Abstract
Glucocorticoid receptor (GR) is cytoplasmic in the absence of ligand and localizes to the nucleus after steroid binding. Previous evidence demonstrated that the hsp90-based heterocomplex bound to GR is required for the efficient retrotransport of the receptor to the nuclear compartment. We examined the putative association of GR and its associated chaperone heterocomplex with structures of the nuclear pore. We found that importin β and the integral nuclear pore glycoprotein Nup62 interact with hsp90, hsp70, p23, and the TPR domain proteins FKBP52 and PP5. Nup62 and GR were able to interact in a more efficient manner when chaperoned by the hsp90-based heterocomplex. Interestingly, the binding of hsp70 and p23 to Nup62 does not require the presence of hsp90, whereas the association of FKBP52 and PP5 is hsp90 dependent, as indicated by the results of experiments where the hsp90 function was disrupted with radicicol. The ability of both FKBP52 and PP5 to interact with Nup62 was abrogated in cells overexpressing the TPR peptide. Importantly, GR cross-linked to the hsp90 heterocomplex was able to translocate to the nucleus in digitonin-permeabilized cells treated with steroid, suggesting that GR could pass through the pore in its untransformed state.
Glucocorticoid receptor (GR) is a ligand-activated transcription factor that belongs to the nuclear receptor superfamily. GR controls a large variety of physiological functions, such as metabolism, development, differentiation, and reproduction (27). In the absence of steroid, GR is primarily located in the cytoplasm of several cell types (4, 15, 20, 50, 51). Upon hormone binding, GR rapidly translocates to its sites of action in the nucleus.
GR exists as an oligomer associated with hsp90, hsp70, p23, and one hsp90-binding TPR (tetratricopeptide repeat) protein (43), this heterocomplex being essential for steroid binding since the chaperones maintain GR in a folded, competent state. hsp90 interacts with TPR proteins, such as the high-molecular-weight immunophilins (IMMs) FKBP51 and FKBP52, Cyp40, and the IMM-like Ser/Thr-phosphatase PP5, and other cochaperones, such as Hop and Tpr2. The hsp90-TPR interaction is highly conserved and takes place in many molecular arrays from the animal and plant kingdoms (1, 11, 25, 33, 44).
The cytoplasmic movement of GR and other steroid receptors toward the nucleus is thought to be achieved by two different mechanisms. The most efficient mechanism is rapid (half-life, ∼5 min) and depends on the hsp90-FKBP52-based heterocomplex (10, 16, 21, 26, 40). The alternative mechanism is hsp90 independent and translocates the receptor to the nucleus with a half-life equal to 40 to 60 min, which allows the formation of degradasomes and the subsequent targeting of the receptor to proteasomal degradation (15). In contrast to the rapidity of the steroid-dependent retrograde movement, GR cycles back very slowly to the cytoplasm (half-life, ∼8 to 12 h) upon steroid withdrawal (18).
Regardless of the primary subcellular localization, all steroid receptors and most of the nuclear factors involved in signaling cascades are constantly shuttling between the nucleus and the cytoplasm (31). This dynamic nucleocytoplasmic shuttling takes place through the nuclear pore complex (NPC), a macromolecular multimeric structure of 125 MDa that is embedded in the nuclear envelope. While small molecules are able to diffuse freely through this structure, molecules larger than 40 kDa require an active transport mechanism (35, 49) mediated by an adapter receptor, importin α (Impα). Impα binds the nuclear localization signal (NLS) of the substrate and forms a trimeric complex with Impβ, the transport receptor that favors the passage of many cargoes through the nuclear pore (see reference 47 for a recent review). Two NLS's have been identified in GR, the bipartite NLS-1 that overlaps the hinge region at the C-terminal end of the DNA-binding domain and is recognized by Impα and the more diffuse NLS-2 that comprises a less defined sequence in the ligand-binding domain (39). It is accepted that the nuclear import mechanism of GR is mediated by the classical pathway that employs Impα (45). Nonetheless, an Impα-independent mechanism has also been postulated for GR (3) and the mineralocorticoid receptor (MR) (40). It has recently been demonstrated that GR is translocated to the nucleus along with Impα (48), but Impβ does not change its perinuclear localization. While it has been shown that upon steroid binding, the cytoplasmic isoform of GR interacts directly with Impα and both proteins are detached shortly after nuclear import, direct interaction between GR and Impβ could not be demonstrated (48).
The novel hsp90-IMM-dependent model postulated for GR retrotransport clearly collides with the classical model that has posed the heuristic notion that the hsp90-based heterocomplex must dissociate immediately from GR upon ligand binding to permit its retrotransport and subsequent nuclear translocation. Actually, there is an emerging body of experimental evidence that agrees with a model by which the hsp90-IMM complex should not dissociate from GR because this complex is still required for the retrotransport mechanism and, perhaps, for the passage of GR through the nuclear pore. If this model is correct, it is highly possible that the chaperone machinery might interact with importins and other key components of the NPC, such as nucleoporins (Nups). In this work, we analyzed this hypothesis and characterized some molecular aspects of the import system of GR.
MATERIALS AND METHODS
Antibodies.
Mouse monoclonal immunoglobulin G (IgG) anti-hsp70 was from StressGen (Ann Arbor, MI); mouse monoclonal IgG anti-Impβ1 and anti-glutathione S-transferase (anti-GST) were from Sigma Chemical Co. (St. Louis, MO); mouse IgG anti-p23 (JJ3 clone), rabbit polyclonal IgG anti-FKBP51, and mouse IgG anti-GR (BuGR2 clone) were from Affinity BioReagents (Golden, CO); mouse IgG clone monoclonal antibody (MAb) MAb414 anti-Nup was from Covance (Berkeley, CA); rabbit antiserum against hsp90 was described previously (13); rabbit antiserum against insect hsp70/hsp90 was a kind gift from J.-M. Renoir; rabbit antiserum against PP5 was a kind gift from M. Chinkers; UP30 rabbit antiserum against FKBP52 was a kind gift from W. B. Pratt; and rabbit IgG antiactin antibody was a gift from L. Jiménez de Asúa.
Cell culture and indirect immunofluorescence assays.
Human HEK293T cells, L929 mouse fibroblasts, and the L929-derived cell line E82.A3 (GR−/−) were cultured in Dulbecco's modified Eagle's medium (Invitrogen Argentina) supplemented with 10% bovine calf serum (Internegocios, Argentina). The nuclear translocation rate of endogenous GR was measured in L929 cells as described in previous works (9, 15). Nuclear accumulation was triggered by adding 100 nM dexamethasone to the medium (zero time). Cells were fixed in cold (−20°C) methanol at different periods of time, and GR was visualized by indirect immunofluorescence using the BuGR2 antibody. Knockdown experiments for Impβ were performed by using specific small interfering RNA (siRNA) (Santa Cruz Biotech, Santa Cruz, CA) according to the manufacturer's instructions. When the quantification of fluorescence was required, cells were analyzed with Image-Pro Plus Media Cybernetics software, and both the nuclear and cytoplasmic fluorescence were quantified as the mean of the intensity for each compartment. The nuclear fraction was calculated as the nuclear/total pixel ratio. Confocal microscopy was performed using a Zeiss LSM5 Pa confocal microscope.
Immunoprecipitations.
Nups were immunoprecipitated according to the method described by Grandi et al. (24). Whole-cell lysate extracts were obtained in Triton X-100 lysis buffer (20 mM Tris hydrochloride, pH 7.4, 150 mM KCl, 0.2 mM MgCl2, 1 mM dithiothreitol, 2% Triton X-100). Cells were maintained on ice for 15 min and cleared by centrifugation at 12,000 rpm in an Eppendorf minifuge at 4°C for 30 min. Approximately 2 mg of proteins of the supernatant (in 2 ml) were sequentially incubated with the anti-Nup antibody MAb414 for 3 h at 4°C and with protein A-Sepharose for 1 h at 4°C. After the pellet was washed with TEGM buffer [10 mM N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid, pH 7.6, 50 mM NaCl, 4 mM EDTA, 10% (vol/vol) glycerol, 20 mM Na2MoO4, and Complete Mini protease inhibitor cocktail (Roche Molecular Biochemicals)], the pellets were divided into two fractions and proteins resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Western blotting. Blots were stripped for 30 min at 55°C with Tris-buffered saline containing 5% 2-β-mercaptoethanol and 2% sodium dodecyl sulfate and reprobed. To determine the presence of GR in the immunopellets, the Triton X-100 in the lysis buffer was replaced with 0.5% NP-40 to avoid the dissociation of the receptor.
Nup-hsp90 heterocomplex reconstitution.
We followed a previously described protocol for Nup-hsp90 heterocomplex reconstitution (19, 42). Briefly, Nups were immunoprecipitated and stripped of associated proteins by incubation of the pellet with 0.5 M NaCl (2 h at 4°C). After the pellet was washed with 10 mM HEPES, pH 7.5, the stripped Nups were incubated with 50 μl of reticulocyte lysate and 5 μl of an ATP-regenerating system (50 mM ATP, 250 mM creatine phosphate, 20 mM magnesium acetate, and 100 units/ml creatine phosphokinase). The pellets were incubated for 20 min at 30°C with the tubes being flicked every 2 min. The pellets were washed three times with 1 ml of ice-cold TEGM buffer. The proteins in the reconstituted pellets were visualized by Western blotting.
Binding of GST-GR to Nup62.
Sf9 insect cells were infected with baculovirus encoding GST-hGR as described in previous works (46). Cytosol was extracted in 10 mM HEPES buffer at pH 7.5, and 50 μl was immobilized on 25 μl of 50% (wt/vol) glutathione-agarose (Sigma). The mixture was rotated at 4°C for 2 h and washed three times with phosphate-buffered saline buffer. The pellets were suspended in TEG buffer containing 1.0 M NaCl and protease inhibitors and incubated at 30°C for 2 h to dissociate insect chaperones. The pellets were washed three times with radioimmunoprecipitation assay buffer and five times with 10 mM HEPES at pH 7.5. Where indicated below, a reconstitution assay with reticulocyte lysate was performed. GST-GR was released with a neutralized solution of reduced glutathione (20 mM), and the resultant supernatants were used as a source of GR or GR-hsp90 heterocomplex. Nup62 pellets were obtained as described above and reconstituted (or not) with reticulocyte lysate. Incubations of GST-GR and pellets of Nup62 were performed on ice for 30 min in HEK buffer (10 mM HEPES, pH 7.5, 1 mM dithiothreitol, 25 mM KCl, 0.02% NP-40). At the end of the incubation, pellets were washed with TEGM buffer and proteins resolved by Western blotting.
Cross-linking of mouse GR with the insect chaperone complex.
Mouse GR was produced in Sf9 cells infected with baculovirus. The GR-insect chaperone system was cross-linked by treating the Sf9 cytosol obtained in 10 mM HEPES buffer at pH 7.5 with 2 mM dithiosuccinimidyl propionate (DSP; Pierce, Rockford, IL) for 2 min at 25°C. The cross-linked receptor was cleared of free GR, excess reagents, and other free factors by a quick centrifugation for 30 s at 12,000 rpm in a minicolumn packed with Sephacryl S200 equilibrated in Adam's buffer (2).
Digitonin-permeabilized cells.
E82.A3 fibroblasts grown on coverslips were permeabilized with 25 μg/ml of digitonin solution as described in previous papers (40, 50). These permeabilized cells were incubated with cross-linked mouse GR in 40 μl of Adam's buffer supplemented with an ATP-regenerating system and 10 μl of E82.A3 cytosol (∼40 μg protein). After 20 min at 30°C (with and without 100 nM dexamethasone in the medium), the coverslips were rapidly washed with Adam's buffer and fixed with cold methanol, and the localization of mouse GR was visualized by indirect immunofluorescence using a confocal microscope. The perinuclear ring was stained with the MAb414 antibody.
RESULTS
Constructing a protein-protein interaction network for the GR-hsp90-TPR protein heterocomplex and the nuclear import/export machinery.
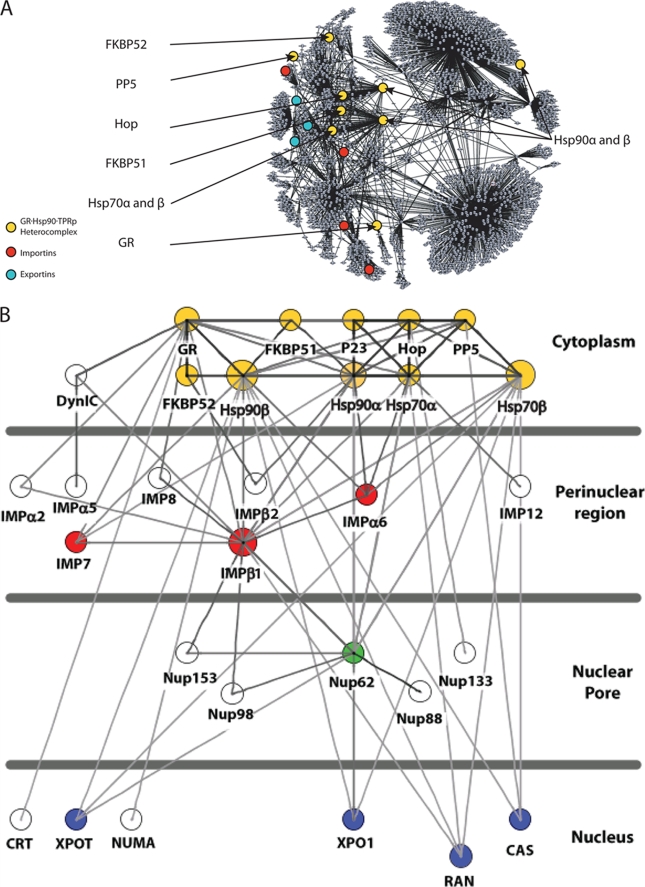
The application of high-throughput technologies, such as large-scale yeast two-hybrid and microarray analyses, has generated a vast amount of data that have allowed the construction of many protein-protein interaction databases. The use of these databases represents a very powerful tool for generating hypotheses for the empirical study of biological protein networks. To perform a predictive analysis of the interactive biology of the GR-hsp90-TPR protein heterocomplex, the available information in several databases that were generated from large-scale experiments (i.e., yeast two-hybrid assays, affinity purification, protein chips, and microarray studies) was accessed to recreate networks centered on the heterocomplex. This protein complex includes GR; hsp90; hsp70; p23; and the TPR proteins Hop, FKBP51, FKBP52, and PP5. The construction of this model involved a number of bioinformatics steps, the first one involving an extensive search in the literature to acquire the currently published experimental data for interaction networks centered on the GR-hsp90-TPR protein complex. In addition to conventional methods, we used an automated text-mining tool, the “Agilent Literature Search Software” plugin for Cytoscape (http://www.agilent.com/labs/research/litsearch.html) for finding interaction data in PubMed abstracts. The next step was the establishment of the protein-protein interaction network by analysis of publicly accessible databases. The database used for this study was cPath (7), an open source database and software suite which compiles data from other databases for many organisms. The cPath software works as a web-based platform integrated inside Cytoscape, a bioinformatic software platform for visualizing molecular interaction networks that summarizes interactions with other biological data (http://www.cytoscape.org/). The interactome data for each protein of the GR-hsp90-TPR protein heterocomplex was collected for mammalian sources (human, mouse, and rat), and then, each individual network was merged in a unique network that contains the combined interactome of the heterocomplex and its first-level interactors. As a result, the complete interactome for the GR-hsp90-TPR protein complex is shown in Fig. 1A (full protein identifications, interaction sources, and extra features of the network are available on request).
FIG. 1.
In silico interactome for the GR-hsp90-TPR domain protein complex. (A) cPath-generated interactome (experimental). The query proteins are highlighted in yellow, and the importins and exportins found inside the network in red and blue, respectively. (B) Schematic of intracellular localization of the potentially associated proteins (interolog-predicted interactome). The proteins that show three or more connections with the heterocomplex are colored yellow for the GR-hsp90-TPR heterocomplex, red for importins, green for Nups, and blue for proteins of the export machinery. The proteins showing six or more connections joining the heterocomplex or going out of the heterocomplex to lower levels in the scheme are oversized.
Most of the interactors found in this search are related to proteins associated with the cytoskeleton, motor proteins, translational and transcription factors, mitochondrial proteins, and the nuclear import/export machinery. This reveals a broad functionality of the heterocomplex. To extend the study of the relationship of the GR-hsp90-TPR protein complex with the import/export machinery and the nuclear pore, it was necessary to filter unnecessary nodes and increase the number of interactors with data from other sources. Consequently, we used the database POINT (28) for the prediction of human protein-protein interactions. This database employs the concept of interologs (orthologous pairs of interacting proteins in different organisms), increasing the number of potential interactions in each case. Data from the literature were also used to correct the absence of some interactors in the cPath-generated network. These results are schematized in Fig. 1B, where the interactome map also shows the annotated and/or predicted subcellular localizations and the putative associated proteins (interaction source data are available on request). This drawing was created by using the Cytoscape plug-in Cerebral (5). The GR-hsp90-TPR protein heterocomplex displays a high degree of connectivity with several import receptors and Nups. Proteins that show three or more connections with the heterocomplex or six or more connections entering the heterocomplex or going out of the heterocomplex to lower levels in the scheme are indicated (Fig. 1B).
Interaction of the chaperone system with proteins of the nuclear pore.
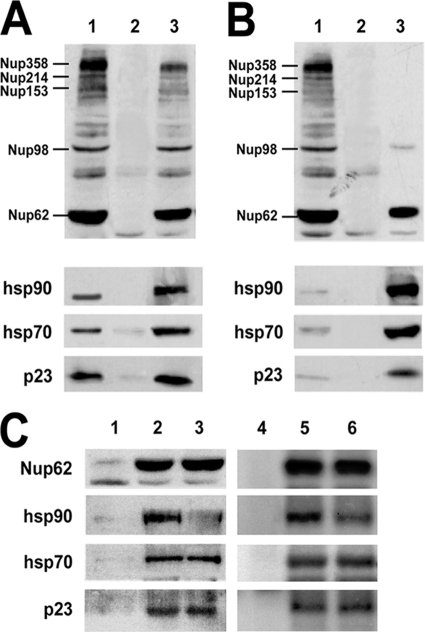
Based on the biological observations that support a novel experimental model for steroid receptor trafficking and the studies performed in silico (Fig. 1), the predicted association of chaperones was explored by immunoprecipitation of Nups with the antibody MAb414. This antibody recognizes the conserved FXFG (FG) domain of several Nups. Figure 2A shows the most prominent bands detected in a whole lysate from L929 cells (lane 1), which are the same as those shown in the immunoprecipitation assay. Thus, the specific immune pellet (lane 3 versus lane 2) yielded five recognizable bands, corresponding to Nup358, Nup214, Nup153, Nup98, and Nup62, the last Nup being the most abundant. Importantly, the Nups with this FG domain coimmunoprecipitate with hsp90, hsp70, and p23.
FIG. 2.
Nup62 interacts with chaperones. (A) Coimmunoprecipitation of hsp90, hsp70, and p23 with Nups. Lane 1, whole-cell lysate from L929 fibroblasts; lane 2, nonimmune pellet; lane 3, immunoprecipitation of Nups with MAb414 antibody. (B) Nup62 is the major Nup interacting with the chaperone complex. Nup62 was immunoprecipitated with MAb414 antibody (lanes 1 and 3) or a nonimmune antibody (lane 2). The pellet was stripped of associated proteins (lanes 2 and 3), and the Nup-chaperone complex was reconstituted with reticulocyte lysate in the presence of an ATP-regenerating system. (C) Binding of hsp70 and p23 to Nup62 is independent of hsp90. Lanes 1 to 3, Nup62 was immunoprecipitated, stripped, and reconstituted with reticulocyte lysate. Lane 1, nonimmune pellet; lane 2, complex reconstituted with reticulocyte lysate; lane 3, complex reconstituted in the presence of 2 μM radicicol. Lanes 4 to 6, Nup62 was immunoprecipitated from L929 cells. Lane 4, nonimmune pellet; lane 5, MAb414 immune pellet; lane 6, immune pellet from cells pretreated with 2 μM radicicol for 3 h.
In order to determine whether Nup62 is the major interacting Nup among those recognized by the MAb414 antibody, the immune pellet was stripped of associated factors with a high-ionic-strength solution. Then, the Nup62-chaperone complex was reconstituted by incubating the immunopurified Nup62 with reticulocyte lysate and an ATP-regenerating system. This regeneration system is identical to that used in previous studies to reconstitute heterocomplexes with GR (34), MR (41), and p53 (17). The results presented in Fig. 2B show that all three factors, hsp90, hsp70, and p23, are bound to the integral protein of the NPC, Nup62.
In view of the fact that the Nup62-chaperone complex could be reconstituted with the reticulocyte lysate system, the assay whose results are shown in Fig. 2B was repeated in the presence of the hsp90-disrupting agent radicicol. The results presented in Fig. 2C show that, as expected, there was a significant loss of hsp90 in the complex (lane 3). Nevertheless, hsp70 and p23 bound to Nup62 were still recovered in amounts similar to those seen without radicicol (lane 2), suggesting that these two proteins are capable of interacting with the Nup in an hsp90-independent manner. In order to explore whether or not this property is an artifact generated during the reconstitution assay, L929 cells were treated with radicicol and Nups were immunoprecipitated with the MAb414 antibody. The treatment of intact cells with radicicol also yielded decreased amounts of hsp90 bound to the Nup (lane 6 versus lane 5), indicating that hsp90 is the only chaperone sensitive to the treatment and that hsp70 and p23 can bind directly to Nups.
Impβ1 interacts with Nup62, hsp90, and GR.
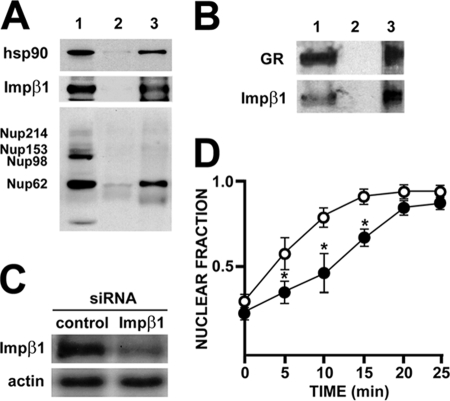
Inasmuch as Nup62 interacts dynamically with hsp90, we asked whether or not any of the other Nups with the FG domain recognized by the MAb414 antibody is also able to interact with the chaperone similarly to Nup62. The results presented in Fig. 3A show that, when hsp90 was immunoprecipitated from L929 lysate, Nup62 was the only Nup among those recognized by the antibody that was coimmunoprecipitated with the chaperone. Importantly, Impβ1 was also recovered in the immune pellet, confirming the interaction predicted by the in silico analysis whose results are depicted in Fig. 1B. Impβ1 bound to GR was also recovered (Fig. 3B). Impβ-type transport receptors account for most, but not all, nuclear transport pathways. Therefore, to assess the functional importance of this interaction for the GR import mechanism, Impβ1 was knocked down with a specific siRNA (Fig. 3C) and the steroid-dependent nuclear import rate of green fluorescent protein (GFP)-GR was measured in HEK293T cells. The results presented in Fig. 3D show that the nuclear translocation rate of GFP-GR was significantly delayed in those cells where the expression of Impβ1 was abolished (this was evidenced by indirect immunofluorescent staining). Nevertheless, the nuclear import of GFP-GR was not fully abrogated. This observation indicates that the association of GR with Impβ1 does favor GR nuclear import but is not absolutely required for the process.
FIG. 3.
GR and hsp90 bind to Impβ. (A) Nup62 and Impβ1 coimmunoprecipitate with hsp90. Lane 1, L929 cell lysate; lane 2, nonimmune pellet; lane 3, immunoprecipitation of hsp90. (B) Impβ1 coimmunoprecipitates with GR. Lane 1, L929 cell lysate; lane 2, nonimmune pellet; lane 3, immunoprecipitation of GR. (C) Knockdown of Impβ with specific siRNA in HEK293T cell lysates. (D) Nuclear translocation rate of GFP-GR in normal HEK293T cells (○) and cells treated with the siRNA against Impβ (•). Receptor translocation was triggered with 100 nM dexamethasone added to the medium at zero time. Results are the means ± standard errors of the means from three independent experiments where ∼100 cells were counted for each time point. *, significantly different at P < 0.005.
It must be emphasized that, in the assays whose results are shown in Fig. 2A, the coimmunoprecipitation of GR and IMMs with Nups was very erratic, most likely due to the high concentration of Triton X-100 required for the extraction and solubilization of Nups. However, when the extractions were performed in a buffer containing NP-40 rather than Triton X-100 (see Materials and Methods), GR was immunoprecipitated and Nup62 was specifically recovered in the immune pellet along with Nup98, Nup153, and Nup214 (Fig. 4). We then analyzed in L929 cells the putative colocalization of Nups with endogenous GR by confocal microscopy. The results in Fig. 4 show that after 12 min of treatment with 10 nM cortisol, the GR signal overlapped with that obtained for the perinuclear ring with the MAb414 anti-Nup antibody. This merged image was not observed without steroid or after 20 min with steroid (data not shown). On the other hand, E82.A3 fibroblasts, a GR−/− cell line, showed the perinuclear ring of Nups and no GR signal, which rules out the possibility that the image obtained with L929 cells is due to cross-reaction between the antibodies. Unfortunately, the anti-Impβ antibody did not provide a reliable specific signal by indirect immunofluorescence, and we were unable to demonstrate a similar pattern after costaining for GR and Impβ.
FIG. 4.
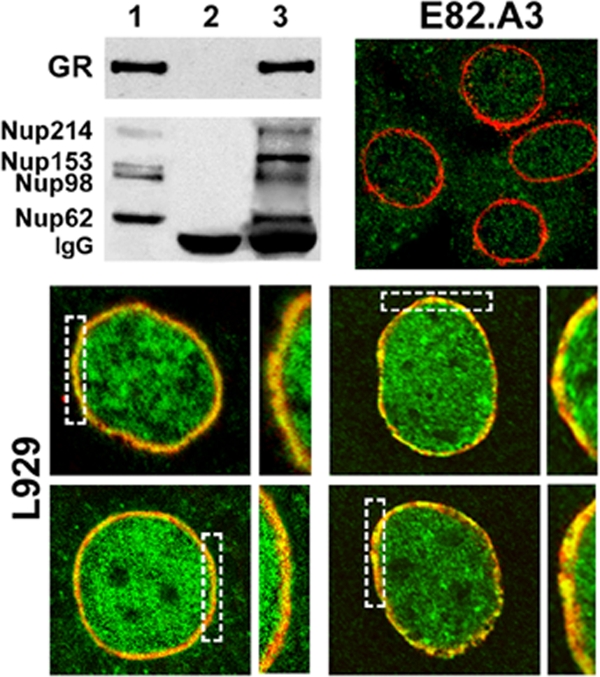
GR interacts with Nup62. Top left, coimmunoprecipitation of Nup62 with GR. Lane 1, L929 cell lysate; lane 2, nonimmune pellet; lane 3, immunoprecipitation of GR. Top right and bottom, confocal microscopy. L929 cells or the L929-derived GR−/− cell line E82.A3 were treated with 10 nM cortisol for 12 min, fixed with cold methanol, and stained for endogenous GR with BuGR2 antibody and for Nups with MAb414 antibody (counterstained with Alexa 488-labeled and rhodamine-labeled secondary antibody, respectively). GR (green signal) overlapping with the perinuclear ring (red signal) obtained with the MAb414 anti-Nup antibody shows a yellow signal (dashed boxes). This yellow merged image was not observed without steroid or after 20 min with steroid (data not shown).
hsp90 favors the interaction of GR with Nup62.
In view of the fact that Nup is chaperoned by hsp90, we asked whether this association affects the interaction between Nup62 and GR. Therefore, high-salt and detergent solution-stripped immune pellets of Nup62 were reconstituted with a reticulocyte lysate system, incubated with recombinant GST-GR purified on glutathione-agarose, stripped of associated proteins, and released from the solid phase with 20 mM reduced glutathione. The results presented in Fig. 5A show that, in contrast to the case with stripped Nup62 (lane 2), reconstituted Nup62-hsp90 complexes (lane 3) interact more efficiently with stripped GR. GR-hsp90 complexes showed even higher efficiency of interaction with Nup62-hsp90 (lane 4), whereas the presence of radicicol (lane 5) greatly disrupted that interaction. These results clearly show that, at least in a cell-free system, the presence of hsp90 in both complexes favors the interaction of GR with Nup62. This finding was somewhat unexpected, because a high level of interaction may represent a very slow translocation rate due to a longer half-life of intermediate interactors and also a potential delay in releasing the cargo from the integral Nup channel in the nucleoplasmic basket structure. In view of the fact that under physiological conditions there are other soluble factors involved in the translocation process through the NPC, we repeated the above-described studies in the presence of E82.A3 cytosol (as a source of soluble factors) and GTP. The results presented in Fig. 5B show that the presence of both GTP and cytosolic factors reduced the amounts of GR-hsp90 complexes capable of interacting with Nup62 compared to the amounts interacting under the optimal conditions for which results are shown in Fig. 5A, although the interaction was still in evidence. Therefore, we may conclude that the GR-hsp90 complex should dynamically associate with and dissociate from Nup62-hsp90 complexes, the strength and/or half-life of these associations being greatly dependent on and regulated by the presence of other soluble factors and GTP.
FIG. 5.
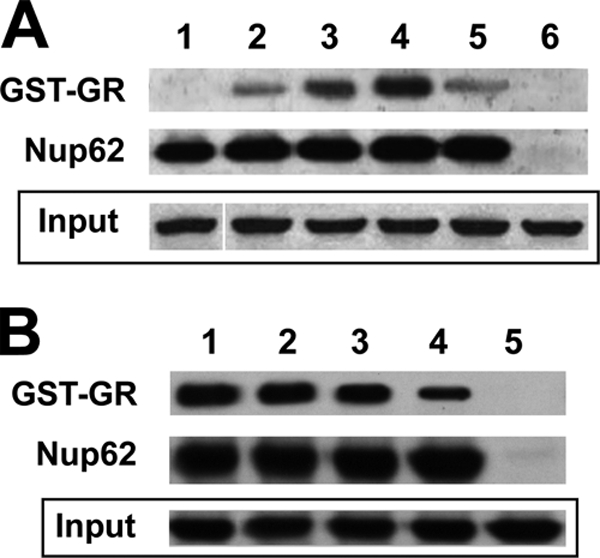
GR interacts with Nup62 directly. (A) Nup62 was immunoprecipitated, stripped, and reincubated with pure GST (lane 1) or pure GST-GR (lane 2), or it was incubated with reticulocyte lysate, washed, and then treated with pure GST-GR (lane 3); Nup62 pellet treated as described for lane 3 was incubated with the GST-GR heterocomplex previously reconstituted with reticulocyte lysate (lane 4); Nup62 pellet treated as described for lane 4 was incubated in the presence of 2 μM radicicol (lane 5); and nonimmune Nup62 pellet was incubated with pure GST-GR (lane 6). GST-GR was revealed with an anti-GST antibody. (B) Effects of soluble cytosolic factors and GTP on GR-Nup62 binding. Nup62 immunopellets were reconstituted with reticulocyte lysate and incubated with GST-GR-hsp90 heterocomplex reconstituted as described for lane 4 of panel A (lane 1), 50 μM GTP (lane 2), 40 μg of cytosolic proteins from E82.A3 lysates (lane 3), or cytosolic proteins and GTP combined (lane 4), and nonimmune Nup62 pellet was incubated with pure GST-GR (lane 5). “Input” represents one-fifth of the total amount of GST (lane 1) or GST-GR (lanes 2 to 6) used in the incubation with the Nup62 immunopellet.
Involvement of hsp90-binding TPR proteins in the Nup-GR interaction.
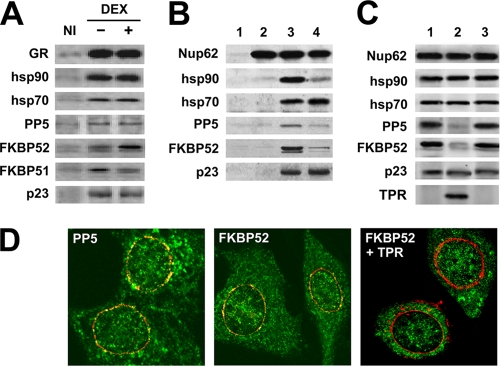
In previous reports by other groups (10) and our own laboratory (21), it was shown that there is a swapping of IMMs upon steroid binding. Thus, FKBP51 is replaced by FKBP52 in the steroid receptor-hsp90 complex. In the experiment whose results are shown in Fig. 6A, L929 cells were incubated on ice with 10 nM dexamethasone for 1 h to allow ligand binding but not receptor movement, which is a temperature- and energy-dependent process. Then, GR was immunoprecipitated and the associated chaperones hsp90 and hsp70, the cochaperones p23 and PP5, and both FKBPs were visualized by Western blotting. FKBP52 was recruited to the complex, but PP5, an IMM that has been shown to be recruited to the MR upon binding of a synthetic agonist (21), was not. On the other hand, FKBP51 was released from the complex, probably due to competition by FKBP52 for the only TPR acceptor site present in the hsp90 dimer (46). Because of this observation, we inferred that IMMs may also be Nup62-interacting proteins, in particular because hsp90 is associated with the Nup. However, we were unable to detect the presence of these IMMs bound to Nup62 in L929 cell extracts where buffers containing detergents were used. This is because IMMs are extracted from the complexes under these conditions.
FIG. 6.
Nup62 binds hsp90-binding TPR proteins. (A) L929 cells were incubated on ice with ethanol (−) or dexamethasone (+). After 1 h of incubation, GR was immunoprecipitated and the associated proteins were visualized by Western blotting. NI, nonimmune antibody. (B) TPR protein association is hsp90 dependent. Nup62 was immunoprecipitated with MAb414 antibody (lanes 2 to 4) or nonimmune antibody (lane 1) and incubated with buffer (lane 2), reticulocyte lysate (lanes 1 and 3), or reticulocyte lysate and 2 μM radicicol (lane 4). (C) IMMs bind to Nup62 via their TPR domains. Stripped Nup62 immunopellets were incubated with reticulocyte lysate supplemented with buffer (lane 1), TPR peptide (lane 2), or peptidyl-prolyl isomerase peptide (lane 3). (D) Confocal microscopy following indirect immunofluorescence assay for Nup62 (red) and the indicated IMMs (green). “TPR” indicates cells overexpressing the TPR peptide. The yellow in the merged images shows colocalization of Nup62 with FKBP2 and was impaired by overexpression of the TPR domain of PP5.
Therefore, we studied the putative IMM-Nup62 interaction in a more stable, cell-free milieu, the reticulocyte lysate reconstitution system. Nup62 was immunoprecipitated, stripped of associated proteins, and reincubated with reticulocyte lysate and an energy-regenerating system. The results in Fig. 6B show that hsp90, hsp70, PP5, FKBP52, and p23 were recovered in association with Nup62 (Fig. 6B, lane 3), whereas we were unable to detect FKBP51. The association of the hsp90-binding TPR proteins FKBP52 and PP5 was abrogated by incubation with the hsp90 inhibitor radicicol (Fig. 6B, compare lane 4 to lane 3). Consistent with the results of the reconstitution experiments shown in Fig. 2, neither hsp70 nor p23 was significantly dissociated, suggesting that their capacity for binding to Nup62 is hsp90 independent. Moreover, the results presented in Fig. 6C show that both FKBP52 and PP5 were released from the complex when the reticulocyte lysate was supplemented with TPR peptide (lane 2). An identical experiment using the peptidyl-prolyl isomerase peptide of the IMM showed no effect (lane 3) compared to the results for the control in lane 1.
To confirm that both IMMs are indeed able to interact with Nups, an indirect immunofluorescence assay was done using the MAb414 antibody for Nups (Fig. 6D). The merged image generated by colocalization of Nup62 with FKBP2 was impaired by overexpression of the TPR domain of PP5. Because the antiserum against PP5 recognizes the TPR domain of endogenous PP5, we could not demonstrate the same type of competition of the TPR peptide with PP5. Nonetheless, the experimental evidence supports the existence of hsp90-IMM-Nup complexes in the NPC.
The GR-hsp90 heterocomplex translocates intact through the NPC.
The classic model for steroid receptor transformation upon steroid binding is being questioned because the steroid receptor-hsp90-IMM complex seems to be required for dynein-dependent retrotransport of steroid receptors (20, 21, 26, 52). Therefore, it might be possible that the entire GR-hsp90 heterocomplex is translocated through the nuclear pore, such that the transformation process could be a nuclear event rather than a necessary cytoplasmic first step to trigger the nuclear localization of GR.
In view of the fact that the chaperones associated with GR are able to interact with components of the NPC, we tested the above-proposed hypothesis by using a cross-linked GR-hsp90 heterocomplex, a condition where receptor transformation upon ligand binding is not an option. Mouse GR was produced in Sf9 cells infected by baculovirus, and the cytosolic form of GR folded by insect chaperones was treated with 2 mM DSP. The Western blot shown in Fig. 7 demonstrates that the mouse GR treated with high-ionic-strength solution was still bound to insect hsp90 and hsp70 and, consequently, did not appear in a 10% acrylamide gel (lane 3) unless the cross-linked complex was previously disrupted with 2-mercaptoethanol (lane 2). Lane 1 shows a standard marker for the proteins in cytosol that was not cross-linked.
FIG. 7.
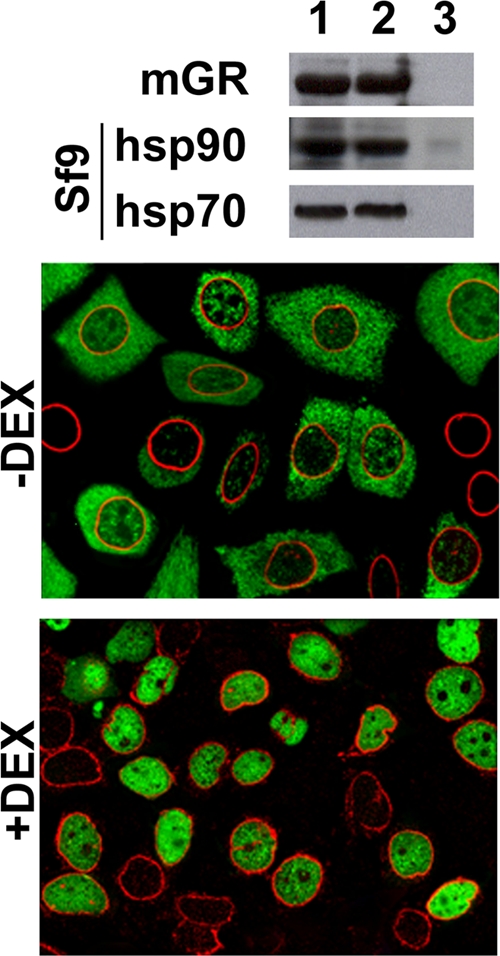
The GR-hsp90-based heterocomplex passes intact through the NPC. Cytosolic mouse GR (mGR) produced by baculovirus infection of Sf9 cells was cross-linked to the insect chaperone heterocomplex with 2 mM DSP. The Western blot shows the association of insect hsp90 and hsp70 with GR in untreated cytosol (lane 1), cytosol heated in the presence of an excess of β-mercaptoethanol (lane 2), or sample buffer without β-mercaptoethanol (lane 3). Confocal microscopy was performed on permeabilized E82.A3 cells incubated with GR cross-linked with DSP in Sf9 cytosol in the absence or presence of 100 nM dexamethasone. GR is seen as green, and the perinuclear ring of Nups is seen as red. Controls incubated without ATP and cytosol showed GR, with steroid, entirely cytoplasmic (data not shown).
To avoid the interference of endogenous GR, we used E82.A3 fibroblasts. The fibroblasts were permeabilized with digitonin by following a standard protocol (2) used in previous studies (50). Figure 7 shows indirect immunofluorescence results for GR and Nups as perinuclear markers. Not all the cells were equally permeabilized, since some of them did not show green fluorescence (∼15 to 20% of the total). Those cells that were permeabilized showed a strong cytoplasmic fluorescence for GR in the absence of steroid, an image that was identical to that observed for control incubations performed in the presence of dexamethasone when ATP and cytosol were omitted from the medium (data not shown). On the other hand, GR became entirely nuclear in the presence of dexamethasone with a full incubation medium. Inasmuch as the GR-hsp90 complex was cross-linked, such nuclear localization of GR is possible only if the entire GR-hsp90-heterocomplex could translocate intact through the NPC.
DISCUSSION
This study was focused on some aspects of the nuclear translocation step of GR, conceiving the receptor to be still bound to the hsp90-based heterocomplex in association with TPR proteins rather than as an isolated factor. This model is the result of empirical observations (10, 20-22, 26, 52) that suggest the requirement of the steroid receptor-hsp90 heterocomplex for the retrotransport of steroid receptors. Moreover, the fact that GR-hsp90 complexes also exist in the nucleus raises the idea that the heterocomplex could pass intact through the NPC. This model is also supported by in silico predictions for putative interactions of chaperones belonging to the heterocomplex and GR itself with some components of the nuclear import machinery (Fig. 1). In this sense, it is accepted that the transport of macromolecules across the nuclear pore involves the assembly of an importin-cargo complex that docks to the NPC via Nups. Our findings demonstrate that proteins belonging to the untransformed GR complex and GR itself are able to interact with key structures of the NPC, such as the integral nucleoporin Nup62 and Impβ1, a class of transport receptor involved in most but not all nuclear transport pathways of soluble factors carrying an NLS signal (for reviews, see references 8 and 32). The interaction of GR with Impβ observed in the experiments whose results are shown in Fig. 3B is important because such an association could not be demonstrated before (48). Previous works (14, 45) have also demonstrated that Impα recognizes the NLS of GR and also internalizes into the nucleus with the receptor (48). Here, we show the association of GR, hsp90, Impβ, and Nup62 in a complex, but we were unable to detect cointernalization of Impβ and GR. However, the knockdown of Impβ with siRNA significantly delayed the nuclear translocation rate of GR (Fig. 3D).
It has been reported that many importins, including Impβ, not only mediate active transport through NPCs but also effectively suppress the aggregation of cargoes (29). The antiaggregation activity of these importins involves shielding of basic patches on the cargo and requires a precise match between cargo and receptor. However, it is hard to explain how a single type of factor could shield each of thousands of different protein-, RNA-, and DNA-binding domains that are import substrates. Based on our novel findings, it may be envisioned that the presence of chaperones and cochaperones in association with importin, Nups, and the cargo itself may act as a whole, cooperative system to prevent the aggregation of cargoes when relatively hydrophobic domains are exposed during the translocation step. This may explain why when GR and Nup62 are properly folded with the hsp90 complex (Fig. 5), there is a more efficient interaction than that seen for the “naked” proteins that should favor the translocation step in vivo. When this complex is disrupted by hsp90 inhibitors, such as radicicol or geldanamycin, the nuclear translocation rate of GR (20, 52), MR (21, 40), and AR (22) undergoes a substantial delay.
Interestingly, it has been reported that proteins carrying an NLS bound to the Impα-Impβ complex dissociate slowly, whereas the release of the cargo in the nuclear basket structure facing the nucleoplasm milieu is faster, such that it was postulated that the rate-limiting step in the Impα-Impβ-Nup-mediated import pathway is the dynamic assembly and disassembly of the importin-cargo complex rather than the translocation process per se (23). Recent studies of the role of Nups that have the FG domain as functional elements of the NPC permeability barrier showed that these proteins are highly flexible and devoid of an ordered secondary structure (12), but those related to the NPC center are able to bind each other via hydrophobic attraction, generating a type of cohesive meshwork that may model the architecture of the pore (37). If integral Nups are chaperoned by hsp90, hsp70, p23, and/or TPR cochaperones as Nup62 is, it is possible that the putative permeability barrier may be regulated by protein-protein interactions allowing (or not) the passage of certain cargoes.
The association of TPR proteins, such as FKBP52 and PP5, with Nup62 seems to be hsp90 dependent, as was shown by the almost-complete dissociation of these IMMs from Nup62 in the presence of radicicol (Fig. 6). However, indirect immunofluorescence assays performed with intact cells treated with radicicol still show the presence of both IMMs in the perinuclear ring (data not shown), suggesting that these IMMs may also bind in an hsp90-independent manner to other perinuclear structures, for example, other Nups. Nonetheless, competition experiments with the TPR domain overexpressed in intact cells showed that the perinuclear signal of FKBP52 was totally abolished (Fig. 6D), indicating that most if not all types of association of this IMM with any structure of the nuclear envelope require the TPR domain.
The chaperone hsp70 and the cochaperone p23, both regular components of the GR-hsp90 heterocomplex, are also Nup62-associated proteins. This is supported by the experimental results shown in Fig. 2C, 6B, and 6C, where hsp90 binding to Nup62 was prevented by radicicol both in intact cells and a reticulocyte lysate-reconstituted system, whereas hsp70 and p23 remain bound to the Nup. This suggests that these proteins may be required for the proper architecture of Nup62. Inasmuch as hsp70 uses its ATPase cycle to control substrate binding and release (6, 34), substrate binding to importins is likewise coupled to RanGTP cycles. However, for some receptor-substrate pairs, the presence of RanGTP is not sufficient for cargo release; instead, an appropriate binding site for the cargo is also required (38). Therefore, it is possible that hsp70 may be related to the substrate binding-release equilibrium in the NPC.
Because steroid receptors are always shuttling between cytoplasm and nucleus, it is possible that GR-importin and GR-Nup complexes like those described in this work are formed constantly, even in the absence of hormone. In the experiments whose results are shown in Fig. 5, we were unable to detect a greater GR-Nup62 interaction when dexamethasone was added to the incubation medium (data not shown). Since this is an in vitro system, we cannot rule out this possibility in vivo. Nevertheless, there is compelling evidence that the mere exposure of the NLS does not automatically result in the full nuclear translocation of the receptor (40).
The conventional view hitherto has been that, in receptors primarily located in the cytoplasm, such as MR or GR, the NLS is hidden when hsp90 is part of the complex. Therefore, it has always been believed that receptor transformation must be the first mandatory step prior to nuclear translocation. More recently, a compelling amount of evidence indicates that hsp90 is required for receptor retrotransport (20-22, 52). Experiments with molecules hybridized from the primarily nuclear PR and hsp90 revealed that the receptor may be relocated in the cytoplasm in a manner that is not altered by the exposure of its NLS (36). Also, nuclear-targeted hsp90 shifts cytoplasmic steroid receptor mutants into the nucleus (30) and hsp90 bound to the MR was recovered immediately after its nuclear translocation (40), suggesting that the complex could remain intact during the process of translocation through the NPC. There is, therefore, no clear relationship between NLS availability and nuclear translocation. Instead, it is likely that nuclear translocation is the result of a mechanism operating in concert between the NLS sequence of the receptor and its interaction with the nuclear import machinery, with both systems, receptor and import machinery, regulated by chaperones and cochaperones, among other potential or unknown factors.
Consistent with a model where the GR-hsp90-TPR protein is able to translocate intact through the NPC, the results of the experiment using a digitonin-permeabilized cell system that are shown in Fig. 7 argue strongly in favor of this hypothesis because the GR-hsp90-based complex was previously cross-linked and there is no possibility of chaperone dissociation. Therefore, the phenomenon of transformation (dissociation of hsp90 from the receptor) could be a nuclear event, rather than a cytoplasmic event as has always been thought.
Acknowledgments
This work was supported by grants from Agencia de Promoción Científica y Tecnológica de Argentina (grants PICT-2006-02109 and PICT-2004-26495), Universidad de Buenos Aires (grant UBACyT-X085), and the FIRCA program of the NIH (grant R03TW007162-01A2).
Footnotes
Published ahead of print on 6 July 2009.
REFERENCES
- 1.Abbas-Terki, T., O. Donze, P. A. Briand, and D. Picard. 2001. Hsp104 interacts with Hsp90 cochaperones in respiring yeast. Mol. Cell. Biol. 217569-7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam, S. A., R. S. Marr, and L. Gerace. 1990. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J. Cell Biol. 111807-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albermann, L., V. Shahin, Y. Ludwig, C. Schafer, H. Schillers, and H. Oberleithner. 2004. Evidence for importin alpha independent nuclear translocation of glucocorticoid receptors in Xenopus laevis oocytes. Cell. Physiol. Biochem. 14343-350. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee, A., S. Periyasamy, I. M. Wolf, T. D. Hinds, Jr., W. Yong, W. Shou, and E. R. Sanchez. 2008. Control of glucocorticoid and progesterone receptor subcellular localization by the ligand-binding domain is mediated by distinct interactions with tetratricopeptide repeat proteins. Biochemistry 4710471-10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barsky, A., J. L. Gardy, R. E. Hancock, and T. Munzner. 2007. Cerebral: a Cytoscape plugin for layout of and interaction with biological networks using subcellular localization annotation. Bioinformatics 231040-1042. [DOI] [PubMed] [Google Scholar]
- 6.Bukau, B., and A. L. Horwich. 1998. The Hsp70 and Hsp60 chaperone machines. Cell 92351-366. [DOI] [PubMed] [Google Scholar]
- 7.Cerami, E. G., G. D. Bader, B. E. Gross, and C. Sander. 2006. cPath: open source software for collecting, storing, and querying biological pathways. BMC Bioinformatics 7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conti, E., and E. Izaurralde. 2001. Nucleocytoplasmic transport enters the atomic age. Curr. Opin. Cell Biol. 13310-319. [DOI] [PubMed] [Google Scholar]
- 9.Czar, M. J., M. D. Galigniana, A. M. Silverstein, and W. B. Pratt. 1997. Geldanamycin, a heat shock protein 90-binding benzoquinone ansamycin, inhibits steroid-dependent translocation of the glucocorticoid receptor from the cytoplasm to the nucleus. Biochemistry 367776-7785. [DOI] [PubMed] [Google Scholar]
- 10.Davies, T. H., Y. M. Ning, and E. R. Sanchez. 2002. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J. Biol. Chem. 2774597-4600. [DOI] [PubMed] [Google Scholar]
- 11.Davies, T. H., and E. R. Sanchez. 2005. Fkbp52. Int. J. Biochem. Cell Biol. 3742-47. [DOI] [PubMed] [Google Scholar]
- 12.Denning, D. P., S. S. Patel, V. Uversky, A. L. Fink, and M. Rexach. 2003. Disorder in the nuclear pore complex: the FG repeat regions of nucleoporins are natively unfolded. Proc. Natl. Acad. Sci. USA 1002450-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Echeverria, P. C., M. Matrajt, O. S. Harb, M. P. Zappia, M. A. Costas, D. S. Roos, J. F. Dubremetz, and S. O. Angel. 2005. Toxoplasma gondii Hsp90 is a potential drug target whose expression and subcellular localization are developmentally regulated. J. Mol. Biol. 350723-734. [DOI] [PubMed] [Google Scholar]
- 14.Freedman, N. D., and K. R. Yamamoto. 2004. Importin 7 and importin alpha/importin beta are nuclear import receptors for the glucocorticoid receptor. Mol. Biol. Cell 152276-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galigniana, M. D., J. M. Harrell, P. R. Housley, C. Patterson, S. K. Fisher, and W. B. Pratt. 2004. Retrograde transport of the glucocorticoid receptor in neurites requires dynamic assembly of complexes with the protein chaperone hsp90 and is linked to the CHIP component of the machinery for proteasomal degradation. Brain Res. Mol. Brain Res. 12327-36. [DOI] [PubMed] [Google Scholar]
- 16.Galigniana, M. D., J. M. Harrell, P. J. Murphy, M. Chinkers, C. Radanyi, J. M. Renoir, M. Zhang, and W. B. Pratt. 2002. Binding of hsp90-associated immunophilins to cytoplasmic dynein: direct binding and in vivo evidence that the peptidylprolyl isomerase domain is a dynein interaction domain. Biochemistry 4113602-13610. [DOI] [PubMed] [Google Scholar]
- 17.Galigniana, M. D., J. M. Harrell, H. M. O'Hagen, M. Ljungman, and W. B. Pratt. 2004. Hsp90-binding immunophilins link p53 to dynein during p53 transport to the nucleus. J. Biol. Chem. 27922483-22489. [DOI] [PubMed] [Google Scholar]
- 18.Galigniana, M. D., P. R. Housley, D. B. DeFranco, and W. B. Pratt. 1999. Inhibition of glucocorticoid receptor nucleocytoplasmic shuttling by okadaic acid requires intact cytoskeleton. J. Biol. Chem. 27416222-16227. [DOI] [PubMed] [Google Scholar]
- 19.Galigniana, M. D., Y. Morishima, P. A. Gallay, and W. B. Pratt. 2004. Cyclophilin-A is bound through its peptidylprolyl isomerase domain to the cytoplasmic dynein motor protein complex. J. Biol. Chem. 27955754-55759. [DOI] [PubMed] [Google Scholar]
- 20.Galigniana, M. D., C. Radanyi, J. M. Renoir, P. R. Housley, and W. B. Pratt. 2001. Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J. Biol. Chem. 27614884-14889. [DOI] [PubMed] [Google Scholar]
- 21.Gallo, L. I., A. A. Ghini, G. P. Pilipuk, and M. D. Galigniana. 2007. Differential recruitment of tetratricopeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity. Biochemistry 4614044-14057. [DOI] [PubMed] [Google Scholar]
- 22.Georget, V., B. Terouanne, J. C. Nicolas, and C. Sultan. 2002. Mechanism of antiandrogen action: key role of hsp90 in conformational change and transcriptional activity of the androgen receptor. Biochemistry 4111824-11831. [DOI] [PubMed] [Google Scholar]
- 23.Gilchrist, D., B. Mykytka, and M. Rexach. 2002. Accelerating the rate of disassembly of karyopherin-cargo complexes. J. Biol. Chem. 27718161-18172. [DOI] [PubMed] [Google Scholar]
- 24.Grandi, P., T. Dang, N. Pane, A. Shevchenko, M. Mann, D. Forbes, and E. Hurt. 1997. Nup93, a vertebrate homologue of yeast Nic96p, forms a complex with a novel 205-kDa protein and is required for correct nuclear pore assembly. Mol. Biol. Cell 82017-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrell, J. M., I. Kurek, A. Breiman, C. Radanyi, J. M. Renoir, W. B. Pratt, and M. D. Galigniana. 2002. All of the protein interactions that link steroid receptor-hsp90-immunophilin heterocomplexes to cytoplasmic dynein are common to plant and animal cells. Biochemistry 415581-5587. [DOI] [PubMed] [Google Scholar]
- 26.Harrell, J. M., P. J. Murphy, Y. Morishima, H. Chen, J. F. Mansfield, M. D. Galigniana, and W. B. Pratt. 2004. Evidence for glucocorticoid receptor transport on microtubules by dynein. J. Biol. Chem. 27954647-54654. [DOI] [PubMed] [Google Scholar]
- 27.Heitzer, M. D., I. M. Wolf, E. R. Sanchez, S. F. Witchel, and D. B. DeFranco. 2007. Glucocorticoid receptor physiology. Rev. Endocr. Metab. Disord. 8321-330. [DOI] [PubMed] [Google Scholar]
- 28.Huang, T. W., A. C. Tien, W. S. Huang, Y. C. Lee, C. L. Peng, H. H. Tseng, C. Y. Kao, and C. Y. Huang. 2004. POINT: a database for the prediction of protein-protein interactions based on the orthologous interactome. Bioinformatics 203273-3276. [DOI] [PubMed] [Google Scholar]
- 29.Jakel, S., J. M. Mingot, P. Schwarzmaier, E. Hartmann, and D. Gorlich. 2002. Importins fulfill a dual function as nuclear import receptors and cytoplasmic chaperones for exposed basic domains. EMBO J. 21377-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang, K. I., J. Devin, F. Cadepond, N. Jibard, A. Guiochon-Mantel, E. E. Baulieu, and M. G. Catelli. 1994. In vivo functional protein-protein interaction: nuclear targeted hsp90 shifts cytoplasmic steroid receptor mutants into the nucleus. Proc. Natl. Acad. Sci. USA 91340-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madan, A. P., and D. B. DeFranco. 1993. Bidirectional transport of glucocorticoid receptors across the nuclear envelope. Proc. Natl. Acad. Sci. USA 903588-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattaj, I. W., and L. Englmeier. 1998. Nucleocytoplasmic transport: the soluble phase. Annu. Rev. Biochem. 67265-306. [DOI] [PubMed] [Google Scholar]
- 33.Moffatt, N. S., E. Bruinsma, C. Uhl, W. M. Obermann, and D. Toft. 2008. Role of the cochaperone Tpr2 in Hsp90 chaperoning. Biochemistry 478203-8213. [DOI] [PubMed] [Google Scholar]
- 34.Murphy, P. J., Y. Morishima, H. Chen, M. D. Galigniana, J. F. Mansfield, S. S. Simons, Jr., and W. B. Pratt. 2003. Visualization and mechanism of assembly of a glucocorticoid receptor-Hsp70 complex that is primed for subsequent Hsp90-dependent opening of the steroid binding cleft. J. Biol. Chem. 27834764-34773. [DOI] [PubMed] [Google Scholar]
- 35.Nigg, E. A. 1997. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature 386779-787. [DOI] [PubMed] [Google Scholar]
- 36.Passinen, S., J. Valkila, T. Manninen, H. Syvala, and T. Ylikomi. 2001. The C-terminal half of Hsp90 is responsible for its cytoplasmic localization. Eur. J. Biochem. 2685337-5342. [DOI] [PubMed] [Google Scholar]
- 37.Patel, S. S., B. J. Belmont, J. M. Sante, and M. F. Rexach. 2007. Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex. Cell 12983-96. [DOI] [PubMed] [Google Scholar]
- 38.Pemberton, L. F., J. S. Rosenblum, and G. Blobel. 1999. Nuclear import of the TATA-binding protein: mediation by the karyopherin Kap114p and a possible mechanism for intranuclear targeting. J. Cell Biol. 1451407-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picard, D., and K. R. Yamamoto. 1987. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 63333-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pilipuk, G. P., G. P. Vinson, C. G. Sanchez, and M. D. Galigniana. 2007. Evidence for NL1-independent nuclear translocation of the mineralocorticoid receptor. Biochemistry 461389-1397. [DOI] [PubMed] [Google Scholar]
- 41.Piwien-Pilipuk, G., and M. D. Galigniana. 2000. Oxidative stress induced by L-buthionine-(S,R)-sulfoximine, a selective inhibitor of glutathione metabolism, abrogates mouse kidney mineralocorticoid receptor function. Biochim. Biophys. Acta 1495263-280. [DOI] [PubMed] [Google Scholar]
- 42.Piwien-Pilipuk, G., K. C. Kanelakis, A. A. Ghini, C. P. Lantos, G. Litwack, G. Burton, and M. D. Galigniana. 2002. Modification of an essential amino group in the mineralocorticoid receptor evidences a differential conformational change of the receptor protein upon binding of antagonists, natural agonists and the synthetic agonist 11,19-oxidoprogesterone. Biochim. Biophys. Acta 158931-48. [DOI] [PubMed] [Google Scholar]
- 43.Pratt, W. B., M. D. Galigniana, Y. Morishima, and P. J. Murphy. 2004. Role of molecular chaperones in steroid receptor action. Essays Biochem. 4041-58. [DOI] [PubMed] [Google Scholar]
- 44.Pratt, W. B., P. Krishna, and L. J. Olsen. 2001. Hsp90-binding immunophilins in plants: the protein movers. Trends Plant Sci. 654-58. [DOI] [PubMed] [Google Scholar]
- 45.Savory, J. G., B. Hsu, I. R. Laquian, W. Giffin, T. Reich, R. J. Hache, and Y. A. Lefebvre. 1999. Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol. Cell. Biol. 191025-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverstein, A. M., M. D. Galigniana, K. C. Kanelakis, C. Radanyi, J. M. Renoir, and W. B. Pratt. 1999. Different regions of the immunophilin FKBP52 determine its association with the glucocorticoid receptor, hsp90, and cytoplasmic dynein. J. Biol. Chem. 27436980-36986. [DOI] [PubMed] [Google Scholar]
- 47.Stewart, M. 2007. Molecular mechanism of the nuclear protein import cycle. Nat. Rev. Mol. Cell Biol. 8195-208. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka, M., M. Nishi, M. Morimoto, T. Sugimoto, and M. Kawata. 2003. Yellow fluorescent protein-tagged and cyan fluorescent protein-tagged imaging analysis of glucocorticoid receptor and importins in single living cells. Endocrinology 1444070-4079. [DOI] [PubMed] [Google Scholar]
- 49.Terry, L. J., E. B. Shows, and S. R. Wente. 2007. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science 3181412-1416. [DOI] [PubMed] [Google Scholar]
- 50.Vicent, G. P., A. Pecci, A. Ghini, G. Piwien-Pilipuk, and M. D. Galigniana. 2002. Differences in nuclear retention characteristics of agonist-activated glucocorticoid receptor may determine specific responses. Exp. Cell Res. 276142-154. [DOI] [PubMed] [Google Scholar]
- 51.Witchel, S. F., and D. B. DeFranco. 2006. Mechanisms of disease: regulation of glucocorticoid and receptor levels—impact on the metabolic syndrome. Nat. Clin. Pract. Endocrinol. Metab. 2621-631. [DOI] [PubMed] [Google Scholar]
- 52.Wochnik, G. M., J. Ruegg, G. A. Abel, U. Schmidt, F. Holsboer, and T. Rein. 2005. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2804609-4616. [DOI] [PubMed] [Google Scholar]