Abstract
CaV1.2 voltage-gated calcium channels play critical roles in the control of membrane excitability, gene expression, and muscle contraction. These channels show diverse functional properties generated by alternative splicing at multiple sites within the CaV1.2 pre-mRNA. The molecular mechanisms controlling this splicing are not understood. We find that two exons in the CaV1.2 channel are controlled in part by members of the Fox family of splicing regulators. Exons 9* and 33 confer distinct electrophysiological properties on the channel and show opposite patterns of regulation during cortical development, with exon 9* progressively decreasing its inclusion in the CaV1.2 mRNA over time and exon 33 progressively increasing. Both exons contain Fox protein binding elements within their adjacent introns, and Fox protein expression is induced in cortical neurons in parallel with the changes in CaV1.2 splicing. We show that knocking down expression of Fox proteins in tissue culture cells has opposite effects on exons 9* and 33. The loss of Fox protein increases exon 9* splicing and decreases exon 33, as predicted by the positions of the Fox binding elements and by the pattern of splicing in development. Conversely, overexpression of Fox1 and Fox2 proteins represses exon 9* and enhances exon 33 splicing in the endogenous CaV1.2 mRNA. These effects of Fox proteins on exons 9* and 33 can be recapitulated in transfected minigene reporters. Both the repressive and the enhancing effects of Fox proteins are dependent on the Fox binding elements within and adjacent to the target exons, indicating that the Fox proteins are directly regulating both exons. These results demonstrate that the Fox protein family is playing a key role in tuning the properties of CaV1.2 calcium channels during neuronal development.
CaV1.2 L-type voltage-gated calcium channels are widely distributed in brain, heart, smooth muscle, and endocrine cells and play essential roles in gene expression, muscle contraction, and hormone release (6, 13, 16, 39, 47). These channels are composed of three subunits, with the α1 subunit being the largest and incorporating the conduction pore, the voltage sensor and gating apparatus, as well as sites for channel regulation by second messengers, drugs, and toxins (Fig. 1A) (9, 14, 17). This CaV1.2 subunit is subject to extensive alternative splicing that generates multiple functionally distinct isoforms (1, 29, 33, 49, 62). At least twenty of the 56 exons in the human CaV1.2 transcript are alternatively spliced (29, 50, 51, 55). In particular, alternative exon 9* within the cytoplasmic I-II loop and exon 33 within the IVS3-IVS4 transmembrane segments confer different electrophysiological and pharmacological properties on the channel and exhibit tissue-specific differences in inclusion (30, 31, 54, 55). Changes in exon 9* (also named exon 9A) splicing are seen in human arterial smooth muscle cells that have developed atherosclerosis (57) and in hypertrophied cardiomyocytes of spontaneously hypertensive rats (56). Alternative exons 9* and 33 are conserved across vertebrate species demonstrating their functional importance to the CaV1.2 channel. However, the molecular mechanisms controlling their splicing have not been studied.
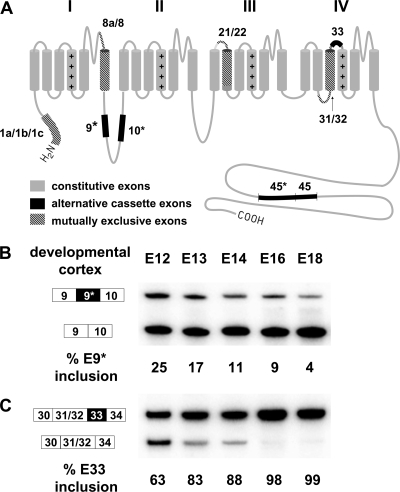
FIG. 1.
(A) Diagram of the CaV1.2 subunit. The protein is composed of four membrane-spanning domains (I to IV), with each domain consisting of six transmembrane segments (S1 to S6). Protein segments encoded by alternatively spliced exons are indicated by black or hatched boxes. Exons 1a, 1b, and 1c derive from three alternative promoters (15, 42, 45); exons 8a/8, 21/22, and 31/32 are spliced in a mutually exclusive manner (51, 61, 62, 67); and exons 9*, 10*, 33, 45*, and 45 are alternative cassette exons (5, 18, 19, 24, 25). (B and C) RT-PCR assay of changes of exons 9* and 33 in embryonic mouse cortex from embryonic day 12 (E12) to day 18 (E18). Exon 9* gradually decreases in inclusion (B) and exon 33 gradually increases in developing mouse cortex (C). The PCR amplified region for exon 9* encompasses exons 9 through 10. The exon 9* included band is 354 bp and the exon 9* skipped band is 279 bp. The PCR-amplified region for exon 33 encompasses exons 30, 31, 32, 33, and 34. The exon 33 included and excluded fragments are 260 and 227 bp. The values for percent exon 9* or exon 33 inclusion are the upper band intensity divided by the summed intensities of upper and lower bands. Approximately 7 to 10 embryonic cortices were pooled for RNA extraction in each group.
Members of the Fox protein family, homologs of the Feminizing on the X gene product from Caenorhabditis elegans (21, 41, 48), regulate the splicing of many neuron- and muscle-specific splicing events (22, 40, 59, 63-66). There are three mammalian family members, Fox1 (A2BP1), Fox2 (RBM9), and Fox3 (hnrbp3), each containing a nearly identical RNA-binding domain that recognizes the hexanucleotide element UGCAUG (2). These proteins bind the introns adjacent to their target exons where they generally repress splicing when bound upstream of the exon but enhance splicing from a downstream binding site (22, 40, 59, 63, 64, 66). In addition to the RNA-binding domain, all three proteins have similar N and C-terminal domains that are extensively modified by alternative promoter use and alternative splicing to produce a large family of related proteins. Fox1 is expressed in neurons and muscle, and Fox3 is expressed only in neurons (22, 23, 36, 59). Fox2 shows somewhat broader expression, being found in embryonic stem cells and in the embryo in addition to neurons and muscle (3, 44, 63).
We identified multiple (U)GCAUG elements in the sequence surrounding CaV1.2 exons 9* and 33 of human, rat, mouse, chicken, and other species. We show here that the mammalian Fox proteins have opposite effects on the alternative splicing of exons 9* and 33. In developing mouse cortex, these exons change their pattern of splicing in parallel with increases in Fox protein expression. This establishes roles for the Fox proteins in tuning CaV1.2 channel physiology during development.
MATERIALS AND METHODS
Cell culture, treatments, and transfections.
N2a and HEK293T cells were grown according to the American Type Culture Collection-recommended protocols. Myoblast C2C12 cells were grown in high-glucose Dulbecco modified Eagle medium (Invitrogen) with 1.5 g of sodium bicarbonate/liter and 1 mM sodium pyruvate, supplemented with 10% fetal bovine serum. Transfections were done with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Plasmids.
The Dup-rat E9* and Dup-rat E33 reporter minigenes were constructed by amplifying rat genomic DNA containing exons 9* and 33 with flanking intron sequences from rat genomic DNA using Pfu DNA polymerase and primers designed from the GenBank genomic sequence NW_047696. The PCR fragments were cloned into the template plasmid pDUP4-1 (37, 38) between the ApaI and BglII sites that are engineered into 5′ and 3′ ends of the amplicons. The primers are presented in Table S1 in the supplemental material (primers 1 to 4). Mutations in Fox binding elements were generated by using the QuikChange site-directed mutagenesis kit (Stratagene) and followed by subcloning into pDUP4-1 using the ApaI and BglII sites. All constructs were confirmed by sequencing. The Fox1 and Fox2 cDNA expression plasmids were constructed in pcDNA3.1 (Invitrogen) as described previously (59). Each carried an N-terminal FLAG tag.
shRNAs for knockdown of Fox proteins.
Short hairpin RNAs (shRNAs) pBlsH1Fox-1 and pBlsH1Fox-2 were designed to target the 3′ untranslated region of mouse Fox1 or Fox2 genes. The template vector was pBlsH1 and the design of the shRNA was similar to what was described previously (12). The oligonucleotides used were primers 9 to 12 in Table S1 in the supplemental material. The shRNA expression plasmids pBlsH1Fox-1 and pBlsH1Fox-2 were transfected into C2C12 and N2A cells grown in six-well plates using Lipofectamine 2000 (Invitrogen). The efficiency of knockdown was verified by Western blot.
Reverse transcription-PCR (RT-PCR) and primer extension.
Cytoplasmic RNA was isolated from cells or mouse embryonic cortexes with an RNeasy minikit (Qiagen) and reverse transcribed with random hexamers and Superscript III (Invitrogen) according to the manufacturer's instruction. Splice products were PCR amplified (25 cycles for N2A cells and 29 cycles for C2C12 cells) using a 32P-labeled primer in the 3′ constitutive exon and an unlabeled primer in the 5′ constitutive exon. The primers (primers 5 to 8) are listed in Table S1 in the supplemental material. Primer extensions were performed as described previously (28, 38), using a 32P-labeled DUP3 reverse primer (5′-AACAGCATCAGGAGTGGACAGATCCC-3′). The annealing temperature was 55°C. The primer gives rise to a 230-bp primer extension product when the middle exon 9* or exon 33 from the minigene is not included. The length of the exon 9*-included product is 305 bp and exon 33-included product is 263 bp. 32P-labeled PCR and primer extension products were separated by 8% polyacrylamide-7.5 M urea denaturing gels. Gels were dried, visualized by using a Typhoon phosphorimager (Amersham Biosciences), and quantified by using ImageQuant TL software (Amersham Biosciences).
Antibodies and Western blotting.
Anti-Fox2 antibody was obtained from Bethel Laboratories. Pan-anti-Fox RRM antibody was raised against an 86-amino-acid peptide sequence containing the Fox1 RRM and 3 amino acids upstream and 12 amino acids downstream. The peptide sequence is PKRLHVSNIPFRFRDPDLRQMFGQFGKILDVEIIFNERGSKGFGFVTFENSADADRAREKLHGTVVEGRKIEVNNATARVMTNKKM. This sequence is identical in the human and mouse Fox1 and Fox2 proteins except for the last amino acid. It has five amino acid changes in Fox3. The antibody was affinity purified on the antigen. Its specificity was confirmed by probing blots of recombinant Fox1 and 2 proteins and native Fox proteins in cell lysates before and after Fox protein knockdown.
The mouse monoclonal anti-Fox1 antibody was raised against bacterially expressed recombinant mouse Fox1 fused with glutathione S-transferase. Immunization in BALB/c mice was done according to standard protocols (27). Myeloma and spleen cell fusion was carried out by Susan Oh at the Caltech Monoclonal Antibody Facility using HL-1 myeloma cells. Hybridomas were initially screened by enzyme-linked immunosorbent assay using the original antigen. Positive hybridomas supernatants were retested on Western blots of total protein from HEK293T cells transfected with Flag-tagged mouse Fox1. One of the positive clones, 1D10, was subsequently cloned by limiting dilution. The anti-Fox3 rabbit polyclonal antibody was raised against the Fox3 peptide CQTPVPPEHGMTLYT at Pacific Immunology and purified from rabbit serum on peptide coupled to Sulfo-Link resin (Pierce).
Tissue culture cell lysates for Western blot were isolated in radioimmunoprecipitation assay buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and 50 mM Tris at pH 8.0) as previously described (11). Whole-cell lysates were resolved on 4 to 20% Tris-glycine gels (Invitrogen) in Tris-glycine-SDS running buffer. The proteins were transferred onto Protran nitrocellulose membranes (Whatman, Germany) or Immobilon-FL polyvinylidene difluoride membranes (Millipore). Membranes were probed under standard conditions with anti-Fox RNA recognition motif (RRM; 1:10,000), anti-Fox1 (1:5,000), anti-Fox2 (1:1,000; Bethyl Laboratories, Inc.), anti-Fox3 (1:3,000), anti-Flag (1:3,000; Sigma), and anti-glyceraldehyde phosphate dehydrogenase (anti-GAPDH; 1:200,000; Research Diagnostics, Inc.) antibodies. Blots were then probed with horseradish peroxidase-conjugated secondary antibodies (1:10,000; GE Healthcare) and developed by using Femto Supersignal reagents (Pierce Biotechnology) and Kodak BioMax XAR film. For blotting with fluorophore-conjugated secondary antibodies, the polyvinylidene difluoride membranes were probed with ECL Plex Cy3-conjugated goat anti-mouse (1:5,000; GE Healthcare) and Cy5-conjugated goat anti-rabbit (1:2,500; GE Healthcare) secondary antibodies, dried, and scanned on a Typhoon PhosphorImager (GE Healthcare). Quantification of fluorescent signals was performed by using ImageQuant TL software (Amersham Biosciences).
Immunostaining.
Brain tissues from 18-day-old mouse embryos (CD-1 mice; Charles River Laboratories, Inc.) were cryosectioned at 10 μm in thickness. Sections attached to glass slides were fixed with 4% paraformaldehyde (pH 7) for 20 min, washed three times with phosphate-buffered saline (PBS) with 0.1% Triton (PBST), and permeabilized with 0.5% Triton in PBS for 10 min at room temperature. Sections were then blocked in PBST, 2% bovine serum albumin (Sigma-Aldrich), and 3% normal goat serum for 1 h. The samples were incubated with primary antibodies overnight at 4°C. Primary antibodies were used at the following concentrations: anti-Fox RRM (1:500), anti-Fox1 (1:1,000), anti-Fox2 (1:200; Bethyl Laboratories, Inc.), anti-NeuN (1:100; Chemicon), anti-MAP2 (1:200; Chemicon), and anti-GFAP (1:1,000; Chemicon). After being washed three times with PBST, sections were incubated with Alexa 568 (red)-conjugated anti-rabbit or anti-mouse immunoglobulin G, and Alexa 488 (green)-conjugated anti-mouse or anti-rabbit immunoglobulin G (1:1,000; Molecular Probes) secondary antibodies at room temperature for 1 h. Sections were rinsed three times with PBST and mounted with ProLong Gold antifade reagent with DAPI (4′,6′-diamidino-2-phenylindole; Invitrogen). Control staining performed in parallel without adding primary antibodies yielded no labeling.
RESULTS
CaV1.2 exons 9* and 33 show divergent regulation in developing cortex coinciding with the expression of Fox proteins.
Exon 9* of CaV1.2 modifies the cytoplasmic I-II loop of the protein, where it alters the voltage-dependent gating of the channel (Fig. 1A) (31, 54). We find that in mouse embryonic cortex (E12) this exon is included in 25% of the CaV1.2 mRNA but is progressively repressed over development, reaching only 4% inclusion by embryonic day 18 (Fig. 1B). This behavior is in contrast to CaV1.2 exon 33. Exon 33 modifies the linker separating the domain IV transmembrane segments S3 and S4 and alters both the voltage dependence of the channel and its sensitivity to dihydropyridine drugs (30, 55). At embryonic day 12, exon 33 is 63% included in the cortical CaV1.2 mRNA. Unlike exon 9*, this splicing progressively increases to near 100% inclusion over the next 6 days of development (Fig. 1C).
We have found that many proteins involved in calcium signaling and homeostasis exhibit alternative splicing that is regulated by members of the Fox protein family (our unpublished data). In mammals, there are three members of this group of RNA-binding proteins Fox1 (A2BP1), Fox2 (RBM9), and Fox3 (hnrbp3). Examining CaV1.2 exon 9* in the rat and mouse, we found potential Fox binding sites (UGCAUG) in three positions, upstream and downstream of the exon and within the exon itself (Fig. 2A). For exon 33, we found one Fox recognition site 111 nucleotides downstream of the 5′ splice site. In the human CaV1.2 genes, these elements are also present although with some variation (see Fig. S1 and S2 in the supplemental material). Thus, both exons 9* and 33 are candidates for Fox protein regulation.
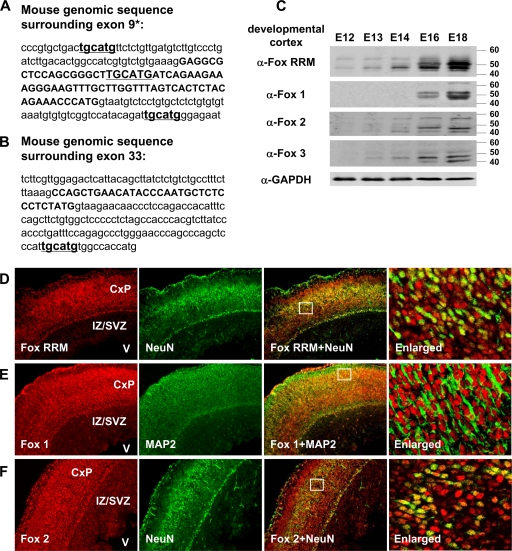
FIG. 2.
(A and B) Mouse genomic sequences encompassing exons 9* and 33. Exonic sequences are in uppercase and intronic sequence in lowercase. The exonic or intronic Fox binding elements “tgcatg” are underlined. (C) Immunoblots of protein in developing cortex show increasing Fox protein expression between embryonic day 12 (E12) to embryonic day 18 (E18). The anti-Fox1, anti-Fox2, and anti-Fox3 antibodies each recognize an individual Fox protein, and the anti-Fox RRM antibody binds to the RRM found in all three proteins. Approximately 7 to 10 embryonic cortices were pooled for protein extraction at each time point. The pooled cortices were weighed and homogenized in radioimmunoprecipitation assay buffer at 1:20 (wt/vol), yielding samples of approximately equal protein concentration. The final loading volumes of cortical lysates were adjusted to obtain similar GAPDH immunoblot signals. α, anti. (D to F) Immunostaining of coronal sections from E18 mouse cortex was visualized on an epifluorescence microscope (Eclipse-TE2000S; Nikon), at 10×10-fold magnification to cover the cortical plate (CxP), intermediate zone/subventricular zone (IZ/SVZ), and ventricle (V) regions within a same image. Fox RRM, Fox1 and Fox2 staining are shown in red (far left panels), while NeuN and MAP2 are shown in green (inner left panels). The overlaid staining of Fox and the neuronal markers (NeuN and MAP2) are shown in the inner right panels. The boxes in the overlay panels indicate the approximate regions shown at higher resolution in the far right panels, which were examined at 10×40-fold magnification by confocal microscope (LSM 510 META; Zeiss). As seen previously (59), Fox protein staining is primarily nuclear (data not shown). (D) Costaining of Fox proteins with the postmitotic neuronal marker NeuN. Fox proteins are expressed in NeuN-positive layers of postmitotic neurons but not in proliferating cells of the intermediate zone/subventricular zone. The majority of neurons are costained by anti-Fox RRM and NeuN, with some stained only by anti-Fox RRM. (E) Immunostaining for Fox1 and the neuronal marker MAP2. Both Fox1 and MAP2 staining is observed in the postmitotic neuronal layers. In the enlarged panel, the nuclear Fox1 staining is surrounded by cytoplasmic MAP2. (F) Immunostaining for Fox2 and NeuN. Similar to anti-Fox RRM and anti-Fox1 staining, Fox2 staining is found in postmitotic neurons.
It was previously shown that in the adult nervous system, the Fox proteins are specifically expressed in postmitotic neurons (36, 59). We next examined the expression of the three Fox proteins during the developmental period that exons 9* and 33 change in splicing. Using antibodies to each individual Fox protein, as well as an antibody that reacts with all three proteins, we probed immunoblots of the proteins from the embryonic cortex (Fig. 2C). We found that each protein is at best weakly expressed at E12 but is induced over the next 6 days, showing strong expression by E18. Thus, the induction of Fox protein expression during cortical development parallels the changes in exon 9* and exon 33 splicing.
To identify cells in the developing brain that express the Fox proteins, sections of mouse cortex from embryonic day 18 were immunostained with α-Fox RRM, α-Fox1, and α-Fox2 antibodies and costained for the neuronal markers MAP2 or NeuN. We found that Fox proteins are strongly expressed in the postmitotic neurons of the developing cortical plate but are only weakly expressed in the proliferating cells of the intermediate zone and subventricular zone (Fig. 2D, E, and F). The expression in the undifferentiated cells is primarily Fox2, a finding in agreement with its earlier induction during development and with its known expression in neuronal progenitor cells (63). As seen at higher magnification of the postmitotic cells in the outer cortical layers, nearly all cells are expressing both the Fox proteins and the terminal differentiation markers NeuN and MAP2. However, there is some heterogeneity in the amount of staining and cells can be identified expressing primarily one protein or another. This heterogeneity is resolved during postnatal development since nearly all mature neurons in the cortex express both Fox1 and Fox2 proteins (59; data not shown).
Fox proteins repress CaV1.2 exon 9* and activate exon 33.
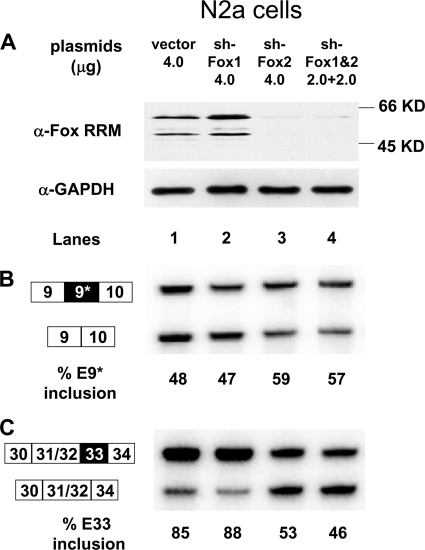
If the Fox proteins expressed during cortical development are inducing changes in CaV1.2 exon splicing, it is predicted that they would act to repress exon 9* and to enhance exon 33. To test whether these exons are indeed affected by Fox proteins, we performed RNA interference knockdown and protein overexpression experiments and measured the response of CaV1.2 exon splicing. The N2a (Neuro-2a) mouse neuroblastoma cell line expresses CaV1.2 transcripts and Fox2 protein, but very little Fox1 or Fox3. This line is amenable to relatively efficient RNA interference knockdown and exhibits a neuronal phenotype for the splicing of many gene transcripts. Transfecting these cells with an shRNA expression construct targeting Fox1 had no effect on the total Fox protein levels as measured with the Fox RRM antibody (Fig. 3A, lanes 1 and 2). Expression of an shRNA targeting Fox2 nearly eliminates Fox protein expression, as did coexpression of the Fox1 and Fox2 shRNAs (Fig. 3A, lanes 3 and 4). In the control cells, exon 9* is included in 48% of the endogenous CaV1.2 mRNA, as measured by RT-PCR. This is unchanged by the Fox1 shRNA (Fig. 3B, lanes 1 and 2). In contrast, the loss of Fox2 leads to an increase in exon 9* splicing to 59%, indicating a repressive effect of Fox2 on exon 9* splicing. The opposite is seen with exon 33. This exon is 85% included in the CaV1.2 mRNA in control treated N2A cells (Fig. 3C lanes 1 and 2). Depletion of Fox2 reduces exon 33 inclusion to ca. 50% (Fig. 3C, lanes 3 and 4).
FIG. 3.
Depletion of Fox proteins increases exon 9* and decreases exon 33 splicing in N2a cells. (A) Immunoblot of Fox proteins in cells treated with control vector (lane 1) and cells treated with short hairpin vectors targeting Fox1, Fox2, or both (lanes 2, 3, and 4). GAPDH expression is monitored as the loading control. (B) RT-PCR assay of exon 9* splicing in the control or Fox-depleted cells. (C) RT-PCR assay of exon 33 splicing in the control or Fox-depleted cells. α, anti.
The Fox2 depletion experiments indicate that Fox proteins repress exon 9* and enhance exon 33, as predicted by the time course of expression and splicing during development. To confirm this, we performed Fox protein overexpression experiments. Untreated N2A cells show high levels of exon 33 inclusion, making it difficult to observe strong enhancement by Fox proteins. Instead, we used C2C12 myoblast cells that show lower basal levels of exon 33 inclusion. C2C12 cells were transfected with Fox1 or Fox2 expression plasmids or with control vector. Immunoblot analysis indicated that in the transfected cells, the Fox protein was increased by 55 to 60% over the control cells (Fig. 4A). Exon 9* shows 33% inclusion in the vector treated cells but is repressed to 24% inclusion by Fox1 expression and to 15% inclusion by Fox2 (Fig. 4B, lanes 2 and 3). Again, the opposite pattern is observed for exon 33. Fox1 expression moderately increases exon 33 splicing from 64 to 70%, whereas Fox2 has a stronger effect, increasing inclusion to 85% (Fig. 4C, lanes 2 and 3). Thus, both Fox protein depletion and overexpression indicate that these regulators repress exon 9* but enhance exon 33 splicing in the CaV1.2 mRNA.
FIG. 4.
Overexpression of Fox1 and Fox2 proteins represses exon 9* but enhances exon 33 splicing. (A) Immunoblot of Fox proteins in C2C12 cells transfected with Fox1 and Fox2 expression plasmids. The bands for endogenous Fox proteins and coexpressed tagged Fox1 and Fox2 proteins are indicated. The total Fox expression (endogenous plus recombinant) was normalized to the amount of GAPDH and indicated below. (B) RT-PCR assay of exon 9* in C2C12 cells transfected with pcDNA3.1 control vector or with Fox1 and Fox2 expression plasmids. (C) RT-PCR assay of exon 33 in the same cells. (D) RT-PCR assay of exon 9* in N2a cells after transfection with increasing amounts of Fox1 and Fox2 expression plasmids. (E) Immunoblot of Fox1 and 2 proteins in cells transfected with increasing doses of Fox1 and Fox2 encoding plasmids. GAPDH expression served as the loading control. Note that this blot was exposed for a short time to avoid saturating the signal from the transiently expressed protein and measure its increasing expression with increasing plasmid. The lower levels of endogenous Fox2 in N2A cells can be observed upon longer exposure (see Fig. 3). α, anti.
We also measured the dose response of exon 9* splicing to increasing amounts of transfected Fox1 or Fox2 plasmid in the N2a cells (Fig. 4D and E). Increasing amounts of Fox1 plasmid progressively reduced exon 9* splicing from 46 to 24%. Similarly increasing levels of Fox2 expression reduced exon 9* inclusion from 44 to 27%. These exons respond to even small changes in the concentration of either Fox protein.
Regulation of exons 9* and 33 is dependent on Fox binding elements.
There are many examples of RNA-binding proteins affecting the expression of other regulators, which could in turn affect the splicing of their own target exons. If CaV1.2 exons 9* and 33 are direct targets of the Fox proteins, then their splicing should be dependent on Fox binding sites within the adjacent RNA. Several studies have indicated that the position of a Fox binding site relative to a target exon will determine whether the protein will enhance or repress splicing. Upstream binding sites are repressive, but downstream Fox sites generally act as enhancers (22, 59, 64). This is in agreement with what we observe for exon 33, which contains a downstream Fox binding site and is enhanced by the protein. However, it is more difficult to predict the behavior of exon 9*, which contains both upstream and downstream Fox sites, as well as a Fox site in the exon itself. To demonstrate a direct role for the Fox proteins in the splicing of these exons and to determine which Fox binding sites are most important for their regulation, we constructed minigene splicing reporters for each exon. Each exon was cloned into the splicing reporter construct pDUP4-1 along with several hundred nucleotides of flanking intron sequence, including all the most likely Fox binding sites (Fig. 5A and 6A; also see Fig. S3 in the supplemental material).
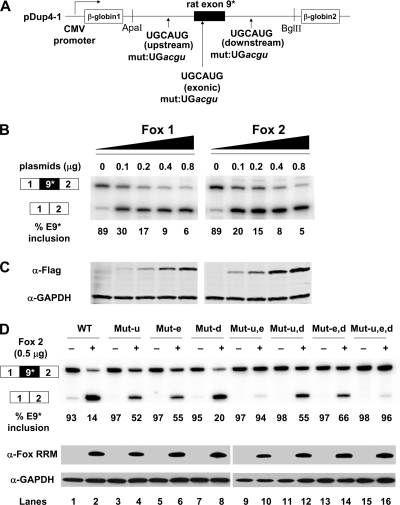
FIG. 5.
Fox1 and Fox2 proteins repress exon 9* splicing in Dup-E9* minigene transcripts expressed in HEK293T cells. (A) Diagram of Dup-E9* minigene shows the positions of three Fox binding elements. The Dup-E9* minigene contains 927 nucleotides of rat genomic sequence including 205 nucleotides of upstream intron, the 75-nucleotide exon and 647 nucleotides of downstream intron (see Fig. S3A in the supplemental material). (B) Primer extension assay of exon 9* splicing in HEK293T cells cotransfected with Dup-E9* and increasing amounts of Fox1 and Fox2 plasmids. (C) Western blot of tagged Fox1 or Fox2 expression in transfected HEK293T cells. (D) Mutation of Fox binding elements in the exon 9* minigene abolishes the Fox2 repressive effect on splicing. WT, Dup-E9* minigene without mutations in Fox-binding element. Mut-u, Mut-e, and Mut-d indicate mutations (UGCAUG→ UGacgu) in the upstream (u), exonic (e), or downstream (d) Fox binding elements, respectively. These were combined in Mut-u,e, Mut-u,d, Mut-e,d, and Mut-u,e,d. Minigene reporters were cotransfected with 0.5 μg of empty vector pCDNA3.1+ (−) or Fox2 expression vector (+). Immunoblot assay of Fox2 expression in the transfected cells using anti-Fox RRM antibody is shown at the bottom. α, anti.
FIG. 6.
Fox1 proteins enhance Exon 33 splicing in the Dup-E33 minigene transcripts. (A) Diagram of the Dup-E33 minigene. The downstream Fox binding element and its mutations (mut 1 or mut 2) are indicated. Dup-E33 minigene contains 1079 nt of rat genomic sequence surrounding exon 33, including 721 nucleotides of the upstream intron, the 33-nucleotide exon, and 325 nucleotides of the downstream intron (see Fig. S3B in the supplemental material). (B) Primer extension assay of exon 33 splicing in HEK293T cells cotransfected with Dup-E33 or its mutants, together with increasing amounts of Fox2 plasmids. Coexpressed Fox2 protein enhances exon 33 splicing in transcripts from the Dup-E33 minigene (lanes 1 to 4). This enhancement was eliminated by the Fox binding element mutations (lanes 5 to 8 and lanes 9 to 12). Fox2 overexpression was assayed by immunoblotting with anti-Fox RRM antibody, shown at the bottom. α, anti.
When expressed from the minigene in HEK293T cells, exon 9* is 89% included in the RNA (Fig. 5B). Cotransfection of a Fox1 or Fox2 expression plasmid strongly repressed this splicing in a dose-dependent manner, with exon 9* inclusion reduced to 5 or 6% at the final titration point (Fig. 5B and C). Thus, in the minigene context the exon is highly sensitive to the Fox protein concentration.
Each of the putative Fox binding elements upstream (u), within (e), or downstream (d) of exon 9* was mutated either singly or in combination with the other elements (Fig. 5A and D). These mutant minigenes were cotransfected with the Fox2 expression plasmid or with a vector control; the splicing was assayed by primer extension, and Fox protein expression was assayed by immunoblotting. The wild-type minigene splicing was reduced from 93% exon 9* inclusion in the vector control to 14% inclusion with Fox2 coexpression (Fig. 5D lanes 1 and 2). Exons carrying mutations in either the upstream or the exonic Fox sites were much less responsive to Fox2 protein, with exon inclusion being reduced to 52 or 55% with Fox2 expression (Fig. 5D, lanes 3 to 6). Combining the upstream and exonic mutations eliminates nearly all response to the Fox protein; exon inclusion decreases from 97 to 94% with Fox2 expression (Fig. 5D, lanes 9 and 10). Mutation of the downstream element had little effect on the Fox response, with exon inclusion decreasing to 20% upon Fox2 expression (Fig. 5D, lanes 7 and 8). Combining this mutation with the upstream and exonic mutations also had only minimal effects (Fig. 5D, lanes 11 to 16). Thus, the Fox-dependent repression of exon 9* is highly dependent on Fox-binding elements within the exon and the upstream intron. This repression is likely a direct effect of the Fox protein acting on the CaV1.2 RNA.
A minigene reporter for exon 33 was also constructed (Fig. 6A). In HEK293 cells, this exon was mostly repressed in the absence of coexpressed Fox protein (8% inclusion, Fig. 6B lane 1). Upon Fox2 coexpression, exon 33 is strongly induced and shows increased splicing with increased Fox2 expression, reaching 51% inclusion at the highest Fox2 protein concentration (Fig. 6B, lanes 1 to 4). Either of two point mutations in the downstream Fox binding site nearly eliminates the Fox-dependent splicing enhancement (Fig. 6B, lanes 5 to 8 and lanes 9 to 12). This is particularly striking for a single nucleotide change from UGCAUG to UGCGUG. Thus, both exons 9* and 33 are directly regulated by Fox proteins. Exon 9* is repressed by Fox binding to upstream and exonic binding sites, and exon 33 is enhanced from a downstream binding site.
DISCUSSION
Developmental regulation of calcium channel physiology through changes in splicing.
We found that the Fox proteins control two exons in the CaV1.2 pre-mRNA. The induction of Fox protein expression during neurogenesis leads to the progressive repression of exon 9* and the progressive inclusion of exon 33. Both of these exons have been shown to affect the electrophysiological properties CaV1.2 channels (29). Exon 33 encodes an 11-amino-acid insert into the IVS3-IVS4 extracellular loop, adjacent to IVS4 voltage sensor region of the channel. Lipscombe et al. have noted that changes in this linker may alter the movement of the adjoining S4 helix and thus influence the kinetics of channel activation (33). Studies of L-type, P/Q-type, and N-type calcium channels, as well as of potassium channels have all shown that the IVS3-IVS4 linker is an important region in coupling voltage-sensing to channel opening (10, 20, 32-34, 55, 58).
When expressed in HEK293T cells, CaV1.2 channels containing the exon 9* encoded peptide exhibit a ∼10-mV hyperpolarized shift (left shift) in their current/voltage relationship curve compared to channels lacking the 9* peptide (31, 55). The exclusion of exon 33 in channels that contain exon 9* (CaV1.2+E9*ΔE33) shifts both the activation and the inactivation potential of the channel by 8 to 10 mV in the same hyperpolarized direction (30, 54). The exclusion of exon 33 also makes the channel more sensitive to nifedipine inhibition. Thus, both the decreased splicing of exon 9* and the increased splicing of exon 33, seen in response to Fox proteins, should lead to channels with a more positive depolarizing potential for activation and inactivation. The effect of these changes on cellular activity will need examination in model systems with better defined physiology and in the context of single cells. Although the cellular consequences of these changes in channel properties are not known, it is clear that the Fox proteins are playing significant roles in fine-tuning calcium channel physiology during neuronal development and likely in mature cells.
In addition to the CaV1.2 channel, there are three other L-type calcium channel genes: CaV1.1, CaV1.3, and CaV1.4 (14, 17, 33). The gene structures of these pore-forming subunits are highly conserved and exhibit alternative splicing in many of the same regions. The CaV1.1, CaV1.3, and CaV1.4 transcripts also contain alternative exons in the IVS3-IVS4 linker region similar to CaV1.2 exon 33 (4, 26, 43, 51, 55, 58). Interestingly, Fox binding elements ((U)GCAUG) are found downstream of all of these IVS3-IVS4 exons (Fig. 7) (64). CaV1.3 also contains an alternatively spliced 26-amino-acid exon 9a in the I-II loop similar to CaV1.2 exon 9* (26, 46). This 9a exon has one TGCATG and one GCATG element in its downstream intron, suggesting regulation by Fox proteins but perhaps in the opposite direction than exon 9* of CaV1.2. We also found conserved Fox sites adjacent to alternative exons in other types of calcium channels, as well as exons in calcium transport proteins. These observations indicate that beyond their alteration of the L-type calcium channel, the Fox proteins are likely affecting other aspects of calcium signaling and calcium ion homeostasis in excitable cells.
FIG. 7.
Alternative cassette exons in the IVS3-IVS4 regions of the four human L-type calcium channel genes all contain conserved Fox binding elements in downstream intron. Alternative exons are in uppercase and the 300 nucleotides of downstream intronic sequence is in lowercase. There are no “tgcatg” or “gcatg” elements in the 200 nucleotides of intronic sequence upstream these alternative exons.
An alternatively spliced exon is generally under the control of multiple splicing regulators, and it is likely that other splicing factors are affecting exons 9* and 33 (7, 8, 35, 37, 60). The Fox binding sites studied here are within larger highly conserved intronic regions that presumably include binding sites for other proteins. Indeed, we found that the PTB/nPTB splicing factors also affect these exons, although less strongly (Z. Z. Tang and D. L. Black, unpublished data). Notably, CaV1.2 channels containing exon 9* and excluding exon 33 are expressed in smooth muscle cells that also express Fox1 and Fox2. In this cellular context, there must be other factors determining the use of these exons. In addition, there are at least 12 other CaV1.2 alternative exons, and many more protein regulators will be needed to generate the complex splicing patterns seen for this gene (1, 54, 55). Of particular interest are the factors controlling exons 8a and 8, which are mutated in the Timothy Syndrome (52, 53) and factors controlling exons altered in cardiovascular disease.
Supplementary Material
Acknowledgments
We thank Andrey Damianov and Peter Stoilov for the anti-Fox RRM antibody and the pBlsH1Fox-1 and pBlsH1Fox-2 plasmid constructs.
This study was supported in part by NIH grant RO1 GM49662 to D.L.B. D.L.B. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Published ahead of print on 29 June 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abernethy, D. R., and N. M. Soldatov. 2002. Structure-functional diversity of human L-type Ca2+ channel: perspectives for new pharmacological targets. J. Pharmacol. Exp. Ther. 300724-728. [DOI] [PubMed] [Google Scholar]
- 2.Auweter, S. D., R. Fasan, L. Reymond, J. G. Underwood, D. L. Black, S. Pitsch, and F. H. Allain. 2006. Molecular basis of RNA recognition by the human alternative splicing factor Fox-1. EMBO J. 25163-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baraniak, A. P., J. R. Chen, and M. A. Garcia-Blanco. 2006. Fox-2 mediates epithelial cell-specific fibroblast growth factor receptor 2 exon choice. Mol. Cell. Biol. 261209-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry, E. L., F. A. Gesek, S. C. Froehner, and P. A. Friedman. 1995. Multiple calcium channel transcripts in rat osteosarcoma cells: selective activation of alpha 1D isoform by parathyroid hormone. Proc. Natl. Acad. Sci. USA 9210914-10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biel, M., P. Ruth, E. Bosse, R. Hullin, W. Stuhmer, V. Flockerzi, and F. Hofmann. 1990. Primary structure and functional expression of a high-voltage activated calcium channel from rabbit lung. FEBS Lett. 269409-412. [DOI] [PubMed] [Google Scholar]
- 6.Bito, H., K. Deisseroth, and R. W. Tsien. 1997. Ca2+-dependent regulation in neuronal gene expression. Curr. Opin. Neurobiol. 7419-429. [DOI] [PubMed] [Google Scholar]
- 7.Black, D. L. 2003. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 72291-336. [DOI] [PubMed] [Google Scholar]
- 8.Blencowe, B. J. 2006. Alternative splicing: new insights from global analyses. Cell 12637-47. [DOI] [PubMed] [Google Scholar]
- 9.Bodi, I., G. Mikala, S. E. Koch, S. A. Akhter, and A. Schwartz. 2005. The L-type calcium channel in the heart: the beat goes on. J. Clin. Investig. 1153306-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourinet, E., T. W. Soong, K. Sutton, S. Slaymaker, E. Mathews, A. Monteil, G. W. Zamponi, J. Nargeot, and T. P. Snutch. 1999. Splicing of α1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat. Neurosci. 2407-415. [DOI] [PubMed] [Google Scholar]
- 11.Boutz, P. L., G. Chawla, P. Stoilov, and D. L. Black. 2007. MicroRNAs regulate the expression of the alternative splicing factor nPTB during muscle development. Genes Dev. 2171-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boutz, P. L., P. Stoilov, Q. Li, C. H. Lin, G. Chawla, K. Ostrow, L. Shiue, M. Ares, Jr., and D. L. Black. 2007. A posttranscriptional regulatory switch in polypyrimidine tract-binding proteins reprograms alternative splicing in developing neurons. Genes Dev. 211636-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cartin, L., K. M. Lounsbury, and M. T. Nelson. 2000. Coupling of Ca2+ to CREB activation and gene expression in intact cerebral arteries from mouse: roles of ryanodine receptors and voltage-dependent Ca2+ channels. Circ. Res. 86760-767. [DOI] [PubMed] [Google Scholar]
- 14.Catterall, W. A., E. Perez-Reyes, T. P. Snutch, and J. Striessnig. 2005. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 57411-425. [DOI] [PubMed] [Google Scholar]
- 15.Cheng, X., J. Liu, M. Asuncion-Chin, E. Blaskova, J. P. Bannister, A. M. Dopico, and J. H. Jaggar. 2007. A novel Ca(V)1.2 N terminus expressed in smooth muscle cells of resistance size arteries modifies channel regulation by auxiliary subunits. J. Biol. Chem. 28229211-29221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolmetsch, R. E., U. Pajvani, K. Fife, J. M. Spotts, and M. E. Greenberg. 2001. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science 294333-339. [DOI] [PubMed] [Google Scholar]
- 17.Ertel, E. A., K. P. Campbell, M. M. Harpold, F. Hofmann, Y. Mori, E. Perez-Reyes, A. Schwartz, T. P. Snutch, T. Tanabe, L. Birnbaumer, R. W. Tsien, and W. A. Catterall. 2000. Nomenclature of voltage-gated calcium channels. Neuron 25533-535. [DOI] [PubMed] [Google Scholar]
- 18.Fearon, I. M., G. Varadi, S. Koch, I. Isaacsohn, S. G. Ball, and C. Peers. 2000. Splice variants reveal the region involved in oxygen sensing by recombinant human L-type Ca2+ channels. Circ. Res. 87537-539. [DOI] [PubMed] [Google Scholar]
- 19.Graf, E. M., M. Bock, J. F. Heubach, I. Zahanich, S. Boxberger, W. Richter, J. H. Schultz, and U. Ravens. 2005. Tissue distribution of a human Ca v 1.2 α1 subunit splice variant with a 75-bp insertion. Cell Calcium 3811-21. [DOI] [PubMed] [Google Scholar]
- 20.Hans, M., A. Urrutia, C. Deal, P. F. Brust, K. Stauderman, S. B. Ellis, M. M. Harpold, E. C. Johnson, and M. E. Williams. 1999. Structural elements in domain IV that influence biophysical and pharmacological properties of human α1A-containing high-voltage-activated calcium channels. Biophys. J. 761384-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodgkin, J., J. D. Zellan, and D. G. Albertson. 1994. Identification of a candidate primary sex determination locus, fox-1, on the X chromosome of Caenorhabditis elegans. Development 1203681-3689. [DOI] [PubMed] [Google Scholar]
- 22.Jin, Y., H. Suzuki, S. Maegawa, H. Endo, S. Sugano, K. Hashimoto, K. Yasuda, and K. Inoue. 2003. A vertebrate RNA-binding protein Fox-1 regulates tissue-specific splicing via the pentanucleotide GCAUG. EMBO J. 22905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiehl, T. R., H. Shibata, T. Vo, D. P. Huynh, and S. M. Pulst. 2001. Identification and expression of a mouse ortholog of A2BP1. Mamm. Genome 12595-601. [DOI] [PubMed] [Google Scholar]
- 24.Klockner, U., G. Mikala, J. Eisfeld, D. E. Iles, M. Strobeck, J. L. Mershon, A. Schwartz, and G. Varadi. 1997. Properties of three COOH-terminal splice variants of a human cardiac L-type Ca2+-channel α1-subunit. Am. J. Physiol. 272H1372-H1381. [DOI] [PubMed] [Google Scholar]
- 25.Koch, W. J., A. Hui, G. E. Shull, P. Ellinor, and A. Schwartz. 1989. Characterization of cDNA clones encoding two putative isoforms of the alpha 1 subunit of the dihydropyridine-sensitive voltage-dependent calcium channel isolated from rat brain and rat aorta. FEBS Lett. 250386-388. [DOI] [PubMed] [Google Scholar]
- 26.Kollmar, R., L. G. Montgomery, J. Fak, L. J. Henry, and A. J. Hudspeth. 1997. Predominance of the alpha1D subunit in L-type voltage-gated Ca2+ channels of hair cells in the chicken's cochlea. Proc. Natl. Acad. Sci. USA 9414883-14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane, H. A. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 28.Lee, J. A., Y. Xing, D. Nguyen, J. Xie, C. J. Lee, and D. L. Black. 2007. Depolarization and CaM kinase IV modulate NMDA receptor splicing through two essential RNA elements. PLoS Biol. 5e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao, P., T. F. Yong, M. C. Liang, D. T. Yue, and T. W. Soong. 2005. Splicing for alternative structures of Cav1.2 Ca2+ channels in cardiac and smooth muscles. Cardiovasc. Res. 68197-203. [DOI] [PubMed] [Google Scholar]
- 30.Liao, P., D. Yu, G. Li, T. F. Yong, J. L. Soon, Y. L. Chua, and T. W. Soong. 2007. A smooth muscle Cav1.2 calcium channel splice variant underlies hyperpolarized window current and enhanced state-dependent inhibition by nifedipine. J. Biol. Chem. 28235133-35142. [DOI] [PubMed] [Google Scholar]
- 31.Liao, P., D. Yu, S. Lu, Z. Tang, M. C. Liang, S. Zeng, W. Lin, and T. W. Soong. 2004. Smooth muscle-selective alternatively spliced exon generates functional variation in Cav1.2 calcium channels. J. Biol. Chem. 27950329-50335. [DOI] [PubMed] [Google Scholar]
- 32.Lin, Z., Y. Lin, S. Schorge, J. Q. Pan, M. Beierlein, and D. Lipscombe. 1999. Alternative splicing of a short cassette exon in α1B generates functionally distinct N-type calcium channels in central and peripheral neurons. J. Neurosci. 195322-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipscombe, D., J. Q. Pan, and A. C. Gray. 2002. Functional diversity in neuronal voltage-gated calcium channels by alternative splicing of Ca(v) α1. Mol. Neurobiol 2621-44. [DOI] [PubMed] [Google Scholar]
- 34.Mathur, R., J. Zheng, Y. Yan, and F. J. Sigworth. 1997. Role of the S3-S4 linker in Shaker potassium channel activation. J. Gen. Physiol. 109191-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matlin, A. J., F. Clark, and C. W. Smith. 2005. Understanding alternative splicing: toward a cellular code. Nat. Rev. Mol. Cell. Biol. 6386-398. [DOI] [PubMed] [Google Scholar]
- 36.McKee, A. E., E. Minet, C. Stern, S. Riahi, C. D. Stiles, and P. A. Silver. 2005. A genome-wide in situ hybridization map of RNA-binding proteins reveals anatomically restricted expression in the developing mouse brain. BMC Dev. Biol. 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Modafferi, E. F., and D. L. Black. 1999. Combinatorial control of a neuron-specific exon. RNA 5687-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Modafferi, E. F., and D. L. Black. 1997. A complex intronic splicing enhancer from the c-src pre-mRNA activates inclusion of a heterologous exon. Mol. Cell. Biol. 176537-6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moosmang, S., P. Lenhardt, N. Haider, F. Hofmann, and J. W. Wegener. 2005. Mouse models to study L-type calcium channel function. Pharmacol. Ther. 106347-355. [DOI] [PubMed] [Google Scholar]
- 40.Nakahata, S., and S. Kawamoto. 2005. Tissue-dependent isoforms of mammalian Fox-1 homologs are associated with tissue-specific splicing activities. Nucleic Acids Res. 332078-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicoll, M., C. C. Akerib, and B. J. Meyer. 1997. X-chromosome-counting mechanisms that determine nematode sex. Nature 388200-204. [DOI] [PubMed] [Google Scholar]
- 42.Pang, L., G. Koren, Z. Wang, and S. Nattel. 2003. Tissue-specific expression of two human Ca(v)1.2 isoforms under the control of distinct 5′ flanking regulatory elements. FEBS Lett. 546349-354. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Reyes, E., X. Y. Wei, A. Castellano, and L. Birnbaumer. 1990. Molecular diversity of L-type calcium channels. Evidence for alternative splicing of the transcripts of three non-allelic genes. J. Biol. Chem. 26520430-20436. [PubMed] [Google Scholar]
- 44.Ponthier, J. L., C. Schluepen, W. Chen, R. A. Lersch, S. L. Gee, V. C. Hou, A. J. Lo, S. A. Short, J. A. Chasis, J. C. Winkelmann, and J. G. Conboy. 2006. Fox-2 splicing factor binds to a conserved intron motif to promote inclusion of protein 4.1R alternative exon 16. J. Biol. Chem. 28112468-12474. [DOI] [PubMed] [Google Scholar]
- 45.Saada, N. I., E. D. Carrillo, B. Dai, W. Z. Wang, C. Dettbarn, J. Sanchez, and P. Palade. 2005. Expression of multiple CaV1.2 transcripts in rat tissues mediated by different promoters. Cell Calcium 37301-309. [DOI] [PubMed] [Google Scholar]
- 46.Safa, P., J. Boulter, and T. G. Hales. 2001. Functional properties of Cav1.3 (α1D) L-type Ca2+ channel splice variants expressed by rat brain and neuroendocrine GH3 cells. J. Biol. Chem. 27638727-38737. [DOI] [PubMed] [Google Scholar]
- 47.Sinnegger-Brauns, M. J., A. Hetzenauer, I. G. Huber, E. Renstrom, G. Wietzorrek, S. Berjukov, M. Cavalli, D. Walter, A. Koschak, R. Waldschutz, S. Hering, S. Bova, P. Rorsman, O. Pongs, N. Singewald, and J. Striessnig. 2004. Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by L-type Ca2+ channels. J. Clin. Investig. 1131430-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skipper, M., C. A. Milne, and J. Hodgkin. 1999. Genetic and molecular analysis of fox-1, a numerator element involved in Caenorhabditis elegans primary sex determination. Genetics 151617-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snutch, T. P., W. J. Tomlinson, J. P. Leonard, and M. M. Gilbert. 1991. Distinct calcium channels are generated by alternative splicing and are differentially expressed in the mammalian CNS. Neuron 745-57. [DOI] [PubMed] [Google Scholar]
- 50.Soldatov, N. M. 1994. Genomic structure of human L-type Ca2+ channel. Genomics 2277-87. [DOI] [PubMed] [Google Scholar]
- 51.Soldatov, N. M. 1992. Molecular diversity of L-type Ca2+ channel transcripts in human fibroblasts. Proc. Natl. Acad. Sci. USA 894628-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Splawski, I., K. W. Timothy, N. Decher, P. Kumar, F. B. Sachse, A. H. Beggs, M. C. Sanguinetti, and M. T. Keating. 2005. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc. Natl. Acad. Sci. USA 1028086-8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Splawski, I., K. W. Timothy, L. M. Sharpe, N. Decher, P. Kumar, R. Bloise, C. Napolitano, P. J. Schwartz, R. M. Joseph, K. Condouris, H. Tager-Flusberg, S. G. Priori, M. C. Sanguinetti, and M. T. Keating. 2004. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 11919-31. [DOI] [PubMed] [Google Scholar]
- 54.Tang, Z. Z., X. Hong, J. Wang, and T. W. Soong. 2007. Signature combinatorial splicing profiles of rat cardiac- and smooth-muscle Cav1.2 channels with distinct biophysical properties. Cell Calcium 41417-428. [DOI] [PubMed] [Google Scholar]
- 55.Tang, Z. Z., M. C. Liang, S. Lu, D. Yu, C. Y. Yu, D. T. Yue, and T. W. Soong. 2004. Transcript scanning reveals novel and extensive splice variations in human L-type voltage-gated calcium channel, Cav1.2 α1 subunit. J. Biol. Chem. 27944335-44343. [DOI] [PubMed] [Google Scholar]
- 56.Tang, Z. Z., P. Liao, G. Li, F. L. Jiang, D. Yu, X. Hong, T. F. Yong, G. Tan, S. Lu, J. Wang, and T. W. Soong. 2008. Differential splicing patterns of L-type calcium channel Ca(v)1.2 subunit in hearts of spontaneously hypertensive rats and Wistar Kyoto rats. Biochim. Biophys. Acta 1783118-130. [DOI] [PubMed] [Google Scholar]
- 57.Tiwari, S., Y. Zhang, J. Heller, D. R. Abernethy, and N. M. Soldatov. 2006. Atherosclerosis-related molecular alteration of the human CaV1.2 calcium channel α1C subunit. Proc. Natl. Acad. Sci. USA 10317024-17029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tuluc, P., N. Molenda, B. Schlick, G. J. Obermair, B. E. Flucher, and K. Jurkat-Rott. 2009. A Ca(V)1.1 Ca2+ channel splice variant with high conductance and voltage-sensitivity alters EC coupling in developing skeletal muscle. Biophys. J. 9635-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Underwood, J. G., P. L. Boutz, J. D. Dougherty, P. Stoilov, and D. L. Black. 2005. Homologues of the Caenorhabditis elegans Fox-1 protein are neuronal splicing regulators in mammals. Mol. Cell. Biol. 2510005-10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, Z., and C. B. Burge. 2008. Splicing regulation: from a parts list of regulatory elements to an integrated splicing code. RNA 14802-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welling, A., Y. W. Kwan, E. Bosse, V. Flockerzi, F. Hofmann, and R. S. Kass. 1993. Subunit-dependent modulation of recombinant L-type calcium channels: molecular basis for dihydropyridine tissue selectivity. Circ. Res. 73974-980. [DOI] [PubMed] [Google Scholar]
- 62.Welling, A., A. Ludwig, S. Zimmer, N. Klugbauer, V. Flockerzi, and F. Hofmann. 1997. Alternatively spliced IS6 segments of the α1C gene determine the tissue-specific dihydropyridine sensitivity of cardiac and vascular smooth muscle L-type Ca2+ channels. Circ. Res. 81526-532. [DOI] [PubMed] [Google Scholar]
- 63.Yeo, G. W., N. G. Coufal, T. Y. Liang, G. E. Peng, X. D. Fu, and F. H. Gage. 2009. An RNA code for the FOX2 splicing regulator revealed by mapping RNA-protein interactions in stem cells. Nat. Struct. Mol. Biol. 16130-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang, C., Z. Zhang, J. Castle, S. Sun, J. Johnson, A. R. Krainer, and M. Q. Zhang. 2008. Defining the regulatory network of the tissue-specific splicing factors Fox-1 and Fox-2. Genes Dev. 222550-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou, H. L., A. P. Baraniak, and H. Lou. 2007. Role for Fox-1/Fox-2 in mediating the neuronal pathway of calcitonin/calcitonin gene-related peptide alternative RNA processing. Mol. Cell. Biol. 27830-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou, H. L., and H. Lou. 2008. Repression of prespliceosome complex formation at two distinct steps by Fox-1/Fox-2 proteins. Mol. Cell. Biol. 285507-5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuhlke, R. D., A. Bouron, N. M. Soldatov, and H. Reuter. 1998. Ca2+ channel sensitivity toward the blocker isradipine is affected by alternative splicing of the human α1C subunit gene. FEBS Lett. 427220-224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.