Abstract
Caspase-8 is the main initiator caspase in death receptor-induced apoptosis. Procaspase-8 is activated at the death-inducing signaling complex (DISC). Previous studies suggested a two-step model of procaspase-8 activation. The first cleavage step occurs between the protease domains p18 and p10. The second cleavage step takes place between the prodomain and the large protease subunit (p18). Subsequently, the active caspase-8 heterotetramer p182-p102 is released into the cytosol, starting the apoptotic signaling cascade. In this report, we have further analyzed procaspase-8 processing upon death receptor stimulation directly at the DISC and in the cytosol. We have found an alternative sequence of cleavage events for procaspase-8. We have demonstrated that the first cleavage can also occur between the prodomain and the large protease subunit (p18). The resulting cleavage product, p30, contains both the large protease subunit (p18) and the small protease subunit (p10). p30 is further processed to p10 and p18 by active caspases. Furthermore, we show that p30 can sensitize cells toward death receptor-induced apoptosis. Taken together, our data suggest an alternative mechanism of procaspase-8 activation at the DISC.
Apoptosis can be triggered by a number of factors, including UV or γ-irradiation, chemotherapeutic drugs, and signaling from death receptors (11, 12). CD95 (APO-1/Fas) is a member of the death receptor family, a subfamily of the tumor necrosis factor receptor (TNF-R) superfamily (1, 30). Eight members of the death receptor subfamily have been characterized so far: TNF-R1 (DR1, CD120a, p55, p60), CD95 (DR2, APO-1, Fas), DR3 (APO-3, LARD, TRAMP, WSL1), TRAIL-R1 (APO-2, DR4), TRAIL-R2 (DR5, KILLER, TRICK2), DR6, EDA-R, and NGF-R (13). Cross-linking of CD95 by its natural ligand, CD95L (CD178) (29), or by agonistic antibodies induces apoptosis in sensitive cells (31, 36). The death-inducing signaling complex (DISC) is formed within seconds after CD95 stimulation (9). The DISC consists of oligomerized, probably trimerized CD95 receptors, the adaptor molecule FADD, two isoforms of procaspase-8 (procaspase-8a and -8b), procaspase-10, and c-FLIPL/S/R (6, 19, 21, 25, 27). The interactions between molecules at the DISC are based on homotypic contacts. The death domain of the receptor interacts with the death domain of FADD, while the death effector domain (DED) of FADD interacts with the N-terminal tandem DEDs of procaspase-8 and -10 and c-FLIPL/S/R.
Two isoforms of procaspase-8 (procaspase-8a and procaspase-8b) were reported to be bound to the DISC (24). Both isoforms possess two tandem DEDs, as well as the catalytic subunits p18 and p10 (see Fig. 1A). Procaspase-8a contains an additional 2-kDa (15-amino-acid [aa]) fragment, which results from the translation of exon 9. This small fragment is located between the second DED and the large catalytic subunit, resulting in different lengths of procaspase-8a and -8b (p55 and p53 kDa), respectively.
FIG. 1.
A new 30-kDa protein is detected by the anti-caspase-8 MAb C15. (A) Scheme of procaspase-8 and its cleavage products. The binding sites of the anti-caspase-8 MAbs C5 and C15 are indicated. (B) The B-lymphoblastoid cell lines SKW6.4, Raji, and BJAB and the T-cell lines CEM, Jurkat 16, and caspase-8-deficient Jurkat (clone JI9.2) were stimulated with LZ-CD95L for the indicated times, followed by caspase-8 immunoprecipitation (C8-IP) using the anti-caspase-8 MAb C15 directed against the p18 subunit of procaspase-8. Western blotting of immunoprecipitates was performed using the anti-caspase-8 MAb C15 (**, Ig heavy chain; *, unspecific band). (C) SKW6.4 cells were stimulated with LZ-CD95L for different times, and procaspase-8 processing in total cellular lysates was analyzed by Western blotting using the anti-caspase-8 MAb C15. (D) B-lymphoblastoid BJAB cells were stimulated with LZ-TRAIL for different times, and procaspase-8 processing was analyzed as described for panel C. (E) Primary human T cells (day 6) were stimulated with LZ-CD95L, and procaspase-8 processing was analyzed as described for panel C (*, unspecific band).
Activation of procaspase-8 is believed to follow an “induced-proximity” model in which high local concentrations and a favorable mutual orientation of procaspase-8 molecules at the DISC lead to their autoproteolytic processing (2, 3, 20). There is strong evidence from several in vitro studies that autoproteolytic activation of procaspase-8 occurs after oligomerization at the receptor complex (20). Furthermore, it has been shown that homodimers of procaspase-8 have proteolytic activity and that proteolytic processing of procaspase-8 occurs between precursor homodimers (3).
Procaspase-8a/b (p55/p53) processing at the DISC has been described to involve two sequential cleavage steps (see Fig. 1A). This process is referred to as the “two-step model” (3, 17). The first cleavage step occurs between the two protease domains, and the second cleavage step takes place between the prodomain and the large protease subunit (see Fig. 1A) (15). During the first cleavage step, the cleavage at Asp374 generates the two subunits p43/p41 and p12. Both cleavage products remain bound to the DISC: p43/p41 by DED interactions and p12 by interactions with the large protease domain of p43/p41. The second cleavage step takes place at Asp216 and Asp384, producing the active enzyme subunits p18, p10, and the prodomain p26/p24. As a result of procaspase-8 processing, the active caspase-8 heterotetramer p182-p102 is formed at the DISC. This heterotetramer is subsequently released into the cytosol, starting the apoptotic signaling cascade (14).
Recent studies have shown that processing of procaspase-8 at the DISC is more complicated and can involve additional steps like the generation of a prolonged prodomain of procaspase-8, termed CAP3 (p27), that is quickly converted to p26 (see Fig. 1A) (7).
In addition to its central role in death receptor-induced apoptosis, caspase-8 was reported to be required for proliferation of lymphocytes (12, 23). Recently caspase-8 was shown to be an important factor for NF-κB activation following T-cell receptor stimulation (28). The mechanism underlying the dual role of caspase-8 activity and its regulation is largely unknown.
In the present study, we show that upon death receptor stimulation, p30 is formed by cleavage at Asp210, a yet-unknown cleavage product of procaspase-8, which comprises the C terminus of procaspase-8. p30 turned out to be a key intermediate product in the course of procaspase-8 processing. Furthermore, we suggest that the p30-mediated activation of procaspase-8 plays an important role in the amplification of the death signal. Taken together, our findings provide a new mechanism of procaspase-8 activation and extend the current two-step cleavage model by an alternative activation pathway.
MATERIALS AND METHODS
Cell lines and cell culture.
The T-cell lines CEM, Jurkat 16 (J16), and caspase-8-deficient Jurkats (clone JI9.2) and the B-lymphoblastoid cell lines SKW6.4, Raji, and BJAB were maintained in RPMI 1640 (Life Technologies, Germany), 10 mM HEPES (Life Technologies, Germany), 50 μg/ml gentamicin (Life Technologies, Germany), and 10% fetal calf serum (Life Technologies, Germany) in 5% CO2.
Antibodies and reagents.
Anti-CD95 polyclonal antibodies (C20) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-caspase-8 monoclonal antibodies (MAbs) C15 and C5, the anti-FADD MAb 1C4, the anti-FLIP MAb NF6 and anti-APO-1 MAb, and the isotype control MAb FII23C have been described elsewhere (5, 24, 26, 31). Hemagglutinin (HA)-tagged proteins were detected by the anti-HA MAb 12CA5 (Roche, Switzerland) or the anti-HA MAb 3F10 (Roche, Switzerland). FLAG-tagged proteins were detected by the anti-FLAG MAb M2 (Sigma, Germany). The horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G1 (IgG1), -2a, and -2b, goat anti-rabbit IgG, and chicken anti-rat IgG were from Southern Biotechnology Associates (United Kingdom). Leucine zipper (LZ)-CD95L and LZ-TRAIL were produced as described previously (34). Fluorescein isothiocyanate-coupled anti-mouse IgG1 MAbs were from Caltag Laboratories. zVAD-fmk was purchased from Merck (Germany). Biotin-zVAD-fmk was purchased from Sigma (Germany). All other chemicals used were of analytical grade and purchased from Merck or Sigma (Germany).
Cloning of procaspase-8 mutants and cleavage fragments.
p30-210 (aa 210 to 418) and p30-216 (aa 216 to 418) were cloned into the pEF4 expression vector (Invitrogen, Germany) using the PCR. The FLAG- or HA-tagged procaspase-8a cleavage products were cloned into the pEF4 expression vector (Invitrogen, Germany) using the PCR: p30 (aa 210 to 418), p18 (aa 210 to 274), and p10 (384 to 418). The cleavage mutants of procaspase-8a were cloned via overlap PCR into the pEF4 expression vector (Invitrogen, Germany).
Flow cytometry analysis.
The percentage of viable cells was determined by forward scatter/side scatter using a FACSscan cytometer (BD). A minimum of 10,000 cells per sample was analyzed. Specific cell death was calculated as follows: (percentage of experimental cell death − percentage of spontaneous cell death)/(100 − percentage of spontaneous cell death) × 100.
CD95 surface staining.
To analyze the surface expression of CD95, 2 × 105 cells were harvested by centrifugation for 5 min at 4,000 rpm. The pellet was resuspended in buffer (10% fetal calf serum in phosphate-buffered saline [PBS]) and incubated with 10 μg/ml of anti-APO-1 MAb or FII23C MAb as an isotype control on ice. The cells were washed with buffer and incubated with fluorescein isothiocyanate-conjugated anti-mouse IgG MAb on ice. The cells were washed and resuspended in buffer for analysis via flow cytometry. The population was gated for the living cells, and the staining of the isotype control was compared to the surface staining with anti-APO-1 MAb.
Preparation of primary human T cells.
Human peripheral T cells were prepared as described previously (10). For activation, resting primary human T cells (day 0) were cultured at 2 × 106 cells/ml with 1 μg/ml phytohemagglutinin (PHA) for 16 h (day 1). T cells were then washed three times and cultured for an additional 5 days in the presence of 25 U/ml interleukin 2 (day 6).
Western blot.
For Western blot analysis, the cells were lysed in buffer A (20 mM Tris-HCl, pH 7.4, 1% Triton X-100, 10% glycerol, 150 mM NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [Sigma], protease inhibitor cocktail [Roche, Switzerland]) for 15 min on ice and centrifuged (15 min, 14,000 × g). Postnuclear supernatants were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto a Hybond nitrocellulose membrane (Amersham Pharmacia Biotech, Germany). The membrane was incubated with the primary antibody for 1 h at room temperature or overnight at 4°C. The horseradish peroxidase-conjugated goat anti-mouse IgG, goat anti-rabbit IgG, or chicken anti-rat IgG was incubated in 1% milk for 1 h at room temperature, and development was done with the Western Lightning Plus reagent (Perkin Elmer). For quantitative Western blot analysis, the blots were developed in a chemiluminescence system (Vilber Loumat, Germany) and the quantification was performed with the Bio1D software (Vilber Loumat, Germany).
DISC analysis by immunoprecipitation and Western blotting.
Cells (1 × 108) were either treated with 1 μg of anti-APO-1 MAb (IgG3) for the indicated periods of time at 37°C, washed twice with 1× PBS, and subsequently lysed in buffer A (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [Sigma], protease inhibitor cocktail [Roche], 1% Triton X-100 [Serva] and 10% glycerol) (stimulated condition) or lysed without treatment (unstimulated condition). The CD95 DISC was immunoprecipitated overnight with 2 μg of anti-APO-1 MAb and protein A Sepharose. Beads were washed five times with 20 volumes of lysis buffer. The immunoprecipitates were analyzed by 12% SDS-PAGE. Subsequently, the gels were transferred onto a Hybond nitrocellulose membrane (Amersham Pharmacia Biotech), blocked with 5% nonfat dry milk in PBS-Tween (PBS plus 0.05% Tween 20) for 1 h, washed with PBS-Tween, and incubated with primary antibody in PBS-Tween at 4°C overnight. Blots were developed with a chemoluminescence method, following the manufacturer's protocol (Perkin Elmer Life Sciences, Germany).
In vitro FLICE assays.
The CD95 DISC was immunoprecipitated from 5 × 107 cells, and immunoprecipitates were incubated with in vitro-translated 35S-labeled procaspase-8 (TNT, T7-coupled reticulocyte lysate system; Promega) in caspase-8 cleavage buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate [CHAPS], 10 mM dithiothreitol, and 10% sucrose) at 4°C overnight. Cleavage reaction products were separated using 15% SDS-PAGE gels, blotted, and subjected to autoradiography.
CD95 stimulation of cell lines.
Cells (1 × 107) were stimulated with 1 μg/ml anti-APO1 or a 1:10 dilution of LZ-CD95L-containing supernatant for the indicated times if not stated otherwise. The cells were lysed, subjected to SDS-PAGE, and analyzed by Western blotting. For the caspase inhibition experiment, 20 μM zVAD-fmk (panspecific caspase inhibitor) was added to the cells 30 min before the stimulation.
Detection of activated caspases by bioVAD-fmk.
The detection of activated caspases by bioVAD-fmk was done according to the method of Tu et al. (32). Jurkat T cells (5 × 107) were incubated with 50 μM bVAD-fmk (Sigma) or dimethylsulfoxide for 2 h at 37°C. Cells were then treated for 4 h with LZ-CD95L and subsequently lysed in CHAPS lysis buffer (150 mM KCl, 50 mM HEPES, 0.1% CHAPS, pH 7.4). After clearance by centrifugation (20 min, 13,000 rpm), Streptavidin-agarose (30 μl) was added to the supernatant and agitated at 4°C. The beads were washed five times in CHAPS lysis buffer, boiled, and resolved by SDS-PAGE. Caspase-8 was detected by immunoblotting with the anti-caspase-8 MAb C15.
Stable transfection of BJAB cells.
Stable transfection of BJAB cells was performed using the pEF4 expression vectors encoding p30-210 and p30-216 and the empty vector by electroporation (960 μF, 200 V). Selection pressure was applied 48 h after transfection (100 μg/ml Zeocin) for 2 weeks. Expression was controlled by Western blotting using the anti-caspase-8 MAb C15.
RESULTS
A new 30-kDa protein is detected with the anti-caspase-8 MAb C15.
Upon stimulation of CD95, we detected a new prominent protein band with the anti-caspase-8 MAb C15 in addition to the previously described procaspase-8a/b products p43/p41 and p18 (Fig. 1A and B) (24). The molecular mass of this protein was about 30 kDa, and therefore, it was termed p30 (Fig. 1B). p30 was observed upon CD95 stimulation with LZ-CD95L in caspase-8 immunoprecipitates from the B-lymphoblastoid cell lines SKW6.4, Raji, and BJAB and the T-cell lines CEM and Jurkat 16 but not in caspase-8-deficient Jurkat (clone JI9.2) (Fig. 1B).
To shed more light on the generation of p30, we stimulated SKW6.4 cells with LZ-CD95L and followed processing of procaspase-8a/b using the anti-caspase-8 MAb C15. p30 appeared in a stimulation-dependent way along with the other well-known cleavage products of procaspase-8 p43/p41 and p18 (Fig. 1C). p30 and p43/p41 were detected at the same time points of stimulation. The amount of p30 was substantially smaller than the amount of p43/p41. This may explain why we overlooked p30 in previous studies.
To analyze whether p30 is a common product of procaspase-8 processing upon death receptor triggering, we stimulated BJAB cells with LZ-TRAIL (Fig. 1D). The same results as for CD95 stimulation were obtained.
We also analyzed procaspase-8 processing upon CD95 stimulation in a number of other T- and B-cell lines (Fig. 1B) and primary human T cells (Fig. 1E). p30 was detected in all these cell lines; however, the amount of p30 was always substantially smaller than the amount of p43/p41.
Thus, we have detected a new protein of 30 kDa, p30, which appears upon death receptor-induced processing of procaspase-8 and is most likely a new cleavage product of procaspase-8.
p30 comprises the C terminus of procaspase-8.
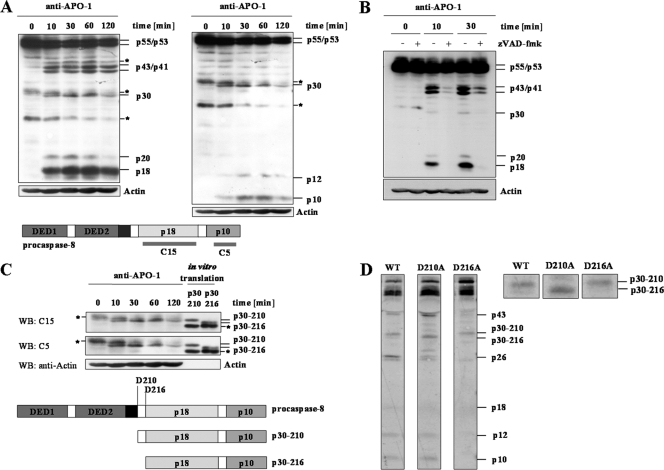
The detection of p30 with the anti-caspase-8 MAb C15, raised against p18 of procaspase-8, indicated the presence of the p18 subunit within the p30 protein (Fig. 1B). To identify the structure of p30, we analyzed procaspase-8 processing upon CD95 stimulation in SKW6.4 cells using two MAbs against the C terminus of procaspase-8: C15 and C5 (Fig. 2A). The anti-caspase-8 MAbs C15 and C5 recognize the p18 and p10 subunits of procaspase-8, respectively (24). Since p30 was recognized by both antibodies, we concluded that p30 comprises the p18 and p10 subunits of procaspase-8.
FIG. 2.
p30 comprises the C terminus of procaspase-8. (A) SKW6.4 cells were stimulated with anti-APO-1 MAb for different times, and procaspase-8 cleavage was analyzed by Western blotting with the anti-caspase-8 MAbs C15 (left panel) or C5 (right panel) and the antiactin MAb. (*, unspecific band). (B) SKW6.4 cells were pretreated with 20 μM of zVAD-fmk and stimulated with anti-APO-1 MAb for different times. Procaspase-8 processing was analyzed by Western blotting using the anti-caspase-8 MAb C15. Antiactin Western blotting was used as a loading control (*, unspecific band). (C) Lower part: scheme of procaspase-8a/b and the putative structures of p30 resulting from cleavage at Asp210 and Asp216. Upper part: in vitro-translated p30-216 and p30-210 were loaded on the same gel as total cellular lysates of CD95-stimulated SKW6.4 cells. Western blotting (WB) was performed using the anti-caspase-8 MAbs C15 and C5. Antiactin Western blotting was used as a loading control (*, unspecific band). (D) Wild-type (WT) procaspase-8a and mutants of procaspase-8a (D210A and D216A) were translated in vitro in the presence of [35S]methionine and added to the CD95 DISCs isolated from SKW6.4 cells. Processing of procaspase-8a by the DISCs was monitored by 35S autoradiography. The enlargement of the 30-kDa region is presented at the right side of the figure.
If p30 is a cleavage product generated by procaspase-8 activity, the formation of p30 should be inhibited by the pan-caspase inhibitor zVAD-fmk. Indeed, preincubation of SKW6.4 cells with zVAD-fmk inhibited the formation of p30 as well as the formation of the known cleavage products p43/p41 and p18 (Fig. 2B). This suggests that p30 is a caspase-dependent cleavage product of procaspase-8.
Analysis of the primary structure of procaspase-8a revealed that cleavage at Asp210 and Asp216 would result in the formation of a C-terminal p10- and p18-containing cleavage product with a molecular mass of about 30 kDa (Fig. 2C, lower part). To test this hypothesis, we generated two cDNAs corresponding to the potential procaspase-8 C-terminal fragments resulting from cleavage at Asp210 and Asp216, which we named p30-210 and p30-216, respectively. p30-210 and p30-216 were translated in vitro and loaded on the same gel as total cellular lysates from CD95-stimulated SKW6.4 cells, followed by Western blot analysis with the anti-caspase-8 MAbs C5 and C15 (Fig. 2C, upper part). Interestingly, the molecular mass of endogenous p30 was identical to the in vitro-translated product p30-210 but not to p30-216. In summary, these data provide evidence that p30 is a C-terminal cleavage product of procaspase-8 generated by cleavage at Asp210.
To verify that p30 is generated by cleavage at Asp210, we compared the processing of wild-type procaspase-8a and cleavage mutants of procaspase-8a: D210A and D216A (Fig. 2D). All three constructs were translated in vitro in the presence of [35S]methionine. CD95 DISCs were isolated from SKW6.4 cells and added to the in vitro-translated protein, and the processing of procaspase-8a was monitored by 35S autoradiography. Besides the described cleavage products (p43, p26, p18, p12, and p10), the processing of wild-type procaspase-8a resulted in the formation of p30-210 and p30-216. p30-210 was abundant compared to p30-216, which was formed only in minor amounts. Upon processing of mutated procaspase-8a (D210A), only p30-216 was generated. Mutation at Asp216 did not influence the formation of p30-210 but impaired the generation of p30-216. Thus, we conclude that p30 is generated predominantly by cleavage at Asp210. However, when Asp210 is mutated, cleavage might also occur at position 216.
p30 is formed at the DISC and interacts with procaspase-8, p30, p18, p10, and c-FLIPL.
To determine at which step in CD95 signaling p30 is generated, the CD95 DISC was immunoprecipitated prior to and at early times after addition of anti-APO-1 MAb. The CD95 DISC was immunoprecipitated from SKW6.4 cells using anti-APO-1 MAb and analyzed by Western blotting with the anti-caspase-8 MAb C15. p30 was detected at the CD95 DISC along with the other known cleavage products of procaspase-8 p43/p41 and p18 (Fig. 3A). As in total cellular lysates, we observed that the amount of p30 at the CD95 DISC was lower than the amount of p43/p41. Thus, p30 is formed at the CD95 DISC during early procaspase-8 processing. The amount of p30 in the lysate (Fig. 1C and 2A) and at the DISC (Fig. 3A) decreased with increasing stimulation time, indicating a possible DISC-mediated cleavage of p30 into p18 and p10.
FIG. 3.
p30 is formed at the DISC. (A) SKW6.4 cells were stimulated with anti-APO-1 MAb for the indicated times. Subsequently, CD95 DISC immunoprecipitation was performed. Procaspase-8 processing at the CD95 DISC was analyzed by Western blotting using the anti-caspase-8 MAb C15. *, unspecific band. (B) Model of p30 generation and its interactions with the CD95 DISC.
We have also detected p30 at complex II, the other caspase-8-activating complex (see Fig. S1 in the supplemental material), which has been reported recently (16). Complex II is formed in the cytosol upon CD95 stimulation and contains DED proteins of the DISC but lacks CD95.
p30 can potentially be bound to the DISC and complex II via interactions with the protease domains of another procaspase-8 molecule, forming a heterodimer, procaspase-8/p30 (Fig. 3B). As soon as the C-terminal part of full-length procaspase-8 in the heterodimer is cleaved, p30 is released into the cytosol. In addition, p30 could be further processed in this procaspase-8/p30 heterodimer to p18 and p10.
To check the hypothesis that p30 interacts with the protease domain of procaspase-8, we transfected 293T cells with HA-tagged procaspase-8 (HAprocaspase-8) and FLAG-tagged p30 (FLAGp30). This was followed by HA immunoprecipitation and FLAG immunoprecipitation (Fig. 4A, upper panel). We can observe FLAGp30 in the HA immunoprecipitation and HAprocaspase-8 in the FLAG immunoprecipitation, indicating an interaction of the proteins. In this way, the proposed interaction between p30 and procaspase-8 was validated.
FIG. 4.
p30 interacts with procaspase-8, p30, p18, p10, and c-FLIPL. (A) 293T cells were transfected with FLAG-tagged p30 (FLAGp30) along with HAprocaspase-8 (upper panel) or FLAGprocaspase-8 and HAp30 (lower panel). Interactions between these molecules were analyzed using HA and FLAG immunoprecipitations with subsequent Western blotting (WB) with the anti-HA MAb 3F10 and the anti-FLAG MAb M2 (**, Ig heavy chain). (B) 293T cells were transfected with HAp30 along with FLAGp30 (upper panel), FLAG-tagged p18 (FLAGp18) (middle panel), or FLAG-tagged p10 (FLAGp10) (lower panel). Interactions between these molecules were analyzed as described for panel A. (C) 293T cells were transfected with HAp30 along with CD95, FADD, or c-FLIPL. Interactions between these molecules were analyzed using coimmunoprecipitation, followed by Western blotting with anti-HA (12CA), anti-CD95 (C20), anti-FADD (1C4), and anti-c-FLIP (NF6) MAbs.
To further verify the interaction between p30 and procaspase-8, we performed independent coimmunoprecipitation experiments using HAp30 and FLAGprocaspase-8 (Fig. 4A, lower panel). We detected FLAGprocaspase-8 and HAp30 in the HA and FLAG immunoprecipitations, respectively. Therefore, we can conclude that p30 interacts with procaspase-8 and forms heterodimers.
To define the site of interaction with procaspase-8, we transfected 293T cells with HAp30 along with the C-terminal fragments of procaspase-8: FLAGp30, FLAGp18, and FLAGp10 (Fig. 4B). We can coimmunoprecipitate HAp30 with FLAGp30, demonstrating that p30 associates with the C terminus of procaspase-8. This was further confirmed by the observed interactions between HAp30 and the protease subunits of caspase-8, FLAGp18 and FLAGp10 (Fig. 4B). Thus, we could show that p30 interacts with the C terminus of procaspase-8 via both the p10 and p18 fragments. Furthermore, we could coimmunoprecipitate dimers of p30 by using differentially tagged p30 constructs in 293T cells (Fig. 4B, upper panel). This is important because the artificially dimerized C-terminal part of procaspase-8 was reported to have catalytic activity (22). Therefore, it might be suggested that dimers of p30 could possess catalytic activity in the cells promoting the apoptotic signal.
To determine other interaction partners of p30 at the CD95 DISC, 293T cells were transfected with HA-tagged p30 (HAp30) along with the main DISC components: CD95, FADD, and c-FLIPL. Subsequently, coimmunoprecipitation experiments were performed. In these experiments, we observed an interaction of p30 with c-FLIPL (Fig. 4C). Since previous reports have demonstrated stable association of protease domains of procaspase-8 and c-FLIPL, it might be suggested that p30 also interacts with c-FLIPL and procaspase-8 via their protease domains (4, 18). As expected, direct interactions of p30 with FADD and CD95 were not detected. Thus, we observed that p30 is formed at the CD95 DISC and interacts with procaspase-8 and c-FLIPL. The interactions with procaspase-8 occur via the C-terminal protease domains: p30, p18, and p10.
We have shown that p30 is the C terminus of procaspase-8 formed at the DISC simultaneously with p43/p41. However, processing via the p30 pathway seems to occur to a lesser extent than that via the p43/p41 pathway, since we can detect only smaller amounts of p30 by Western blotting.
To estimate the relative amounts of procaspase-8 cleaved via the p30 pathway versus the p43/p41 pathway, we applied quantitative Western blotting, measuring the ratio of p43/p41 cleavage products to the p30 cleavage product in SKW6.4 cells upon CD95 stimulation (Fig. 5). The amount of p30 generated was always about 10 times less than the amount of p43/p41. This was observed for different time points (Fig. 5A) and for different amounts of stimulating anti-APO-1 antibodies (Fig. 5B). However, to estimate the real amount of procaspase-8 cleaved to p43/p41 versus that cleaved to p30, it is important to know the degradation and cleavage rates of p43/p41 versus p30 products. This will be a topic of future studies.
FIG. 5.
Quantification of procaspase-8 processing. SKW6.4 cells were stimulated for different times with 1 μg/ml of anti-APO-1 MAb (A) or with different amounts of anti-APO-1 MAb for 1 h (B). Procaspase-8 processing at the CD95 DISC was analyzed by Western blotting using the anti-caspase-8 MAb C15. The relative distribution of procaspase-8 cleavage products detected by the anti-caspase-8 MAb C15 was quantified (*, unspecific band).
p30 can be processed to p10 and p18 by active caspase-8 and -9.
Next, we studied whether p30 can be further processed to the active protease subunits p18 and p10 and can form the active heterotetramer p102-p182. To determine the protease activity that can process p30 into p10 and p18, recombinant caspase-3, -8, and -9 were added to in vitro-translated 35S-labeled HAp30 and an uncleavable mutant (D374/384A) of HAp30 (HAp30 mut). The results of processing were analyzed by autoradiography. Both caspase-8 and caspase-9 processed HAp30 to the small protease subunits p10 and p18, indicating a cleavage at Asp374 and Asp384 (Fig. 6A). We also detected the intermediate product of procaspase-8 processing, p20. However, the uncleavable mutant of HAp30 (D374/384A) was not processed. Thus, p30 is processed to p10 and p18 by cleavage at Asp374 and Asp384.
FIG. 6.
p30 can be processed to p10 and p18 by active caspase-8 and -9. (A) Recombinant caspase-3, -8, or -9 was added to in vitro-translated 35S-labeled HAp30 and the uncleavable mutant D374/384A of HAp30 (HAp30mut). Processing was visualized by autoradiography (*, unspecific band). (B) 293T cells were transfected with HAp30 and HAp30 mut. Recombinant caspase-3, -8, or -9 or stimulated lysate (lys) of SKW6.4 cells was added to HA immunoprecipitates, and processing of p30 was analyzed by Western blotting using the anti-caspase-8 MAbs C15 and C5 and the anti-HA MAb 12CA (*, unspecific band).
To confirm this result, HAp30 and HAp30 mut (D374/384A) were overexpressed in 293T cells and immunoprecipitated using the anti-HA MAb 12CA (Fig. 6B). Recombinant caspase-3, -8, and 9 were added to immunoprecipitates, and the results of processing were detected by Western blotting using the anti-caspase-8 MAbs C15 and C5. We saw only very weak bands of the processed forms of p30, which was probably due to rapid processing of the cleavage products by other proteases or the proteasome. Again, p30 was processed to p10 and p18 by recombinant caspase-8 and -9 but not by caspase-3.
Thus, p30 can be cleaved by caspase-8 and -9 at Asp374 and Asp384. This cleavage results in the formation of the active protease subunits p10 and p18.
p30 sensitizes cells toward CD95-induced apoptosis.
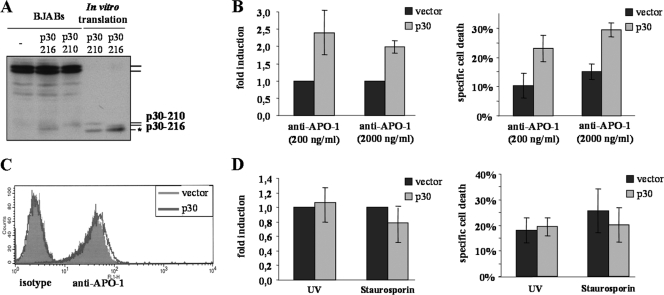
To examine the role of p30 in CD95-mediated apoptosis, we generated BJAB cell lines overexpressing p30-210 and p30-216. The amount of p30 in these cell lines was validated by Western blotting (Fig. 7A). We further analyzed p30-overexpressing cells (p30-210) for their apoptosis phenotype. Even under mild overexpression conditions, p30-overexpressing BJAB cells showed increased sensitivity toward CD95-induced apoptosis compared to the vector-transfected control (Fig. 7B). The surface expression of CD95 was not altered (Fig. 7C). The background apoptosis was only slightly higher in p30-overexpressing BJAB cells. In contrast, we did not observe any sensitization in these p30-overexpressing BJAB cell lines toward UV-induced or staurosporine-induced apoptosis relying on the intrinsic mitochondrial pathway (Fig. 7D). The same results were obtained with p30-216-overexpressing BJAB cells (see Fig. S2 in the supplemental material). These data provide direct evidence that p30 sensitizes cells toward CD95-induced death receptor-mediated apoptosis.
FIG. 7.
p30 sensitizes cells toward CD95-induced apoptosis. (A) Western blot analysis of expression levels of p30-210 or p30-216 in transfected BJAB cell lines with C15 MAb. In vitro-translated p30-210 and p30-216 were used as a positive control (*, unspecific band). (B) Analysis of the sensitivity of p30-overexpressing BJAB cells toward CD95-induced apoptosis after 16 h of treatment with the indicated concentrations of anti-APO-1 MAb. Vector-transfected BJAB cells were used as a negative control. (C) Analysis of the surface expression of CD95 in p30-overexpressing BJAB cells. (D) Analysis of the sensitivity of p30-overexpressing BJAB cells toward staurosporine- or UV-induced apoptosis after 16 h. Vector-transfected BJAB cells were used as a negative control.
To get more insight into the mechanism of sensitization toward CD95-mediated apoptosis induced by p30, we used active site labeling and subsequent pulldown experiments using biotinylated zVAD. Besides the known activated forms of procaspase-8, a band corresponding to p30 could be detected in the pulldown (Fig. 8A). Considering that typically p30 is generated in very small amounts, it is remarkable that we could detect p30 in this pulldown and thereby show that it is catalytically active. This demonstrates that p30 has enzymatic activity and explains how p30 can amplify CD95-induced apoptosis.
FIG. 8.
p30 is enzymatically active and the new model of procaspase-8 activation at the CD95 DISC. (A) SKW6.4 cells were stimulated with LZ-CD95L for 1 h. BioVAD pulldown was analyzed by Western blotting using the anti-caspase-8 MAb C15 (*, unspecific band). (B) Procaspase-8 activation at the DISC involves two parallel cleavage pathways, which occur simultaneously and lead to the generation of the active p102-p182 heterotetramer. The first pathway of procaspase-8 processing comprises the well-described two-step mechanism (right side). The first cleavage occurs between the p18 and p10 subunits, which is followed by the second cleavage between the prodomain and the p18 subunit. The alternative second pathway of procaspase-8 activation involves the novel pathway described in this article (left side). In this pathway, the first cleavage occurs between the prodomain and the protease subunit of p18, which results in p30 generation. Subsequently, p30 is processed to p10 and p18. As the final step in both activation pathways, the active heterotetramer p102-p182 is formed, which is then released into the cytosol to propagate the apoptotic signal.
DISCUSSION
Caspase-8 is a critical component of the death receptor-apoptotic pathway (8, 19, 33). The activation of procaspase-8 at the DISC plays a central role in the regulation of this type of apoptosis. In this article, we describe a new procaspase-8 cleavage product, p30, which comprises the C terminus of procaspase-8, containing both protease subunits p10 and p18. p30 is generated at the DISC upon stimulation of the death receptor CD95 or TRAIL-R1/R2 and can be further processed to the active caspase-8 subunits p10 and p18. Overexpression of p30 sensitizes cells toward the CD95-induced apoptotic cascade.
The amount of p30 generated upon both CD95 and TRAIL-R1/R2 stimulation is lower than those of the cleavage products p43/p41 and p18. This might be explained by p30 being an intermediate cleavage product of procaspase-8 that is quickly converted to p10 and p18. Therefore, p30 was overlooked in previous studies of procaspase-8 processing.
p30 formation takes place fast after CD95 and TRAIL-R1/R2 stimulation (Fig. 1 and 2). In a number of previous publications, the two-step cleavage model was described and it was suggested that the two procaspase-8 cleavage steps occur in strict order: the first cleavage step occurs between the large and small subunits of procaspase-8 and the second cleavage step between the large subunit and the prodomain (3, 17). p30 appears simultaneously with the other cleavage products of procaspase-8 p43/p41 and p18. From these data, we conclude that cleavage between the protease domains (D374/D384) that generates p43/p41 and cleavage between the prodomain of procaspase-8 and the large protease subunit (D210/D216) that generates p30 can occur at the same time. Since the generation of p43/p41 and the generation of p30 exclude each other, the appearance of p43/p41 and p30 in the course of procaspase-8 processing shows simultaneous cleavage at D374/D384 and D210/D216.
The difference between our results and previous data might be explained by the fact that previous studies of procaspase-8 activation were based on artificial constructs in which the DEDs of procaspase-8 were replaced by the FK506 binding protein or by the Fv protein to induce dimerization (3, 35). In contrast to these models, our studies are based on oligomerization of endogenous procaspase-8 at the CD95 DISC.
Recently it has been shown that procaspase-8 processing also takes place in complex II, which is formed upon CD95 stimulation and contains the DED-containing proteins: procaspase-8, FADD, and FLIP (16). This complex has been suggested to play an important role in caspase-8 processing, contributing to the amplification of caspase activation in apoptosis. Besides the known caspase-8 cleavage products, p30 can also be found at complex II (see Fig. S1 in the supplemental material). Therefore, p30 is found not only at the DISC but also at other caspase-8 activating complexes.
Based on our experimental data, we suggest a novel model of procaspase-8 activation at the DISC. This model involves two parallel cleavage pathways that occur simultaneously and lead to the generation of an active p102-p182 heterotetramer (Fig. 8B). The first pathway of procaspase-8 processing comprises the well-known two-step mechanism as described above (Fig. 8B, right side), whereas the alternative pathway of procaspase-8 activation involves an alternative mechanism (Fig. 8B, left side). In this pathway, the first cleavage occurs between the prodomain and the protease subunit p18, resulting in p30 generation. Subsequently, p30 is further processed into the p10 and p18 subunits. At the final step in both cleavage pathways, the heterotetramer p102-p182 is formed, which propagates the apoptotic signal.
We have shown that upon overexpression, p30 can sensitize cells toward CD95-induced apoptosis. From our data, three main pathways of p30-mediated sensitization might be suggested. First, p30 might form dimers which possess catalytic activity. p30 probably can form dimers in the cytosol or at the DISC, since we could show dimer formation in an overexpression system (Fig. 4B). In addition, we have shown that p30 is enzymatically active upon CD95 stimulation (Fig. 8A). It has been reported before that dimers of artificially dimerized C-terminal fragments of procaspase-8 possess catalytic activity while monomers do not (22). Therefore, it might be proposed that p30-mediated sensitization toward CD95-mediated apoptosis involves formation of enzymatically active dimers. The second way of sensitization could be the further processing of p30 to the active heterotetramer p102-p182. We have demonstrated that p30 is processed to the active subunits in vitro (Fig. 6). This might lead to an increase in the amount of catalytically active caspase-8. The third possibility is that p30 might act as a scaffold for procaspase-8 activation at the DISC. It was reported that the association of protease domains is crucial for the activation of caspase-8 (3). We have shown that p30 interacts with procaspase-8 (Fig. 4A) and might therefore be able to form a catalytically active heterodimer with procaspase-8 at the DISC, initiating proteolytic activity of procaspase-8. However, the exact mechanism of the sensitization induced by p30 will be a subject of further studies.
We have described a novel mechanism of procaspase-8 activation, which involves simultaneous cleavage between prodomain and protease subunits, leading to the formation of p30, the C-terminal cleavage product. p30 sensitizes cells toward CD95-induced apoptosis and likely plays an important role in procaspase-8 activation at the DISC. It is essential to understand all processing steps of procaspase-8 at the DISC to eventually regulate CD95-induced apoptosis. In addition, these data might have further implications for the development of CD95-specific apoptosis inhibitors. Thus, these findings might be a basis for drug design in diseases connected with dysregulation in CD95 signaling (11).
Supplementary Material
Acknowledgments
We thank D. Brenner, C. Frey, T. Mock, and C. Pforr for discussion and for critically reading the manuscript, T. Lerchl and S. Röhling for technical assistance, and H. Sauter for expert secretarial assistance. We thank Uta Schäfer and Henning Walzcak for LZ-TRAIL.
This work was supported by grants from the Deutsche Forschungsgemeinschaft, the Deutsche Krebshilfe, the European Community, SBCancer, HRJRG 102, and the Wilhelm Sander Stiftung.
Footnotes
Published ahead of print on 15 June 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 2811305-1308. [DOI] [PubMed] [Google Scholar]
- 2.Boatright, K. M., M. Renatus, F. L. Scott, S. Sperandio, H. Shin, I. M. Pedersen, J. E. Ricci, W. A. Edris, D. P. Sutherlin, D. R. Green, and G. S. Salvesen. 2003. A unified model for apical caspase activation. Mol. Cell 11529-541. [DOI] [PubMed] [Google Scholar]
- 3.Chang, D. W., Z. Xing, V. L. Capacio, M. E. Peter, and X. Yang. 2003. Interdimer processing mechanism of procaspase-8 activation. EMBO J. 224132-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, D. W., Z. Xing, Y. Pan, A. Algeciras-Schimnich, B. C. Barnhart, S. Yaish-Ohad, M. E. Peter, and X. Yang. 2002. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 213704-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhein, J., P. T. Daniel, B. C. Trauth, A. Oehm, P. Moller, and P. H. Krammer. 1992. Induction of apoptosis by monoclonal antibody anti-APO-1 class switch variants is dependent on cross-linking of APO-1 cell surface antigens. J. Immunol. 1493166-3173. [PubMed] [Google Scholar]
- 6.Golks, A., D. Brenner, C. Fritsch, P. H. Krammer, and I. N. Lavrik. 2005. c-FLIPR, a new regulator of death receptor-induced apoptosis. J. Biol. Chem. 28014507-14513. [DOI] [PubMed] [Google Scholar]
- 7.Golks, A., D. Brenner, I. Schmitz, C. Watzl, A. Krueger, P. H. Krammer, and I. N. Lavrik. 2006. The role of CAP3 in CD95 signaling: new insights into the mechanism of procaspase-8 activation. Cell Death Differ. 13489-498. [DOI] [PubMed] [Google Scholar]
- 8.Juo, P., C. J. Kuo, J. Yuan, and J. Blenis. 1998. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr. Biol. 81001-1008. [DOI] [PubMed] [Google Scholar]
- 9.Kischkel, F. C., S. Hellbardt, I. Behrmann, M. Germer, M. Pawlita, P. H. Krammer, and M. E. Peter. 1995. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 145579-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klas, C., K. M. Debatin, R. R. Jonker, and P. H. Krammer. 1993. Activation interferes with the APO-1 pathway in mature human T cells. Int. Immunol. 5625-630. [DOI] [PubMed] [Google Scholar]
- 11.Krammer, P. H. 2000. CD95's deadly mission in the immune system. Nature 407789-795. [DOI] [PubMed] [Google Scholar]
- 12.Krammer, P. H., R. Arnold, and I. N. Lavrik. 2007. Life and death in peripheral T cells. Nat. Rev. Immunol. 7532-542. [DOI] [PubMed] [Google Scholar]
- 13.Lavrik, I., A. Golks, and P. H. Krammer. 2005. Death receptor signaling. J. Cell Sci. 118265-267. [DOI] [PubMed] [Google Scholar]
- 14.Lavrik, I., A. Krueger, I. Schmitz, S. Baumann, H. Weyd, P. H. Krammer, and S. Kirchhoff. 2003. The active caspase-8 heterotetramer is formed at the CD95 DISC. Cell Death Differ. 10144-145. [DOI] [PubMed] [Google Scholar]
- 15.Lavrik, I. N., A. Golks, and P. H. Krammer. 2005. Caspases: pharmacological manipulation of cell death. J. Clin. Investig. 1152665-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavrik, I. N., T. Mock, A. Golks, J. C. Hoffmann, S. Baumann, and P. H. Krammer. 2008. CD95 stimulation results in the formation of a novel death effector domain protein-containing complex. J. Biol. Chem. 28326401-26408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medema, J. P., C. Scaffidi, F. C. Kischkel, A. Shevchenko, M. Mann, P. H. Krammer, and M. E. Peter. 1997. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 162794-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Micheau, O., M. Thome, P. Schneider, N. Holler, J. Tschopp, D. W. Nicholson, C. Briand, and M. G. Grutter. 2002. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J. Biol. Chem. 27745162-45171. [DOI] [PubMed] [Google Scholar]
- 19.Muzio, M., A. M. Chinnaiyan, F. C. Kischkel, K. O'Rourke, A. Shevchenko, J. Ni, C. Scaffidi, J. D. Bretz, M. Zhang, R. Gentz, M. Mann, P. H. Krammer, M. E. Peter, and V. M. Dixit. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85817-827. [DOI] [PubMed] [Google Scholar]
- 20.Muzio, M., B. R. Stockwell, H. R. Stennicke, G. S. Salvesen, and V. M. Dixit. 1998. An induced proximity model for caspase-8 activation. J. Biol. Chem. 2732926-2930. [DOI] [PubMed] [Google Scholar]
- 21.Peter, M. E., and P. H. Krammer. 2003. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 1026-35. [DOI] [PubMed] [Google Scholar]
- 22.Pop, C., P. Fitzgerald, D. R. Green, and G. S. Salvesen. 2007. Role of proteolysis in caspase-8 activation and stabilization. Biochemistry 464398-4407. [DOI] [PubMed] [Google Scholar]
- 23.Salmena, L., B. Lemmers, A. Hakem, E. Matysiak-Zablocki, K. Murakami, P. Y. Au, D. M. Berry, L. Tamblyn, A. Shehabeldin, E. Migon, A. Wakeham, D. Bouchard, W. C. Yeh, J. C. McGlade, P. S. Ohashi, and R. Hakem. 2003. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 17883-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scaffidi, C., J. P. Medema, P. H. Krammer, and M. E. Peter. 1997. FLICE is predominantly expressed as two functionally active isoforms, caspase-8/a and caspase-8/b. J. Biol. Chem. 27226953-26958. [DOI] [PubMed] [Google Scholar]
- 25.Scaffidi, C., I. Schmitz, P. H. Krammer, and M. E. Peter. 1999. The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 2741541-1548. [DOI] [PubMed] [Google Scholar]
- 26.Scaffidi, C., J. Volkland, I. Blomberg, I. Hoffmann, P. H. Krammer, and M. E. Peter. 2000. Phosphorylation of FADD/MORT1 at serine 194 and association with a 70-kDa cell cycle-regulated protein kinase. J. Immunol. 1641236-1242. [DOI] [PubMed] [Google Scholar]
- 27.Sprick, M. R., M. A. Weigand, E. Rieser, C. T. Rauch, P. Juo, J. Blenis, P. H. Krammer, and H. Walczak. 2000. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 12599-609. [DOI] [PubMed] [Google Scholar]
- 28.Su, H., N. Bidere, L. Zheng, A. Cubre, K. Sakai, J. Dale, L. Salmena, R. Hakem, S. Straus, and M. Lenardo. 2005. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science 3071465-1468. [DOI] [PubMed] [Google Scholar]
- 29.Suda, T., T. Takahashi, P. Golstein, and S. Nagata. 1993. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 751169-1178. [DOI] [PubMed] [Google Scholar]
- 30.Tartaglia, L. A., T. M. Ayres, G. H. Wong, and D. V. Goeddel. 1993. A novel domain within the 55 kd TNF receptor signals cell death. Cell 74845-853. [DOI] [PubMed] [Google Scholar]
- 31.Trauth, B. C., C. Klas, A. M. Peters, S. Matzku, P. Moller, W. Falk, K. M. Debatin, and P. H. Krammer. 1989. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 245301-305. [DOI] [PubMed] [Google Scholar]
- 32.Tu, S., G. P. McStay, L. M. Boucher, T. Mak, H. M. Beere, and D. R. Green. 2006. In situ trapping of activated initiator caspases reveals a role for caspase-2 in heat shock-induced apoptosis. Nat. Cell Biol. 872-77. [DOI] [PubMed] [Google Scholar]
- 33.Varfolomeev, E. E., M. Schuchmann, V. Luria, N. Chiannilkulchai, J. S. Beckmann, I. L. Mett, D. Rebrikov, V. M. Brodianski, O. C. Kemper, O. Kollet, T. Lapidot, D. Soffer, T. Sobe, K. B. Avraham, T. Goncharov, H. Holtmann, P. Lonai, and D. Wallach. 1998. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9267-276. [DOI] [PubMed] [Google Scholar]
- 34.Walczak, H., R. E. Miller, K. Ariail, B. Gliniak, T. S. Griffith, M. Kubin, W. Chin, J. Jones, A. Woodward, T. Le, C. Smith, P. Smolak, R. G. Goodwin, C. T. Rauch, J. C. Schuh, and D. H. Lynch. 1999. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 5157-163. [DOI] [PubMed] [Google Scholar]
- 35.Yang, X., H. Y. Chang, and D. Baltimore. 1998. Autoproteolytic activation of pro-caspases by oligomerization. Mol. Cell 1319-325. [DOI] [PubMed] [Google Scholar]
- 36.Yonehara, S., A. Ishii, and M. Yonehara. 1989. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen codownregulated with the receptor of tumor necrosis factor. J. Exp. Med. 1691747-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.