Abstract
PTP1B−/− mice are resistant to diet-induced obesity due to leptin hypersensitivity and consequent increased energy expenditure. We aimed to determine the cellular mechanisms underlying this metabolic state. AMPK is an important mediator of leptin's metabolic effects. We find that α1 and α2 AMPK activity are elevated and acetyl-coenzyme A carboxylase activity is decreased in the muscle and brown adipose tissue (BAT) of PTP1B−/− mice. The effects of PTP1B deficiency on α2, but not α1, AMPK activity in BAT and muscle are neuronally mediated, as they are present in neuron- but not muscle-specific PTP1B−/− mice. In addition, AMPK activity is decreased in the hypothalamic nuclei of neuronal and whole-body PTP1B−/− mice, accompanied by alterations in neuropeptide expression that are indicative of enhanced leptin sensitivity. Furthermore, AMPK target genes regulating mitochondrial biogenesis, fatty acid oxidation, and energy expenditure are induced with PTP1B inhibition, resulting in increased mitochondrial content in BAT and conversion to a more oxidative muscle fiber type. Thus, neuronal PTP1B inhibition results in decreased hypothalamic AMPK activity, isoform-specific AMPK activation in peripheral tissues, and downstream gene expression changes that promote leanness and increased energy expenditure. Therefore, the mechanism by which PTP1B regulates adiposity and leptin sensitivity likely involves the coordinated regulation of AMPK in hypothalamus and peripheral tissues.
Protein tyrosine phosphatase 1B (PTP1B) belongs to a family of tyrosine phosphatases with diverse roles in eukaryotes (2, 4). PTP1B attenuates insulin signaling by dephosphorylating the insulin receptor (19, 22, 61) and possibly IRS-1 (9, 23) and leptin signaling by dephosphorylating JAK2, which phosphorylates the leptin receptor and associated substrates (10, 45, 67). PTP1B-deficient mice are insulin hypersensitive, lean, and resistant to diet-induced obesity (20, 36) due, at least in part, to increased energy expenditure (36). The leanness can be explained by the absence of PTP1B in neurons, because neuron-specific PTP1B−/− mice also have reduced body weight and adiposity and increased energy expenditure (6). In contrast, muscle- and liver-specific PTP1B-deficient mice have normal body weight with improved insulin sensitivity, whereas adipose-PTP1B-deficient mice have increased body weight (6, 15, 16). These data suggest that PTP1B in peripheral tissues such as muscle and liver is an important mediator of peripheral insulin sensitivity, whereas PTP1B in the nervous system plays a critical role in regulating energy expenditure and adiposity (6).
The adipocyte-derived hormone leptin plays an essential role in regulating energy homeostasis by acting on multiple tissues, most importantly the hypothalamus, to regulate food intake and energy expenditure (1). PTP1B−/− mice have enhanced basal and leptin-stimulated hypothalamic STAT3 phosphorylation and are hypersensitive to leptin's effect on food intake and body weight (10, 67). The overexpression of PTP1B in heterologous cells dose dependently reduces the leptin-induced phosphorylation of JAK2 and STAT3 and inhibits leptin-stimulated STAT3-dependent reporter gene activation (10, 35, 39, 67). These and other data established that enhanced leptin sensitivity contributes to the leanness in PTP1B−/− mice. We sought to determine the cellular mechanisms underlying the altered energy homeostasis in the setting of PTP1B deficiency.
AMP-activated protein kinase (AMPK) is a major mediator of leptin's metabolic effects (43, 44). AMPK is a fuel-sensing enzyme complex activated by cellular stresses that increase AMP or deplete ATP, including hypoxia, ischemia, glucose deprivation, uncouplers of oxidative phosphorylation, exercise, and muscle contraction (66). AMPK also is activated by the antidiabetic drugs metformin (68) and the thiazolidinediones (21). Mechanisms involved in AMPK activation include (i) the binding of AMP to an allosteric site on the γ subunit, which renders the holoenzyme resistant to inactivating serine phosphatases and also may have direct allosteric effects on kinase activity (55), and (ii) phosphorylation by upstream AMPK kinases of the α (catalytic) subunits on Thr172, which is essential for kinase activity (29). Once activated, AMPK phosphorylates multiple downstream substrates, leading to the inhibition of ATP-utilizing pathways, such as fatty acid synthesis, and the activation of ATP-generating pathways, including fatty acid oxidation (34).
The phosphorylation of acetyl coenzyme A (acetyl-CoA) carboxylase (ACC) by AMPK results in the inhibition of ACC activity, decreased malonyl-CoA content, and a subsequent increase in fatty acid oxidation in skeletal muscle caused by the disinhibition of carnitine palmitoyltransferase 1 (27, 52, 62). The leptin stimulation of muscle fatty acid oxidation is mediated by AMPK (44). AMPK also is an important regulator of muscle mitochondrial biogenesis and function (7, 37, 48, 58, 63). This may, in part, be mediated by peroxisome proliferator-activated receptor γ (PPARγ)-coactivator 1α (PGC-1α), because AMPK induces the expression and phosphorylation of PGC-1α, which regulates mitochondrial biogenesis and muscle fiber type (31).
In addition to a role for AMPK in leptin action in peripheral tissues, the inhibition of hypothalamic AMPK activity by leptin plays an important role in mediating leptin's effect on food intake and energy homeostasis (43). This appears to involve neurons that express neuropeptide Y (NPY) and agouti-related peptide (AgRP), since the expression of constitutively active AMPK in the basomedial hypothalamus augments NPY/AgRP expression (43). Furthermore, the deletion of the AMPK α2 catalytic subunit specifically in these neurons results in leanness, whereas deletion in proopiomelanocortin (POMC)-expressing neurons results in mild obesity (13).
To determine whether alterations in AMPK contribute to increased energy expenditure and leanness in PTP1B−/− mice, we investigated the AMPK pathway in peripheral tissues and hypothalamus. We demonstrate that the global absence of PTP1B alters AMPK and downstream biological processes in multiple tissues, and that neuronal PTP1B regulates AMPK activity in peripheral tissues in an isoform-specific manner. Our data establish a novel link between PTP1B and AMPK, two signaling molecules that are critical in the regulation of energy homeostasis.
MATERIALS AND METHODS
Animals.
The generation of PTP1B−/− mice (36), neuron-specific PTP1B−/− (Nes-PTP1B−/−) mice (6), and muscle specific-PTP1B−/− (MCK-PTP1B−/−) mice (15) was described previously. All experiments used male mice on a mixed C57BL/6Jx129 background that were housed with a 12-h light/12-h dark cycle in a temperature-controlled barrier facility with free access to water and standard laboratory chow (Purina no. 5053; fat content of 4.5% by weight/11.9% by calories). For some studies, 5- to 6-week-old PTP1B−/− mice were put on a high-fat diet (Harlan Teklad 93075; fat content of 29.3% by weight/54.8% by calories) for 8 weeks and sacrificed at 13 to 14 weeks of age. Mice were sacrificed in the fed state, except for the neuropeptide measurements, for which mice were fasted for 48 h. All aspects of animal care were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee and the animal welfare committee at Harvard Medical School.
Measurement of enzyme activities.
Tissue lysates were prepared as described in the supplemental material. To measure AMPK activity, protein (100 μg) was immunoprecipitated with sheep polyclonal antibodies specific to α1 or α2 AMPK (kindly provided by David Carling, Imperial College, London, United Kingdom) bound to protein G-Sepharose overnight. The kinase activity of the immunoprecipitates was measured using SAMS peptide and [γ-32P]ATP (30, 43, 44).
ACC activity measurements were based on acetyl-CoA-dependent 14CO2 fixation (24, 44). Detailed procedures are described in the supplemental material.
Immunoblotting.
Protein (20 μg) was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and phosphorylation and total levels of specific proteins were measured by immunoblotting. Thr172 phospho-AMPK and MO25α antibodies (rabbit polyclonal) were from Cell Signaling Technology (Beverly, MA). α2 AMPK, troponin I (slow), UCP1, β-actin (goat polyclonal), and LKB1 (mouse monoclonal) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against α1 AMPK and Ser79 phospho-ACC (rabbit polyclonal) were from Upstate Biotechnology (Lake Placid, NY). Mouse monoclonal antibody against cell death-inducing DFFA-like effector A (CIDEA) was from Novus Biologicals (Littleton, CO). Total ACC was detected using Streptavidin-conjugated horseradish peroxidase (Amersham Biosciences, Piscataway, NJ) (44).
Dissection of hypothalamic regions.
For AMPK activity measurements, hypothalamic regions were dissected according to a previous description (43). Briefly, each hypothalamic region was dissected from 1-mm-thick sagittal sections of fresh brain. Arcuate (ARC), paraventricular (PVN), and ventromedial/dorsomedial (VMH/DMH) hypothalamus were dissected from the first sections from the midline of the brain. Lateral hypothalamus (LH) was dissected from the next lateral sections. Coordinates for each hypothalamic region were described previously (43).
Quantitative real-time RT-PCR.
Total RNA was isolated from extensor digitorum longus (EDL) skeletal muscle, interscapular brown adipose tissue (BAT), and hypothalamus using TRIReagent according to the manufacturer's instructions (Molecular Research Center, Cincinnati, OH). Messenger RNAs of specific genes were quantified by real-time reverse transcription-PCR (RT-PCR) using the TaqMan one-step RT-PCR Master mix (Applied Biosystems) and a Stratagene Mx3000P or Mx4000P system (Stratagene, La Jolla, CA) as described previously (43). The sequences for each primer/probe set used are listed in Table S1 in the supplemental material.
Hypothalamic neuropeptide measurements.
Neuropeptides were measured in total hypothalamus by quantitative RT-PCR as described above. To measure leptin's effects on neuropeptide expression, PTP1B−/− and wild-type (WT) mice were fasted for 48 h starting at 8 a.m. on day 1. Leptin (1 μg/g of body weight) was injected intraperitoneally at 6 p.m. and 8 a.m. from day 1 to day 3. One hour after the last leptin injection on day 3, mice were sacrificed and whole hypothalamus was removed for neuropeptide measurements.
Light microscopy and immunohistochemistry.
Mice were perfused transcardially with 4% paraformaldehyde-0.1 M phosphate buffer (pH 7.4). BAT and EDL muscle were dissected, fixed, paraffin embedded, and cut into 3-μm sections. Sections of BAT were used for the localization of UCP1, and transverse sections of EDL muscle were used for the detection of cytochrome c by immunohistochemistry (32), as described in the supplemental material.
Electron microscopy.
Small fragments of BAT were fixed in 2% glutaraldehyde-2% paraformaldehyde-0.1 M phosphate buffer (pH 7.4) for 4 h, postfixed in 1% osmium tetroxide, and embedded in an Epon-Araldite mixture. Semithin sections (2 μm) were stained with toluidine blue, and thin sections were obtained with an MT-X ultratome (RMC, Tucson, Arizona), stained with lead citrate, and examined with a CM10 transmission electron microscope (Philips, Eindhoven, Netherlands).
Morphometric analysis.
To evaluate the number of cytochrome c-positive muscle cells in EDL muscle, we studied one transverse section for each animal. About 1,600 muscle cells were analyzed per section. Muscle morphometry was assessed without prior knowledge of the genotype. Results are reported as the percentage of muscle cells exhibiting different degrees of cytochrome c positivity versus the total number of muscle cells in the section.
The morphometric evaluation of BAT mitochondria was performed by image analysis software (Lucia IMAGE, version 4.82; Laboratory Imaging, CZ) without the prior knowledge of the genotype. Six electron micrographs from the interscapular depot of each mouse were obtained at a magnification of ×9,800 and were acquired as digital images by a scanner at 200% enlargement. Mitochondrial (calculated as the number of mitochondria in a cytoplasmic area free of lipid droplets) and crista (the number of cristae per mitochondrial area) density were quantified.
Adenine nucleotide measurement.
BAT and EDL tissues were obtained by freeze-clamping (40) in mice under ketamine-xylazine anesthesia, and AMP, ADP, and ATP content was measured by high-performance liquid chromatography (5) using a Waters high-performance liquid chromatography system (Waters Co., Milford, MA) as described in the supplemental material.
Statistical analysis.
Results are presented as means ± standard errors of the means (SEM). Differences between groups were examined for statistical significance by Student's t test or analysis of variance.
RESULTS
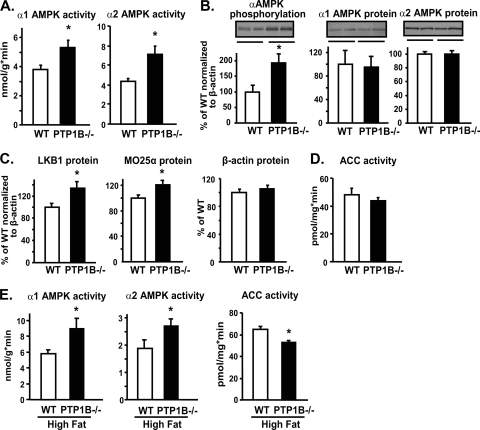
At 3 months of age, PTP1B−/− mice on a chow diet were ∼3 g lighter than WT littermates (WT, 28.8 ± 0.5; PTP1B−/−, 25.9 ± 0.5 g; P < 0.05), consistent with previous observations (20, 36). α1 and α2 AMPK activities were increased by 40 and 65%, respectively, in EDL muscle of PTP1B−/− mice compared to those in WT mice (P < 0.05) (Fig. 1A). This was associated with increased AMPK α subunit phosphorylation at Thr172 without changes of α1 and α2 AMPK protein levels (Fig. 1B); thus, the specific activity of both isoforms was increased. Similar increases in α1 and α2 AMPK activities were seen in EDL muscle in an independent cohort of PTP1B−/− mice at 5 months of age (data not shown). LKB1 is one of the upstream kinases that activates AMPK by phosphorylating it at Thr172 (28, 57, 65). LKB1 associates with Ste20-related adaptor protein (STRADα/β) and mouse protein 25 (MO25α/β), forming a heterotrimeric complex (64). Although we could not measure STRAD levels owing to the lack of available reagents, both LKB1 (30%) and MO25α (20%) protein levels were elevated in the muscle of PTP1B−/− mice (Fig. 1C) (P < 0.05) and are likely to contribute to constitutive AMPK activation. Consistent with the increased AMPK activity, the activity of ACC tended to be lower in EDL muscle of PTP1B−/− mice than in WT mice on a chow diet (Fig. 1D). In PTP1B−/− mice on a high-fat diet for 8 weeks (body weight: WT, 32.4 ± 1.1 g; PTP1B−/−, 28.1 ± 0.6 g; P < 0.05), α1 and α2 AMPK activities in EDL muscle were increased by 55 and 45%, respectively, and ACC activity was decreased by 21% (Fig. 1E).
FIG. 1.
Effect of PTP1B deficiency on the AMPK pathway in EDL muscle. (A) α1 and α2 AMPK activities. (B and C) Immunoblotting of α AMPK subunit phosphorylation on Thr172, α1 and α2 AMPK, LKB1, MO25α, and β-actin protein levels. Data are normalized to β-actin. (D) ACC activity. (E) α1 and α2 AMPK and ACC activities. All experiments were carried out using 3-month-old male WT and whole-body PTP1B−/− mice on a chow (A to D) or high-fat (E) diet. Data are expressed as means ± SEM; n = 6 to 8 for each genotype. *, P < 0.05 compared to results for WT mice.
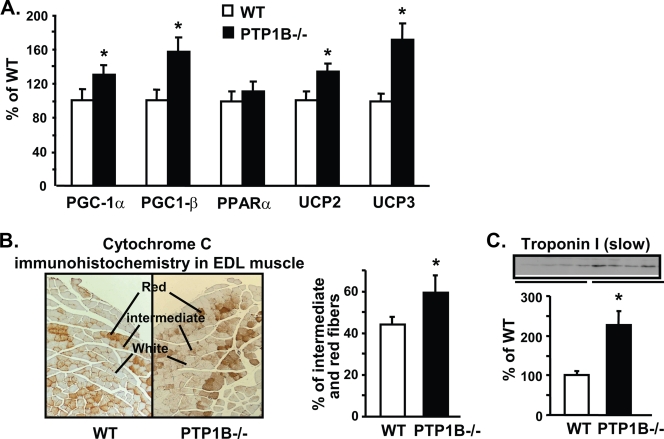
Activation of AMPK in muscle of PTP1B−/− mice was accompanied by the upregulation of AMPK target genes involved in mitochondrial biogenesis and muscle fiber type composition (PGC-1α, 35%; PGC-1β, 57%) and mitochondrial uncoupling (UCP2, 27%; UCP3, 71%) (Fig. 2A) (P < 0.05). EDL muscle normally is a fast-twitch muscle primarily composed of type II fibers, which have lower mitochondrial content and oxidative capacity than type I fibers. The proportion of red (type I) and intermediate fibers in EDL muscle of PTP1B−/− mice was increased by 35% compared to that of WT mice, as reflected in immunohistochemical staining for the mitochondrial marker cytochrome c (Fig. 2B). In parallel, the level of the inhibitory subunit of the troponin complex, troponin I (slow), a classic marker of type I slow-twitch fibers, was increased by more than twofold in EDL muscle of PTP1B−/− mice. These data indicate that the absence of PTP1B leads to a shift to more slow-twitch type I fibers, which likely results from increased AMPK-mediated PGC-1α expression.
FIG. 2.
Effect of PTP1B deficiency on gene expression and fiber type composition in EDL muscle. (A) Levels of PGC-1α, PGC-1β, PPARα, UCP2, and UCP3 mRNAs were measured by quantitative RT-PCR. (B) Left, cytochrome c immunohistochemical staining in EDL muscle; right, quantification of red and intermediate fiber composition determined by cytochrome c staining in EDL muscle. (C) Immunoblotting of the slow-twitch type I fiber marker troponin I (slow) in muscle. All experiments were carried out using 3- to 4-month-old male mice on a chow diet. Data are expressed as means ± SEM; n = 6 to 8 for each genotype for panels A and C and 5 per genotype for panel B. *, P < 0.05 compared to results for WT mice.
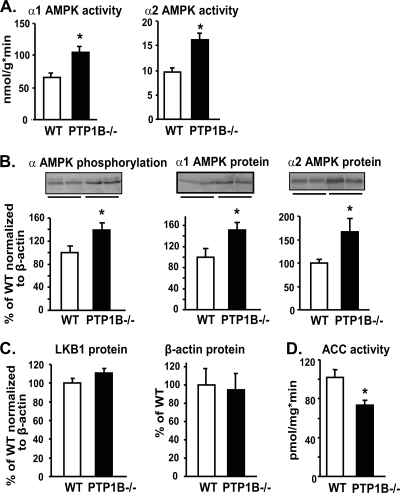
BAT is a major thermogenic organ that regulates energy expenditure. α1 and α2 AMPK activities were elevated by 60% in the BAT of PTP1B−/− mice compared to that of WT mice at both 3 (Fig. 3A) (P < 0.001) and 5 months (data not shown) of age. The phosphorylation of the AMPK α subunits at Thr172 was increased by 30% in PTP1B−/− mice (P < 0.05) (Fig. 3B). Unlike the case for muscle tissue, α1 and α2 AMPK protein levels were increased by 40 to 60% in BAT of PTP1B−/− mice (P < 0.05) (Fig. 3B). Also in contrast to results for muscle, the levels of LKB1 protein in BAT were similar between WT and PTP1B−/− mice (Fig. 3C). Thus, increased α1 and α2 AMPK protein levels likely contribute to enhanced AMPK activity in BAT. The latter was accompanied by, and most likely causes, an approximately 30% decrease in ACC activity (P < 0.05) (Fig. 3D). In BAT, CIDEA binds to the AMPK β subunit, leading to its ubiquitination and degradation. This results in instability and decreased protein levels of the α, β, and γ complex (49). However, neither CIDEA RNA nor protein levels were altered in the BAT of PTP1B−/− mice compared to those of WT mice (data not shown).
FIG. 3.
Effect of PTP1B deficiency on the AMPK pathway in BAT. (A) α1 and α2 AMPK activities. (B and C) Immunoblotting of α AMPK subunit phosphorylation on Thr172, α1 and α2 AMPK, LKB1, and β-actin protein levels. Data are normalized to β-actin. (D) ACC activity. All experiments were carried out using 3-month-old male mice on a chow diet. Data are expressed as means ± SEM; n = 6 to 8 for each genotype. *, P < 0.05 compared to results for WT mice.
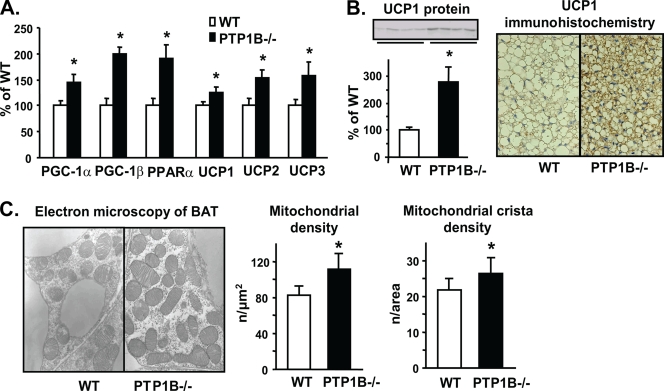
Furthermore, the expression of several AMPK target genes was induced in the BAT of PTP1B−/− mice. These included genes involved in fatty acid oxidation, mitochondrial biogenesis, and thermogenesis, including PGC-1α (44%), PGC-1β (98%), PPARα (91%), mitochondrial UCP1 (24%), UCP2 (53%), and UCP3 (57%) (P < 0.05) (Fig. 4A). In addition, UCP1 protein levels were increased, as revealed by immunoblotting (1.8-fold increase; P < 0.05) (Fig. 4B, left) and immunohistochemistry (Fig. 4B, right). Lipid droplets in brown adipocytes of PTP1B−/− mice were smaller, indicating enhanced lipid oxidation and/or reduced lipogenesis (Fig. 4B). The electron microscopic examination of BAT showed that the mean mitochondrial density (21%) and mean mitochondrial crista density (35%) were increased in PTP1B−/− mice (P < 0.05) (Fig. 4C), indicating enhanced net mitochondrial biogenesis. The increased mitochondrial density along with increased UCP expression is expected to increase energy expenditure, as is observed in PTP1B−/− mice (36).
FIG. 4.
Effect of PTP1B deficiency on BAT gene expression, morphology, and mitochondrial content. (A) Levels of PGC-1α, PGC-1β, PPARα, UCP1, UCP2, and UCP3 mRNAs were measured by quantitative RT-PCR; n = 7 to 17 for each genotype. (B) Left, UCP1 protein level in BAT; right, UCP1 immunohistochemical staining in BAT; n = 6 to 8 per genotype [left] or 5 per genotype [right]. (C) Left, electron microscopy of BAT in WT and PTP1B−/− mice. Right, quantification of mitochondrial density and mitochondrial crista density in BAT of WT and PTP1B−/− mice. Five mice were used for each genotype, and six electron micrographs from each mouse were examined. All experiments were carried out on 3- to 4-month-old male mice on a chow diet. Data are expressed as means ± SEM. *, P < 0.05 compared to results for WT mice.
We sought to further investigate the mechanisms by which the absence of PTP1B regulates AMPK activity. PTP1B deficiency resulted in a 93% elevation of AMP content in EDL muscle and a 49% elevation in BAT of PTP1B−/− mice compared to those in WT mice, resulting in an increased AMP/ATP ratio in both tissues (Table 1).
TABLE 1.
Adenine nucleotide content and AMP/ATP and ATP/ADP ratios in EDL muscle and BAT of WT and PTP1B−/− micea
| Tissue and mouse type | Adenine nucleotide content (nmol/mg protein)
|
AMP/ATP | ATP/ADP | ||
|---|---|---|---|---|---|
| AMP | ADP | ATP | |||
| EDL muscle | |||||
| WT | 0.29 ± 0.03 | 3.66 ± 0.43 | 55.61 ± 0.79 | 0.005 ± 0.001 | 17.57 ± 2.54 |
| PTP1B−/− | 0.56 ± 0.08* | 3.43 ± 0.46 | 54.71 ± 1.03 | 0.01 ± 0.001* | 19.16 ± 3 |
| BAT | |||||
| WT | 3.08 ± 0.18 | 4.99 ± 0.35 | 8.92 ± 0.88 | 0.38 ± 0.04 | 1.91 ± 0.24 |
| PTP1B−/− | 4.57 ± 0.3* | 5.75 ± 0.33 | 7.66 ± 0.3 | 0.6 ± 0.03* | 1.37 ± 0.05 |
Male mice on a chow diet were sacrificed in the fed state. Data are expressed as means ± SEM. n = 10 for each genotype. *, P < 0.05 compared to results for WT mice.
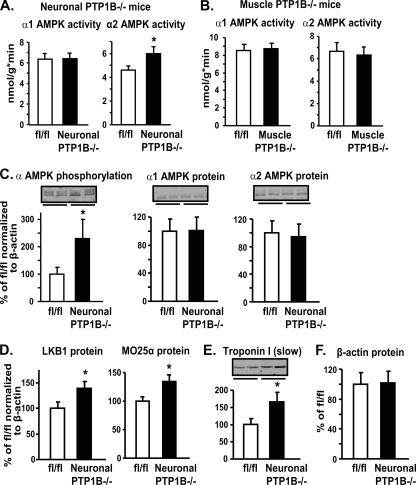
We next asked whether increased AMPK activity in muscle and BAT of whole-body PTP1B−/− mice is mediated through the deletion of PTP1B in the nervous system or more directly through PTP1B deficiency in muscle or BAT. We found no differences in α1 AMPK activity in EDL muscle of either neuron-specific (Nes-PTP1B−/−) or muscle-specific (MCK-PTP1B−/−) mice (Fig. 5A and B). However, α2 AMPK activity was increased by 30% in EDL of neuronal PTP1B−/− mice compared to that in control littermates (Fig. 5A) but was not increased in muscle PTP1B−/− mice (Fig. 5B). Accompanying the increased α2 AMPK activity, AMPK α subunit phosphorylation was 2.3-fold increased in muscle of neuronal PTP1B−/− mice compared to that in control littermates, without changes in α2 protein levels (Fig. 5C). LKB1 (40%) and MO25α (34%) levels in EDL muscle of neuronal PTP1B−/− mice also were increased compared to those in control mice (Fig. 5D). In addition, the neuronal deletion of PTP1B resulted in the upregulation of the slow-twitch fiber type marker troponin I (slow) (Fig. 5E), paralleling the shift to more oxidative muscle fibers seen in whole-body PTP1B−/− mice. The comparable effects of whole-body and neuronal PTP1B deficiency on the LKB1-α2AMPK pathway and downstream muscle fiber type switching indicate that these alterations are caused by the absence of PTP1B in neurons (see the model in Fig. 8). In contrast, the increased α1 AMPK activity in muscle does not appear to be regulated by neuronal PTP1B.
FIG. 5.
Effect of neuron- and muscle-specific PTP1B deficiency on the AMPK pathway in EDL muscle. (A and B) α1 and α2 AMPK activities in EDL muscle of neuronal PTP1B−/− (A) and muscle PTP1B−/− (B) mice. (C to F) Immunoblotting of α AMPK phosphorylation, α1 and α2 AMPK, LKB1, MO25α, troponin I (slow), and β-actin protein levels in neuronal PTP1B−/− mice. Data in panels C to E are normalized to β-actin. All experiments were carried out using 2-month-old male flox/flox (fl/fl) control, neuronal PTP1B−/−, and muscle PTP1B−/− mice on a chow diet. Data are expressed as means ± SEM. n = 6 to 8 for each genotype. *, P < 0.05 compared to results for WT mice.
FIG. 8.
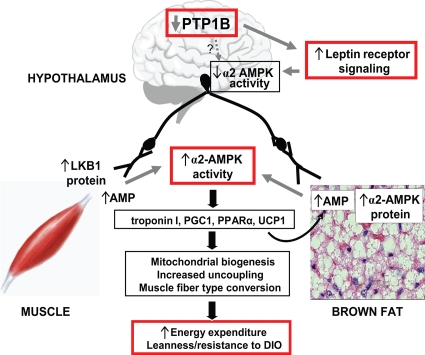
Proposed model for leanness and resistance to diet-induced obesity following PTP1B inhibition. The reduction of PTP1B in neurons leads to suppression of hypothalamic α2 AMPK activity and activation of α2 AMPK in muscle and BAT. Multiple mechanisms are involved, including increased AMP levels and increased LKB1 and AMPK α2 subunit protein expression in peripheral tissues. These alterations in AMPK activity are accompanied by the induction of genes that regulate muscle fiber type, mitochondrial biogenesis, the uncoupling of oxidative phosphorylation, and fatty acid oxidation, with resultant biologic changes that contribute to increased energy expenditure, leanness, and resistance to diet-induced obesity (DIO).
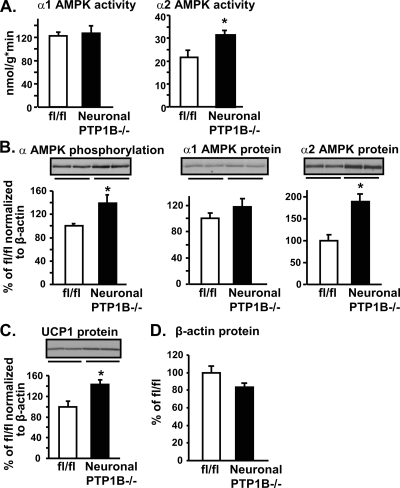
Likewise, α2, but not α1, AMPK activity was increased in BAT of neuronal PTP1B−/− mice, establishing that the nervous system regulates BAT α2 AMPK activity (Fig. 6A). As observed in whole-body PTP1B−/− mice, the increase in α2 AMPK activity in BAT of neuronal PTP1B−/− mice could be accounted for by an increase (∼90%) in α2 protein levels (Fig. 6B). UCP1 protein content also was increased (by 42%) with the neuronal deletion of PTP1B, reflecting the regulation of UCP1 by sympathetic innervation (17, 47) (Fig. 6C). Thus, in BAT as well as in muscle of whole-body PTP1B−/− mice, alterations in α2, but not α1, AMPK activity are mediated by PTP1B in neurons.
FIG. 6.
Effect of neuron-specific PTP1B deficiency on the AMPK pathway and UCP1 protein expression in BAT. (A) α1 and α2 AMPK activities. (B to D) Immunoblotting of α AMPK phosphorylation, α1 and α2 AMPK, UCP1, and β-actin protein levels. Data in panels B and C are normalized to β-actin. All experiments were carried out using 2-month-old male flox/flox (fl/fl) control and neuronal PTP1B−/− mice on a chow diet. Data are expressed as means ± SEM; n = 6 to 8 for each genotype. *, P < 0.05 compared to results for fl/fl mice.
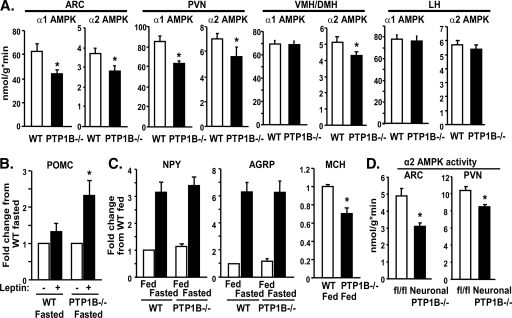
Because PTP1B−/− mice are lean and have increased energy expenditure yet do not have a compensatory increase in food intake (36), and AMPK activity in the hypothalamus regulates these processes (43), we investigated AMPK activity in specific hypothalamic regions. In PTP1B−/− mice, α1 and α2 AMPK activities were decreased 25 to 40% in ARC and PVN nuclei, and α2 AMPK activity was also decreased in the VMH/DMH (P < 0.05) (Fig. 7A). In contrast, α1 and α2 AMPK activities were not affected in LH, consistent with the lack of leptin effect on AMPK in the LH of normal mice (43). These changes in hypothalamic AMPK α2 activity could explain the enhanced leptin-induced biologic effects in PTP1B−/− mice (10, 67).
FIG. 7.
Effect of PTP1B deficiency on hypothalamic AMPK activity and neuropeptide expression. Panels A to C show data from whole-body PTP1B−/− mice, and panel D shows data from neuronal PTP1B−/− mice. (A) α1 and α2 AMPK activities in ARC, PVN, VMH/DMH, and LH of whole-body PTP1B−/− mice in the fed state. (B) POMC expression in hypothalamus of fasted mice injected with saline or leptin (intraperitoneally). (C) NPY and AgRP mRNA expression in hypothalamus of fasted and fed mice and MCH mRNA expression in hypothalamus of fed mice. (D) α2 AMPK activity in ARC and PVN hypothalamus of neuronal PTP1B−/− mice in the fed state. All experiments were carried out using 12-week-old (A) or 8- to 10-week-old (B to D) male WT and whole-body (A to C) or neuronal (D) PTP1B−/− mice on a chow diet. For panel A, n = 6 to 8 for each genotype; panels B and C, n = 12 per genotype; and panel D, n = 11 to 12 per genotype. Data are expressed as means ± SEM. *, P < 0.05 compared to results for WT mice.
To determine the functional significance of these alterations in AMPK activity, we measured neuropeptide expression. In the fasted state, leptin-induced POMC expression was augmented in whole-body PTP1B−/− mice (Fig. 7B). Fasting induced similar (not enhanced) increases in the orexigenic neuropeptides AgRP and NPY in PTP1B−/− mice (compared to levels in WT mice) (Fig. 7C) despite their lower body weight and serum leptin levels (36), consistent with increased leptin sensitivity. Further evidence of leptin hypersensitivity was the decreased expression of the orexigenic neuropeptide melanin-concentrating hormone (MCH) in PTP1B−/− mice (Fig. 7C). These alterations in neuropeptide expression are consistent with the reduced hypothalamic AMPK activity in these mice and may mediate the enhanced leptin action caused by PTP1B deficiency (10, 67).
To investigate whether alterations in hypothalamic AMPK activity result from the absence of PTP1B in neurons or indirectly from the lack of PTP1B in nonneuronal tissues, we measured AMPK activity in hypothalamus of neuronal PTP1B−/− mice. α2 AMPK activity was reduced in both ARC and PVN of neuronal PTP1B−/− mice (Fig. 7D), similar to the effects in whole-body PTP1B−/− mice (Fig. 7A). Consistent with these findings, we previously demonstrated alterations in hypothalamic neuropeptide expression in neuronal PTP1B−/− mice that would be expected as a result of reduced hypothalamic AMPK activity and that are indicative of leptin hypersensitivity (6).
DISCUSSION
Mice with either the whole-body or neuronal deletion of PTP1B are lean and hypersensitive to leptin. Furthermore, agents that inhibit PTP1B expression or activity (11, 25, 26, 50, 69) cause weight loss and enhance insulin sensitivity in obese mice. Such agents are under development for the treatment or prevention of obesity and type 2 diabetes in humans, but the mechanisms underlying their physiologic effects are unknown. In the present study, we investigated the cellular mechanisms responsible for the effects of PTP1B inhibition on body mass. We found altered regulation of AMPK activity in hypothalamus and in peripheral tissues such as muscle and BAT (see Table S2 in the supplemental material), all of which are key sites for the regulation of fuel homeostasis. Because neuronal PTP1B is critical for the control of adiposity, our results indicate that the altered regulation of peripheral α2 AMPK by neuronal PTP1B is a major mechanism for the beneficial effects of PTP1B inhibition on energy balance and adiposity (Fig. 8). Our data also provide novel insights into the tissue-specific regulation of LKB1 and the isoform-specific activation of AMPK.
To assess the significance of these alterations in AMPK activity in PTP1B−/− mice, we investigated downstream biologic effects known to be regulated by AMPK, such as muscle mitochondrial biogenesis (70) and conversion to a more slow-twitch type I oxidative fiber type (58). We demonstrate that both of these effects are enhanced in PTP1B−/− mice. Given that AMPK induces the expression of PGC1α and that mice overexpressing PGC-1α in muscle show fiber type switching (38), the AMPK-induced activation of PGC-1α in PTP1B−/− mice may contribute to these phenotypic changes. Increased AMPK activity also can account for the induction of genes involved in fatty acid oxidation in PTP1B−/− mice, given that the pharmacological or genetic activation of AMPK upregulates the activity and expression of these genes. These alterations, combined with the increased expression of genes encoding uncoupling proteins, undoubtedly play a major role in the increased energy dissipation in the absence of PTP1B. Interestingly, increased AMPK phosphorylation and activity were also reported in liver of mice deficient in stearoyl-CoA desaturase 1 (SCD1) (18), in white adipose tissue (WAT) of UCP1-overexpressing mice (41), and in muscle of mice with muscle-specific overexpression of human UCP3 (56). The increased AMPK activities in these animal models are associated with increased mitochondrial biogenesis (51), genes involved in fatty acid oxidation (46), and enhanced fatty acid oxidation (18, 41), and they likely contribute to reduced adiposity, increased energy expenditure (12, 18, 41), and improved glucose homeostasis (56).
Multiple cellular mechanisms contribute to the observed increase in AMPK activity in the setting of PTP1B inhibition. The whole-body deletion of PTP1B increases the AMP/ATP ratio in BAT and EDL muscle, which would acutely activate AMPK. The magnitude of the increase in tissue AMP content in PTP1B−/− mice is remarkable and similar to that seen under ischemic conditions (3). The enhanced expression of UCPs in BAT and EDL muscle and the resultant increased uncoupling of oxidative phosphorylation (41, 42) would be expected to increase tissue AMP content. Because UCP1 is regulated by PGC-1α, increased UCP expression could be both downstream and upstream (14, 42) of the increased AMPK activity. In addition, LKB1 and MO25 protein levels are increased in the muscle of PTP1B−/− mice, which is likely to contribute to increased AMPK activity. Increased LKB1 expression in skeletal muscle has been reported only in endurance training (59, 60) and hyperthyroidism (8). Both of these conditions also are associated with increased MO25 protein levels and enhanced AMPK activity. In contrast, in BAT of PTP1B−/− mice, LKB1 expression is normal and increased AMPK activity can be explained by the enhanced expression of the α1 and α2 catalytic subunits.
Although both α1 and α2 AMPK activities are increased in whole-body PTP1B−/− mice, our genetic data show that these isoforms are regulated by different physiologic mechanisms (see Table S2 in the supplemental material). The activation of α2 AMPK in both muscle and BAT is mediated indirectly through the neuronal deletion of PTP1B. In contrast, α1 AMPK activity is normal in neuronal PTP1B−/− mice, indicating that it is regulated by an alternative mechanism. Consistent with this conclusion, preliminary data show that denervation of BAT in normal FVB mice results in a selective decrease in α2 (and not α1) AMPK protein levels (data not shown). Interestingly, leptin, acting through the hypothalamus, selectively stimulates α2 (but not α1) AMPK activity in muscle (43, 44). Furthermore, preliminary experiments indicate that the expression of dominant-negative AMPK in the hypothalamus is sufficient to augment leptin action on α2 AMPK in skeletal muscle, whereas constitutively active AMPK in the hypothalamus is sufficient to block the activation of muscle α2 AMPK by leptin (data not shown). Therefore, the increased α2 AMPK activity in peripheral tissues in neuronal PTP1B−/− mice is a likely outcome, at least in part, of enhanced hypothalamic leptin-induced signaling, which results in reduced hypothalamic AMPK activity under ambient conditions (Fig. 8).
Our data, combined with previous observations, suggest that LKB1 selectively regulates α2 AMPK in vivo. For example, the deficiency of LKB1 in murine skeletal muscle and heart blunts contraction- and ischemia-induced α2 but not α1 AMPK activation (53, 54). Our findings that neuronal PTP1B−/− mice have increased LKB1 protein levels and increased α2, but not α1, AMPK activity in muscle are consistent with the notion that LKB1 preferentially promotes α2 AMPK activity.
Several lines of evidence suggest that increased α2, and not α1, AMPK activity in muscle and BAT is important for the altered energy homeostasis seen upon PTP1B inhibition. Most importantly, leanness in PTP1B−/− mice is due to a deficiency of PTP1B in neurons (6), and in the current study, neuronal PTP1B deficiency affected only α2 AMPK activity in muscle and BAT. The biologic significance of this is reflected in the fact that α2 AMPK inhibition alone results in impaired mitochondrial biogenesis (33, 70). The neuronal regulation of α2 AMPK also may influence energy balance through promoting mitochondrial uncoupling in BAT and fiber type switching in muscle, because we find comparable induction of UCP1 in BAT and troponin I (slow) in muscle of neuronal and whole-body PTP1B−/− mice.
Alterations in hypothalamic AMPK activity also are likely to contribute to the leanness in PTP1B−/− mice. Compared to controls, mice with the whole-body deletion of PTP1B have lower AMPK activity in ARC, PVN, and VMH/DMH but not LH, a distribution that is characteristic of leptin action (10, 43, 67). These changes in AMPK activity in hypothalamic nuclei that are critical for leptin action are also seen in neuronal PTP1B−/− mice, indicating that they emanate from the absence of PTP1B in neurons. Leptin decreases the expression of orexigenic neuropeptides while it increases the expression of anorexigenic neuropeptides. The inhibition of AMPK activity in the hypothalamus of normal mice is sufficient to produce leptin-like effects on neuropeptide expression (43). In whole-body PTP1B−/− mice, leanness is associated with lower leptin levels than those in WT mice, consistent with enhanced leptin sensitivity (36). Lower leptin levels normally result in augmented induction of AgRP and NPY upon fasting. However, the response in PTP1B−/− mice is similar to that of WT mice. In addition, PTP1B−/− mice have augmented leptin-induced POMC expression, and MCH expression is decreased. All of these observations are consistent with increased leptin sensitivity in PTP1B−/− mice and undoubtedly contribute to their enhanced metabolic responses to leptin (10, 67).
Collectively, our data provide a model that can explain the altered energy homeostasis caused by PTP1B inhibition (Fig. 8). The inhibition of PTP1B in the hypothalamus results in increased leptin signaling and augmented inhibition of hypothalamic AMPK activity (see Table S2 in the supplemental material). This, in turn, alters hypothalamic neuropeptide expression and leads to altered neuronal signaling in skeletal muscle and BAT. In muscle, these neuronal signals result in increased LKB1 and AMP levels and selective enhancement of α2 AMPK activity. Neuronal PTP1B inhibition also results in the selective activation of α2-AMPK in BAT, but this comes as a consequence of increased AMP and α2 subunit levels. In both muscle and BAT, the increased AMPK activity leads to gene expression changes that result in enhanced energy dissipation: switching to a more oxidative fiber type in muscle and increased uncoupling and mitochondrial biogenesis in BAT. Together, these changes provide a plausible explanation for how PTP1B inhibition in neurons causes leanness and increased sensitivity to the metabolic effects of leptin. Currently, PTP1B inhibitors as well as AMPK modulators are being developed for the treatment of obesity and type 2 diabetes, and our data demonstrate that such agents may share overlapping therapeutic mechanisms.
Supplementary Material
Acknowledgments
We are grateful to O. D. Peroni, P. Prada, and J. Kawashima for technical expertise, D. Carling and D. Ricquier for antibodies, and D. Olson and B. B. Lowell for comments on the manuscript.
This work was supported by NIH grants R01 DK60839 (to B.B.K. and B.G.N.), P01 DK56116 (to B.B.K.), the Physiology Core of P30 DK57521 (to B.B.K.), R37CA49152 (to B.G.N.), and grant FIRB n RBIN047PZY (to S.C.). T.P. was supported by fellowships from the Heart and Stroke Foundation of Canada and Canadian Diabetes Association. K.J.C. was supported by an NIH NRSA.
Footnotes
Published ahead of print on 15 June 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ahima, R. S., and J. S. Flier. 2000. Leptin. Annu. Rev. Physiol. 62413-437. [DOI] [PubMed] [Google Scholar]
- 2.Alonso, A., J. Sasin, N. Bottini, I. Friedberg, A. Osterman, A. Godzik, T. Hunter, J. Dixon, and T. Mustelin. 2004. Protein tyrosine phosphatases in the human genome Cell 117699-711. [DOI] [PubMed] [Google Scholar]
- 3.Altarejos, J. Y., M. Taniguchi, A. S. Clanachan, and G. D. Lopaschuk. 2005. Myocardial ischemia differentially regulates LKB1 and an alternate 5′-AMP-activated protein kinase kinase. J. Biol. Chem. 280183-190. [DOI] [PubMed] [Google Scholar]
- 4.Andersen, J. N., O. H. Mortensen, G. H. Peters, P. G. Drake, L. F. Iversen, O. H. Olsen, P. G. Jansen, H. S. Andersen, N. K. Tonks, and N. P. Moller. 2001. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol. Cell. Biol. 217117-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bak, M. I., and J. S. Ingwall. 1994. Acidosis during ischemia promotes adenosine triphosphate resynthesis in postischemic rat heart. In vivo regulation of 5′-nucleotidase. J. Clin. Investig. 9340-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bence, K. K., M. Delibegovic, B. Xue, C. Z. Gorgun, G. S. Hotamisligil, B. G. Neel, and B. B. Kahn. 2006. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat. Med. 12917-924. [DOI] [PubMed] [Google Scholar]
- 7.Bergeron, R., J. M. Ren, K. S. Cadman, I. K. Moore, P. Perret, M. Pypaert, L. H. Young, C. F. Semenkovich, and G. I. Shulman. 2001. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am. J. Physiol. Endocrinol. Metab. 281E1340-E1346. [DOI] [PubMed] [Google Scholar]
- 8.Branvold, D. J., D. R. Allred, D. J. Beckstead, H. J. Kim, N. Fillmore, B. M. Condon, J. D. Brown, S. N. Sudweeks, D. M. Thomson, and W. W. Winder. 2008. Thyroid hormone effects on LKB1, MO25, phospho-AMPK, phospho-CREB, and PGC-1α in rat muscle. J. Appl. Physiol. [DOI] [PubMed]
- 9.Calera, M. R., G. Vallega, and P. F. Pilch. 2000. Dynamics of protein-tyrosine phosphatases in rat adipocytes. J. Biol. Chem. 2756308-6312. [DOI] [PubMed] [Google Scholar]
- 10.Cheng, A., N. Uetani, P. D. Simoncic, V. P. Chaubey, A. Lee-Loy, C. J. McGlade, B. P. Kennedy, and M. L. Tremblay. 2002. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev. Cell 2497-503. [DOI] [PubMed] [Google Scholar]
- 11.Clampit, J. E., J. L. Meuth, H. T. Smith, R. M. Reilly, M. R. Jirousek, J. M. Trevillyan, and C. M. Rondinone. 2003. Reduction of protein-tyrosine phosphatase-1B increases insulin signaling in FAO hepatoma cells. Biochem. Biophys. Res. Commun. 300261-267. [DOI] [PubMed] [Google Scholar]
- 12.Clapham, J. C., J. R. Arch., H. Chapman, A. Haynes, C. Lister, G. B. Moore, V. Piercy, S. A. Carter, I. Lehner, S. A. Smith, L. J. Beeley, R. J. Godden, N. Herrity, M. Skehel, K. K. Changani, P. D. Hockings, D. G. Reid, S. M. Squires, J. Hatcher, B. Trail, J. Latcham, S. Rastan, A. J. Harper, S. Cadenas, J. A. Buckingham, M. D. Brand, and A. Abuin. 2000. Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature 406415-418. [DOI] [PubMed] [Google Scholar]
- 13.Claret, M., M. A. Smith, R. L. Batterham, C. Selman, A. I. Choudhury, L. G. Fryer, M. Clements, H. Al-Qassab, H. Heffron, A. W. Xu, J. R. Speakman, G. S. Barsh, B. Viollet, S. Vaulont, M. L. Ashford, D. Carling, and D. J. Withers. 2007. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J. Clin. Investig. 1172325-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cline, G. W., A. J. Vidal-Puig, S. Dufour, K. S. Cadman, B. B. Lowell, and G. I. Shulman. 2001. In vivo effects of uncoupling protein-3 gene disruption on mitochondrial energy metabolism. J. Biol. Chem. 27620240-20244. [DOI] [PubMed] [Google Scholar]
- 15.Delibegovic, M., K. K. Bence, N. Mody, E. G. Hong, H. J. Ko, J. K. Kim, B. B. Kahn, and B. G. Neel. 2007. Improved glucose homeostasis in mice with muscle-specific deletion of protein-tyrosine phosphatase 1B. Mol. Cell. Biol. 277727-7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delibegovic, M., D. Zimmer, C. Kauffman, K. Rak, E. G. Hong, Y. R. Cho, J. K. Kim, B. B. Kahn, B. G. Neel, and K. K. Bence. 2009. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes 58590-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denjean, F., J. Lachuer, A. Geloen, F. Cohen-Adad, C. Moulin, H. Barre, and C. Duchamp. 1999. Differential regulation of uncoupling protein-1, -2 and -3 gene expression by sympathetic innervation in brown adipose tissue of thermoneutral or cold-exposed rats. FEBS Lett. 444181-185. [DOI] [PubMed] [Google Scholar]
- 18.Dobrzyn, P., A. Dobrzyn, M. Miyazaki, P. Cohen, E. Asilmaz, D. G. Hardie, J. M. Friedman, and J. M. Ntambi. 2004. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc. Natl. Acad. Sci. USA 1016409-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubé, N., and M. L. Tremblay. 2005. Involvement of the small protein tyrosine phosphatases TC-PTP and PTP1B in signal transduction and diseases: from diabetes, obesity to cell cycle, and cancer. Biochim. Biophys. Acta 1754108-117. [DOI] [PubMed] [Google Scholar]
- 20.Elchebly, M., P. Payette, E. Michaliszyn, W. Cromlish, S. Collins, A. L. Loy, D. Normandin, A. Cheng, J. Himms-Hagen, C. C. Chan, C. Ramachandran, M. J. Gresser, M. L. Tremblay, and B. P. Kennedy. 1999. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 2831544-1548. [DOI] [PubMed] [Google Scholar]
- 21.Fryer, L. G., A. Parbu-Patel, and D. Carling. 2002. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 27725226-25232. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein, B. J. 2001. Protein-tyrosine phosphatase 1B (PTP1B): a novel therapeutic target for type 2 diabetes mellitus, obesity and related states of insulin resistance. Curr. Drug Targets Immun. Endocr. Metab. Disord. 1265-275. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein, B. J., A. Bittner-Kowalczyk, M. F. White, and M. Harbeck. 2000. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J. Biol. Chem. 2754283-4289. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin, G. W., and H. Taegtmeyer. 1999. Regulation of fatty acid oxidation of the heart by MCD and ACC during contractile stimulation. Am. J. Physiol. 277E772-E777. [DOI] [PubMed] [Google Scholar]
- 25.Gum, R. J., L. L. Gaede, M. A. Heindel, J. F. Waring, J. M. Trevillyan, B. A. Zinker, M. E. Stark, D. Wilcox, M. R. Jirousek, C. M. Rondinone, and R. G. Ulrich. 2003. Antisense protein tyrosine phosphatase 1B reverses activation of p38 mitogen-activated protein kinase in liver of ob/ob mice. Mol. Endocrinol. 171131-1143. [DOI] [PubMed] [Google Scholar]
- 26.Gum, R. J., L. L. Gaede, S. L. Koterski, M. Heindel, J. E. Clampit, B. A. Zinker, J. M. Trevillyan, R. G. Ulrich, M. R. Jirousek, and C. M. Rondinone. 2003. Reduction of protein tyrosine phosphatase 1B increases insulin-dependent signaling in ob/ob mice. Diabetes 5221-28. [DOI] [PubMed] [Google Scholar]
- 27.Hardie, D. G., D. Carling, and M. Carlson. 1998. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67821-855. [DOI] [PubMed] [Google Scholar]
- 28.Hawley, S. A., J. Boudeau, J. L. Reid, K. J. Mustard, L. Udd, T. P. Makela, D. R. Alessi, and D. G. Hardie. 2003. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawley, S. A., M. Davison, A. Woods, S. P. Davies, R. K. Beri, D. Carling, and D. G. Hardie. 1996. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 27127879-27887. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi, T., M. F. Hirshman, N. Fujii, S. A. Habinowski, L. A. Witters, and L. J. Goodyear. 2000. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes 49527-531. [DOI] [PubMed] [Google Scholar]
- 31.Jäger, S., C. Handschin, J. St-Pierre, and B. M. Spiegelman. 2007. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 10412017-12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jimenez, M., G. Barbatelli, R. Allevi, S. Cinti, J. Seydoux, J. P. Giacobino, P. Muzzin, and F. Preitner. 2003. Beta 3-adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. Eur. J. Biochem. 270699-705. [DOI] [PubMed] [Google Scholar]
- 33.Jørgensen, S. B., J. T. Treebak, B. Viollet, P. Schjerling, S. Vaulont, J. F. Wojtaszewski, and E. A. Richter. 2007. Role of AMPKα2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am. J. Physiol. Endocrinol. Metab. 292E331-E339. [DOI] [PubMed] [Google Scholar]
- 34.Kahn, B. B., T. Alquier, D. Carling, and D. G. Hardie. 2005. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 115-25. [DOI] [PubMed] [Google Scholar]
- 35.Kaszubska, W., H. D. Falls, V. G. Schaefer, D. Haasch, L. Frost, P. Hessler, P. E. Kroeger, D. W. White, M. R. Jirousek, and J. M. Trevillyan. 2002. Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol. Cell Endocrinol. 195109-118. [DOI] [PubMed] [Google Scholar]
- 36.Klaman, L. D., O. Boss, O. D. Peroni, J. K. Kim, J. L. Martino, J. M. Zabolotny, N. Moghal, M. Lubkin, Y. B. Kim, A. H. Sharpe, A. Stricker-Krongrad, G. I. Shulman, B. G. Neel, and B. B. Kahn. 2000. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 205479-5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, W. J., M. Kim, H. S. Park, H. S. Kim, M. J. Jeon, K. S. Oh, E. H. Koh, J. C. Won, M. S. Kim, G. T. Oh, M. Yoon, K. U. Lee, and J. Y. Park. 2006. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARα and PGC-1. Biochem. Biophys. Res. Commun. 340291-295. [DOI] [PubMed] [Google Scholar]
- 38.Lin, J., H. Wu, P. T. Tarr, C. Y. Zhang, Z. Wu, O. Boss, L. F. Michael, P. Puigserver, E. Isotani, E. N. Olson, B. B. Lowell, R. Bassel-Duby, and B. M. Spiegelman. 2002. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418797-801. [DOI] [PubMed] [Google Scholar]
- 39.Lund, I. K., J. A. Hansen, H. S. Andersen, N. P. Moller, and N. Billestrup. 2005. Mechanism of protein tyrosine phosphatase 1B-mediated inhibition of leptin signalling. J. Mol. Endocrinol. 34339-351. [DOI] [PubMed] [Google Scholar]
- 40.Ma, S. W., and D. O. Foster. 1984. Redox state of brown adipose tissue as a possible determinant of its blood flow. Can. J. Physiol. Pharmacol. 62949-956. [DOI] [PubMed] [Google Scholar]
- 41.Matejkova, O., K. J. Mustard, J. Sponarova, P. Flachs, M. Rossmeisl, I. Miksik, M. Thomason-Hughes, D. Grahame Hardie, and J. Kopecky. 2004. Possible involvement of AMP-activated protein kinase in obesity resistance induced by respiratory uncoupling in white fat. FEBS Lett. 569245-248. [DOI] [PubMed] [Google Scholar]
- 42.Matthias, A., A. Jacobsson, B. Cannon, and J. Nedergaard. 1999. The bioenergetics of brown fat mitochondria from UCP1-ablated mice. Ucp1 is not involved in fatty acid-induced de-energization (“uncoupling”). J. Biol. Chem. 27428150-28160. [DOI] [PubMed] [Google Scholar]
- 43.Minokoshi, Y., T. Alquier, N. Furukawa, Y. B. Kim, A. Lee, B. Xue, J. Mu, F. Foufelle, P. Ferre, M. J. Birnbaum, B. J. Stuck, and B. B. Kahn. 2004. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428569-574. [DOI] [PubMed] [Google Scholar]
- 44.Minokoshi, Y., Y. B. Kim, O. D. Peroni, L. G. Fryer, C. Muller, D. Carling, and B. B. Kahn. 2002. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415339-343. [DOI] [PubMed] [Google Scholar]
- 45.Myers, M. P., J. N. Andersen, A. Cheng, M. L. Tremblay, C. M. Horvath, J. P. Parisien, A. Salmeen, D. Barford, and N. K. Tonks. 2001. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 27647771-47774. [DOI] [PubMed] [Google Scholar]
- 46.Ntambi, J. M., M. Miyazaki, J. P. Stoehr, H. Lan, C. M. Kendziorski, B. S. Yandell, Y. Song, P. Cohen, J. M. Friedman, and A. D. Attie. 2002. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 9911482-11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park, I. R., and J. Himms-Hagen. 1988. Neural influences on trophic changes in brown adipose tissue during cold acclimation. Am. J. Physiol. 255R874-R881. [DOI] [PubMed] [Google Scholar]
- 48.Pedersen, S. B., S. Lund, E. S. Buhl, and B. Richelsen. 2001. Insulin and contraction directly stimulate UCP2 and UCP3 mRNA expression in rat skeletal muscle in vitro. Biochem. Biophys. Res. Commun. 28319-25. [DOI] [PubMed] [Google Scholar]
- 49.Qi, J., J. Gong, T. Zhao, J. Zhao, P. Lam, J. Ye, J. Z. Li, J. Wu, H. M. Zhou, and P. Li. 2008. Downregulation of AMP-activated protein kinase by Cidea-mediated ubiquitination and degradation in brown adipose tissue. EMBO J. 271537-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rondinone, C. M., J. M. Trevillyan, J. Clampit, R. J. Gum, C. Berg, P. Kroeger, L. Frost, B. A. Zinker, R. Reilly, R. Ulrich, M. Butler, B. P. Monia, M. R. Jirousek, and J. F. Waring. 2002. Protein tyrosine phosphatase 1B reduction regulates adiposity and expression of genes involved in lipogenesis. Diabetes 512405-2411. [DOI] [PubMed] [Google Scholar]
- 51.Rossmeisl, M., G. Barbatelli, P. Flachs, P. Brauner, M. C. Zingaretti, M. Marelli, P. Janovska, M. Horakova, I. Syrovy, S. Cinti, and J. Kopecky. 2002. Expression of the uncoupling protein 1 from the aP2 gene promoter stimulates mitochondrial biogenesis in unilocular adipocytes in vivo. Eur. J. Biochem. 26919-28. [DOI] [PubMed] [Google Scholar]
- 52.Ruderman, N. B., A. K. Saha, D. Vavvas, and L. A. Witters. 1999. Malonyl-CoA, fuel sensing, and insulin resistance. Am. J. Physiol. 276E1-E18. [DOI] [PubMed] [Google Scholar]
- 53.Sakamoto, K., A. McCarthy, D. Smith, K. A. Green, D. Grahame Hardie, A. Ashworth, and D. R. Alessi. 2005. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 241810-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakamoto, K., E. Zarrinpashneh, G. R. Budas, A. C. Pouleur, A. Dutta, A. R. Prescott, J. L. Vanoverschelde, A. Ashworth, A. Jovanovic, D. R. Alessi, and L. Bertrand. 2006. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKα2 but not AMPKα1. Am. J. Physiol. Endocrinol. Metab. 290E780-E788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanders, M. J., P. O. Grondin, B. D. Hegarty, M. A. Snowden, and D. Carling. 2007. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem. J. 403139-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schrauwen, P., D. G. Hardie, B. Roorda, J. C. Clapham, A. Abuin, M. Thomason-Hughes, K. Green, P. M. Frederik, and M. K. Hesselink. 2004. Improved glucose homeostasis in mice overexpressing human UCP3: a role for AMP-kinase? Int. J. Obes. Relat. Metab. Disord. 28824-828. [DOI] [PubMed] [Google Scholar]
- 57.Shaw, R. J., M. Kosmatka, N. Bardeesy, R. L. Hurley, L. A. Witters, R. A. DePinho, and L. C. Cantley. 2004. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 1013329-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suwa, M., H. Nakano, and S. Kumagai. 2003. Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J. Appl. Physiol. 95960-968. [DOI] [PubMed] [Google Scholar]
- 59.Taylor, E. B., D. Hurst, L. J. Greenwood, J. D. Lamb, T. D. Cline, S. N. Sudweeks, and W. W. Winder. 2004. Endurance training increases LKB1 and MO25 protein but not AMP-activated protein kinase kinase activity in skeletal muscle. Am. J. Physiol. Endocrinol Metab. 287E1082-E1089. [DOI] [PubMed] [Google Scholar]
- 60.Taylor, E. B., J. D. Lamb, R. W. Hurst, D. G. Chesser, W. J. Ellingson, L. J. Greenwood, B. B. Porter, S. T. Herway, and W. W. Winder. 2005. Endurance training increases skeletal muscle LKB1 and PGC-1alpha protein abundance: effects of time and intensity. Am. J. Physiol. Endocrinol Metab. 289E960-E968. [DOI] [PubMed] [Google Scholar]
- 61.Tonks, N. K. 2003. PTP1B: from the sidelines to the front lines! FEBS Lett. 546140-148. [DOI] [PubMed] [Google Scholar]
- 62.Winder, W. W., and D. G. Hardie. 1999. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am. J. Physiol. 277E1-E10. [DOI] [PubMed] [Google Scholar]
- 63.Winder, W. W., B. F. Holmes, D. S. Rubink, E. B. Jensen, M. Chen, and J. O. Holloszy. 2000. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J. Appl. Physiol. 882219-2226. [DOI] [PubMed] [Google Scholar]
- 64.Witczak, C. A., C. G. Sharoff, and L. J. Goodyear. 2008. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol. Life Sci. 653737-3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woods, A., S. R. Johnstone, K. Dickerson, F. C. Leiper, L. G. Fryer, D. Neumann, U. Schlattner, T. Wallimann, M. Carlson, and D. Carling. 2003. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 132004-2008. [DOI] [PubMed] [Google Scholar]
- 66.Xue, B., and B. B. Kahn. 2006. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J. Physiol. 57473-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zabolotny, J. M., K. K. Bence-Hanulec, A. Stricker-Krongrad, F. Haj, Y. Wang, Y. Minokoshi, Y. B. Kim, J. K. Elmquist, L. A. Tartaglia, B. B. Kahn, and B. G. Neel. 2002. PTP1B regulates leptin signal transduction in vivo. Dev. Cell 2489-495. [DOI] [PubMed] [Google Scholar]
- 68.Zhou, G., R. Myers, Y. Li, Y. Chen, X. Shen, J. Fenyk-Melody, M. Wu, J. Ventre, T. Doebber, N. Fujii, N. Musi, M. F. Hirshman, L. J. Goodyear, and D. E. Moller. 2001. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 1081167-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zinker, B. A., C. M. Rondinone, J. M. Trevillyan, R. J. Gum, J. E. Clampit, J. F. Waring, N. Xie, D. Wilcox, P. Jacobson, L. Frost, P. E. Kroeger, R. M. Reilly, S. Koterski, T. J. Opgenorth, R. G. Ulrich, S. Crosby, M. Butler, S. F. Murray, R. A. McKay, S. Bhanot, B. P. Monia, and M. R. Jirousek. 2002. PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proc. Natl. Acad. Sci. USA 9911357-11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zong, H., J. M. Ren, L. H. Young, M. Pypaert, J. Mu, M. J. Birnbaum, and G. I. Shulman. 2002. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. USA 9915983-15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.