Abstract
Activation of p38 mitogen-activated protein kinase (MAPK) plays an important role in the G2/M cell cycle arrest induced by DNA damage, but little is known about the role of this signaling pathway in the G1/S transition. Upregulation of the cyclin-dependent kinase inhibitor p21Cip1 is thought to make a major contribution to the G1/S cell cycle arrest induced by γ radiation. We show here that inhibition of p38 MAPK impairs p21Cip1 accumulation and, as a result, the ability of cells to arrest in G1 in response to γ radiation. We found that p38 MAPK induces p21Cip1 mRNA stabilization, without affecting its transcription or the stability of the protein. In particular, p38 MAPK phosphorylates the mRNA binding protein HuR on Thr118, which results in cytoplasmic accumulation of HuR and its enhanced binding to the p21Cip1 mRNA. Our findings help to understand the emerging role of p38 MAPK in the cellular responses to DNA damage and reveal the existence of p53-independent networks that cooperate in modulating p21Cip1 levels at the G1/S checkpoint.
The cellular response to DNA damage involves the activation of checkpoint pathways that impose a delay in cell cycle progression and control DNA repair and replication (28). At the molecular level, key mediators for the initiation of the DNA damage response include the Ser/Thr protein kinases ataxia telangiectasia mutated (ATM) and Rad3-related (ATR), which orchestrate multiple aspects of the DNA damage response via phosphorylation of effectors on S/TQ motifs (5, 41). The tumor suppressor protein p53 is thought to be a major downstream effector of these DNA damage-activated kinase pathways (22). In normal cells, the phosphorylation and nuclear accumulation of p53 result in G1 arrest, mainly mediated by transcriptional upregulation of the cyclin-dependent kinase (CDK) inhibitor p21Cip1 (46). Another signaling pathway that is activated downstream of ATM and ATR in response to some DNA-damaging agents, such as cisplatin and doxorubicin, involves the stress-activated kinases p38 mitogen-activated protein kinase (MAPK) and MAPK-activated protein kinase 2 (MK2) (39).
The levels of p21Cip1 should be tightly regulated in order to ensure an efficient cellular response to DNA damage and to avoid the persistence of fatal lesions in the genetic material. Several mechanisms have been proposed to regulate p21Cip1 expression levels, with the p53-dependent control of p21Cip1 transcription being the most thoroughly investigated (21). Nevertheless, high levels of p21Cip1 mRNA do not always correlate with enhanced protein expression levels. In fact, it has been shown that low, but not high, doses of UV radiation rapidly decrease the p21Cip1 protein half-life, and this UV-induced p21Cip1 degradation is essential to ensure optimal DNA repair (7). In addition, since p21Cip1 is a short-lived protein, it can be regulated posttranslationally by phosphorylation (29, 40, 51) and protein stabilization (9, 13, 25).
Steady-state mRNA levels are the sum of mRNA transcription and degradation, and the regulation of p21Cip1 mRNA stability is also a rate-limiting step for p21Cip1 expression in processes such as UV light response and the differentiation of muscle or leukemic cells (11, 42, 48). The stability of mRNAs can be controlled by diverse stabilizing and destabilizing proteins, which bind to specific mRNA sequences. The Hu/ELAV family member HuR binds to and regulates many mRNAs that encode stress-response and proliferation-related proteins such as cyclins, tumor suppressors, oncogenes, and CDK inhibitors (reviewed in reference 31). HuR is a nuclear protein whose stress-dependent translocation to the cytoplasm is thought to be fundamental for its mRNA stabilizing function. Interestingly, HuR phosphorylation has been reported to regulate both cytoplasmic accumulation and the formation of HuR-mRNA complexes (1, 19, 26, 30, 31, 48).
In contrast to the DNA damage-specific activation of ATM and ATR, the p38 MAPK pathway responds to many types of stress stimuli, including cytokines, hyperosmolarity, UV radiation, and oxidative stress (16). Nevertheless, the ability of ionizing radiation to activate p38 MAPK has been controversial (29, 38, 44, 49). Of note, recent evidence has suggested that the role of p38 MAPK in the responses to DNA damage may be especially important in p53-defective cells (39).
We show here that in response to γ radiation, p38 MAPK is rapidly and transiently activated in an ATM-dependent manner and is required for p21Cip1 accumulation without affecting p21Cip1 protein half-life. Moreover, p38 MAPK upregulates p21Cip1 mRNA levels without affecting the recruitment of p53 to the p21Cip1 promoter. Our results indicate that the accumulation of p21Cip1 protein in response to ionizing radiation requires both transcriptional activation by p53 and the p38 MAPK-regulated posttranscriptional stabilization of the p21Cip1 mRNA.
MATERIALS AND METHODS
DNA cloning and mutagenesis.
The human HuR cDNA was a gift from Alberto Muñoz (IIB-CSIC, Madrid, Spain). For the expression of N-terminally hemagglutinin (HA)-tagged HuR, the pGEX-HuR plasmid was digested with EcoRI followed by BamHI partial digestion and the cDNA was subcloned into the HA-pCDNA3.1 vector (provided by Giulio Superti-Furga, CeMM, Vienna, Austria). HuR mutants were prepared using the QuikChange site-directed mutagenesis kit (Stratagene) and were verified by DNA sequencing. To activate p38α in mammalian cells, the constructs pEFmlink-p38α (4) and pEFmlink-MKK6DD (3) were used.
Cell culture and siRNA transfection.
RKO, HCT116, NIH 3T3-TopBp1-ER, and U2OS cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Wild-type (wt) ATM cells (GM00367) and ATM−/− (AT) cells (GM090607) were obtained from the Coriell Cell Repositories (Camden, NJ). HCT116 p53−/− and p21Cip1−/− cells were provided by Bert Vogelstein (Johns Hopkins University School of Medicine, Baltimore, MD). FuGene (Roche Applied Science) and Lipofectamine 2000 (Invitrogen) were used for cell transfection according to the manufacturer's protocol. Cells were treated with γ radiation (3 to 10 Gy) as indicated. Neocarzinostatin (NCS; 100 nM), 4α-phorbol 12-myristate 13-acetate (TPA; 0.5 μM), and cycloheximide (CHX; 30 μg/ml) were from Sigma-Aldrich, SB203580 (10 μM) was from Calbiochem, and KU55933 (20 μM) was from KuDOS Pharmaceuticals, Ltd.
The small interfering RNAs (siRNAs) for human p38α (SMART-pool duplex D-003512-14) and lamin A (siGLO), used as a control, were obtained from Dharmacon (Lafayette, CO). siRNAs targeting HuR (AAGAGGCAAUUACCAGUUUCA) and KSRP (K homology splicing regulator protein; GAUCAACCGGAGAGCAAGA) were obtained from Qiagen. U2OS and RKO cells were grown to 75% of confluence and transfected with Dharmafect reagent 1 (Dharmacon) according to the manufacturer's protocol. Cells were treated with γ radiation or actinomycin D 48 h after siRNA transfection.
Immunoblotting, IP, and kinase assays.
The following commercial antibodies were used for immunoblotting: anti-phospho-p38 (9211; Cell Signaling), anti-phospho-Hsp27 (Ser78; Stressgen), anti-phospho-MK2 (Thr222; Cell Signaling), anti-α-tubulin (T9026; Sigma), anti-phospho-Thr (9381; Cell Signaling), and anti-HA (2362; Roche). Anti-p38 (C-20), anti-Myc (9E10), anti-human-p53 (DO-1), anti-p21Cip1 (C-19), anti-HuR (3A2), and antinucleolin (C23) were from Santa Cruz Biotechnology.
Total cell lysates were prepared in immunoprecipitation (IP) buffer: 50 mM Tris-HCl (pH 7.5); 150 mM NaCl; 1% Nonidet P-40; 5 mM EDTA; 0.1 mM Na vanadate; 1 mM phenylmethylsulfonyl fluoride; 2 μM microcystin; 2.5 mM benzamidine; and 2 μg each of aprotinin, leupeptin, and pepstatin A per ml. For the IPs, 20 μl of anti-HA agarose beads (sc-7392 AC, Santa Cruz Biotechnology) was incubated with 1 mg of cell lysates for 4 h at 4°C. The beads were then washed three times in IP buffer and analyzed by immunoblotting. Immunoblots were developed and quantified using Alexa Fluor 680 (Molecular Probes) or Li-Cor IRDye 800 (Rockland)-labeled antibodies with the Odyssey infrared imaging system (Li-Cor).
Recombinant glutathione S-transferase (GST) fusion proteins were expressed in Escherichia coli BL21(DE3) and purified using standard protocols. p38 MAPK activity assays using as substrates GST-fused wt or mutant HuR proteins were carried out as described previously (3).
Flow cytometry analysis.
Cells were trypsinized, washed with phosphate-buffered saline (PBS), fixed with chilled 70% ethanol for 30 min at 4°C, and then incubated in PBS containing RNase (0.1 mg/ml) and propidium iodide (25 μg/ml) for 30 min at 37°C.
mRNA stability assays.
Actinomycin D (1 μg/ml) was added to the culture medium, and at the indicated times, total RNA was prepared and used for quantitative reverse transcription (qRT)-PCR. mRNA half-lives were calculated by being normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels and were plotted on logarithmic scales using Excel. The time required for a given transcript to undergo a 50% reduction of its initial abundance (time zero before actinomycin D) was calculated using nonlinear regression analysis. Two independent experiments were performed in all cases.
Protein stability assays.
CHX (30 μg/ml) was added to the culture medium 14 h after γ irradiation, and at the indicated times, cells were collected and total lysates were analyzed by immunoblotting.
Quantitative real-time PCR.
Total RNA was isolated using the Qiagen RNeasy kit, and cDNA was synthesized with Superscript-II reverse transcriptase (Invitrogen) using random hexamer primers following the manufacturer's instructions. PCRs were done in triplicates. An Applied Biosystems 7900HT fast real-time PCR system was used to determine the mRNA levels. Data analysis was done by normalization to GAPDH mRNA levels. The following oligonucleotides were used: 5′-TGCACCACCAACTGCTTAGC-3′ and 5′-GAGGGGCCATCCACAGTCTTC-3′ for GAPDH, 5′-GGTTATGAAGACCACATGGCC-3′ and 5′-GCGAGCATACGACACCTTAAT-3′ for HuR, 5′-CCCCCTCCTCACCAGTACC-3′ and 5′-CCCAGGCCTTAGTGTAGTCC-3′ for KSRP, 5′-TGTCCGTCAGAACCCATG-3′ and 5′-TGCCTCCTCCCAACTCATC-3′ for human p21Cip1, and 5′-CCAGGCCAAGATGGTGTCTT and 5′-TGAGAAAGGATCAGCCATTGC-3′ for mouse p21Cip1.
ChIP analysis.
For chromatin IP (ChIP) assays, U2OS cells (5 × 106) were seeded in 15-cm dishes and 24 h later were γ radiated in the presence or absence of SB203580, as indicated, and incubated 4 h more before collection.
Cells were fixed by incubation in medium containing 1% formaldehyde for 15 min, and the cross-linking reaction was stopped by addition of glycine to a final concentration of 125 mM. Cells were washed twice with ice-cold PBS containing a protease inhibitor cocktail (Roche), scraped on ice, and harvested by centrifugation at 2,000 rpm at 4°C. The resulting pellet was lysed in an appropriate volume (106 cells per 100 μl) of lysis buffer (1% sodium dodecyl sulfate [SDS], 10 mM EDTA, 50 mM Tris-HCl [pH 8.1] plus protease inhibitor cocktail) and chromatin was sheared in a bath sonicator (Diagenode Bioruptor) to an average length of 0.2 to 0.8 kb. Samples were then centrifuged at 13,000 rpm for 10 min at 13°C, and supernatants containing the fragmented chromatin were collected. Fragmented DNA (200 μg per antibody and condition, including a no-antibody control sample) was diluted in 10× dilution buffer (1% Triton X-100, 2 mM EDTA [pH 8.0], 150 mM NaCl, 20 mM Tris-HCl [pH 8.1]), precleared for 1 h at 4°C with 60 μl of a 50% slurry of salmon sperm DNA and protein A or protein G agarose (Upstate), and incubated overnight at 4°C with 2 to 5 μg of the p53 antibody (DO-1, Santa Cruz Biotechnology). Thirty micrograms of each sample (input) was frozen and kept at −80°C until the reverse-cross-link step. After 14 h at 4°C in a rotating platform, samples were incubated in the presence of 60 μl of a 50% slurry of salmon sperm DNA-protein A/G agarose for 1 h. Immune complexes were then washed once each in low-salt wash buffer (0.1% sodium dodecyl sulfate [SDS], 1% Triton X-100, 2 mM EDTA [pH 8.0], 20 mM Tris-HCl [pH 8.1], 150 mM NaCl), high-salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA [pH 8.0], 20 mM Tris-HCl [pH 8.1], 500 mM NaCl) and LiCl wash buffer (0.25 M LiCl, 1% NP-40, 1% deoxycholate, 1 mM EDTA, 10 mM Tris-HCl [pH 8.1]) and twice in Tris-EDTA (TE). Immune complexes were eluted after two rounds of incubation with 250 μl of freshly prepared elution buffer (1% SDS, 0.1 M NaHCO3), and cross-linking was reverted by addition of 20 μl of 5 M NaCl and incubation at 65°C for 4 h. After 1 h of proteinase K digestion, DNA was recovered by phenol-chloroform extraction and ethanol precipitation.
For semiquantitative PCR, reactions were performed in the exponential range of amplification that varied from 23 to 32 cycles. Amplification products (all in the size range of 200 to 300 bp) were analyzed by electrophoresis in 2% agarose gels and visualized by ethidium bromide. For quantitative analysis, real-time PCR was performed using the SYBR green master mix (Invitrogen) with triplicates of each sample. Data reported in the figures are values normalized to input DNA. The primers used for PCR of GAPDH were previously described (15). For p21Cip1 promoter amplification, we used the following primers: 5′-TTTTCTGGGCTTGTTTACCC-3′ and 5′-TACAAAACGAGGTGGGAGGA-3′.
Immunofluorescence and confocal microscopy.
Cells were rinsed in PBS, fixed in 4% paraformaldehyde for 30 min, and washed again with PBS. Nonspecific sites were blocked by incubation in PBS containing 1% bovine serum albumin and 0.5% Triton X-100 for 1 h at room temperature. Cells were then washed four times in PBS and incubated for 1 h with anti-HuR antibody (1:200) or anti-HA antibody (1:100, Santa Cruz Biotechnology). After four washes with PBS, cells were incubated for 1 h with Alexa Fluor 488 and 594 secondary antibodies (1:500; Molecular Probes) and mounted in Mowiol. Samples were examined by confocal microscopy (Zeiss, Germany).
The quantitative analysis of fluorescence intensities was performed using a confocal laser microscope and the Analysis of Image system (LSM 510; Zeiss, Germany). To measure fluorescence intensities within cytoplasmic domains, regions of interest were positioned using a 63× (1.4-numerical aperture) immersion oil objective. The same confocal settings were used for all cell series. Images were background corrected by reference regions outside the cells. Fluorescence intensities of 100 to 300 cells γ irradiated in the presence or absence of SB203580 were measured. Data were analyzed with Microsoft Excel.
IP of RNP complexes.
The association of endogenous HuR with target mRNAs was assessed by IP of HuR-mRNA complexes essentially as described previously (1). Briefly, cytoplasmic lysates from RKO cells (100 × 106) were prepared in polysome lysis buffer: 100 mM KCl, 5 mM MgCl2, 10 mM HEPES [pH 7], 0.5% NP-40, 1 mM dithiothreitol, containing 100 U/ml of RNase OUT (Invitrogen) and a protease inhibitor cocktail (Roche). After centrifugation, the supernatants were precleared for 30 min at 4°C using 15 μg of immunoglobulin G (IgG) and 50 μl of protein A-agarose (both from Santa Cruz Biotechnology), which had been previously swollen in NT2 buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1 mM MgCl2, 0.05% NP-40). Beads (100 μl) were incubated for 18 h at 4°C with 30 μg of antibody (either mouse IgG or anti-HuR from Santa Cruz Biotechnology) and then for 1 h at 4°C with 3 mg of cell lysate. After extensive washes and digestion of the proteins in the immunoprecipitates (37), the RNA was extracted and used to perform RT followed by semiquantitative PCR or qPCR to detect the presence of particular target mRNAs using gene-specific primers. GAPDH mRNA was used to normalize the data.
In the case of overexpressed proteins, RKO cells were transfected with the appropriate expression constructs based on the HA-pCDNA3 vector and 36 h later were irradiated with 10 Gy and then incubated further for 3 h before processing. HA-tagged beads (Santa Cruz Biotechnology) were used for the IP. The following oligonucleotides were used: 5′-TGCACCACCAACTGCTTAGC-3′ and 5′-GGCATGGACTGTGGTCATGA-3′ for GAPDH and 5′-GGGGAAGGGACACACAAGA-3′ and 5′-AATGAACTGGGGAGGGATGG-3′ for p21Cip1 mRNA.
RESULTS
ATM-dependent activation of p38 MAPK in response to γ radiation.
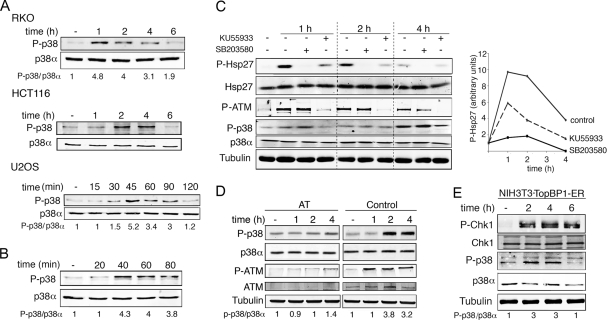
Activation of p38 MAPK is a well-established response to many types of genotoxic damage, but there are conflicting reports on the ability of γ radiation to activate this stress kinase. We found that treatment with 10 Gy of γ radiation induced a rapid and transient activating phosphorylation of p38 MAPK in several types of cell lines, including human osteosarcoma (U2OS) and colon cancer (RKO) cells as well as in human colon carcinoma HCT116 cells treated with 3 Gy γ radiation (Fig. 1A). Moreover, incubation of U2OS cells with NCS, a drug that mimics γ-radiation-induced DNA damage, similarly increased p38 MAPK phosphorylation levels (Fig. 1B).
FIG. 1.
p38α is activated by γ radiation. (A) U2OS and RKO cells were irradiated with 10 Gy and HCT116 cells with 3 Gy, and phospho-p38 levels were analyzed by immunoblotting at the indicated times. (B) U2OS cells were treated with NCS (0.3 μg/ml) and p38 phosphorylation was analyzed by immunoblotting. (C) RKO cells were pretreated for 1 h with KU55933 or 20 min with SB203580 and irradiated with 10 Gy, and the total cell lysates were analyzed by immunoblotting. (D) ATM-deficient (AT) and wt (control) cells were γ irradiated, and p38 phosphorylation was analyzed by immunoblotting at the indicated time points. (E) NIH 3T3 cells expressing TopBP1-ER were incubated with OHT (500 nM) for the indicated times and analyzed by immunoblotting.
Treatment with γ radiation generates double strand breaks in the DNA, which lead to the activation of ATM and ATR (34). This cascade is generally known as the DNA damage response (DDR). To investigate the potential implication of these protein kinases upstream of p38 MAPK, we first used the ATM inhibitor KU55933 (23). We found that KU55933 significantly inhibited the γ-radiation-induced activation of p38 MAPK, as determined by the levels of phosphorylation of Hsp27, a substrate of the p38 MAPK-activated kinase MK2 (Fig. 1C). On the contrary, treatment with the inhibitor of p38α and p38β, SB203580, did not affect γ-radiation-induced ATM phosphorylation, indicating that p38 MAPK activation occurs downstream of ATM. In support of the specificity of KU55933, we observed that the γ-radiation-induced activation of p38 MAPK was abrogated in ATM-defective cells (Fig. 1D). To determine whether the activation of DDR kinases was not only necessary but also sufficient to promote p38 MAPK activation, we took advantage of a recently published cellular system in which ATR can be activated at will, in the absence of any actual DNA damage (45). The system is based on the 4-hydroxy tamoxifen (OHT)-mediated nuclear translocation of a fragment of TopBP1, which behaves as an allosteric activator of ATR (32). By using this system, we directly correlated ATR activation with the phosphorylation of p38 MAPK (Fig. 1E). Altogether, our results indicate the existence of an active cross talk between DDR kinases and p38 MAPK.
Induction of p21Cip1 in response to γ radiation requires p38 MAPK.
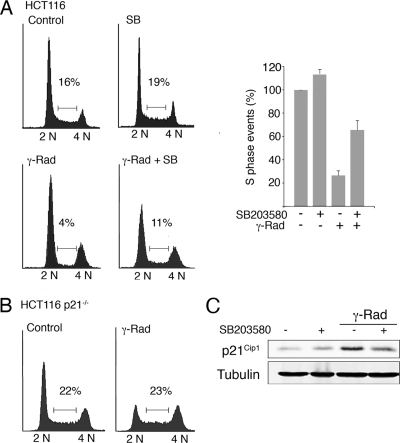
In response to γ radiation, cells engage G1 and G2 phase checkpoints to arrest the cell cycle and try to repair the damaged DNA. To determine the importance of p38 MAPK in the activation of these checkpoints, we used HCT116 cells, a system broadly used for this type of cell cycle studies. As expected, γ radiation induced a dramatic reduction in the S-phase population of HCT116 cells (from 16% to 4%), with a clear arrest in G1 and a less prominent arrest in G2/M. However, preincubation with SB203580 inhibited the G1 cell cycle arrest, with the consequent increase in S-phase events (11%) compared with γ irradiation alone (4%) (Fig. 2A).
FIG. 2.
p38α activity is necessary for the establishment of the G1/S checkpoint in response to γ radiation (γ-Rad). HCT116 wt (A) and p21Cip1−/− (B) cells were irradiated with 3 Gy and 16 h later were analyzed by flow cytometry. The percentage of cells in S phase is indicated in the histogram, which shows the average of two independent experiments. (C) Total cell lysates were analyzed by immunoblotting. SB, SB203580.
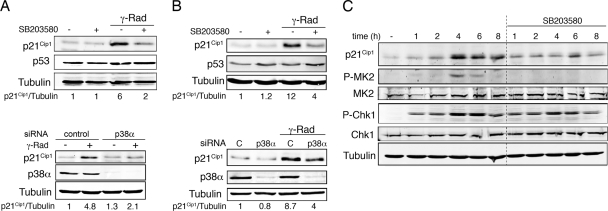
The CDK inhibitor p21Cip1 is thought to be a key regulator of the G1 checkpoint (47), and we confirmed that p21Cip1-defective HCT116 cells were impaired in γ-radiation-induced G1 cell cycle arrest (Fig. 2B). We therefore investigated the induction of p21Cip1 in γ-irradiated HCT116 cells both in the absence and presence of SB203580. Consistent with the above results, the inhibition of p38 MAPK markedly reduced p21Cip1 protein levels (Fig. 2C). This effect was not restricted to HCT116 cells, since we also observed impaired upregulation of γ-radiation-induced p21Cip1 in RKO cells treated either with SB203580 (Fig. 3A, top panel) or with siRNA specific for p38α, the most abundant p38 MAPK family member (Fig. 3A, bottom panel), as well as in U2OS cells (Fig. 3B). Furthermore, TopBP1-induced activation of ATR failed to increase p21Cip1 protein levels in the presence of SB203580 (Fig. 3C).
FIG. 3.
γ-radiation-induced p21Cip1 expression depends on p38 MAPK activity. RKO cells (A) and U2OS cells (B) were incubated with SB203580 or were transfected with p38α and control siRNAs before treatment with 10 Gy of γ radiation (γ-Rad). After 16 h, total cell lysates were analyzed by immunoblotting. (C) NIH 3T3 cells expressing TopBP1-ER were treated with OHT alone or together with SB203580 for the indicated times. Total cell lysates were analyzed by immunoblotting.
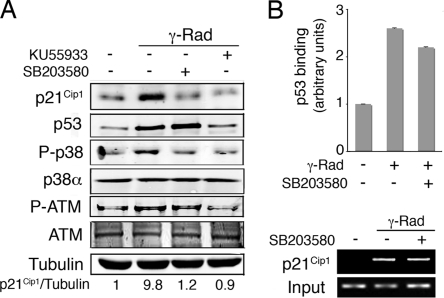
The DNA damage-induced upregulation of p21Cip1 is known to depend on p53 activity, which in turn can be regulated by p38 MAPK via different mechanisms (10). To determine if the effect of p38 MAPK on p21Cip1 levels was mediated by p53, RKO cells were γ irradiated in the presence of either ATM or p38 MAPK inhibitors. As expected, the inhibition of ATM prevented the accumulation of both p53 and p21Cip1; however, p38 MAPK inhibition interfered with p21Cip1 upregulation without affecting total p53 levels (Fig. 4A). Inhibition of p38 MAPK did not affect the γ-radiation-induced binding of p53 to the p21Cip1 promoter (Fig. 4B).
FIG. 4.
p38α regulates p21Cip1 expression levels independently of p53 transcriptional activation. (A) RKO cells were pretreated with KU55933 or SB203580 and then irradiated with 10 Gy. After 6 h, total cell lysates were analyzed by immunoblotting. (B) U2OS cells were incubated in the presence or absence of SB203580 from 30 min before treatment with γ radiation (γ-Rad; 5 Gy). Samples were analyzed by ChIP followed by qRT-PCR (histogram) and semiquantitative RT-PCR (agarose gel).
p38 MAPK regulates p21Cip1 mRNA stability.
Once established that p38 MAPK regulates p21Cip1 expression independently of p53-mediated transcription, we performed a series of experiments to determine the mechanisms underlying p21Cip1 upregulation. It has been reported that p21Cip1 can be degraded via the ubiquitin-proteasome pathway and the phosphorylation of Ser130 by p38 MAPK stabilizes the p21Cip1 protein in response to transforming growth factor β1 (29). To investigate if p38 MAPK regulates p21Cip1 protein stability in response to γ radiation, we treated RKO cells with CHX and then analyzed p21Cip1 protein levels in the presence or absence of SB203580. We found that the inhibition of protein synthesis did not affect p21Cip1 protein half-life, neither under basal conditions (Fig. 5A) nor in response to γ radiation (Fig. 5B). In contrast, p38 MAPK inhibition significantly reduced p21Cip1 protein accumulation in the presence of the transcriptional inhibitor actinomycin D (data not shown), suggesting a possible effect of p38 MAPK on p21Cip1 mRNA stability. To investigate this possibility, we analyzed the contribution of p38 MAPK to the γ-radiation-induced upregulation of p21Cip1 mRNA. We found that either p38α downregulation or the pharmacological inhibition of its kinase activity strongly reduced p21Cip1 mRNA levels in RKO cells treated with γ radiation (Fig. 6A and B). Similar results were observed in γ-irradiated U2OS and HCT116 cells (data not shown) as well as in OHT-treated NIH 3T3-TopBP1-ER cells (Fig. 6C). Moreover, the inhibition of p38 MAPK significantly reduced the half-life of the p21Cip1 mRNA in RKO, U2OS, and HCT116 cells upon γ radiation (Fig. 6D) (data not shown) as well as in NIH 3T3-TopBP1-ER cells treated with OHT (data not shown), strongly supporting a role of p38 MAPK in the regulation of p21Cip1 mRNA stability.
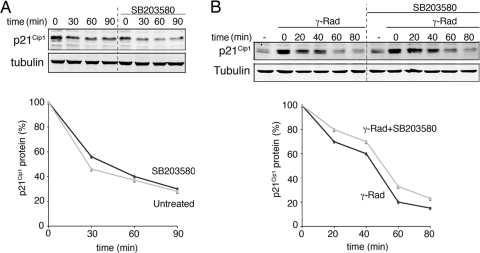
FIG. 5.
p38α does not regulate p21Cip1 protein half-life. (A) RKO cells were pretreated with SB203580 (10 μM) and then incubated with CHX (30 μg/μl) for the indicated times. Total cell lysates were analyzed by immunoblotting. (B) RKO cells were treated with γ radiation (γ-Rad) and 4 h later were processed as in panel A. Quantification of p21Cip1 protein half-life is shown in the graphics below.
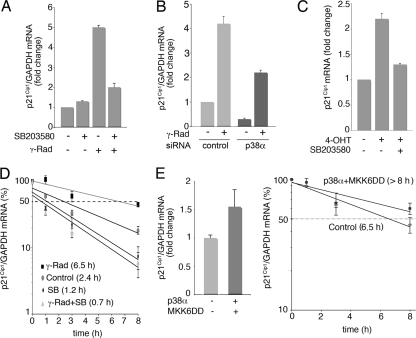
FIG. 6.
p38α regulates p21Cip1 mRNA half-life. (A and B) RKO cells were treated with SB203580 (SB) or transfected with p38α and control siRNAs and γ irradiated (γ-Rad; 10 Gy), and 4 h later, total RNA was purified. p21Cip1 and GAPDH mRNA levels were analyzed by qRT-PCR. (C) NIH 3T3-TopBP1-ER cells were treated with OHT alone or together with SB203580, and p21Cip1 mRNA levels were measured by qRT-PCR. (D) RKO cells were irradiated (10 Gy) and 3 h later were incubated with actinomycin D in the presence or absence of SB203580 (SB). Total RNA was obtained at the indicated times, and p21Cip1 and GAPDH mRNA levels were analyzed by qRT-PCR. (E) U2OS cells were transfected with vector alone or with both p38α and MKK6DD expression vectors (1 μg each) and 48 h later were treated with actinomycin D for the indicated times. p21Cip1 and GAPDH mRNA levels were measured by qRT-PCR.
We then cotransfected U2OS cells, which yield a high transfection efficiency, with p38α and its activator MKK6DD to specifically activate this pathway. Interestingly, activation of p38α by itself was sufficient to increase p21Cip1 total mRNA levels as well as the half-life of the p21Cip1 mRNA (Fig. 6E).
To further support the ability of p38 MAPK to regulate p21Cip1 mRNA stability independently of p53, we used HCT116 p53−/− cells. These cells barely increased p21Cip1 mRNA levels in response to γ radiation (data not shown), as expected from the essential role of p53 in p21Cip1 transcription. We therefore treated HCT116 p53−/− cells with TPA, which is known to induce a robust and p53-independent upregulation of p21Cip1 (50). We first confirmed that TPA treatment activated the p38 MAPK pathway in HCT116 p53−/− cells (Fig. 7A). Moreover, TPA also upregulated p21Cip1 mRNA total levels and enhanced p21Cip1 mRNA stability, but this effect was impaired in the presence of SB203580 (Fig. 7B). These results support a p53-independent role of p38 MAPK in p21Cip1 mRNA stabilization in response to different stimuli.
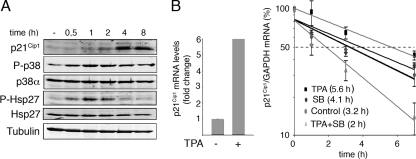
FIG. 7.
p38 MAPK regulates p21Cip1 mRNA stability independently of p53. (A) HCT116 p53−/− cells were treated with TPA, and total cell lysates were analyzed by immunoblotting at the indicated times. (B) HCT116 p53−/− cells were treated with TPA and 4 h later were incubated with actinomycin D in the presence or absence of SB203580 (SB). Total RNA was obtained at the indicated times, and p21Cip1 and GAPDH mRNA levels were analyzed by qRT-PCR.
The mRNA stability-promoting factor HuR is phosphorylated by p38 MAPK.
The half-life of the p21Cip1 mRNA has been reported to be regulated by the RNA-binding proteins HuR and KSRP in response to UV radiation and muscle differentiation, respectively (11, 48). To determine the importance of these proteins in the γ-radiation-induced upregulation of p21Cip1 mRNA levels, we knocked down their expression with siRNAs and then analyzed p21Cip1 mRNA half-life in γ-radiation-treated RKO cells. We observed that although the mRNAs encoding HuR and KSRP were both downregulated with similar efficiencies (Fig. 8A, left panel), the downregulation of KSRP did not significantly affect p21Cip1 mRNA levels, while the knockdown of HuR reduced the p21Cip1 mRNA half-life by more than 50% (Fig. 8A, right panel). These results strongly suggest the implication of HuR in the γ-radiation-induced accumulation of p21Cip1 mRNA.
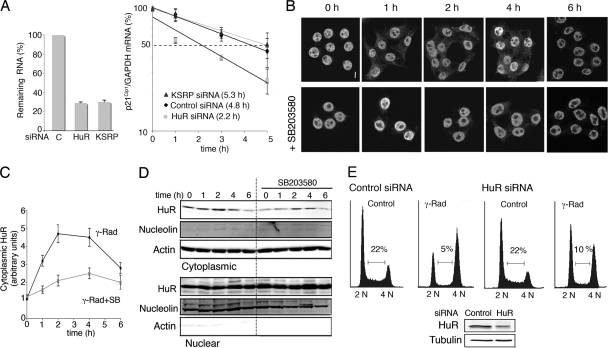
FIG. 8.
Cytoplasmic localization of HuR after γ radiation (γ-Rad) regulates p21Cip1 mRNA stability. (A) RKO cells were transfected with HuR, KSRP, or control (C) siRNAs and 48 h later were γ irradiated with 10 Gy for 4 h and then treated with actinomycin D for the indicated times. Total RNA was obtained at the indicated times, and mRNA levels were analyzed by qRT-PCR. (B) RKO cells were treated with γ radiation (10 Gy) in the presence or absence of SB203580, and HuR localization was visualized by confocal microscopy at the indicated times after irradiation. Bar, 5 μm. (C) Quantification of fluorescence intensities for the cytosolic HuR of the samples in panel B obtained using a confocal laser microscope and the Analysis of Image system. (D) Immunoblotting analysis of HuR levels in cytoplasmic and nuclear fractions of RKO cells at the indicated times after γ radiation (10 Gy). Antibodies against actin and nucleolin were used to confirm equal loading of the cytoplasmic and nuclear samples, respectively. (E) U2OS cells were transfected with HuR and control siRNAs and 48 h later were treated with 3 Gy of γ radiation, incubated for 16 h, and analyzed by flow cytometry. The immunoblot shows HuR protein expression.
To stabilize mRNAs, HuR should be transported from the nucleus to the cytoplasm (20, 27), which is not a constitutive process, although it is subjected to modulation by different cues, including extracellular stimuli. We found by both immunofluorescence and immunoblotting that the amount of cytoplasmic HuR significantly increased in a transient and p38 MAPK-dependent manner in response to γ radiation (Fig. 8B, C, and D). Interestingly, donwregulation of HuR protein expression impaired the γ-radiation-induced G1 cell cycle arrest in U2OS cells (Fig. 8E).
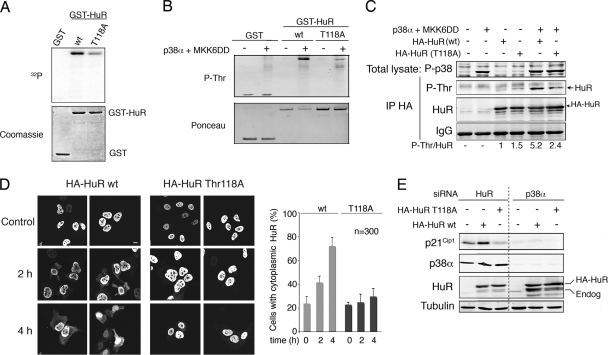
Several reports have correlated the phosphorylation of HuR with its subcellular redistribution (19, 30, 31). We found that p38α phosphorylated recombinant HuR protein in vitro (Fig. 9A). The analysis of HuR sequence with NetPhosK 1.0 (http://www.cbs.dtu.dk/services/NetPhos) revealed three potential phosphorylation sites (Thr118, Ser202, and Ser221). We generated HuR mutants with Ala substitutions at each of these sites and then performed in vitro kinase assays with active p38α. We found that mutation of Thr118 significantly reduced the phosphorylation of HuR by p38α in vitro (Fig. 9A), while mutation of the other two residues did not affect so much HuR phosphorylation (data not shown). We confirmed the phosphorylation of HuR by p38 MAPK by using a phospho-Thr antibody that recognized HuR phosphorylated on Thr118 (Fig. 9B). We then expressed HuR wt and the T118A mutant proteins in RKO cells, either alone or in combination with p38α and its specific activator MKK6DD, and confirmed that HuR was phosphorylated on Thr118 upon p38 MAPK activation in cells (Fig. 9C).
FIG. 9.
p38α phosphorylates HuR on Thr118. (A) Kinase assays were performed in the presence of [γ-32P]ATP using MKK6DD-activated GST-p38α (200 ng) and GST, GST-HuR wt or GST-HuR-T118A as substrates (1 μg). The gels were stained with Coomassie blue and then exposed to autoradiography. (B) GST-HuR wt and GST-HuR-T118A proteins (500 ng) were incubated with MKK6DD-activated GST-p38α and then analyzed by immunoblotting with a phospho-Thr antibody. (C) RKO cells were transfected with HA-tagged HuR wt or mutant T118A and MKK6DD plus p38α, as indicated. After 48 h, HuR phosphorylation was analyzed by HA IP followed by immunoblotting with a phospho-Thr antibody. Total cell lysates were also analyzed by immunoblotting. (D) U2OS cells were transfected with HA-HuR wt or T118A and 24 h later were treated with γ radiation. HuR localization was visualized by confocal microscopy at the indicated times after γ radiation using an HA antibody to avoid the endogenous HuR protein. Bar, 5 μm. The percentage of cells (out of 300 counted) with a cytoplasmic HuR signal is shown in the right panel. (E) U2OS cells were transfected with p38α or HuR siRNAs and 48 h later were transfected again with HA-tagged HuR wt or the T118A mutant. Total cell lysates were analyzed by immunoblotting.
Next, we expressed wt or T118A HA-HuR in U2OS cells to analyze the contribution of Thr118 phosphorylation to the accumulation of cytosolic HuR mediated by p38 MAPK in response to γ radiation. We observed that γ radiation induced the translocation of a fraction of wt HA-HuR to the cytosol, whereas the T118A mutant remained mostly nuclear, indicating that Thr118 phosphorylation contributes to the cytosolic localization of HuR induced by γ radiation (Fig. 9D).
HuR phosphorylation on Thr118 by p38 MAPK is essential for p21Cip1 mRNA stabilization in response to γ radiation.
To better support the functional relevance of p38 MAPK-mediated phosphorylation of HuR on Thr118, we expressed both HuR wt and the T118A mutant proteins in U2OS cells. For this experiment, we first knocked down the endogenous HuR using siRNAs and 48 h later transfected the exogenous proteins (1). Interestingly, we found that expression of the T118A mutant did no affect endogenous p21Cip1 expression, whereas wt HuR significantly increased p21Cip1 protein levels, and this effect was abolished by p38α downregulation (Fig. 9E). These results support that phosphorylation of Thr118 by p38 MAPK is important for the positive effect of HuR on p21Cip1 expression.
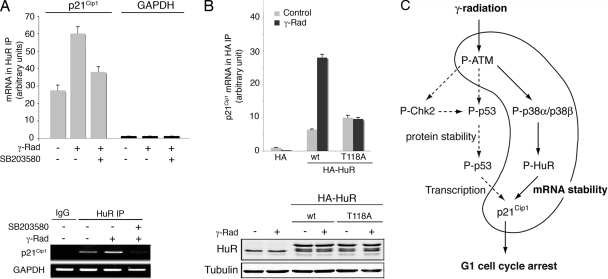
Since HuR has been shown to bind directly to the p21Cip1 mRNA (48), we investigated the regulation of this interaction by p38 MAPK. First, we tested if γ radiation stimulated the association of p21Cip1 mRNA with HuR protein by performing HuR IP followed by qRT-PCR. We observed that binding of endogenous HuR to p21Cip1 mRNA in vivo increased by more than twofold upon treatment with γ radiation in a p38 MAPK-dependent manner (Fig. 10A). In contrast, GAPDH mRNA was found at similar low levels in HuR and control IgG immunoprecipitates, as expected for a non-HuR target, and no changes were detected with the different treatments. Importantly, when the binding levels of p21Cip1 mRNA to wt HA-HuR and the T118A mutant were compared, we found that γ radiation dramatically increased the association of wt, but not of T118A, HuR with the p21Cip1 mRNA, while the binding levels of both HuR proteins were comparable in untreated cells (Fig. 10B). These results strongly support the importance of Thr118 phosphorylation for the binding of HuR to p21Cip1 mRNA.
FIG. 10.
γ radiation induces the binding of HuR to p21Cip1 mRNA. (A) RKO cells were γ irradiated (γ-Rad; 10 Gy) and treated with SB203580, as indicated, and total cell lysates were then immunoprecipitated with either HuR antibodies or control IgGs. RNA was purified from the immunoprecipitates and used for qRT-PCRs. The graph shows fold differences in transcript abundance in HuR immunoprecipitates compared with the IgG immunoprecipitates. The image shows a representative PCR visualized by Syber-safe staining of the agarose gel. (B) RKO cells were transfected with constructs encoding HA-tagged HuR wt and T118A mutant proteins, and total cell lysates were then immunoprecipitated with HA antibody. RNA was purified from the immunoprecipitates, and the amount of p21Cip1 mRNA was determined by qRT-PCR. The expression levels of the different HA-HuR proteins were analyzed by immunoblotting. (C) A model for the p38 MAPK-dependent upregulation of p21Cip1 in response to γ radiation.
DISCUSSION
The G1 phase of the cell cycle is a period when cells make critical decisions about their fate, including the optional commitment to replicate DNA and proceed through cell division. There are two events that play a major role in the G1 checkpoint: an initial, rapid, and transient response that targets Cdc25A activity and regulates CDK2 phosphorylation, followed by the delayed and more sustained G1 arrest imposed by the p53-dependent upregulation of p21Cip1 (6). The p38 MAPK signaling pathway plays an important role in the regulation of the G2/M transition when cells are challenged with many types of DNA-damaging agents. For example, Cdc25B phosphorylation by p38 MAPK regulates the initiation of the G2/M checkpoint in response to UV radiation (12). Moreover, phosphorylation of Cdc25B and Cdc25C by the p38 MAPK-activated kinase MK2 contributes to maintain not only the G2/M but also the G1 and S checkpoints in UV-irradiated cells (36). The p38 MAPK family member p38γ has been also reported to regulate the G2 arrest induced by γ radiation (49). In contrast, the role of the p38 MAPK pathway in controlling the G1 arrest induced by ionizing radiations remains unclear.
We report here a fundamental role for p38 MAPK in the G1 checkpoint induced by DNA double-strand breaks. Thus, γ radiation induces the activation of p38 MAPK, which phosphorylates the mRNA binding protein HuR and induces its cytoplasmic accumulation. Once in the cytosol, HuR binds to and stabilizes the p21Cip1 mRNA, allowing generation of the p21Cip1 protein levels required to ensure an efficient G1 arrest (Fig. 10C).
Cross talk between DDR and p38 MAPK in response to DNA damage.
Activation of ATM and ATR is essential to transduce the DNA damage checkpoint signals, but the extent to which these kinases cross talk with the p38 MAPK pathway is still poorly defined. For example, both signaling pathways appear to function independently in the response to genotoxic stresses such as UV (12), whereas the p38 MAPK pathway has been reported to mediate ATM and ATR signaling in response to several chemotherapeutic drugs that induce DNA damage (39). Our results indicate that γ radiation induces p38 MAPK activation, but transiently and to a lower extent than other types of stresses, such as UV radiation or osmotic agents, which may explain previous apparent discrepancies in the activation of p38 MAPK by γ radiation when single time points were analyzed (17). Our observations also extend recent work assaying p38 MAPK activity in immunoprecipitates from γ-irradiated cells (38). We found that ATM activity is required for the γ-radiation-induced phosphorylation of p38 MAPK in cells, which is consistent with ATM being the main DDR kinase that promotes the G1/S checkpoint. Importantly, the TopBp1-dependent activation of ATR has allowed us to demonstrate that activation of DDR kinases is not only necessary but also sufficient to activate p38 MAPK. It will therefore be worth exploring whether p38 MAPK activity plays a role in other DDR-activating conditions such as the still ill-defined replicative stress that is induced by oncogenes (18).
p38 MAPK induces the accumulation of p21Cip1.
The ATM-Chk2 and ATR-Chk1 pathways can induce p53 phosphorylation, which interferes with the degradation of p53 mediated by the Mdm2 ubiquitin ligase (14, 24). The resultant accumulation of the p53 protein is essential for p21Cip1 transcription and maintenance of the G1 checkpoint. However, p53-dependent transcriptional activation is not enough to ensure high levels of p21Cip1 protein in response to some types of DNA damage, such as low doses of UV irradiation, which induce a rapid degradation of p21Cip1 protein despite high levels of p21Cip1 mRNA (7). We have also recently reported that p21Cip1 protein levels do not increase in response to genotoxic agents such as cisplatin, in contrast to other p53-dependent targets such as Noxa or PUMA (15). In the case of γ radiation, ATM inhibition dramatically affects p53 induction, which correlates with a decrease in p21Cip1 protein levels. In contrast, we show that p38 MAPK activity is essential to obtain the p21Cip1 protein levels required for G1/S cell cycle arrest, but this p38 MAPK function is independent of p53 accumulation. Altogether, these results indicate that the p53-dependent transcriptional program is necessary but not sufficient to ensure appropriate p21Cip1 protein levels in response to γ radiation and that p38 MAPK plays a fundamental role in this process. Importantly, p38 MAPK can also regulate p21Cip1 mRNA stability when p21Cip1 transcription does not depend on p53 activity, as in the case of TPA treatment (50).
We have previously reported that the p38 MAPK substrate ZnHIT-1/p18Hamlet contributes to the γ-radiation-induced recruitment of p53 to the p21Cip1 promoter (33). However, this p18Hamlet function appears to be independent of p38 MAPK, since the levels of p53 at the p21Cip1 promoter are not affected by p38 MAPK inhibition.
p38 MAPK regulates p21Cip1 mRNA stability through HuR phosphorylation.
As expected for a protein with a crucial role in cell cycle control, p21Cip1 expression is tightly regulated by several mechanisms, including p53-dependent and -independent transcription (35), subcellular localization (8), mRNA stability (48), and protein degradation (43). Our results indicate that γ-radiation-induced accumulation of p21Cip1 requires the stabilization of p21Cip1 mRNA mediated by p38 MAPK. A possible link between this signaling pathway and p21Cip1 expression has been previously reported in different cellular processes. For example, incubation of HD3 human colon carcinoma cells with transforming growth factor β1 activates p38α, which in turn induces the accumulation of p21Cip1 protein through its phosphorylation on Ser130 (29). In addition, p38 MAPK has been implicated in the posttranscriptional control of p21Cip1 during muscle differentiation, through phosphorylation of the mRNA-destabilizing protein KSRP (11). We have found that in response to γ radiation, p21Cip1 protein stability does not change, while the half-life of its mRNA increases in a p38 MAPK-dependent manner. Our results indicate that the mRNA-stabilizing factor HuR is important for p21Cip1 upregulation induced by γ radiation. Previous work has proposed a role for HuR in UV-induced p21Cip1 mRNA stabilization, although the underlying mechanism was not elucidated (48). Here we provide evidence supporting a role for this mRNA binding protein in the ATM-p38 MAPK-p21Cip1 axis. It is worth mentioning that TopBp1-induced ATR activation, in the absence of DNA damage, also induces p38 MAPK-mediated stabilization of the p21Cip1 mRNA. This suggests that the mechanism of p21Cip1 regulation that we describe for γ-irradiated cells could be involved in a wider range of DNA damage responses involving ATM and/or ATR activation.
In general, cytoplasmic translocation of HuR is thought to be essential for the binding to and stabilization of target mRNAs, and several phosphorylation sites have been reported to modulate HuR nucleocytoplasmic shuttling (19, 30, 31). For example, Cdk1-mediated phosphorylation of HuR on Ser202 retains it in the nucleus by association with 14-3-3 proteins (30). In addition phosphorylation of Ser221 by protein kinase Cα is essential for cytoplasmic localization of HuR in response to ATP (19). We found that phosphorylation of HuR on Thr118 by p38 MAPK regulates cytosolic HuR accumulation in response to γ radiation. The implication of p38 MAPK in the phosphorylation of other HuR residues, such as Ser202 and Ser221, will be an interesting subject for future studies. A recent report has also proposed that phosphorylation on Thr118 regulates HuR protein degradation after heat shock treatment (2). These results suggest that reduced HuR protein stability might contribute to the impaired cytosolic localization of the T118A mutant upon γ radiation. Interestingly, the binding of HuR to target mRNAs can be also regulated by phosphorylation. Thus, phosphorylation of HuR on Ser100 by Chk2 has been reported to induce the dissociation of HuR from the SIRT1 mRNA, whereas phosphorylation on Thr118 induces HuR-SIRT1 mRNA association and regulates SIRT1 expression in response to oxidative stress (1). Our results demonstrate that p38 MAPK phosphorylates HuR in vitro and in cells on Thr118, and that this phosphorylation regulates the γ-radiation-induced cytoplasmic accumulation of HuR and its binding to the p21Cip1 mRNA. In summary, we report a new mechanism of p21Cip1 regulation in response to γ radiation that complements the well-characterized p53-dependent transcriptional activation. This involves p38 MAPK phosphorylation of HuR, which in turn results in enhanced binding of HuR to p21Cip1 mRNA, mRNA stabilization, and the accumulation of p21Cip1 protein leading to G1 cell cycle arrest.
Acknowledgments
We thank Carolina Aparicio and Laura Doglio for technical support, Maite Berciano and Javier Leon for helpful suggestions, and Luis Toledo for NIH 3T3-TopBp1-ER cells.
V.L. was funded by the Fundación Científica de la AECC and by an FPU fellowship from the Spanish MEC. Work in the laboratory of A.R.N. is funded by the CNIO and by grants from the Spanish MICINN (BFU2007-60575), Fundación La Caixa and ISCIII-RTICC RD06/0020/0083.
Footnotes
Published ahead of print on 15 June 2009.
REFERENCES
- 1.Abdelmohsen, K., R. Pullmann, Jr., A. Lal, H. H. Kim, S. Galban, X. Yang, J. D. Blethrow, M. Walker, J. Shubert, D. A. Gillespie, H. Furneaux, and M. Gorospe. 2007. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol. Cell 25543-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelmohsen, K., S. Srikantan, X. Yang, A. Lal, H. H. Kim, Y. Kuwano, S. Galban, K. G. Becker, D. Kamara, R. de Cabo, and M. Gorospe. 2009. Ubiquitin-mediated proteolysis of HuR by heat shock. EMBO J. 281271-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso, G., C. Ambrosino, M. Jones, and A. R. Nebreda. 2000. Differential activation of p38 mitogen-activated protein kinase isoforms depending on signal strength. J. Biol. Chem. 27540641-40648. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosino, C., G. Mace, S. Galban, C. Fritsch, K. Vintersten, E. Black, M. Gorospe, and A. R. Nebreda. 2003. Negative feedback regulation of MKK6 mRNA stability by p38α mitogen-activated protein kinase. Mol. Cell. Biol. 23370-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakkenist, C. J., and M. B. Kastan. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421499-506. [DOI] [PubMed] [Google Scholar]
- 6.Bartek, J., and J. Lukas. 2007. DNA damage checkpoints: from initiation to recovery or adaptation. Curr. Opin. Cell Biol. 19238-245. [DOI] [PubMed] [Google Scholar]
- 7.Bendjennat, M., J. Boulaire, T. Jascur, H. Brickner, V. Barbier, A. Sarasin, A. Fotedar, and R. Fotedar. 2003. UV irradiation triggers ubiquitin-dependent degradation of p21WAF1 to promote DNA repair. Cell 114599-610. [DOI] [PubMed] [Google Scholar]
- 8.Besson, A., S. F. Dowdy, and J. M. Roberts. 2008. CDK inhibitors: cell cycle regulators and beyond. Dev. Cell 14159-169. [DOI] [PubMed] [Google Scholar]
- 9.Blagosklonny, M. V., G. S. Wu, S. Omura, and W. S. el-Deiry. 1996. Proteasome-dependent regulation of p21WAF1/CIP1 expression. Biochem. Biophys. Res. Commun. 227564-569. [DOI] [PubMed] [Google Scholar]
- 10.Bode, A. M., and Z. Dong. 2004. Post-translational modification of p53 in tumorigenesis. Nat. Rev. Cancer 4793-805. [DOI] [PubMed] [Google Scholar]
- 11.Briata, P., S. V. Forcales, M. Ponassi, G. Corte, C. Y. Chen, M. Karin, P. L. Puri, and R. Gherzi. 2005. p38-dependent phosphorylation of the mRNA decay-promoting factor KSRP controls the stability of select myogenic transcripts. Mol. Cell 20891-903. [DOI] [PubMed] [Google Scholar]
- 12.Bulavin, D. V., Y. Higashimoto, I. J. Popoff, W. A. Gaarde, V. Basrur, O. Potapova, E. Appella, and A. J. Fornace, Jr. 2001. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature 411102-107. [DOI] [PubMed] [Google Scholar]
- 13.Cayrol, C., and B. Ducommun. 1998. Interaction with cyclin-dependent kinases and PCNA modulates proteasome-dependent degradation of p21. Oncogene 172437-2444. [DOI] [PubMed] [Google Scholar]
- 14.Chehab, N. H., A. Malikzay, M. Appel, and T. D. Halazonetis. 2000. Chk2/hCds1 functions as a DNA damage checkpoint in G1 by stabilizing p53. Genes Dev. 14278-288. [PMC free article] [PubMed] [Google Scholar]
- 15.Cuadrado, A., V. Lafarga, P. C. Cheung, I. Dolado, S. Llanos, P. Cohen, and A. R. Nebreda. 2007. A new p38 MAP kinase-regulated transcriptional coactivator that stimulates p53-dependent apoptosis. EMBO J. 262115-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuenda, A., and S. Rousseau. 2007. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 17731358-1375. [DOI] [PubMed] [Google Scholar]
- 17.Dent, P., A. Yacoub, P. B. Fisher, M. P. Hagan, and S. Grant. 2003. MAPK pathways in radiation responses. Oncogene 225885-5896. [DOI] [PubMed] [Google Scholar]
- 18.Di Micco, R., M. Fumagalli, and F. d'Adda di Fagagna. 2007. Breaking news: high-speed race ends in arrest—how oncogenes induce senescence. Trends Cell Biol. 17529-536. [DOI] [PubMed] [Google Scholar]
- 19.Doller, A., A. Huwiler, R. Muller, H. H. Radeke, J. Pfeilschifter, and W. Eberhardt. 2007. Protein kinase C alpha-dependent phosphorylation of the mRNA-stabilizing factor HuR: implications for posttranscriptional regulation of cyclooxygenase-2. Mol. Biol. Cell 182137-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan, X. C., and J. A. Steitz. 1998. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 173448-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes, N. P., G. Bjerke, B. Llorente, S. A. Szostek, B. M. Emerson, and J. M. Espinosa. 2006. Gene-specific requirement for P-TEFb activity and RNA polymerase II phosphorylation within the p53 transcriptional program. Genes Dev. 20601-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris, S. L., and A. J. Levine. 2005. The p53 pathway: positive and negative feedback loops. Oncogene 242899-2908. [DOI] [PubMed] [Google Scholar]
- 23.Hickson, I., Y. Zhao, C. J. Richardson, S. J. Green, N. M. Martin, A. I. Orr, P. M. Reaper, S. P. Jackson, N. J. Curtin, and G. C. Smith. 2004. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 649152-9159. [DOI] [PubMed] [Google Scholar]
- 24.Hirao, A., Y. Y. Kong, S. Matsuoka, A. Wakeham, J. Ruland, H. Yoshida, D. Liu, S. J. Elledge, and T. W. Mak. 2000. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 2871824-1827. [DOI] [PubMed] [Google Scholar]
- 25.Jascur, T., H. Brickner, I. Salles-Passador, V. Barbier, A. El Khissiin, B. Smith, R. Fotedar, and A. Fotedar. 2005. Regulation of p21WAF1/CIP1 stability by WISp39, a Hsp90 binding TPR protein. Mol. Cell 17237-249. [DOI] [PubMed] [Google Scholar]
- 26.Jin, S. H., T. I. Kim, K. M. Yang, and W. H. Kim. 2007. Thalidomide destabilizes cyclooxygenase-2 mRNA by inhibiting p38 mitogen-activated protein kinase and cytoplasmic shuttling of HuR. Eur. J. Pharmacol. 55814-20. [DOI] [PubMed] [Google Scholar]
- 27.Keene, J. D. 1999. Why is Hu where? Shuttling of early-response-gene messenger RNA subsets. Proc. Natl. Acad. Sci. USA 965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanna, K. K., and S. P. Jackson. 2001. DNA double-strand breaks: signaling, repair and the cancer connection. Nat. Genet. 27247-254. [DOI] [PubMed] [Google Scholar]
- 29.Kim, G. Y., S. E. Mercer, D. Z. Ewton, Z. Yan, K. Jin, and E. Friedman. 2002. The stress-activated protein kinases p38 alpha and JNK1 stabilize p21Cip1 by phosphorylation. J. Biol. Chem. 27729792-29802. [DOI] [PubMed] [Google Scholar]
- 30.Kim, H. H., K. Abdelmohsen, A. Lal, R. Pullmann, Jr., X. Yang, S. Galban, S. Srikantan, J. L. Martindale, J. Blethrow, K. M. Shokat, and M. Gorospe. 2008. Nuclear HuR accumulation through phosphorylation by Cdk1. Genes Dev. 221804-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim, H. H., X. Yang, Y. Kuwano, and M. Gorospe. 2008. Modification at HuR(S242) alters HuR localization and proliferative influence. Cell Cycle 73371-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumagai, A., J. Lee, H. Y. Yoo, and W. G. Dunphy. 2006. TopBP1 activates the ATR-ATRIP complex. Cell 124943-955. [DOI] [PubMed] [Google Scholar]
- 33.Lafarga, V., A. Cuadrado, and A. R. Nebreda. 2007. p18Hamlet mediates different p53-dependent responses to DNA-damage inducing agents. Cell Cycle 62319-2322. [DOI] [PubMed] [Google Scholar]
- 34.Lavin, M. F., and Y. Shiloh. 1997. The genetic defect in ataxia-telangiectasia. Annu. Rev. Immunol. 15177-202. [DOI] [PubMed] [Google Scholar]
- 35.Macleod, K. F., N. Sherry, G. Hannon, D. Beach, T. Tokino, K. Kinzler, B. Vogelstein, and T. Jacks. 1995. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 9935-944. [DOI] [PubMed] [Google Scholar]
- 36.Manke, I. A., A. Nguyen, D. Lim, M. Q. Stewart, A. E. Elia, and M. B. Yaffe. 2005. MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol. Cell 1737-48. [DOI] [PubMed] [Google Scholar]
- 37.Pabla, N., S. Huang, Q. S. Mi, R. Daniel, and Z. Dong. 2008. ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J. Biol. Chem. 2836572-6583. [DOI] [PubMed] [Google Scholar]
- 38.Raman, M., S. Earnest, K. Zhang, Y. Zhao, and M. H. Cobb. 2007. TAO kinases mediate activation of p38 in response to DNA damage. EMBO J. 262005-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinhardt, H. C., A. S. Aslanian, J. A. Lees, and M. B. Yaffe. 2007. p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell 11175-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rössig, L., A. S. Jadidi, C. Urbich, C. Badorff, A. M. Zeiher, and S. Dimmeler. 2001. Akt-dependent phosphorylation of p21Cip1 regulates PCNA binding and proliferation of endothelial cells. Mol. Cell. Biol. 215644-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rotman, G., and Y. Shiloh. 1998. ATM: from gene to function. Hum. Mol. Genet. 71555-1563. [DOI] [PubMed] [Google Scholar]
- 42.Schwaller, J., H. P. Koeffler, G. Niklaus, P. Loetscher, S. Nagel, M. F. Fey, and A. Tobler. 1995. Posttranscriptional stabilization underlies p53-independent induction of p21WAF1/CIP1/SDI1 in differentiating human leukemic cells. J. Clin. Investig. 95973-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheaff, R. J., J. D. Singer, J. Swanger, M. Smitherman, J. M. Roberts, and B. E. Clurman. 2000. Proteasomal turnover of p21Cip1 does not require p21Cip1 ubiquitination. Mol. Cell 5403-410. [DOI] [PubMed] [Google Scholar]
- 44.Taher, M. M., C. M. Hershey, J. D. Oakley, and K. Valerie. 2000. Role of the p38 and MEK-1/2/p42/44 MAP kinase pathways in the differential activation of human immunodeficiency virus gene expression by ultraviolet and ionizing radiation. Photochem. Photobiol. 71455-459. [DOI] [PubMed] [Google Scholar]
- 45.Toledo, L. I., M. Murga, P. Gutierrez-Martinez, R. Soria, and O. Fernandez-Capetillo. 2008. ATR signaling can drive cells into senescence in the absence of DNA breaks. Genes Dev. 22297-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vogelstein, B., D. Lane, and A. J. Levine. 2000. Surfing the p53 network. Nature 408307-310. [DOI] [PubMed] [Google Scholar]
- 47.Waldman, T., C. Lengauer, K. W. Kinzler, and B. Vogelstein. 1996. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature 381713-716. [DOI] [PubMed] [Google Scholar]
- 48.Wang, W., H. Furneaux, H. Cheng, M. C. Caldwell, D. Hutter, Y. Liu, N. Holbrook, and M. Gorospe. 2000. HuR regulates p21 mRNA stabilization by UV light. Mol. Cell. Biol. 20760-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, X., C. H. McGowan, M. Zhao, L. He, J. S. Downey, C. Fearns, Y. Wang, S. Huang, and J. Han. 2000. Involvement of the MKK6-p38γ cascade in γ-radiation-induced cell cycle arrest. Mol. Cell. Biol. 204543-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng, Y. X., and W. S. el-Deiry. 1996. Regulation of p21WAF1/CIP1 expression by p53-independent pathways. Oncogene 121557-1564. [PubMed] [Google Scholar]
- 51.Zhou, B. P., Y. Liao, W. Xia, B. Spohn, M. H. Lee, and M. C. Hung. 2001. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 3245-252. [DOI] [PubMed] [Google Scholar]