Dixit, and D. Chakravortty. 2008. Differentially evolved genes of Salmonella pathogenicity islands: insights into the mechanism of host specificity in Salmonella. PLoS One 3e3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folkesson, A., S. Lofdahl, and S. Normark. 2002. The Salmonella enterica subspecies I specific centisome 7 genomic island encodes novel protein families present in bacteria living in close contact with eukaryotic cells. Res. Microbiol. 153537-545. [DOI] [PubMed] [Google Scholar]
- 11.Frazer, K. A., L. Pachter, A. Poliakov, E. M. Rubin, and I. Dubchak. 2004. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 32W273-W279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatta, M., and H. L. Smits. 2007. Detection of Salmonella typhi by nested polymerase chain reaction in blood, urine, and stool samples. Am. J. Trop. Med. Hyg. 76139-143. [PubMed] [Google Scholar]
- 13.Hensel, M. 2004. Evolution of pathogenicity islands of Salmonella enterica. Int. J. Med. Microbiol. 29495-102. [DOI] [PubMed] [Google Scholar]
- 14.Joys, T. M., and B. A. Stocker. 1966. Isolation and serological analysis of mutant forms of flagellar antigen i of Salmonella typhimurium. J. Gen. Microbiol. 44121-138. [DOI] [PubMed] [Google Scholar]
- 15.Joys, T. M., and B. A. Stocker. 1963. Mutation and recombination of flagellar antigen i of Salmonella typhimurium. Nature 197413-414. [DOI] [PubMed] [Google Scholar]
- 16.Kumar, A., V. Arora, A. Bashamboo, and S. Ali. 2002. Detection of Salmonella typhi by polymerase chain reaction: implications in diagnosis of typhoid fever. Infect. Genet. Evol. 2107-110. [DOI] [PubMed] [Google Scholar]
- 17.Massi, M. N., T. Shirakawa, A. Gotoh, A. Bishnu, M. Hatta, and M. Kawabata. 2003. Rapid diagnosis of typhoid fever by PCR assay using one pair of primers from flagellin gene of Salmonella typhi. J. Infect. Chemother. 9233-237. [DOI] [PubMed] [Google Scholar]
- 18.Mayor, C., M. Brudno, J. R. Schwartz, A. Poliakov, E. M. Rubin, K. A. Frazer, L. S. Pachter, and I. Dubchak. 2000. VISTA: visualizing global DNA sequence alignments of arbitrary length. Bioinformatics 161046-1047. [DOI] [PubMed] [Google Scholar]
- 19.Nair, S., S. Alokam, S. Kothapalli, S. Porwollik, E. Proctor, C. Choy, M. McClelland, S. L. Liu, and K. E. Sanderson. 2004. Salmonella enterica serovar Typhi strains from which SPI7, a 134-kilobase island with genes for Vi exopolysaccharide and other functions, has been deleted. J. Bacteriol. 1863214-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nolan, L. K., C. W. Giddings, and J. Brown. 1995. The distribution of invA, pagC and spvC genes among Salmonella isolates from animals. Vet. Res. Commun. 19167-177. [DOI] [PubMed] [Google Scholar]
- 21.Ohl, M. E., and S. I. Miller. 2001. Salmonella: a model for bacterial pathogenesis. Annu. Rev. Med. 52259-274. [DOI] [PubMed] [Google Scholar]
- 22.Olsen, S. J., J. Pruckler, W. Bibb, T. M. Nguyen, M. T. Tran, T. M. Nguyen, S. Sivapalasingam, A. Gupta, T. P. Phan, T. C. Nguyen, V. C. Nguyen, D. C. Phung, and E. D. Mintz. 2004. Evaluation of rapid diagnostic tests for typhoid fever. J. Clin. Microbiol. 421885-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parry, C. M., T. T. Hien, G. Dougan, N. J. White, and J. J. Farrar. 2002. Typhoid fever. N. Engl. J. Med. 3471770-1782. [DOI] [PubMed] [Google Scholar]
- 24.Roumagnac, P., F. X. Weill, C. Dolecek, S. Baker, S. Brisse, N. T. Chinh, T. A. Le, C. J. Acosta, J. Farrar, G. Dougan, and M. Achtman. 2006. Evolutionary history of Salmonella typhi. Science 3141301-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowe, B., L. R. Ward, and E. J. Threlfall. 1997. Multidrug-resistant Salmonella typhi: a worldwide epidemic. Clin. Infect. Dis. 24(Suppl. 1)S106-S109. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Jimenez, M. M., and N. Cardona-Castro. 2004. Validation of a PCR for diagnosis of typhoid fever and salmonellosis by amplification of the hilA gene in clinical samples from Colombian patients. J. Med. Microbiol. 53875-878. [DOI] [PubMed] [Google Scholar]
- 27.Song, J. H., H. Cho, M. Y. Park, D. S. Na, H. B. Moon, and C. H. Pai. 1993. Detection of Salmonella typhi in the blood of patients with typhoid fever by polymerase chain reaction. J. Clin. Microbiol. 311439-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wain, J., T. S. Diep, V. A. Ho, A. M. Walsh, T. T. Nguyen, C. M. Parry, and N. J. White. 1998. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J. Clin. Microbiol. 361683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson, K. C. 1978. Laboratory and clinical investigation of recovery of Salmonella typhi from blood. J. Clin. Microbiol. 7122-126. [DOI] [PMC free article] [PubMed] [Google Scholar]