Abstract
Rapamycin, a selective inhibitor of mTORC1 signaling, blocks terminal myoblast differentiation. We found that downregulation of rictor, a component of the mTORC2 complex, but not downregulation of raptor, a component of the mTORC1 complex, prevented terminal differentiation (fusion) of C2C12 myoblasts. Both rapamycin and rictor downregulation suppressed the phosphorylation of AKT(S473), and rapamycin treatment of C2C12 myoblasts disrupted the mTORC2 complex. Importantly, downregulation of rictor inhibited TORC2 signaling without inhibiting mTORC1 signaling, suggesting that inhibition of mTORC1 by rapamycin may not be the cause of arrested differentiation. In support of this, expression of a phosphomimetic mutant AKT(S473D) in rictor-deficient cells rescued myoblast fusion even in the presence of rapamycin. mTORC2 signaling to AKT appears necessary for downregulation of the Rho-associated kinase (ROCK1) that occurs during myogenic differentiation. Rapamycin treatment prevented ROCK1 inactivation during differentiation, while suppression of ROCK1 activity during differentiation and myoblast fusion was restored through expression of AKT(S473D), even in the presence of rapamycin. Further, the ROCK inhibitor Y-27632 restored terminal differentiation in rapamycin-treated myoblasts. These results provide the first evidence of a specific role for mTORC2 signaling in terminal myogenic differentiation.
Differentiation of skeletal muscle cells involves highly coordinated processes involving myogenic determination of pluripotent mesodermal precursors, withdrawal from the cell cycle, subsequent expression of myotube-specific genes, and cell fusion to form multinucleated myotubes (5, 19, 23, 33). AKT represents a nodal point, signaling to several pathways to positively or negatively regulate growth, proliferation, survival, and myogenic differentiation (34, 35). AKT phosphorylates the FoxO1a transcription factor required for myotube fusion of primary myoblasts (3), causing cytoplasmic localization. However, in primary myoblasts and C2C12 myoblasts, phosphorylation of FoxO1a appears partially independent of the PI3K/AKT pathway, possibly regulated through the Rho-associated kinase ROCK1. Inactivation of ROCK1 has been shown to be necessary for FoxO1a nuclear translocation and C2C12 cell fusion (20). AKT also activates mTOR (mammalian target of rapamycin) through phosphorylation and inactivation of the tuberous sclerosis complex (36) and phosphorylation of PRAS40, an endogenous inhibitor of mTOR (31, 32). Recent studies have shown that the TOR kinase(s) exists in two complexes that are conserved (25, 36). In mammalian cells, TORC1 comprises mTOR, raptor, and mLST8 and is part of a pathway that senses nutrient (amino acids, O2, AMP) and growth factor status. mTORC1 activates S6K1 and phosphorylates and inactivates 4E-BPs, promoting association of eIF4E, the RNA cap-binding protein, with the eIF4G scaffolding protein and assembly of the eIF4F preinitiation translation complex (36). Importantly, rapamycin in complex with the immunophilin FKBP12 is a potent and selective inhibitor of mTORC1 and potently inhibits myogenic differentiation in vitro (8, 29). Whether the kinase function of mTOR is required for myogenic differentiation is controversial (9). Park and Chen have proposed that neither S6K1 nor mTOR kinase activity is required for initiation of myogenic differentiation, although mTOR catalytic activity is required for a second-stage fusion that results in mature myotubes (22).
These studies, particularly with the mTORC1 inhibitor rapamycin, strongly suggest a role for mTORC1 in myogenic differentiation. However, the mTORC2 complex (rictor, Sin1, mLST8) modulates the phosphorylation of protein kinase Cα and the actin cytoskeleton, an aspect of TOR signaling that is conserved between yeasts and mammals (11). Furthermore, the mTORC2 complex directly phosphorylates AKT/PKB on S473 in vitro and facilitates T308 phosphorylation by PDK1 (27). Sin1 is also required for TORC2 kinase activity in vitro. Sin1 and rictor are key components of mTORC2 and play an essential role in AKT phosphorylation. Although rapamycin is considered a selective inhibitor of mTORC1, there are data to suggest that persistent inhibition by rapamycin can lead to redistribution of mTOR from the mTORC2 complex into the mTORC1 complex, leading to decreased phosphorylation of AKT at S473. While the biological effect of hypophosphorylation of AKT at this residue is not well understood (1, 10, 11), it has been suggested that it may alter signaling to substrates such as FoxO1. Thus, it is possible that rapamycin exerts its inhibitory effect on myogenesis by modulating mTORC2 activity (rather than mTORC1), hence identifying mTORC2 as having an essential function in terminal myogenic differentiation.
MATERIALS AND METHODS
Cell line and cultures.
Mouse C2C12 myoblasts (American Type Culture Collection, Manassas, VA) were routinely grown in antibiotic-free Dulbecco's modified Eagle's medium with 15% fetal calf serum (growth medium [GM]). Cells were induced to differentiate by growth in differentiation medium (DM; Dulbecco's modified Eagle's medium with 2% horse serum supplemented with 4 mM l-glutamine) at 37°C and 5% CO2 (29).
Antibodies and reagents.
Phospho-specific antibodies to AKT(S473), S6K1(T389), S6(S235/236), 4EBP1(T37/46), and mTOR(S2448) and antibodies to AKT, S6K1, S6, Sin1, Myc tag, and ROCK1(C8F7), as well as horseradish peroxidase-labeled anti-mouse and anti-rabbit secondary antibodies, were from Cell Signaling Technology (Beverly, MA). Antibodies to rictor and raptor were from Bethyl Laboratories, Inc. (Montgomery, TX). Mouse monoclonal antibody MF20 to myosin heavy chain (MyHC; immunofluorescence studies), developed by Donald A. Fischman, was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biological Sciences, University of Iowa, Iowa City. Mouse monoclonal anti-MyHC antibody (clone A4.1025; Millipore, Temecula, CA) was used for immunoblotting. Mouse monoclonal antibodies to MyoD (5.8A) and myogenin were obtained from BD Biosciences (San Jose, CA). Antibody to β-tubulin was from Sigma Chemical Co. (St. Louis, MO). Secondary anti-mouse and anti-rabbit antibodies conjugated to Alexa Fluor 488 for immunofluorescence staining were from Molecular Probes (Eugene, OR). Purified normal rabbit or mouse immunoglobulin G (IgG) was from Upstate (Lake Placid, NY). Protein A/G plus-agarose was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The transfection reagent TransIT-LT1 was from Mirus (Madison, WI), and Lipofectamine 2000 was from Invitrogen (Carlsbad, CA). The QuikChange site-directed mutagenesis kit was from Stratagene (La Jolla, CA). The wild-type AKT cDNA clone was from Upstate (Lake Placid, NY).
Subcellular fractionation.
C2C12 cells were cultured in GM or DM in the presence or absence of rapamycin or lacking four amino acids (leucine, lysine, glutamine, and methionine) for 3 days. Subcellular fractionation was done as previously reported (14, 37).
Western blotting and immunoprecipitation.
Cell lysates were prepared as described in reference 17. Electrophoresis (7.5 or 12% Tris-HCl gels) and transfer to Immobilon-P membranes were done as described previously (29). For immunoprecipitation experiments, the procedures previously reported were followed (14).
Immunofluorescence staining and confocal microscopy.
C2C12 cells were seeded on two-chamber slides (Nunc Inc., Naperville, IL) in GM overnight. After plasmid transfection or lentivirus infection for 24 h, the medium was replaced with DM without drug or with rapamycin (100 ng/ml), Y27632 (20 μM), or both on the next day. Cells were incubated for 3 or 7 days. To prevent against potential degradation of the drug, fresh rapamycin or Y27632 was added to the culture on day 3. Immunofluorescence procedures were as described previously, and slides were examined in a Leica TCS NT SP confocal laser scanning microscope (37).
Inhibition of C2C12 cells by the AKT inhibitor API-2.
C2C12 cells were seeded into six-well plates in GM. The next day, the medium was replaced with DM with 0.1% dimethyl sulfoxide as a control or with API-2 (1 or 10 μM). After 3 days, cells were observed by microscopy and photographed.
Packaging of lentivirus with rictor short hairpin RNA (shRNA) or control shRNA.
Rictor shRNA plasmids E5 (CCGGGCCAGTAAGATGGGAATCATTCTCGAGAATGATTCCCATCTTACTGGCTTTTTG) and E6 (CCGGGCCATCTGAATAACTTCACTACTCGAGTAGTGAAGTTATTCAGATGGCTTTTTG) were purchased from Open Biosystems (Huntsville, AL). The helper vector pCMV-dR8.2dvpr and pCMV-VSVG and control shRNA (CCTAAGGTTA AGTCGCCCTCGCTCTAGCGAGGGCGACTTAACCTT AGG) plasmids were from Addgene Inc. (Cambridge, MA). 293T cells were from GenHunter (Nashville, TN), and the QuickTiter lentivirus quantitation kit (human immunodeficiency virus [HIV] p24 enzyme-linked immunosorbent assay [ELISA)] was from Cell Biolabs, Inc. (San Diego, CA). 293T cells (3 × 106) were seeded onto a 10-cm tissue culture plate in GM (DMEM plus 10% heat-inactivated fetal bovine serum and 0.1× penicillin-streptomycin) and incubated for 24 h (37°C, 5% CO2). On the second day, when the cells were 70% confluent, the rictor shRNA or control shRNA plasmid, together with helper vectors pCMV-dR8.2dvpr and pCMV-VSV-G, was cotransfected into 293T cells with TransIT-LT1 reagent. The cells were incubated for 18 h. The medium was changed to 6 ml of high-serum GM (DMEM plus 30% heat-inactivated fetal bovine serum and 1× penicillin-streptomycin). After 24 h of incubation, medium containing lentivirus was harvested by filtering through a 0.4-μm filter. The viral titer was determined by an ELISA method with the QuickTiter lentivirus quantitation kit (HIV p24 ELISA) according to the manufacturer's instructions.
Lentivirus transduction.
C2C12 cells, 0.3 × 106 per well, were seeded into six-well plates. On the second day, the cells were infected with an aliquot of lentivirus to achieve a multiplicity of infection of 250 PFU/cell. Twenty-four hours later, the cells were washed once with serum-free medium and placed in DM. The cell lysates were harvested at appropriate time points for Western blot analysis.
Raptor knockdown with a raptor siRNA pool.
A raptor small interfering RNA (siRNA) pool was purchased from Dharmacon. Transfection was done as recommended by the company.
Construction of the AKT(S473D) phosphomimetic mutant.
A point mutation in AKT1 (Ser473) converting Ser to Asp was performed with the QuikChange site-directed mutagenesis kit (Stratagene, Cedar Creek, TX) by following the manufacturer's instructions.
ROCK1 kinase assay.
C2C12 cells (2.5 × 106) were seeded in 100-mm dishes with GM and incubated at 37°C overnight. After 24 h, the cells were transfected with pUSE AKT(S473D) with LT1 reagent or left untransfected. One day later, the cells were washed once with serum-free DMEM and placed in DM in the presence or absence of rapamycin (100 ng/ml). Whole-cell lysates were collected with M-PER lysate buffer (Thermo Scientific, Rockford, IL). Cell lysates with 200 μg of total proteins were precleared with protein A/G plus-agarose (Santa Cruz, CA). ROCK1 proteins were immunoprecipitated with goat anti-ROCK1 antibody (2 μg/tube; Santa Cruz) or normal goat IgG (2 μg/tube) as a negative control at 4°C overnight with rotation. The immunoprecipitates were washed twice with lysis buffer and three times with kinase buffer (25 mM Tris-HCl [pH 7.5], 5 mM β-glycerophosphate, 2 mM dithiothreitol, 0.1 mM Na3VO4, 10 mM MgCl2) from Cell Signaling. The kinase assay was performed as described by Nishiyama et al. (20). A ROCK2 kinase assay was performed by the same methods as for ROCK1. The peptide substrate used was KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK.
RESULTS
Rapamycin inhibits C2C12 differentiation.
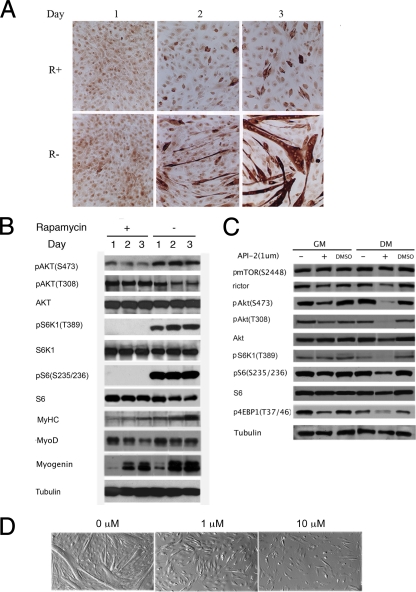
We and others (8, 9, 29) have shown previously that rapamycin potently inhibits the myogenic differentiation of C2C12 myoblasts. However, the mechanism by which rapamycin inhibits differentiation is poorly understood. As shown in Fig. 1A, rapamycin inhibited myotube formation. In the absence of rapamycin, C2C12 cells induced MyHC and became elongated by day 2 in DM. By day 3, there were numerous fused multinucleated MyHC-positive cells. In contrast, rapamycin-treated cells remained mononuclear, with very few multinucleated myotubes, although some cells stained faintly positive for MyHC. The expression of MyHC and myogenin increased in cells maintained for 2 to 3 days in DM, whereas in comparison, rapamycin decreased the induction of both MyHC and myogenin and reduced the expression of MyoD (Fig. 1B). However, the most predominant effect of rapamycin was to suppress myotube formation. As anticipated, rapamycin completely suppressed mTORC1 signaling, as shown by the absence of detectable phospho-S6K1(T389) [pS6K1(T389)] and pS6(S235/236). Of note, however, was that in C2C12 myoblasts rapamycin induced a decrease in pAKT(S473) as reported previously (26), in contrast to its effects on malignant muscle cells or other malignant cells, where rapamycin treatment enhances phosphorylation at this residue (18, 21, 28).
FIG. 1.
Rapamycin and API-2 inhibit C2C12 differentiation and decrease pAKT(S473). (A) C2C12 cells were grown in DM in the presence (R+) or absence (R−) of rapamycin (10 ng/ml) for 3 days. MyHC was detected by immunostaining with anti-MyHC antibody. (B) Cells were grown as described for panel A but with or without rapamycin at 100 ng/ml. Lysates were prepared on days 1 to 3. Equal amounts of total proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Immobilon-P membrane, and proteins were determined by immunoblotting. (C) Lysates of C2C12 cells maintained for 3 days in GM or DM in the presence (+) or absence (−) of API-2 (1 μM) or a vehicle control (0.1% dimethyl sulfoxide [DMSO]) were used to determine the phosphorylation status of proteins in the AKT-mTORC pathway. β-Tubulin was used as a loading control. (D) Morphology of C2C12 cells after 3 days in DM without or with API-2 at the concentration indicated.
The AKT inhibitor API-2 inhibits C2C12 cell differentiation.
The role of AKT signaling in myogenic differentiation has been previously reported (34, 35). Since the mTOR-rictor complex has AKT kinase activity (13, 27) and our results above suggested that mTORC2 signaling was decreased by rapamycin, as shown by decreased pAKT(S473), we explored the role of AKT in the regulation of C2C12 cell differentiation. To address this, C2C12 cells were cultured in GM for 1 day, the medium was replaced with DM, and the cells were incubated in the presence of the AKT inhibitor API-2 (1 or 10 μM) for 3 days. The results show that differentiation was completely inhibited by API-2 (Fig. 1C). The AKT(S473) phosphorylation status in GM and DM in the presence or absence of API-2 was determined by immunoblotting. The results show that pAKT(S473) was decreased by API-2, most markedly under conditions of differentiation (Fig. 1D). API-2 also decreased mTORC1 signaling, as shown by partial suppression of phosphorylation of the 4E-BP1 and S6 proteins. Thus, although these results are consistent with a role for AKT in myogenic differentiation (12), it is not possible to distinguish the roles of mTORC2 and mTORC1 signaling in the regulation of myogenesis by using this inhibitor.
The mTORC2 complex is sensitive to rapamycin.
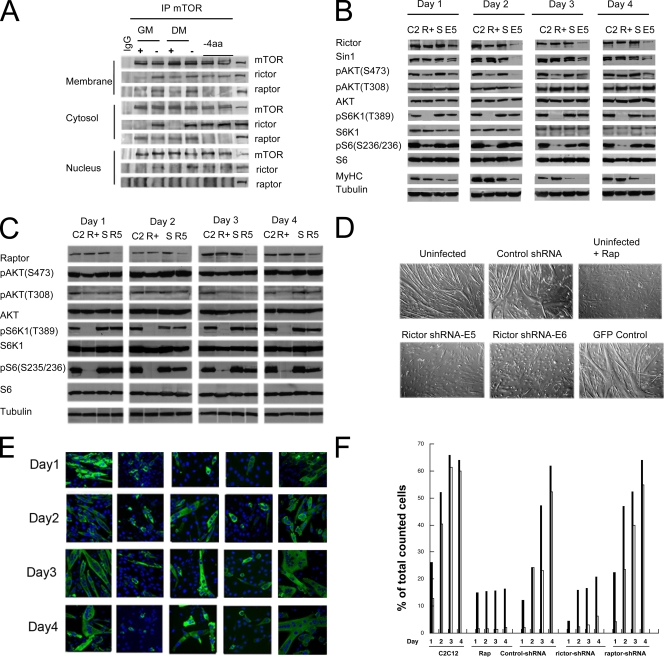
To explore the function of the rictor-mTOR complex in the regulation of C2C12 cell differentiation, myoblasts were grown under GM (proliferation) or DM (differentiation) conditions without or with rapamycin. Alternatively, myoblasts were grown in the absence of four amino acids (leucine, lysine, glutamine, and methionine) for 3 days. Immunoprecipitation with the extracts from membrane, cytosolic, and nuclear fractions of C2C12 cells was performed with an anti-mTOR antibody. The coimmunoprecipitated proteins were detected by anti-rictor and anti-raptor antibodies. As shown in Fig. 2A, mTOR coimmunoprecipitated with rictor in all of the fractions, whereas raptor coimmunoprecipitated only in the membrane and cytosolic fractions. Rapamycin decreased the coprecipitation of rictor from the membrane fractions under GM conditions only. In contrast, rapamycin completely blocked the association of rictor with mTOR in the cytosolic and nuclear fractions under conditions of proliferation (GM) or differentiation (DM). Rapamycin had relatively little effect on the association of raptor with mTOR, whereas amino acid depletion abolished the coprecipitation of raptor with mTOR in both the membrane and cytosolic fractions under either growth condition (GM or DM). These data point to the selective disruption of mTORC2 by rapamycin.
FIG. 2.
The rictor-mTOR complex is sensitive to rapamycin, and differentiation of C2C12 cells is inhibited by depletion of rictor but not raptor. (A) C2C12 myoblasts were maintained in GM or DM with or without rapamycin (100 ng/ml) or deprived of four amino acids (−4aa; Leu, Lys, Met, and Gln) for 3 days. Cells were fractionated as described in Materials and Methods, and proteins were immunoprecipitated (IP) from the membrane, cytosolic, and nuclear fractions with an anti-mTOR antibody. The coimmunoprecipitated proteins were detected by anti-rictor and anti-raptor antibodies. Normal rabbit IgG was used as a control. (B) C2C12 myoblasts were left uninfected (lanes C2) or infected with a lentivirus encoding a control shRNA (lanes S) or rictor shRNA (lanes E5). After 24 h, the medium was replaced with DM. C2C12 myoblasts without rapamycin (lanes C2) or with rapamycin (lanes R+; 100 ng/ml) or lentivirus-infected cells were harvested after incubation in DM for 1 to 4 days. Cell lysates were prepared, and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted as described in Materials and Methods. (C) C2C12 myoblasts were transfected with siRNA against raptor (R5, smart pool) or a control siRNA (lanes S) or mock transfected (lanes C2 and R+). After 24 h, the medium was replaced with DM. C2C12 myoblasts without rapamycin (lanes C2), with rapamycin (lanes R+), with control siRNA (lanes S), or with raptor siRNA (lanes R5) were harvested after incubation in DM for 1 to 4 days. Cell lysates were prepared, and proteins were separated by SDS-PAGE and immunoblotted as described in Materials and Methods. (D) C2C12 cells were infected with lentiviruses with rictor shRNA (E5 or E6, two different plasmids for packaging), control shRNA, or lentivirus encoding GFP for 24 h, the medium was replaced with DM, and the cells were incubated for 5 days. Cells were photographed, and representative microscope fields are shown. (E) Parental C2C12 cells or C2C12 cells infected with lentiviruses encoding rictor shRNA or control shRNA or transfected with raptor siRNA were incubated in DM in the absence (C2C12) or presence of rapamycin 100 ng/ml (Rap+) for 1 to 4 days. MyHC was detected by immunofluorescence. (F) The percentages of the total cells that were MyHC positive (black bars) and multinucleated (gray bars) were calculated by counting 20 random microscope fields for each condition.
Lentivirus-encoded rictor shRNA inhibits rictor protein expression.
To further examine the role of mTORC2 in the regulation of C2C12 differentiation, we constructed two lentiviruses encoding different rictor shRNAs (E5 and E6) to inhibit rictor expression. After transduction of lentivirus with rictor shRNA into C2C12 cells for 24 h, the cells were cultured in DM. The protein expression and phosphorylation of AKT and mTORC1 substrates over 4 days were detected by Western blotting. As shown in Fig. 2B, lanes E5, rictor was inhibited from day1 to day 4 and pAKT(S473) was also markedly decreased in the same time period, though the total AKT level remained constant. Of note also was the failure to induce MyHC in rictor-deficient cells, suggesting a role for mTORC2 earlier in myogenic differentiation. Importantly, pS6(S235/236) was not altered by lentivirus expressing rictor shRNA, though it was inhibited by rapamycin from day1 to day 4 (Fig. 2B, lanes E5 and R). Of note was that Sin1, an interacting protein in the mTORC2 complex, also decreased when rictor was downregulated (Fig. 2B, lanes E5). Thus, consistent with previous results (11), rictor disruption appears to selectively alter mTORC2 signaling without altering mTORC1 function, thus allowing the function of mTORC2 in myoblast differentiation to be assessed.
Raptor siRNA inhibits raptor protein expression.
Under growth conditions similar to those described above, raptor siRNA was used to downregulate raptor (Fig. 2C). While the raptor level was significantly decreased (Fig. 2C, lanes R5), surprisingly, there was only a relatively small decrease in both pS6K1 and pS6, (∼50%), as determined by immunoblotting. Similar results were obtained in three independent experiments. In contrast, rapamycin completely abrogated mTORC1 downstream signaling.
Differentiation of C2C12 cells is inhibited by depletion of rictor.
To determine whether differentiation of C2C12 cells could be inhibited by depletion of rictor, C2C12 cells were infected with lentivirus encoding rictor shRNA, control shRNA, or green fluorescent protein (GFP) and cultured in DM for 3 days. Both GFP-expressing C2C12 cells and those infected with control shRNA differentiated normally compared to uninfected parental C2C12 cells after 3 days in DM. Cells infected with viruses encoding rictor shRNA (E5 and E6) and cells treated with rapamycin failed to form multinucleated myotubes (Fig. 2D). To further evaluate the effect of rictor deficiency, C2C12 cells were seeded into two-chamber microscope slides and infected as described in Materials and Methods. Immunofluorescence microscopy showed that parental C2C12 cells fused to become multinucleated after culture in DM from day 1 (Fig. 2E), whereas differentiation of C2C12 cells in the presence of rapamycin or after infection with lentivirus expressing rictor shRNA was inhibited compared to that of cells infected with lentivirus encoding control shRNA (Fig. 2E). In contrast, C2C12 cells transfected with raptor siRNA differentiated normally to form MyHC-positive myotubes (Fig. 2E). To quantify alterations in MyHC-positive and multinucleated cells, we randomly picked 20 microscope fields to count the total cells, MyHC-positive cells, and multinucleated cells. As shown in Fig. 2F, the effect of rictor downregulation in reducing the proportion of MyHC-positive and multinucleated cells was essentially identical to that of rapamycin treatment. In contrast, the lentivirus-delivered and control shRNAs or transfection with raptor siRNA had relatively little effect on differentiation compared to that of control C2C12 cells.
Phosphomimetic AKT(S473D) rescues differentiation in C2C12 cells when rictor is downregulated.
To determine whether phosphorylation of AKT(S473) is essential for C2C12 myogenesis, mutant phosphomimetic AKT(S473D) was constructed. C2C12 cells were transiently transfected with Myc-tagged AKT(S473D) or vector control pUSEamp(+), and protein expression was confirmed by Western blotting with anti-Myc tag antibody (Fig. 3A). The cells were then infected with lentivirus expressing rictor shRNA for 24 h, the medium was replaced with DM, and the cells were incubated for up to 4 days. C2C12 cells transfected with the control vector and infected with lentivirus expressing rictor shRNA failed to differentiate over 4 days. In contrast, C2C12 cells expressing AKT(S473D) differentiated normally when infected with the virus expressing rictor shRNA (Fig. 3B). In parallel experiments, the proportion of MyHC-positive cells and multinucleated myotubes was determined on days 1 to 4 (Fig. 3C). As quantitated in Fig. 3D, downregulation of rictor suppressed differentiation in C2C12 cells transfected with the control vector, whereas cells expressing mutant AKT(S473D) formed MyHC-positive multinucleated myotubes from day 2. These data support the notion that mTORC2 plays a pivotal role in myogenic differentiation in C2C12 myoblasts.
FIG. 3.
Phosphomimetic mutant AKT(S473D) maintains C2C12 differentiation when rictor is downregulated. (A) C2C12 cells were transiently transfected with a vector control or one of 11 plasmids encoding constitutively active AKT(S473D), respectively, for 24 h, and protein expression was determined by immunoblotting with anti-Myc tag antibody. (B) Cells were then infected with lentiviruses encoding rictor shRNA or control shRNA for 24 h and then incubated in DM for 1 to 4 days. Representative microscopic fields from days 2 and 4 are presented. (C) MyHC was detected by immunofluorescence staining. (D) Quantitation of MyHC-positive (black bars) and multinucleated (open bars) cells was performed as described for Fig. 2F.
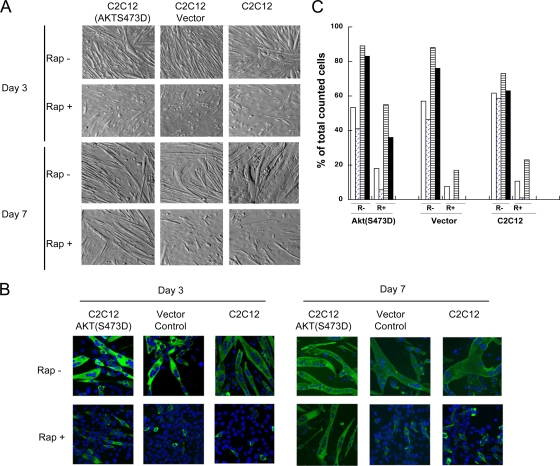
Expression of AKT(S473D) overcomes rapamycin-induced block on myogenic differentiation.
The results above suggest that depletion of rictor suppressed phosphorylation of AKT(S473) without suppressing signaling through mTORC1. Also, expressing phosphomimetic AKT(S473D) restored myogenic differentiation even when rictor was downregulated. To test whether expression of AKT(S473D) could overcome suppression of myogenic differentiation caused by rapamycin, C2C12 cells were transiently transfected with the vector or a plasmid encoding AKT(S473D). After 24 h, the cells were shifted from GM to DM with or without rapamycin (100 ng/ml) and differentiation was examined at days 3 and 7. Rapamycin significantly inhibited myogenic differentiation in parental and vector-transfected C2C12 cells at either time point. In contrast, although at day 3 there was little evidence of myotube formation, by day 7 cells expressing AKT(S473D) had differentiated in the presence of rapamycin (Fig. 4A and B), forming MyHC-positive multinucleated myotubes. The percentage of MyHC-positive and multinucleated myotubes in the cells transfected by AKT(S473D) and treated with rapamycin was significantly higher than that in vector control and parental cells on day 7 (Fig. 4C).
FIG. 4.
Phosphomimetic mutant AKT(S473D) overrides rapamycin-induced inhibition of myotube formation. (A) C2C12 cells were transfected with a plasmid encoding AKT(S473D) or with a control vector or left untransfected (C2C12). After 24 h, GM was replaced with DM with rapamycin (100 ng/ml; Rap+) or without rapamycin (Rap−) and the cells were incubated for 3 or 7 days. Representative microscopic fields are presented. (B) MyHC was detected by immunofluorescence staining as described for Fig. 2E. (C) Quantitation of MyHC-stained cells and multinucleated cells in the absence (R−) or presence (R+) of rapamycin was performed as described for Fig. 2F. Day 3 MyHC, open bars; day 3 multinucleated cells, diagonal hatching; day 7 MyHC, horizontal hatching; day 7 multinucleated cells, black bars.
Inhibition of the Rho-associated kinase ROCK1 restores myogenic differentiation in mTORC2-deficient or rapamycin-treated C2C12 myoblasts.
To determine whether ROCK1 kinase activity is involved in signaling downstream of AKT (16), we asked whether the ROCK kinase inhibitor Y27632 can overcome mTORC2 deficiency. C2C12 myoblasts were infected with lentivirus encoding control or rictor shRNA or mock infected. After 24 h, GM was replaced with DM containing Y27632 (20 μM) and the cells were incubated for an additional 3 to 7 days. Downregulation of rictor blocked myotube formation at day 3, with cells retaining myoblast morphology. In contrast, by day 3 in the presence of Y27632, cells infected with rictor shRNA virus had become spindle shaped, with some multinucleated cells, and by day 7 there was precocious myotube formation only in the presence of Y27632, where multinucleated myotube formation was similar to that in mock infected C2C12 cells (Fig. 5A). In contrast, there were relatively few multinucleated myotubes in C2C12 cells in which rictor had been downregulated in the absence of Y27632 treatment. The data suggest that mTORC2 signals to induce differentiation through negative regulation of ROCK1.
FIG. 5.
Inhibition of the Rho-associated kinase ROCK1 restores myogenic differentiation in cells infected with rictor shRNA or treated with rapamycin. (A) C2C12 cells were infected with lentiviruses encoding rictor shRNA or control shRNA or left uninfected (C2C12) and grown for 24 h. The medium was replaced with DM with (Y+; 20 μM) or without (Y−) the ROCK inhibitor Y27632. Cells were photographed after 3 or 7 days. Representative microscopic fields are shown. (B) C2C12 cells were shifted to DM without (control) or with rapamycin (Rap, 100 ng/ml), Y27632 (20 μM), or both agents. Photomicrographs show representative fields after 3 and 7 days. (C) MyHC was detected by immunofluorescence staining. (D) Quantitation of MyHC-positive and multinucleated cells was performed as described for Fig. 2F. Day 3 MyHC, open bars; day 3 multinucleated cells, diagonal hatching; day 7 MyHC, horizontal hatching; day 7 multinucleated cells, black bars. (E) ROCK1 activity was determined as described in Materials and Methods. Control C2C12 myoblasts (○, □) or cells transfected with AKT(S473D) (▴, ×) were shifted to DM with rapamycin (100 ng/ml; broken lines) or without rapamycin (solid lines), and ROCK1 activity was determined over 10 days. The results shown are representative of two experiments.
Results showing that downregulation of rictor suppresses mTORC2 signaling without altering mTORC1 signaling (Fig. 2B) and the results obtained with Y27632 imply that mTORC2, rather than mTORC1, plays a role in myoblast fusion. It was thus of interest to determine whether Y27632 could induce myoblast fusion in the presence of rapamycin. C2C12 cells were cultured in GM for 1 day, and the medium was replaced with DM in the presence of rapamycin, Y27632, or both agents. Differentiation was inhibited in the presence of rapamycin (Fig. 5B). However, in the presence of Y27632 or both rapamycin and Y27632, cells continued to form multinucleated myotubes, similar to parental control C2C12 cells (Fig. 5C). MyHC-positive and multinucleated myotubes were scored for cultures treated with Y27632 or rapamycin or with both agents and compared to parental C2C12 cells (Fig. 5D).
These results suggest that downregulation of ROCK1 activity, which is a requirement for myogenic differentiation, is prevented by rapamycin treatment through its effects on mTORC2 signaling, whereas the expression of mutant AKT(S473D) would induce downregulation of ROCK1 activity even in the presence of rapamycin. To test this, parental C2C12 cells or C2C12 cells transfected with a plasmid encoding AKT(S473D) were transferred to DM with or without rapamycin. ROCK1 kinase activity was detected from 2 h to 10 days. As shown in Fig. 5E, transferring C2C12 cells to DM induced a rapid and sustained decrease in ROCK1 activity that was prevented by rapamycin. In contrast, in cells expressing mutant AKT(S473D), there was an immediate drop in ROCK1 activity upon transfer to DM and then a sustained progressive reduction in activity that was relatively refractory to rapamycin treatment. The delay in decreased ROCK1 activity paralleled the delay in myogenic differentiation observed in C2C12 myoblasts that were transfected with the AKT(S473D) plasmid (Fig. 4A). In contrast to the results obtained with ROCK1, the activity of ROCK2 did not change during myoblast differentiation and ROCK2 activity was not inhibited by rapamycin treatment under GM or DM growth conditions (data not shown).
DISCUSSION
The observations that rapamycin potently inhibits myogenic differentiation and that expression of rapamycin-resistant mTOR(S2035I) allowed differentiation in the presence of rapamycin (29) implied that signaling through mTORC1 was critical in myogenesis, as the rapamycin-FKBP12 complex is considered to selectively target signaling by the mTORC1 complex. However, prolonged inhibition of mTORC1 by rapamycin may cause redistribution of mTOR from the mTORC2 complex, leading to decreased phosphorylation of AKT(S473). We found previously that expression of constitutively active, rapamycin-insensitive S6K1 or downregulation of 4E-BP1 failed to support C2C12 differentiation in the presence of rapamycin (data not shown). The present data also tend to refute the role of mTORC1 in myoblast differentiation. In C2C12 myoblasts, we observed that rapamycin induced a significant and prolonged decrease in pAKT(S473), suggesting, perhaps, destabilization of the mTORC2 complex and decreased signaling to AKT. Consistent with the idea that AKT signaling is essential for myoblast differentiation (34, 35), the AKT inhibitor API-2 blocked C2C12 differentiation. Rapamycin also appeared to destabilize the mTORC2 complex by inhibiting the interaction of mTOR and rictor in cytosolic and nuclear complexes. To test the role of mTORC2 in myogenesis, we used shRNA approaches to downregulate rictor. Notably, downregulation of rictor abrogated the phosphorylation of AKT(S473) while having no significant effect on mTORC1 signaling. Similar to the effect of rapamycin, the downregulation of rictor completely prevented C2C12 myotube formation, decreased the frequency of MyHC-positive cells, and blocked induction of MyHC under DM conditions. Thus, abrogating rictor function induces a phenotype similar to that induced by rapamycin treatment in that both block MyHC induction. Abrogating rictor decreases AKT(S473) phosphorylation to a greater extent than does rapamycin. In contrast to the effect of downregulating rictor, the siRNA used to silence raptor did not block myotube formation. However, marked suppression of raptor did not completely abrogate signaling, as phosphorylation of both S6K1 and S6 was still detected.
The mTORC2 complex phosphorylates AKT(S473) but potentially regulates other pathways, such as protein kinase Cα and the actin cytoskeleton, which may also play roles in myoblast fusion. To test the significance of AKT(S473) phosphorylation, we expressed phosphomimetic mutant AKT(S473D). Expression of this mutant protein abrogated the inhibitory activity of downregulating rictor in C2C12 myoblasts, allowing myogenic differentiation. These results argue that the target of mTORC2 critical for terminal myogenesis is AKT. Expression of AKT(S473D) also overcame the block on differentiation in the presence of rapamycin, although the process of myotube formation was somewhat delayed in the presence of rapamycin. Regulation of AKT activity is complex. Phosphorylation of T308 by PDK1 increases activity by ∼100-fold, whereas phosphorylation of S473 increases activity a further 10-fold. Our data showing that downregulation of rictor did not inhibit mTORC1 signaling suggest that S473 phosphorylation is not required for AKT to inactivate the TSC complex or phosphorylate PRAS40. In contrast, phosphorylation of S473 appears critical for AKT signaling to other pathways involved in myogenic differentiation.
The FoxO1a transcription factor is required for myoblast fusion in primary myoblasts (3), and dominant negative mutant FoxO1a reduces fusion without changing the expression of the myogenic gene for MyoD or myogenin. In HEK293 cells, AKT phosphorylates FoxO1a (2, 4, 30), leading to its cytoplasmic localization; however, several reports suggest that AKT is not the kinase responsible for modulating FoxO1a in myogenic cells (3, 20). Rather, the Rho-associated kinase ROCK1 has been implicated in FoxO1a posttranslational modification (20). However, the role of ROCK1 in myogenesis is controversial. RhoA signaling upstream of ROCK kinases has been reported to be a positive regulator of myogenic differentiation, as inactivation of RhoA decreases the expression of MyoD and decreases differentiation (6). RhoA or ROCK1 can inhibit myogenesis by preventing cell fusion and myotube formation, whereas inhibition of RhoA/ROCK signaling in differentiating myocytes promoted differentiation (7, 20). These differences have, in part, been resolved recently (15). Inhibition of the RhoA/ROCK signaling pathway in proliferating myoblasts downregulated serum response factor activity and MyoD expression, resulting in decreased myogenic potential. However, inhibition of RhoA/ROCK signaling in differentiating myocytes promoted differentiation (15), consistent with previous studies (7, 20). ROCK2 is upregulated during skeletal muscle myogenesis, whereas ROCK1 activity is downregulated during C2C12 differentiation (20, 24). We determined whether blocking ROCK activity could restore myogenic differentiation in the presence of mTORC2 inactivation. The ROCK inhibitor Y26732 induced precocious myotube formation in cells with downregulated rictor and overcame the effect of rapamycin, allowing full differentiation. Transfer of C2C12 myoblasts to DM induced a rapid and progressive decrease in ROCK1 activity, as previously reported (20). In contrast, ROCK2 activity did not change during myoblast differentiation and was not altered by rapamycin treatment. Importantly, this decrease in ROCK1 activity was completely prevented by rapamycin treatment. In cells transfected with mutant AKT(S473D), this decrease in ROCK1 activity was progressive although somewhat delayed, which correlated with the delay in differentiation. However, the downregulation of ROCK1 activity in cells expressing AKT(S473D) was quite refractory to treatment with rapamycin.
mTOR catalytic activity has been shown to be essential for fusion, which results in mature myotubes (18, 23). Taken together, our results strongly imply a role for mTOR in the mTORC2 complex, rather than in the rapamycin-sensitive mTORC1 complex, in the terminal differentiation of C2C12 myoblasts to form myotubes. Full activation of AKT leads to downregulation of ROCK1 kinase activity and potentially activation of FoxO1a and downregulation of the myogenic inhibitor Id3 (15). That expression of phosphomimetic AKT(S473D) supports differentiation in the absence of mTORC2 activity suggests that AKT is the critical downstream target in this process. Expression of AKT(S473D) or use of Y26732 inhibits ROCK1 activity, overcomes rapamyin-induced inhibition of myogenic differentiation, and suggests that it is the effect of rapamycin on the mTORC2 complex, rather than its primary target, mTORC1, that disrupts the ability of C2C12 myoblasts to form myotubes.
Acknowledgments
This study was supported by U.S. Public Health Service awards CA77776, CA96996, and CA21675 (Cancer Center Support Grant) and by American, Lebanese, Syrian Associated Charities.
Footnotes
Published ahead of print on 29 June 2009.
REFERENCES
- 1.Bhaskar, P. T., and N. Hay. 2007. The two TORCs and Akt. Dev. Cell 12487-502. [DOI] [PubMed] [Google Scholar]
- 2.Biggs, W. H., III, J. Meisenhelder, T. Hunter, W. K. Cavenee, and K. C. Arden. 1999. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA 967421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bois, P. R., and G. C. Grosveld. 2003. FKHR (FOXO1a) is required for myotube fusion of primary mouse myoblasts. EMBO J. 221147-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96857-868. [DOI] [PubMed] [Google Scholar]
- 5.Buckingham, M., L. Bajard, T. Chang, P. Daubas, J. Hadchouel, S. Meilhac, D. Montarras, D. Rocancourt, and F. Relaix. 2003. The formation of skeletal muscle: from somite to limb. J. Anat. 20259-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carnac, G., M. Primig, M. Kitzmann, P. Chafey, D. Tuil, N. Lamb, and A. Fernandez. 1998. RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol. Biol. Cell 91891-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castellani, L., E. Salvati, S. Alema, and G. Falcone. 2006. Fine regulation of RhoA and Rock is required for skeletal muscle differentiation. J. Biol. Chem. 28115249-15257. [DOI] [PubMed] [Google Scholar]
- 8.Coolican, S. A., D. S. Samuel, D. Z. Ewton, F. J. McWade, and J. R. Florini. 1997. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J. Biol. Chem. 2726653-6662. [DOI] [PubMed] [Google Scholar]
- 9.Erbay, E., and J. Chen. 2001. The mammalian target of rapamycin regulates C2C12 myogenesis via a kinase-independent mechanism. J. Biol. Chem. 27636079-36082. [DOI] [PubMed] [Google Scholar]
- 10.Guertin, D. A., and D. M. Sabatini. 2007. Defining the role of mTOR in cancer. Cancer Cell 129-22. [DOI] [PubMed] [Google Scholar]
- 11.Guertin, D. A., D. M. Stevens, C. C. Thoreen, A. A. Burds, N. Y. Kalaany, J. Moffat, M. Brown, K. J. Fitzgerald, and D. M. Sabatini. 2006. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev. Cell 11859-871. [DOI] [PubMed] [Google Scholar]
- 12.Héron-Milhavet, L., D. Mamaeva, A. Rochat, N. J. Lamb, and A. Fernandez. 2008. Akt2 is implicated in skeletal muscle differentiation and specifically binds Prohibitin2/REA. J. Cell. Physiol. 214158-165. [DOI] [PubMed] [Google Scholar]
- 13.Hresko, R. C., and M. Mueckler. 2005. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J. Biol. Chem. 28040406-40416. [DOI] [PubMed] [Google Scholar]
- 14.Huang, S., L. Shu, M. B. Dilling, J. Easton, F. C. Harwood, H. Ichijo, and P. J. Houghton. 2003. Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21(Cip1). Mol. Cell 111491-1501. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki, K., K. Hayashi, T. Fujioka, and K. Sobue. 2008. Rho/Rho-associated kinase signal regulates myogenic differentiation via myocardin-related transcription factor-A/Smad-dependent transcription of the Id3 gene. J. Biol. Chem. 28321230-21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang, K., J. Sun, J. Cheng, J. Y. Djeu, S. Wei, and S. Sebti. 2004. Akt mediates Ras downregulation of RhoB, a suppressor of transformation, invasion, and metastasis. Mol. Cell. Biol. 245565-5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, D. H., and D. M. Sabatini. 2004. Raptor and mTOR: subunits of a nutrient-sensitive complex. Curr. Top. Microbiol. Immunol. 279259-270. [DOI] [PubMed] [Google Scholar]
- 18.Kurmasheva, R. T., F. C. Harwood, and P. J. Houghton. 2007. Differential regulation of vascular endothelial growth factor by Akt and mammalian target of rapamycin inhibitors in cell lines derived from childhood solid tumors. Mol. Cancer Ther. 61620-1628. [DOI] [PubMed] [Google Scholar]
- 19.McKinsey, T. A., C. L. Zhang, and E. N. Olson. 2002. Signaling chromatin to make muscle. Curr. Opin. Cell Biol. 14763-772. [DOI] [PubMed] [Google Scholar]
- 20.Nishiyama, T., I. Kii, and A. Kudo. 2004. Inactivation of Rho/ROCK signaling is crucial for the nuclear accumulation of FKHR and myoblast fusion. J. Biol. Chem. 27947311-47319. [DOI] [PubMed] [Google Scholar]
- 21.O'Reilly, K. E., F. Rojo, Q. B. She, D. Solit, G. B. Mills, D. Smith, H. Lane, F. Hofmann, D. J. Hicklin, D. L. Ludwig, J. Baselga, and N. Rosen. 2006. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 661500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park, I. H., and J. Chen. 2005. Mammalian target of rapamycin (mTOR) signaling is required for a late-stage fusion process during skeletal myotube maturation. J. Biol. Chem. 28032009-32017. [DOI] [PubMed] [Google Scholar]
- 23.Parker, M. H., P. Seale, and M. A. Rudnicki. 2003. Looking back to the embryo: defining transcriptional networks in adult myogenesis. Nat. Rev. Genet. 4497-507. [DOI] [PubMed] [Google Scholar]
- 24.Pelosi, M., F. Marampon, B. M. Zani, S. Prudente, E. Perlas, V. Caputo, L. Cianetti, V. Berno, S. Narumiya, S. W. Kang, A. Musaro, and N. Rosenthal. 2007. ROCK2 and its alternatively spliced isoform ROCK2m positively control the maturation of the myogenic program. Mol. Cell. Biol. 276163-6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarbassov, D. D., S. M. Ali, D. H. Kim, D. A. Guertin, R. R. Latek, H. Erdjument-Bromage, P. Tempst, and D. M. Sabatini. 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 141296-1302. [DOI] [PubMed] [Google Scholar]
- 26.Sarbassov, D. D., S. M. Ali, S. Sengupta, J. H. Sheen, P. P. Hsu, A. F. Bagley, A. L. Markhard, and D. M. Sabatini. 2006. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22159-168. [DOI] [PubMed] [Google Scholar]
- 27.Sarbassov, D. D., D. A. Guertin, S. M. Ali, and D. M. Sabatini. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 3071098-1101. [DOI] [PubMed] [Google Scholar]
- 28.Shi, Y., H. Yan, P. Frost, J. Gera, and A. Lichtenstein. 2005. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol. Cancer Ther. 41533-1540. [DOI] [PubMed] [Google Scholar]
- 29.Shu, L., X. Zhang, and P. J. Houghton. 2002. Myogenic differentiation is dependent on both the kinase function and the N-terminal sequence of mammalian target of rapamycin. J. Biol. Chem. 27716726-16732. [DOI] [PubMed] [Google Scholar]
- 30.Tang, E. D., G. Nunez, F. G. Barr, and K. L. Guan. 1999. Negative regulation of the forkhead transcription factor FKHR by Akt. J. Biol. Chem. 27416741-16746. [DOI] [PubMed] [Google Scholar]
- 31.Vander Haar, E., S. I. Lee, S. Bandhakavi, T. J. Griffin, and D. H. Kim. 2007. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 9316-323. [DOI] [PubMed] [Google Scholar]
- 32.Wang, L., T. E. Harris, R. A. Roth, and J. C. Lawrence, Jr. 2007. PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J. Biol. Chem. 28220036-20044. [DOI] [PubMed] [Google Scholar]
- 33.Weintraub, H. 1993. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell 751241-1244. [DOI] [PubMed] [Google Scholar]
- 34.Wilson, E. M., and P. Rotwein. 2007. Selective control of skeletal muscle differentiation by Akt1. J. Biol. Chem. 2825106-5110. [DOI] [PubMed] [Google Scholar]
- 35.Wilson, E. M., J. Tureckova, and P. Rotwein. 2004. Permissive roles of phosphatidyl inositol 3-kinase and Akt in skeletal myocyte maturation. Mol. Biol. Cell 15497-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wullschleger, S., R. Loewith, and M. N. Hall. 2006. TOR signaling in growth and metabolism. Cell 124471-484. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, X., L. Shu, H. Hosoi, K. G. Murti, and P. J. Houghton. 2002. Predominant nuclear localization of mammalian target of rapamycin in normal and malignant cells in culture. J. Biol. Chem. 27728127-28134. [DOI] [PubMed] [Google Scholar]