Abstract
Oxidative stress is a major source of chromosome single-strand breaks (SSBs), and the repair of these lesions is retarded in neurodegenerative disease. The rate of the repair of oxidative SSBs is accelerated by XRCC1, a scaffold protein that is essential for embryonic viability and that interacts with multiple DNA repair proteins. However, the relative importance of the interactions mediated by XRCC1 during oxidative stress in vivo is unknown. We show that mutations that disrupt the XRCC1 interaction with DNA polymerase β or DNA ligase III fail to slow SSB repair in proliferating CHO cells following oxidative stress. In contrast, mutation of the domain that interacts with polynucleotide kinase/phosphatase (PNK) and Aprataxin retards repair, and truncated XRCC1 encoding this domain fully supports this process. Importantly, the impact of mutating the protein domain in XRCC1 that binds these end-processing factors is circumvented by the overexpression of wild-type PNK but not by the overexpression of PNK harboring a mutated DNA 3′-phosphatase domain. These data suggest that DNA 3′-phosphatase activity is critical for rapid rates of chromosomal SSB repair following oxidative stress, and that the XRCC1-PNK interaction ensures that this activity is not rate limiting in vivo.
Oxidative stress can have a major influence on genome integrity and cell survival and is an etiological factor in a number of neurological human diseases. Of these, several are associated with defects in the repair of DNA damage, including xeroderma pigmentosum (XP), ataxia telangiecatsia (A-T), ataxia oculomotor apraxia 1 (AOA1), and spinocerebellar ataxia with axonal neuropathy 1 (SCAN1) (1, 28, 34, 37). The neuropathology evident in XP most likely reflects an inability to repair one or more single-strand oxidative adducts by nucleotide excision repair. In contrast, A-T is associated with cellular defects in the repair of DNA double-strand breaks (DSBs) and AOA1 and SCAN1 with defects in the repair of DNA single-strand breaks (SSBs). SSBs are the commonest DNA lesions arising in cells, and if they are not rapidly repaired they can inhibit transcription and/or generate replication-associated DSBs (3, 26, 59, 60). The repair of oxidative SSBs involves DNA damage detection by PARP-1 followed by recruitment of the enzymes required for subsequent steps of the repair process, which include DNA end processing, DNA gap filling, and DNA ligation (9, 19). Many of the enzymes implicated in these steps interact physically with XRCC1, including DNA polynucleotide kinase (PNK) (54), Aprataxin (APTX) (13, 14, 18, 31, 44), DNA polymerase β (Pol β) (10, 27), and DNA ligase IIIα (Lig3α) (11, 12). This has prompted the hypothesis that XRCC1 is a scaffold protein that recruits, stabilizes, and/or stimulates SSB repair (SSBR) enzymes at chromosomal SSBs, thereby accelerating the overall process (8, 9). While in vitro analyses generally are consistent with this idea, including the observation that XRCC1 mutation (50, 58), deletion (49), or depletion (6) retards the rate of chromosomal SSBR by approximately fivefold following DNA oxidation or DNA base damage, the relative importance of the protein-protein interactions mediated by XRCC1 for SSBR is unclear.
Here, we have addressed the importance of the protein-protein interactions mediated by XRCC1 during the repair of oxidative SSBs. To do this, we have employed isogenic XRCC1 mutant CHO cells expressing recombinant derivatives of XRCC1 in which specific protein-protein interaction domains are mutated. We find that whereas the interactions between XRCC1 and either Pol β or Lig3α are dispensable for rapid rates of chromosomal SSBR in asynchronous populations of CHO cells following oxidative stress, SSBR rates are markedly slowed in cells expressing XRCC1 that cannot interact with PNK. Importantly, we show that the overexpression of wild-type recombinant PNK, but not 3′-phosphatase-dead PNK, can override the requirement for PNK interaction with XRCC1 for rapid rates of SSBR following oxidative stress. These data indicate that DNA 3′-phosphatase activity is critical for rapid rates of chromosomal SSBR following oxidative stress, and that the XRCC1 interaction with PNK prevents this activity from becoming rate limiting.
MATERIALS AND METHODS
DNA constructs.
pCD2E-XHF67A, pCD2E-XHS485A,T488A, and pCD2E-XHS518A.T519A,T523A were created by the site-directed mutagenesis of the XRCC1 open reading frame (ORF) in pCD2E-XH (12) using a QuikChange mutagenesis kit (Stratagene) and the appropriate primers. pCD2E and pCD2E-XHCKM have been described previously (30, 46). To create pCD2E-XHS408A,S409A,S410A, a KpnI fragment of wild-type XRCC1 cDNA from pCD2E-XH was replaced with the corresponding fragment in pET16B-XHS408A,S409A,S410A (Richard Taylor, unpublished data). To create pAS-XRCC1F67A, a CelII-BlnI fragment of XRCC1 from pAS-XH was replaced with the corresponding fragment from pCD2E-XHF67A. To create pCD2E-PNKD171N, the PNK ORF in pCD2E-PNK (30) was mutated by site-directed mutagenesis as described above. pCD2E-HX161-533 encoding His-XRCC1161-533 was created by PCR amplification, insertion into pCR2.1-TOPO (Invitrogen), and subcloning into the EcoRI sites of pCD2E.
Cell lines.
The XRCC1 mutant CHO cell line EM9 and derivatives were maintained as monolayers in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (vol/vol) fetal calf serum, 100 U/ml penicillin, 2 mM glutamine, and 100 μg/ml streptomycin. Expression constructs were introduced into EM9 cells by calcium phosphate coprecipitation (EM9-XHF67A) or by Genejuice (Novagen) transfection (EM9-XHCKM, EM9-XHS408A,S409A,S410A, EM9-XHS485A,T488A, and EM9-XHS518A,T519A,T523A). Stable cell lines were prepared by the selection of transfected cells with 1.5 mg/ml G418 (Gibco-Invitrogen) for 7 to 10 days.
Antibodies and immunoblotting.
Cells were lysed in sodium dodecyl sulfate (SDS) loading buffer at 90°C, and whole-cell extracts from 5 × 104 to 2.5 × 105 cells were fractionated by SDS-polyacrylamide gel electrophoresis (PAGE). Proteins were transferred to nitrocellulose and immunoblotted with anti-XRCC1 monoclonal (clone 33.2.5), anti-XRCC1 polyclonal (SK3188), anti-PNK (SK3195), anti-Lig3α polyclonal (TL25), anti-Polβ polyclonal (a kind gift from Grigory Dianov), or anti-β-actin monoclonal antibody (Sigma).
Yeast two-hybrid analyses.
Saccharomyces cerevisiae Y190 cells were transformed with the indicated pAS and pACT constructs, and transformants were selected on yeast minimal medium plates (glucose plus yeast nitrogen base without amino acids) supplemented with adenine and histidine. Pooled populations of transformants were streaked onto minimal medium supplemented with either adenine and histidine for β-galactosidase colony-lift filter assays or adenine and 25 mM 3-amino-1,2,4-triazole for the analysis of histidine prototrophy.
Affinity purification of histidine-tagged XRCC1 protein complexes from CHO cells.
For the analysis of the XRCC1-Pol β interaction, whole-cell extract was prepared from a confluent flask (T75) or plate (15 cm) of EM9-XH, EM9-XHF67A, or EM9-V cells by resuspension in lysis buffer (25 mM HEPES, pH 8.0, 325 mM sodium chloride, 0.5% Triton X-100, 10% glycerol, 1 mM dithiothreitol [DTT], 25 mM imidazole, 1/100 dilution of mammalian protease inhibitor cocktail [Sigma]) at a density of 6 × 106 cells/ml and incubated on ice for 15 min. High-molecular-weight DNA was sheered by passage through a narrow-gauge needle, and extracts were clarified by centrifugation (8,832 × g) for 10 min at 2°C. For the analysis of the XRCC1-PNK interaction, EM9 cells were transiently transfected with 8 μg/dish each of pCD2E-PNK and either pCD2E-XH, pCD2E-XHCKM, pCD2E-XHS408A,S409A,S410A, pCD2E-XHS485A,T488A, pCD2E-XHS518A,T519A,T523A, or empty pCD2E using Genejuice (Novagen). Twenty-four hours later, transiently transfected cell populations were selected in medium containing 1.5 mg/ml G418 for 4 days. Cells (5 × 106) were harvested, washed, and resuspended in 0.3 ml lysis buffer (25 mM HEPES, pH 8.0, 325 mM sodium chloride, 10% glycerol, 1 mM DTT, 25 mM imidazole, 1/100 dilution of mammalian protease inhibitor cocktail [Sigma]) and sonicated (five 30-s pulses at high power using a Biorupter sonicator). XRCC1-His complexes then were purified from the clarified extract by metal-chelate affinity chromatography. Briefly, cell extract (0.3 to 1 ml) was incubated with 0.25 to 0.5 ml nickel-nitrilotriacetic acid-agarose (Qiagen) for 20 min on ice with frequent mixing. The resulting suspension was added to a disposable 5-ml chromatography column (Polyprep; Bio-Rad), and the flow rate was adjusted to ∼0.5 ml/min. The column was washed (twice with 5 ml) with lysis buffer and then 5 ml of elution buffer (lysis buffer containing 250 mM imidazole), and 0.5-ml fractions were collected.
Clonogenic survival assays.
For survival assays, five hundred cells were plated in 10-cm dishes in duplicate and incubated for 4 h at 37°C. Cells were rinsed with phosphate-buffered saline (PBS) (twice) and either mock treated or treated with H2O2 (diluted in PBS at the appropriate concentration immediately prior to use) for 15 min at room temperature or with MMS (diluted in complete medium at the appropriate concentration immediately prior to use) for 15 min at 37°C. After treatment, cells were washed twice with PBS and incubated for 10 to 14 days in drug-free medium at 37°C to allow the formation of macroscopic colonies. Colonies were fixed in ethanol (95%) and stained with 1% methylene blue-70% ethanol. Colonies of greater than 50 cells were counted, and the survival (as a percentage) was calculated using the equation 100 × [mean colony number (treated plate)/mean colony number (untreated plate)].
Alkaline single-cell agarose-gel electrophoresis (alkaline comet assay).
To examine EM9-XHF67A and its associated controls by alkaline comet assay, 2 × 105 cells were plated in 3.5-cm culture dishes in complete medium and incubated for 16 to 18 h at 37°C prior to mock treatment or treatment with 0.15 to 0.2 mM H2O2 in PBS for 10 to 20 min on ice. For other cell lines, subconfluent monolayers were trypsinized and diluted to 4 × 105 cells/ml immediately prior to treatment with H2O2 as described above. For exposure with gamma rays, cells were treated in suspension (4 × 105 cells/ml) in complete medium. After treatment with MMS, H2O2, or gamma irradiation (20 Gy), cells were washed once in ice-cold PBS and incubated in fresh drug-free medium for the desired repair period. Adherent cells were harvested in ice-cold PBS by scraping (1 ml/3.5-cm plate), and 0.2 ml of the cell suspension was analyzed by alkaline comet assay as previously described (7). For cells treated in suspension, 1-ml aliquots (4 × 105 cells) were analyzed as described above. For transient-transfection experiments, EM9 cells were transiently cotransfected in 10-cm dishes with 8 μg/dish of either pCD2E-XH, pCD2E-XHS518A,T519A,T523A, or empty pCD2E and additionally with 24 μg/dish of either pCD2E-PNK, pCD2E-PNKD171N, or empty pCD2E using Genejuice (Novagen). Twenty-four hours later cells were selected in medium containing 1.5 mg/ml G418 for 4 days and then H2O2 treated or not treated as described above.
RESULTS
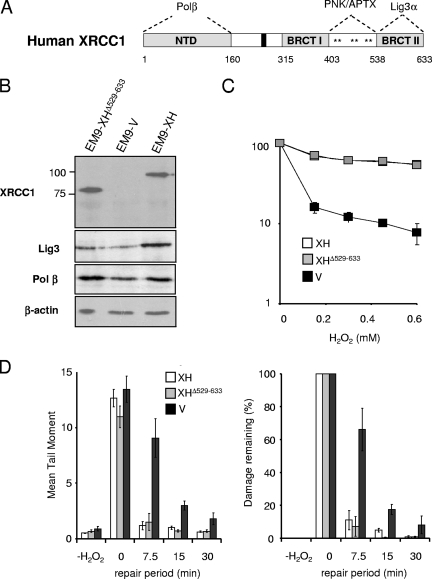
XRCC1 interacts with polypeptide components of each of the three core stages of DNA processing during SSBR (Fig. 1A). However, the relative importance of these interactions for the repair of oxidative SSBs is unknown. To examine the requirement for XRCC1 interaction with Lig3α during DNA ligation, we employed XRCC1 mutant EM9 CHO cells stably expressing either wild-type recombinant human XRCC1-His (EM9-XH cells), XRCC1-Hisδ529-633 protein lacking the C-terminal BRCT domain that binds Lig3α (EM9-XHδ529-633 cells), or an empty expression vector (EM9-V cells) (Fig. 1B). We have shown previously that the deletion of the C-terminal BRCT domain ablates binding to Lig3α (36). Consistent with this, Lig3 levels were no higher in EM9-XHδ529-633 cells than in EM9-V cells, consistent with the requirement for interaction with XRCC1 for Lig3α stability (Fig. 1B) (11, 12). Notably, while EM9-V cells harboring empty vector were hypersensitive to H2O2-induced oxidative stress, the level of sensitivity of EM9-XHδ529-633 cells to H2O2 was similar to that of EM9-XH (Fig. 1C). To measure chromosomal SSBR rates, we employed alkaline comet assays. Although this assay can detect both SSBs and DSBs, the vast majority (>99%) of breaks induced by H2O2 are SSBs (4). Surprisingly, while EM9-V cells harboring empty vector exhibited reduced rates of global SSBR following H2O2-induced oxidative stress, SSBR rates in EM9-XHδ529-633 cells were similar to those of EM9-XH (Fig. 1D). We conclude that the interaction between XRCC1 and Lig3α is dispensable for the repair of oxidative SSBs in proliferating CHO cells.
FIG. 1.
XRCC1-DNA ligase IIIα interaction is dispensable for rapid SSBR rates following oxidative stress in proliferating CHO cells. (A) Schematic depicting human XRCC1 and the protein domains that mediate interaction with Pol β, PNK, APTX, and Lig3α. Asterisks denote the three clusters of consensus CK2 phosphorylation sites. (B) Cell extracts from the indicated cell lines were fractionated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-XRCC1 monoclonal, anti-Lig3 polyclonal, anti-Pol β polyclonal, or anti-β-actin monoclonal antibody. (C) Clonogenic sensitivity of the indicated EM9 CHO cell lines to H2O2. Cells were treated for 15 min at room temperature with the indicated concentrations of H2O2 in PBS, and surviving colonies were stained and counted after 10 to 14 days. Data are the means (± standard errors of the means) of at least three independent experiments. (D) DNA strand break repair rates following H2O2 treatment as measured by alkaline comet assays. The indicated EM9 CHO cell lines were treated with 150 μM H2O2 for 20 min on ice in PBS and then incubated for the indicated repair periods in drug-free media. DNA strand breaks were quantified by alkaline comet assays from three or more independent experiments. (Left) Mean tail moments were quantified from ∼100 cells/sample/experiment and are the averages (± standard errors of the means) of at least three independent experiments. (Right) The data in the left panel were plotted as the mean fractions of DNA strand breaks remaining at the indicated time points.
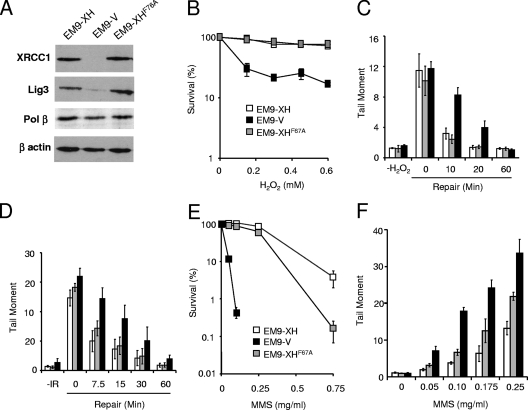
To examine the requirement for XRCC1 interaction with DNA Pol β, we employed EM9 cells expressing XRCC1 protein harboring the point mutation F67A (Fig. 2A). This mutation is located within the N-terminal domain (NTD) that binds Pol β and reduces the interaction of XRCC1 with the DNA polymerase in vitro (33). Consistent with this, the F67A mutation reduced Pol β binding by XRCC1 more than 25-fold in vivo, whereas the interaction with aprataxin (APTX), which is mediated by the C-terminal half of XRCC1, was only weakly (<2-fold) affected (data not shown; also see Fig. S1A in the supplemental material). In addition, whereas Pol β copurified with XRCC1-His from EM9-XH cell extract, it failed to do so measurably from cell extract from EM9 cells expressing XRCC1-HisF67A (denoted EM9-XHF67A cells) (see Fig. S1B in the supplemental material). XRCC1-HisF67A largely corrected the hypersensitivity of XRCC1-deficient EM9 cells to H2O2, suggesting that the XRCC1-Pol β interaction is dispensable for cell survival following oxidative DNA damage (Fig. 2B). XRCC1-HisF67A similarly restored cellular resistance to H2O2 in EM-C11 cells, a second XRCC1 mutant CHO cell line (see Fig. S2 in the supplemental material). XRCC1-HisF67A also complemented the defect in chromosomal SSBR rates in XRCC1 mutant cells, because H2O2-induced DNA breaks declined at normal rates in EM9-XHF67A cells compared to those of EM9-V cells (Fig. 2C). This result was not limited to H2O2-induced oxidative stress, because similar results were observed following ionizing radiation (Fig. 2D). Notably, in contrast to oxidative stress, XRCC1-HisF67A failed to fully correct cellular hypersensitivity and reduced repair rates in EM9 cells following methyl methanesulfonate (MMS) treatment, consistently with the established importance of the XRCC1-Pol β interaction during DNA base excision repair (BER) (15, 38, 56) (Fig. 2E and F). We conclude that while the XRCC1-Pol β interaction is required for efficient BER, it is dispensable for cell survival and rapid rates of chromosomal SSBR in proliferating cells following oxidative stress.
FIG. 2.
XRCC1-Pol β interaction is dispensable for rapid SSBR rates following oxidative stress in proliferating CHO cells. (A) Cell extracts from the indicated cell lines were fractionated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-XRCC1 monoclonal, anti-Lig3 polyclonal, anti-Pol β polyclonal, or anti-β-actin monoclonal antibody. (B) Clonogenic sensitivity of the indicated cell lines to H2O2. Cells were treated for 15 min at room temperature with the indicated concentrations of H2O2 in PBS, and surviving colonies were stained and counted after 10 to 14 days. Data are the means (± standard errors of the means) of at least three independent experiments. (C) DNA strand break repair rates following H2O2 treatment. The indicated CHO cell lines (symbols as in panel B) were treated with 200 μM H2O2 for 10 min on ice and then incubated for the indicated repair periods in drug-free media. DNA strand breaks were quantified by alkaline comet assays as described in the legend to Fig. 1. Mean tail moments were quantified from ∼100 cells/sample/experiment and are the averages (± standard errors of the means) of at least three independent experiments. (D) DNA strand break repair rates following gamma radiation. The indicated CHO cell lines (symbols as in panel B) were treated with irradiation (IR) (20 Gy) and then incubated for the indicated repair periods in drug-free media. DNA strand breaks were quantified as described for panel C. (E) Clonogenic sensitivity of the indicated cell lines to MMS. Cells were treated for 15 min at 37°C with the indicated concentrations of MMS, and surviving colonies stained and counted after 10 to 14 days. Data are the means (± standard errors of the means) of at least three independent experiments. (F) DNA strand break repair rates following MMS treatment. The indicated CHO cell lines (symbols as in panel B) were treated with the indicated concentrations of MMS for 15 min at 37°C, and the accumulated levels of DNA strand breaks were quantified as described for panel C.
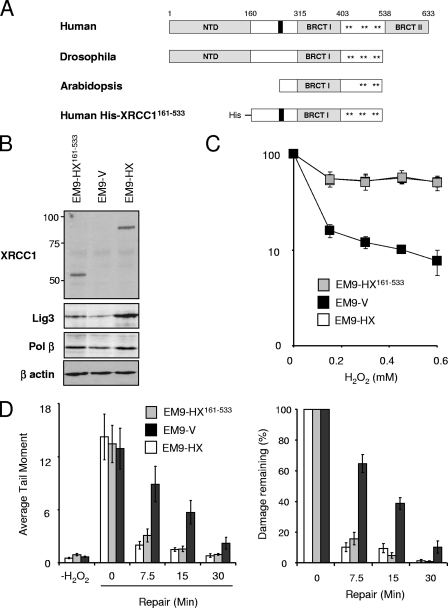
To confirm that the requirement for XRCC1 for rapid rates of SSBR in asynchronous CHO cells did not require interaction with either Pol β or Lig3α, we employed a truncated derivative of human XRCC1 (His-XRCC1161-533) lacking both the amino-terminal (NTD) and C-terminal domains that bind Pol β and Lig3α, respectively (Fig. 3A). This protein mimics the native XRCC1 homolog that is present in the plant Arabidopsis thaliana, and it was expressed in EM9 cells at levels similar to those of wild-type human His-XRCC1 (Fig. 3B). Notably, proliferating EM9-HX161-533 cells expressing the truncated protein exhibited both normal sensitivity (Fig. 3C) and normal rates of SSBR following H2O2 treatment (Fig. 3D and E). These data identify the region spanning amino acids 161 to 533 as sufficient for the ability of XRCC1 to maintain cell survival and rapid global rates of SSBR in proliferating CHO cells.
FIG. 3.
His-XRCC1161-533 restores cellular resistance and rapid SSBR rates in XRCC1 mutant CHO cells following oxidative stress. (A) Schematic depicting the evolutionarily conserved domains in XRCC1. XRCC1 homologues in Homo sapiens, Drosophila melanogaster, and Arabidopsis thaliana are shown, along with the truncated derivative of human XRCC1 employed here (denoted His-XRCC1161-533). Asterisks denote clusters of CK2 consensus phosphorylation sites. (B) XRCC1 protein levels in XRCC1 mutant CHO cells stably transfected with empty expression vector (EM9-V) or with expression constructs encoding either wild-type His-XRCC1 (EM9-HX) or mutant His-XRCC1 lacking the NTD and C-terminal BRCT II domain (EM9-HX161-533). Cell extracts from the indicated cell lines were fractionated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-XRCC1 monoclonal, anti-Lig3 polyclonal, anti-Pol β polyclonal, or anti-β-actin monoclonal antibody. (C) Clonogenic sensitivity of the indicated cell lines to H2O2. Cells were treated for 15 min at room temperature with the indicated concentrations of H2O2 in PBS, and surviving colonies were stained and counted after 10 to 14 days. Data are the means (± standard errors of the means) of at least three independent experiments. (D) DNA strand break repair rates following H2O2 treatment as measured by alkaline comet assays. The indicated CHO cell lines were treated with 150 μM H2O2 for 20 min on ice and then incubated for the indicated repair periods in drug-free media. DNA strand breaks were quantified by alkaline comet assays as described in the legend to Fig. 1. (Left) Mean tail moments were quantified from ∼100 cells/sample/experiment and are the averages (± standard errors of the means) of at least three independent experiments. (Right) The data in the left panel were plotted as the mean fractions of DNA strand breaks remaining at the indicated time points.
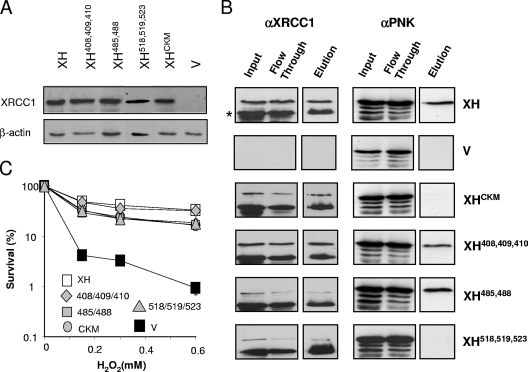
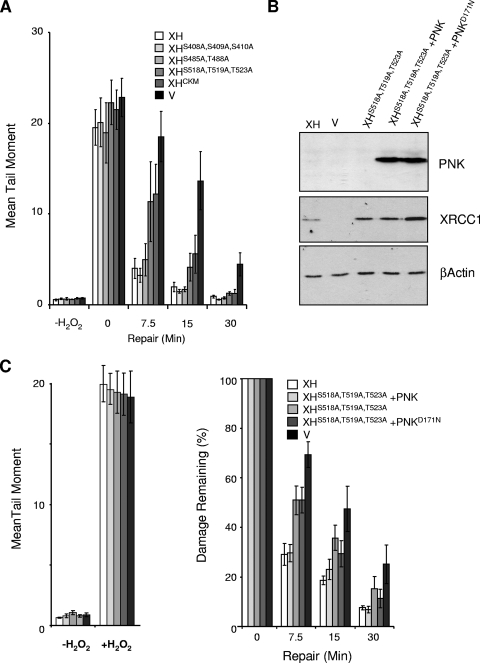
Residues 161 to 533 encompass the most evolutionarily conserved region of XRCC1 and include the inter-BRCT domain linker region of ∼100 amino acids (Fig. 3A). This region, which is phosphorylated multiple times at CK2 consensus sites that are grouped into three clusters at residues 408/409/410, 485/488, and 518/519/523, mediates phosphorylation-specific interactions with the DNA end-processing factors PNK (30) and APTX (13, 31) and also with a recently identified protein of unknown function, denoted APLF (2, 22, 24). Previously, we mutated all eight of the predicted primary CK2 phosphorylation sites in XRCC1 and showed that the mutant protein (denoted XRCC1CKM) was unable to support normal rates of SSBR following oxidative stress (30). However, whether the importance of this region for SSBR reflects the interaction with PNK or with APTX and/or APLF is unknown. To address this issue, we mapped the site of PNK interaction in detail by mutating each of the three clusters of CK2 phosphorylation sites separately and expressing the respective proteins, denoted XRCC1-HisS408A/S409A/S410A, XRCC1-HisS485A/T488A, and XRCC1-HisS518A/T519A/T523A, in EM9 cells (Fig. 4A). PNK copurified with wild-type XRCC1-His, XRCC1-HisS408A/S409A/S410A, and XRCC1-HisS485A/T488A during affinity chromatography but failed to copurify with either XRCC1-HisCKM or XRCC1-HisS518A/T519A/T523A (Fig. 4B). Most of the recombinant XRCC1 recovered in these experiments was truncated by ∼10 kDa due to the proteolytic removal of the labile amino-terminal domain. This did not affect interaction with PNK, however, which occurs toward the C terminus of XRCC1. We conclude from these experiments that the interaction between XRCC1 and PNK requires only the cluster of CK2 phosphorylation sites at S518/T519/T523. Notably, this is the same cluster of CK2 phosphorylation sites that interacts with APTX (31).
FIG. 4.
Mutation of the cluster of CK2 phosphorylation sites at S518/T519/T523 greatly reduces or ablates interaction with PNK. (A) Total cell extracts from 5 × 104 of cells expressing the indicated recombinant XRCC3 protein or harboring empty vector (v) were fractionated by SDS-PAGE, transferred to nitrocellulose, and immunostained with anti-XRCC1 polyclonal antibody or anti-β-actin monoclonal antibody. (B) XRCC1-His protein complexes were recovered from whole-cell extract from 5 × 106 of the indicated cells by affinity chromatography as described in Materials and Methods. Aliquots of the column input (8% of column input), flowthrough, and 250 mM imidazole eluate (13% of total eluate) were fractionated by SDS-PAGE and immunoblotted for the presence of anti-XRCC1 (αXRCC1) or anti-PNK (αPNK) polyclonal antibody. (C) Clonogenic sensitivity of the indicated cell lines to H2O2. Cells were treated for 15 min at room temperature with the indicated concentrations of H2O2 in PBS, and surviving colonies were stained and counted after 10 to 14 days. Data are the means of at least three independent experiments (± standard errors of the means).
None of the CK2 phosphorylation site mutations greatly affected the ability of XRCC1 to correct the hypersensitivity of EM9 cells to H2O2 (Fig. 4C). In contrast, global rates of SSBR were markedly slower in EM9-XHCKM and EM9-XHS518A/T519A/T523A cells than in EM9-XH, EM9-XHS408A/S409A/S410A, or EM9-XHS485A/T488A cells (Fig. 5A). This suggests that, of the three clusters of CK2 phosphorylation sites, only S518/T519/T523 is required for the rapid repair of oxidative SSBs. Although APTX also binds this region of XRCC1, we considered it more likely that the reduced SSBR rate in EM9-XHS518A/T519A/T523A cells resulted from the loss of PNK interaction. This is because, in our hands, the loss of APTX activity by itself does not affect global rates of SSBR following oxidative stress (17, 43), whereas the loss of PNK has been reported to slow the rate of the repair of oxidative SSBs (41). To examine directly whether the mutation of S518/T519/T523 slowed chromosomal SSBR rates by disrupting the XRCC1-PNK interaction, we examined whether normal rates of SSBR could be restored in EM9-XHS518A/T519A/T523A cells by PNK overexpression (Fig. 5B). Indeed, the rate of chromosomal SSBR observed in EM9-XHS518A/T519A/T523A cells was restored to normal by the transient overexpression of wild-type recombinant human PNK protein (Fig. 5C). In contrast, PNKD171N, harboring a mutated DNA 3′-phosphatase domain, was unable to complement the defect in SSBR in EM9-XHS518A/T519A/T523A cells. These data demonstrate, for the first time, that DNA 3′-phosphatase activity is critical for rapid global rates of chromosomal SSBR following oxidative stress, and they suggest that the XRCC1-PNK interaction ensures that this activity does not become rate limiting in vivo.
FIG. 5.
PNK overexpression overrides the requirement for S518/T519/T523 for rapid global rates of chromosomal SSBR following oxidative stress. (A) DNA strand break repair rates following H2O2 treatment as measured by alkaline comet assays. The indicated EM9 CHO cell lines were treated with 150 μM H2O2 for 20 min on ice in PBS and then incubated for the indicated repair periods in drug-free media. DNA strand breaks were quantified by alkaline comet assays as described in the legend to Fig. 1. Mean tail moments were quantified from ∼100 cells/sample/experiment and are the averages (± standard errors of the means) of at least three independent experiments. V, EM9 cells harboring empty vector. (B) Total cell extracts from 3 × 104 EM9-XH (XH) or EM9-V (v) cells or EM9-XHS518A,T519A,T523A cells transiently transfected with empty vector or with the indicated PNK expression construct were fractionated by SDS-PAGE, transferred to nitrocellulose, and immunostained with anti-PNK polyclonal, anti-XRCC1 monoclonal, or anti-β-actin monoclonal antibody. (C) DNA strand break repair rates following H2O2 treatment as measured by alkaline comet assays. The CHO cells described for panel B were mock treated (−H2O2) or treated with 150 μM H2O2 (+H2O2) for 20 min on ice (left) and then incubated for the indicated repair periods in drug-free medium (right). DNA strand breaks were quantified by alkaline comet assays as described above. The data are plotted as the mean fraction of DNA strand breaks remaining and are the averages of at least three independent experiments (± standard errors of the means).
DISCUSSION
XRCC1 is a critical component of the SSBR machinery and functions as a molecular scaffold that interacts with multiple enzymatic components of the repair process (9). While numerous XRCC1 partners and their respective binding sites have been identified, it is unclear which of these interactions is important for rapid SSBR rates following oxidative stress in vivo. An understanding of the protein-protein interactions mediated by XRCC1 and their importance during oxidative stress is likely to affect our understanding of human disease, because oxidative stress is a major source of SSBs. Indeed, mutations in components of the SSBR machinery have been identified in two hereditary neurodegenerative diseases (recently reviewed in references 9 and 42).
Notably, the XRCC1 mutation F67A failed to prevent XRCC1 from restoring cellular resistance and normal SSBR rates in proliferating EM9 cells following H2O2 treatment or ionizing radiation, despite reducing the interaction with Pol β more than 26-fold. It is unlikely that this reflects residual levels of interaction, because this mutation results in cellular hypersensitivity and reduced SSBR rates following treatment with MMS, which is consistent with the established requirement for this interaction during BER at sites of DNA alkylation (15, 38, 56). Also, His-XRCC1161-533 protein fully restored rapid global SSBR rates in EM9 cells following H2O2 despite the complete absence of the Pol β binding N-terminal domain in this protein. We conclude from these data that Pol β can function independently of interaction with XRCC1 following oxidative DNA damage or, more likely, that another DNA polymerase can substitute for Pol β. For example, Pol λ, Pol ι, and Pol Q also are implicated in the cellular response to H2O2, and Pol Q has been reported to physically associate with XRCC1 (5, 39, 47, 51, 57). Alternatively, the long-patch repair DNA polymerases Pol δ and/or Pol ɛ might substitute for Pol β. Consistent with this idea, while early-passage and proliferating populations of Pol β−/− murine embryonic fibroblasts exhibit normal sensitivity and DNA strand break repair rates following oxidative stress, increased sensitivity and decreased repair rates are observed in late-passage or G1-enriched Pol β−/− murine embryonic fibroblasts, conditions under which long-patch repair machinery may be downregulated (21, 52, 53). It remains to be determined whether a similar cell cycle stage-specific requirement exists for the interaction of Pol β with XRCC1.
Surprisingly, the deletion of the entire XRCC1 C-terminal BRCT domain (BRCT II) that binds Lig3α also failed to significantly affect global SSBR rates in asynchronous CHO cells following oxidative stress. Once again, it is unlikely that this reflects residual interaction between XRCC1 and Lig3α. This is because interaction with XRCC1 is required to stabilize the DNA ligase in vivo, yet the cellular level of Lig3α is no higher in EM9-XHδ529-633 cells expressing XRCC1 that lack the BRCT II domain than in EM9 cells that lack XRCC1 altogether (36). Consequently, either Lig3α can function independently of XRCC1 following oxidative stress or, more likely, another DNA ligase can substitute for Lig3α in proliferating CHO cells. One possibility is that Lig1 can fulfill this role, because this DNA ligase is associated with the DNA replication machinery and can conduct BER in vitro (29, 35, 40). It will be of interest to examine whether the requirement for XRCC1-Lig3α interaction during SSBR following oxidative stress exhibits cell cycle specificity, as has been reported for this interaction following ethyl methanesulfonate-induced DNA alkylation (36, 48).
The phosphorylation of XRCC1 by CK2 within a 100-amino-acid region linking the internal (BRCT I) and C-terminal (BRCT II) BRCT domains is required both for PNK interaction and for rapid SSBR rates following H2O2 treatment (30). However, the phosphorylation of this linker region by CK2 also facilitates interactions with the DNA repair proteins APTX and APLF, confusing the issue of which interaction(s) accounts for the importance of this region for rapid SSBR (2, 13, 22, 31). To address this question, we mapped in detail the PNK binding site within the linker region and identified the cluster of CK2 phosphorylation sites in XRCC1 located at S518, T519, and T523 as being critical for this purpose. Intriguingly, S518/T519/T523 is also the APTX binding site, providing an explanation for the observation that XRCC1 associates with these proteins in separate and distinct complexes (31). The mutation of S518/T519/T523 reduced SSBR rates to a level similar to that observed if all eight primary CK2 sites within the linker region were mutated. In contrast, mutation of the remaining CK2 sites by themselves failed to slow SSBR. The CK2 phosphorylation of XRCC1 within the inter-BRCT domain linker region thus accelerates SSBR largely or entirely via S518/T519/T523.
Although S518/T519/T523 mediates interactions with multiple proteins, we considered it likely that it is the PNK interaction that accounts for the importance of these residues for rapid SSBR following H2O2 treatment. This is because a large fraction (>50%) of the breaks induced by oxidative stress are believed to possess 3′-phosphate termini, and thus are putative substrates for the 3′-phosphatase activity of PNK. In addition, so far we have failed to detect an impact of the loss of APTX or APLF alone on rapid global rates of SSBR in proliferating mammalian cells (data not shown). Consistent with this finding, the overexpression of wild-type PNK restored normal rates of SSBR in CHO cells expressing XRCC1-HisS518/T519/T523 following H2O2 treatment, confirming that the requirement for S518/T519/T523 for rapid global SSBR rates reflects the interaction of this region with PNK. Why might the interaction between XRCC1 and PNK be important for rapid chromosomal SSBR rates following oxidative stress? One possibility is that interaction with XRCC1 ensures that PNK activity is not rate limiting during SSBR in vivo. This idea is consistent with the observation that XRCC1 stimulates PNK activity in vitro at limiting concentrations of the latter enzyme, possibly by dissociating PNK from its DNA product and thereby increasing the enzyme turnover rate (32, 54). However, interaction with XRCC1 also might promote the recruitment or accumulation of PNK at chromosomal SSBs, because the appearance of this protein in subnuclear foci is reduced in EM9-XHCKM cells following oxidative stress (30).
Human PNK possesses both 5′-DNA kinase and 3′-DNA phosphatase activities (23, 25). Whereas 5′-hydroxyl termini likely are present at only a minor fraction of DNA strand breaks following oxidative DNA damage, 3′-phosphate termini may be present at 70% or more of all such breaks (20). Consistent with this, the overexpression of PNK harboring a mutated 3′-phosphatase domain failed to complement the SSBR defect in EM9-XHS518A/T519A/T523A cells. Although the mutation of D171 does not directly affect the DNA kinase activity of PNK, it is possible that the repair of 5′-hydroxyl termini also is inhibited by the mutation of the 3′-phosphatase domain. This is because the persistence of a 3′-phosphate terminus may inhibit the phosphorylation of an adjacent 5′-hydroxyl terminus (16). Nevertheless, the inability of PNKD171N to restore rapid SSBR rates demonstrates directly that 3′-DNA phosphatase activity is crucial for rapid rates of SSBR following oxidative stress. Interestingly, the overexpression of AP endonuclease-1 (APE1), another protein that can process damaged 3′ termini, also increases chromosomal SSBR rates in XRCC1 mutant cells following oxidative stress, albeit to less than half the rate present in wild-type cells (45). However, the 3′-phosphatase activity of human APE1 is two to three orders of magnitude less than that of PNK and is unlikely to be a physiologically relevant source of 3′-DNA phosphatase activity in vivo (55). Indeed, the impact of mutating XRCC1 at S518A/T519A/T523A on both PNK binding and chromosomal SSBR rates strongly suggests that PNK, in conjunction with XRCC1, is the major source of this activity in vivo.
In summary, we demonstrate here for the first time that there is a critical requirement for DNA 3′-phosphatase activity during the repair of chromosomal SSBs following oxidative stress, and we suggest that the XRCC1 interaction with PNK ensures that this activity is not rate limiting in vivo.
Supplementary Material
Acknowledgments
This work was funded by the MRC (G0600776), BBSRC (BB/C516595/1), and CR-UK (C6563/A10192).
Footnotes
Published ahead of print on 22 June 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Barzilai, A., S. Biton, and Y. Shiloh. 2008. The role of the DNA damage response in neuronal development, organization and maintenance. DNA Repair (Amsterdam) 71010-1027. [DOI] [PubMed] [Google Scholar]
- 2.Bekker-Jensen, S., K. Fugger, J. R. Danielsen, I. Gromova, M. Sehested, J. Celis, J. Bartek, J. Lukas, and N. Mailand. 2007. Human Xip1 (C2orf13) is a novel regulator of cellular responses to DNA strand breaks. J. Biol. Chem. 28219638-19643. [DOI] [PubMed] [Google Scholar]
- 3.Bendixen, C., B. Thomsen, J. Alsner, and O. Westergaard. 1990. Camptothecin-stabilized topoisomerase I-DNA adducts cause premature termination of transcription. Biochemistry 295613-5619. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, M. O., and K. W. Kohn. 1979. X-ray induced DNA double-strand break production and repair in mammalian cells as measured by neutral filter elution. Nucleic Acids Res. 7793-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braithwaite, E. K., P. S. Kedar, L. Lan, Y. Y. Polosina, K. Asagoshi, V. P. Poltoratsky, J. K. Horton, H. Miller, G. W. Teebor, A. Yasui, and S. H. Wilson. 2005. DNA polymerase lambda protects mouse fibroblasts against oxidative DNA damage and is recruited to sites of DNA damage/repair. J. Biol. Chem. 28031641-31647. [DOI] [PubMed] [Google Scholar]
- 6.Brem, R., and J. Hall. 2005. XRCC1 is required for DNA single-strand break repair in human cells. Nucleic Acids Res. 332512-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breslin, C., P. M. Clements, S. F. El-Khamisy, E. Petermann, N. Iles, and K. W. Caldecott. 2006. Measurement of chromosomal DNA single-strand breaks and replication fork progression rates. Methods Enzymol. 409410-425. [DOI] [PubMed] [Google Scholar]
- 8.Caldecott, K. W. 2001. Mammalian DNA single-strand break repair: an X-ra(y)ted affair. Bioessays 23447-455. [DOI] [PubMed] [Google Scholar]
- 9.Caldecott, K. W. 2008. Single-strand break repair and genetic disease. Nat. Rev. Genet. 9619-631. [DOI] [PubMed] [Google Scholar]
- 10.Caldecott, K. W., S. Aoufouchi, P. Johnson, and S. Shall. 1996. XRCC1 polypeptide interacts with DNA polymerase beta and possibly poly (ADP-ribose) polymerase, and DNA ligase III is a novel molecular ‘nick-sensor’ in vitro. Nucleic Acids Res. 244387-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldecott, K. W., C. K. McKeown, J. D. Tucker, S. Ljungquist, and L. H. Thompson. 1994. An interaction between the mammalian DNA repair protein XRCC1 and DNA ligase III. Mol. Cell. Biol. 1468-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caldecott, K. W., J. D. Tucker, L. H. Stanker, and L. H. Thompson. 1995. Characterization of the XRCC1-DNA ligase III complex in vitro and its absence from mutant hamster cells. Nucleic Acids Res. 234836-4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clements, P. M., C. Breslin, E. D. Deeks, P. J. Byrd, L. Ju, P. Bieganowski, C. Brenner, M. C. Moreira, A. M. Taylor, and K. W. Caldecott. 2004. The ataxia-oculomotor apraxia 1 gene product has a role distinct from ATM and interacts with the DNA strand break repair proteins XRCC1 and XRCC4. DNA Repair (Amsterdam) 31493-1502. [DOI] [PubMed] [Google Scholar]
- 14.Date, H., S. Igarashi, Y. Sano, T. Takahashi, T. Takahashi, H. Takano, S. Tsuji, M. Nishizawa, and O. Onodera. 2004. The FHA domain of aprataxin interacts with the C-terminal region of XRCC1. Biochem. Biophys. Res. Commun. 3251279-1285. [DOI] [PubMed] [Google Scholar]
- 15.Dianova, I. I., K. M. Sleeth, S. L. Allinson, J. L. Parsons, C. Breslin, K. W. Caldecott, and G. L. Dianov. 2004. XRCC1-DNA polymerase beta interaction is required for efficient base excision repair. Nucleic Acids Res. 322550-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobson, C. J., and S. L. Allinson. 2006. The phosphatase activity of mammalian polynucleotide kinase takes precedence over its kinase activity in repair of single strand breaks. Nucleic Acids Res. 342230-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Khamisy, S. F., S. Katyal, P. Patel, L. Ju, P. J. McKinnon, and K. W. Caldecott. 2009. Synergistic decrease of DNA single-strand break repair rates in mouse neural cells lacking both Tdp1 and aprataxin. DNA Repair (Amsterdam) 8760-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gueven, N., O. J. Becherel, A. W. Kijas, P. Chen, O. Howe, J. H. Rudolph, R. Gatti, H. Date, O. Onodera, G. Taucher-Scholz, and M. F. Lavin. 2004. Aprataxin, a novel protein that protects against genotoxic stress. Hum. Mol. Genet. 131081-1093. [DOI] [PubMed] [Google Scholar]
- 19.Hegde, M. L., T. K. Hazra, and S. Mitra. 2008. Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res. 1827-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henner, W. D., S. M. Grunberg, and W. A. Haseltine. 1982. Sites and structure of gamma radiation-induced DNA strand breaks. J. Biol. Chem. 25711750-11754. [PubMed] [Google Scholar]
- 21.Horton, J. K., A. Baker, B. J. Berg, R. W. Sobol, and S. H. Wilson. 2002. Involvement of DNA polymerase beta in protection against the cytotoxicity of oxidative DNA damage. DNA Repair (Amsterdam) 1317-333. [DOI] [PubMed] [Google Scholar]
- 22.Iles, N., S. Rulten, S. F. El-Khamisy, and K. W. Caldecott. 2007. APLF (C2orf13) is a novel human protein involved in the cellular response to chromosomal DNA strand breaks. Mol. Cell. Biol. 273793-3803. (Erratum, 28:3561, 2008.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jilani, A., D. Ramotar, C. Slack, C. Ong, X. M. Yang, S. W. Scherer, and D. D. Lasko. 1999. Molecular cloning of the human gene, PNKP, encoding a polynucleotide kinase 3′-phosphatase and evidence for its role in repair of DNA strand breaks caused by oxidative damage. J. Biol. Chem. 27424176-24186. [DOI] [PubMed] [Google Scholar]
- 24.Kanno, S., H. Kuzuoka, S. Sasao, Z. Hong, L. Lan, S. Nakajima, and A. Yasui. 2007. A novel human AP endonuclease with conserved zinc-finger-like motifs involved in DNA strand break responses. EMBO J. 262094-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karimi-Busheri, F., G. Daly, P. Robins, B. Canas, D. J. Pappin, J. Sgouros, G. G. Miller, H. Fakhrai, E. M. Davis, M. M. Le Beau, and M. Weinfeld. 1999. Molecular characterization of a human DNA kinase. J. Biol. Chem. 27424187-24194. [DOI] [PubMed] [Google Scholar]
- 26.Kathe, S. D., G. P. Shen, and S. S. Wallace. 2004. Single-stranded breaks in DNA but not oxidative DNA base damages block transcriptional elongation by RNA polymerase II in HeLa cell nuclear extracts. J. Biol. Chem. 27918511-18520. [DOI] [PubMed] [Google Scholar]
- 27.Kubota, Y., R. A. Nash, A. Klungland, P. Schar, D. E. Barnes, and T. Lindahl. 1996. Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase beta and the XRCC1 protein. EMBO J. 156662-6670. [PMC free article] [PubMed] [Google Scholar]
- 28.Lavin, M. F., N. Gueven, and P. Grattan-Smith. 2008. Defective responses to DNA single- and double-strand breaks in spinocerebellar ataxia. DNA Repair (Amsterdam) 71061-1076. [DOI] [PubMed] [Google Scholar]
- 29.Levin, D. S., A. E. McKenna, T. A. Motycka, Y. Matsumoto, and A. E. Tomkinson. 2000. Interaction between PCNA and DNA ligase I is critical for joining of Okazaki fragments and long-patch base-excision repair. Curr. Biol. 10919-922. [DOI] [PubMed] [Google Scholar]
- 30.Loizou, J. I., S. F. El-Khamisy, A. Zlatanou, D. J. Moore, D. W. Chan, J. Qin, S. Sarno, F. Meggio, L. A. Pinna, and K. W. Caldecott. 2004. The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks. Cell 11717-28. [DOI] [PubMed] [Google Scholar]
- 31.Luo, H., D. W. Chan, T. Yang, M. Rodriguez, B. P. Chen, M. Leng, J. J. Mu, D. Chen, Z. Songyang, Y. Wang, and J. Qin. 2004. A new XRCC1-containing complex and its role in cellular survival of methyl methanesulfonate treatment. Mol. Cell. Biol. 248356-8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mani, R. S., M. Fanta, F. Karimi-Busheri, E. Silver, C. A. Virgen, K. W. Caldecott, C. E. Cass, and M. Weinfeld. 2007. XRCC1 stimulates polynucleotide kinase by enhancing its damage discrimination and displacement from DNA repair intermediates. J. Biol. Chem. 28228004-28013. [DOI] [PubMed] [Google Scholar]
- 33.Marintchev, A., M. R. Gryk, and G. P. Mullen. 2003. Site-directed mutagenesis analysis of the structural interaction of the single-strand-break repair protein, X-ray cross-complementing group 1, with DNA polymerase beta. Nucleic Acids Res. 31580-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKinnon, P. J., and K. W. Caldecott. 2007. DNA strand break repair and human genetic disease. Annu. Rev. Genomics Hum. Genet. 837-55. [DOI] [PubMed] [Google Scholar]
- 35.Montecucco, A., R. Rossi, D. S. Levin, R. Gary, M. S. Park, T. A. Motycka, G. Ciarrocchi, A. Villa, G. Biamonti, and A. E. Tomkinson. 1998. DNA ligase I is recruited to sites of DNA replication by an interaction with proliferating cell nuclear antigen: identification of a common targeting mechanism for the assembly of replication factories. EMBO J. 173786-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore, D. J., R. M. Taylor, P. Clements, and K. W. Caldecott. 2000. Mutation of a BRCT domain selectively disrupts DNA single-strand break repair in noncycling Chinese hamster ovary cells. Proc. Natl. Acad. Sci. USA 9713649-13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nouspikel, T. 2008. Nucleotide excision repair and neurological diseases. DNA Repair (Amsterdam) 71155-1167. [DOI] [PubMed] [Google Scholar]
- 38.Parsons, J. L., Dianova, I. I., S. L. Allinson, and G. L. Dianov. 2005. DNA polymerase beta promotes recruitment of DNA ligase III alpha-XRCC1 to sites of base excision repair. Biochemistry 4410613-10619. [DOI] [PubMed] [Google Scholar]
- 39.Petta, T. B., S. Nakajima, A. Zlatanou, E. Despras, S. Couve-Privat, A. Ishchenko, A. Sarasin, A. Yasui, and P. Kannouche. 2008. Human DNA polymerase iota protects cells against oxidative stress. EMBO J. 272883-2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prasad, R., R. K. Singhal, D. K. Srivastava, J. T. Molina, A. E. Tomkinson, and S. H. Wilson. 1996. Specific interaction of DNA polymerase beta and DNA ligase I in a multiprotein base excision repair complex from bovine testis. J. Biol. Chem. 27116000-16007. [DOI] [PubMed] [Google Scholar]
- 41.Rasouli-Nia, A., F. Karimi-Busheri, and M. Weinfeld. 2004. Stable down-regulation of human polynucleotide kinase enhances spontaneous mutation frequency and sensitizes cells to genotoxic agents. Proc. Natl. Acad. Sci. USA 1016905-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rass, U., I. Ahel, and S. C. West. 2007. Defective DNA repair and neurodegenerative disease. Cell 130991-1004. [DOI] [PubMed] [Google Scholar]
- 43.Reynolds, J. J., S. F. El-Khamisy, and K. W. Caldecott. 2009. Short-patch single-strand break repair in ataxia oculomotor apraxia-1. Biochem. Soc. Trans. 37577-581. [DOI] [PubMed] [Google Scholar]
- 44.Sano, Y., H. Date, S. Igarashi, O. Onodera, M. Oyake, T. Takahashi, S. Hayashi, M. Morimatsu, H. Takahashi, T. Makifuchi, N. Fukuhara, and S. Tsuji. 2004. Aprataxin, the causative protein for EAOH is a nuclear protein with a potential role as a DNA repair protein. Ann. Neurol. 55241-249. [DOI] [PubMed] [Google Scholar]
- 45.Sossou, M., C. Flohr-Beckhaus, I. Schulz, F. Daboussi, B. Epe, and J. P. Radicella. 2005. APE1 overexpression in XRCC1-deficient cells complements the defective repair of oxidative single strand breaks but increases genomic instability. Nucleic Acids Res. 33298-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takayama, K., E. P. Salazar, A. Lehmann, M. Stefanini, L. H. Thompson, and C. A. Weber. 1995. Defects in the DNA repair and transcription gene ERCC2 in the cancer-prone disorder xeroderma pigmentosum group D. Cancer Res. 555656-5663. [PubMed] [Google Scholar]
- 47.Tano, K., J. Nakamura, K. Asagoshi, H. Arakawa, E. Sonoda, E. K. Braithwaite, R. Prasad, J. M. Buerstedde, S. Takeda, M. Watanabe, and S. H. Wilson. 2007. Interplay between DNA polymerases beta and lambda in repair of oxidation DNA damage in chicken DT40 cells. DNA Repair (Amsterdam) 6869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor, R. M., D. J. Moore, J. Whitehouse, P. Johnson, and K. W. Caldecott. 2000. A cell cycle-specific requirement for the XRCC1 BRCT II domain during mammalian DNA strand break repair. Mol. Cell. Biol. 20735-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tebbs, R. S., M. L. Flannery, J. J. Meneses, A. Hartmann, J. D. Tucker, L. H. Thompson, J. E. Cleaver, and R. A. Pedersen. 1999. Requirement for the Xrcc1 DNA base excision repair gene during early mouse development. Dev. Biol. 208513-529. [DOI] [PubMed] [Google Scholar]
- 50.Thompson, L. H., K. W. Brookman, L. E. Dillehay, A. V. Carrano, J. A. Mazrimas, C. L. Mooney, and J. L. Minkler. 1982. A CHO-cell strain having hypersensitivity to mutagens, a defect in DNA strand-break repair, and an extraordinary baseline frequency of sister-chromatid exchange. Mutat. Res. 95427-440. [DOI] [PubMed] [Google Scholar]
- 51.Vermeulen, C., B. Bertocci, A. C. Begg, and C. Vens. 2007. Ionizing radiation sensitivity of DNA polymerase lambda-deficient cells. Radiat. Res. 168683-688. [DOI] [PubMed] [Google Scholar]
- 52.Vermeulen, C., M. Verwijs-Janssen, A. C. Begg, and C. Vens. 2008. Cell cycle phase dependent role of DNA polymerase beta in DNA repair and survival after ionizing radiation. Radiother. Oncol. 86391-398. [DOI] [PubMed] [Google Scholar]
- 53.Vermeulen, C., M. Verwijs-Janssen, P. Cramers, A. C. Begg, and C. Vens. 2007. Role for DNA polymerase beta in response to ionizing radiation. DNA Repair (Amsterdam) 6202-212. [DOI] [PubMed] [Google Scholar]
- 54.Whitehouse, C. J., R. M. Taylor, A. Thistlethwaite, H. Zhang, F. Karimi-Busheri, D. D. Lasko, M. Weinfeld, and K. W. Caldecott. 2001. XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell 104107-117. [DOI] [PubMed] [Google Scholar]
- 55.Wiederhold, L., J. B. Leppard, P. Kedar, F. Karimi-Busheri, A. Rasouli-Nia, M. Weinfeld, A. E. Tomkinson, T. Izumi, R. Prasad, S. H. Wilson, S. Mitra, and T. K. Hazra. 2004. AP endonuclease-independent DNA base excision repair in human cells. Mol. Cell 15209-220. [DOI] [PubMed] [Google Scholar]
- 56.Wong, H. K., and D. M. Wilson III. 2005. XRCC1 and DNA polymerase beta interaction contributes to cellular alkylating-agent resistance and single-strand break repair. J. Cell Biochem. 95794-804. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimura, M., M. Kohzaki, J. Nakamura, K. Asagoshi, E. Sonoda, E. Hou, R. Prasad, S. H. Wilson, K. Tano, A. Yasui, L. Lan, M. Seki, R. D. Wood, H. Arakawa, J. M. Buerstedde, H. Hochegger, T. Okada, M. Hiraoka, and S. Takeda. 2006. Vertebrate POLQ and POLbeta cooperate in base excision repair of oxidative DNA damage. Mol. Cell 24115-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zdzienicka, M. Z., G. P. Vanderschans, A. T. Natarajan, L. H. Thompson, I. Neuteboom, and J. W. I. M. Simons. 1992. A Chinese-hamster ovary cell mutant (Em-c-11) with sensitivity to simple alkylating-agents and a very high-level of sister chromatid exchanges. Mutagenesis 7265-269. [DOI] [PubMed] [Google Scholar]
- 59.Zhou, W., and P. W. Doetsch. 1993. Effects of abasic sites and DNA single-strand breaks on prokaryotic RNA polymerases. Proc. Natl. Acad. Sci. USA 906601-6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou, W., and P. W. Doetsch. 1994. Transcription bypass or blockage at single-strand breaks on the DNA template strand: effect of different 3′ and 5′ flanking groups on the T7 RNA polymerase elongation complex. Biochemistry 3314926-14934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.