Abstract
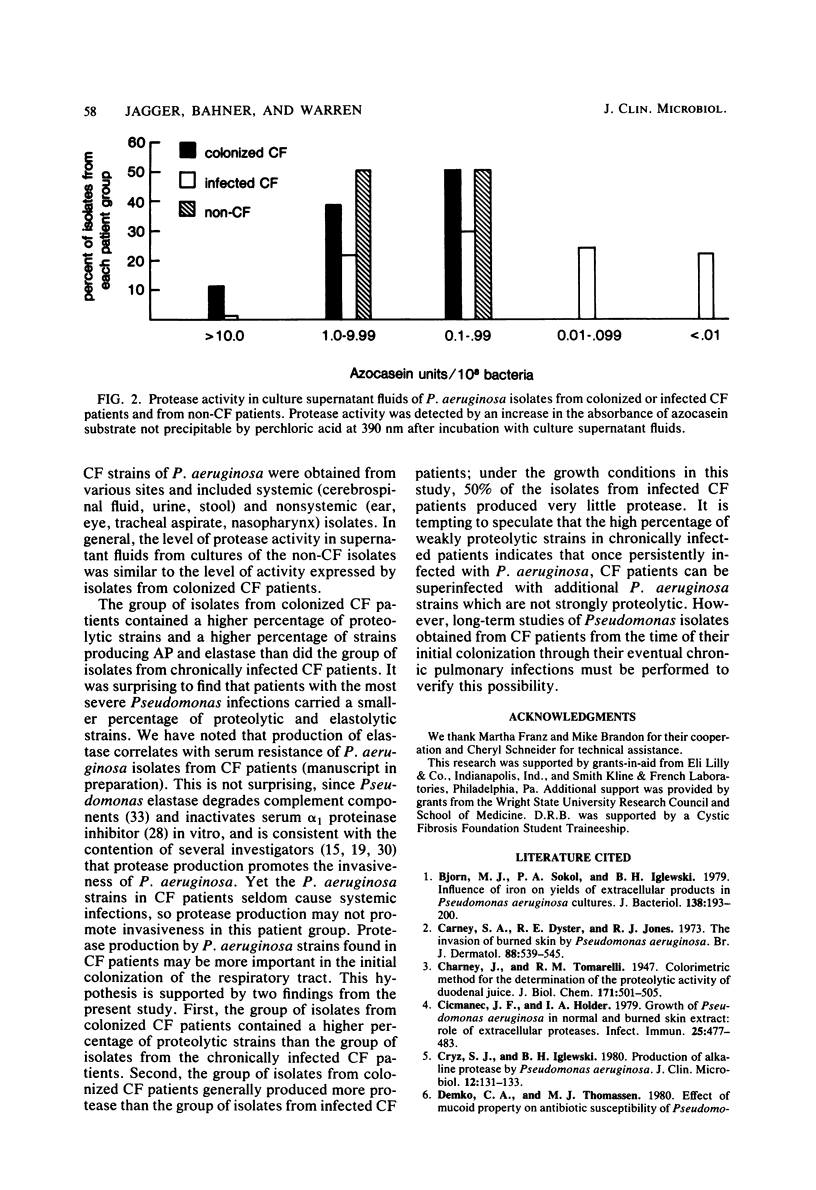
The protease phenotypes expressed by isolates of Pseudomonas aeruginosa from cystic fibrosis (CF) patients were evaluated. The majority of isolates tested produced elastase (65%) or alkaline protease (64%) or both. The mucoid phenotype expressed by many CF isolates of P. aeruginosa did not absolutely restrict the expression of protease activity, although a higher percentage of nonmucoid isolates was proteolytic. When isolates from CF patients chronically infected with P. aeruginosa were compared to isolates from CF patients colonized with this organism, both groups were found to contain comparable percentages of elastase-producing strains and mucoid strains. However, the group of isolates from colonized patients contained a higher percentage of strains producing alkaline protease and expressing general protease activity. In addition, the group of isolates from chronically infected patients contained more weakly proteolytic isolates than either the group from colonized CF patients or a group of isolates from pediatric patients without CF. These data suggest that protease production may be important in the initial colonization of the respiratory tract of CF patients by P. aeruginosa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorn M. J., Sokol P. A., Iglewski B. H. Influence of iron on yields of extracellular products in Pseudomonas aeruginosa cultures. J Bacteriol. 1979 Apr;138(1):193–200. doi: 10.1128/jb.138.1.193-200.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney S. A., Dyster R. E., Jones R. J. The invasion of burned skin by Pseudomonas aeruginosa. Br J Dermatol. 1973 Jun;88(6):539–545. doi: 10.1111/j.1365-2133.1973.tb08016.x. [DOI] [PubMed] [Google Scholar]
- Cicmanec J. F., Holder I. A. Growth of Pseudomonas aeruginosa in normal and burned skin extract: role of extracellular proteases. Infect Immun. 1979 Aug;25(2):477–483. doi: 10.1128/iai.25.2.477-483.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Iglewski B. H. Production of alkaline protease by Pseudomonas aeruginosa. J Clin Microbiol. 1980 Jul;12(1):131–133. doi: 10.1128/jcm.12.1.131-133.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz E., Mosovich L. L., Neter E. Serogroups of Pseudomonas aeruginosa and the immune response of patients with cystic fibrosis. J Infect Dis. 1970 Mar;121(3):269–274. doi: 10.1093/infdis/121.3.269. [DOI] [PubMed] [Google Scholar]
- Doggett R. G., Harrison G. M., Carter R. E. Mucoid Pseudomonas aeruginosa in patients with chronic illnesses. Lancet. 1971 Jan 30;1(7692):236–237. doi: 10.1016/s0140-6736(71)90973-1. [DOI] [PubMed] [Google Scholar]
- Gray L., Kreger A. Microscopic characterization of rabbit lung damage produced by Pseudomonas aeruginosa proteases. Infect Immun. 1979 Jan;23(1):150–159. doi: 10.1128/iai.23.1.150-159.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao Y., Homma J. Y. Therapeutic effect of immunization with OEP, protease toxoid and elastase toxoid on corneal ulcers in mice due to Pseudomonas aeruginosa infection. Jpn J Exp Med. 1978 Feb;48(1):41–51. [PubMed] [Google Scholar]
- Hirao Y., Homma J. Y., Zierdt C. H. Serotyping of Pseudomonas aeruginosa from patients with cystic fibrosis of the pancreas. Jpn J Exp Med. 1977 Aug;47(4):249–254. [PubMed] [Google Scholar]
- Holby N., Olling S. Pseudomonas aeruginosa infection in cystic fibrosis. Bactericidal effect of serum from normal individuals and patients with cystic fibrosis on P. aeruginosa strains from patients with cystic fibrosis or other diseases. Acta Pathol Microbiol Scand C. 1977 Apr;85(2):107–114. [PubMed] [Google Scholar]
- Holder I. A., Haidaris C. G. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: extracellular protease and elastase as in vivo virulence factors. Can J Microbiol. 1979 May;25(5):593–599. doi: 10.1139/m79-085. [DOI] [PubMed] [Google Scholar]
- Homma J. Y., Abe C., Tanamoto K., Hirao Y., Morihara K., Tsuzuki H., Yanagawa R., Honda E., Aoi Y., Fujimoto Y. Effectiveness of immunization with single and multi-component vaccines prepared from a common antigen (OEP), protease and elastase toxoids of Pseudomonas aeruginosa on protection against hemorrhagic pneumonia in mink due to P. aeruginosa. Jpn J Exp Med. 1978 Apr;48(2):111–133. [PubMed] [Google Scholar]
- Høiby N., Rosendal K. Epidemiology of Pseudomonas aeruginosa infection in patients treated at a cystic fibrosis centre. Acta Pathol Microbiol Scand B. 1980 Jun;88(3):125–131. doi: 10.1111/j.1699-0463.1980.tb02617.x. [DOI] [PubMed] [Google Scholar]
- Jagger K. S., Robinson D. L., Franz M. N., Warren R. L. Detection by enzyme-linked immunosorbent assays of antibody specific for Pseudomonas proteases and exotoxin A in sera from cystic fibrosis patients. J Clin Microbiol. 1982 Jun;15(6):1054–1058. doi: 10.1128/jcm.15.6.1054-1058.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda J. M., Atang-Nomo S., Bottone E. J., Desmond E. P. Correlation of proteolytic activity of Pseudomonas aeruginosa with site of isolation. J Clin Microbiol. 1980 Oct;12(4):626–628. doi: 10.1128/jcm.12.4.626-628.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda J. M., Bottone E. J. Pseudomonas aeruginosa enzyme profiling: predictor of potential invasiveness and use as an epidemiological tool. J Clin Microbiol. 1981 Jul;14(1):55–60. doi: 10.1128/jcm.14.1.55-60.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. E., Phillippe L., Teng Tseng J., Stemke G. W., Campbell J. N. Purification and characterization of exocellular proteases produced by a clinical isolate and a laboratory strain of Pseudomonas aeruginosa. Can J Microbiol. 1980 Jan;26(1):77–86. doi: 10.1139/m80-012. [DOI] [PubMed] [Google Scholar]
- Kawaharajo K., Abe C., Homma J. Y., Kawano M., Goto E. Corneal ulcers caused by protease and elastase from Pseudomonas aeruginosa. Jpn J Exp Med. 1974 Oct;44(5):435–442. [PubMed] [Google Scholar]
- Kessler E., Kennah H. E., Brown S. I. Pseudomonas protease. Purification, partial characterization, and its effect on collagen, proteoglycan, and rabbit corneas. Invest Ophthalmol Vis Sci. 1977 Jun;16(6):488–497. [PubMed] [Google Scholar]
- Klinger J. D., Straus D. C., Hilton C. B., Bass J. A. Antibodies to proteases and exotoxin A of Pseudomonas aeruginosa in patients with cystic fibrosis: Demonstration by radioimmunoassay. J Infect Dis. 1978 Jul;138(1):49–48. doi: 10.1093/infdis/138.1.49. [DOI] [PubMed] [Google Scholar]
- MORIHARA K., TSUZUKI H., OKA T., INOUE H., EBATA M. PSEUDOMONAS AERUGINOSA ELASTASE. ISOLATION, CRYSTALLIZATION, AND PRELIMINARY CHARACTERIZATION. J Biol Chem. 1965 Aug;240:3295–3304. [PubMed] [Google Scholar]
- Markowitz S. M., Macrina F. L., Phibbs P. V., Jr Antimicrobial susceptibility of mucoid Pseudomonas aeruginosa and their spontaneously occurring non-mucoid derivatives. J Antimicrob Chemother. 1980 Mar;6(2):251–260. doi: 10.1093/jac/6.2.251. [DOI] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H., Oda K. Protease and elastase of Pseudomonas aeruginosa: inactivation of human plasma alpha 1-proteinase inhibitor. Infect Immun. 1979 Apr;24(1):188–193. doi: 10.1128/iai.24.1.188-193.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H. Production of protease and elastase by Pseudomonas aeruginosa strains isolated from patients. Infect Immun. 1977 Mar;15(3):679–685. doi: 10.1128/iai.15.3.679-685.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mull J. D., Callahan W. S. The role of the elastase of Pseudomonas aeruginosa in experimental infection. Exp Mol Pathol. 1965 Dec;4(6):567–575. doi: 10.1016/0014-4800(65)90037-7. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Wretlind B. Assessment of protease (elastase) as a Pseudomonas aeruginosa virulence factor in experimental mouse burn infection. Infect Immun. 1979 Apr;24(1):181–187. doi: 10.1128/iai.24.1.181-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulverer G., Wegrzynowicz Z., Ko H. L., Jeljaszewicz J. INfluence of Pseudomonas aeruginosa proteases on prothrombin, plasminogen and fibrinogen. Zentralbl Bakteriol A. 1980;248(1):99–109. [PubMed] [Google Scholar]
- Schultz D. R., Miller K. D. Elastase of Pseudomonas aeruginosa: inactivation of complement components and complement-derived chemotactic and phagocytic factors. Infect Immun. 1974 Jul;10(1):128–135. doi: 10.1128/iai.10.1.128-135.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale T. W., Thirkill H., Tarpay M., Flux M., Rennert O. M. Serotypes and antibiotic susceptibilities of Pseudomonas aeruginosa isolates from single sputa of cystic fibrosis patients. J Clin Microbiol. 1979 Jan;9(1):72–78. doi: 10.1128/jcm.9.1.72-78.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Holder I. A., Leppla S. A., Saelinger C. B. Role of exotoxin and protease as possible virulence factors in experimental infections with Pseudomonas aeruginosa. Infect Immun. 1978 Mar;19(3):839–845. doi: 10.1128/iai.19.3.839-845.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen M. J., Demko C. A., Boxerbaum B., Stern R. C., Kuchenbrod P. J. Multiple of isolates of Pseudomonas aeruginosa with differing antimicrobial susceptibility patterns from patients with cystic fibrosis. J Infect Dis. 1979 Dec;140(6):873–880. doi: 10.1093/infdis/140.6.873. [DOI] [PubMed] [Google Scholar]
- di Sant'Agnese P. A., Davis P. B. Research in cystic fibrosis (third of three parts). N Engl J Med. 1976 Sep 9;295(11):597–602. doi: 10.1056/NEJM197609092951105. [DOI] [PubMed] [Google Scholar]




