Abstract
NFATc1 has been characterized as a master regulator of nuclear factor κB ligand-induced osteoclast differentiation. Herein, we demonstrate a novel role for NFATc1 as a positive regulator of nuclear factor κB ligand-mediated osteoclast fusion as well as other fusion-inducing factors such as TNF-α. Exogenous overexpression of a constitutively active form of NFATc1 in bone marrow-derived monocyte/macrophage cells (BMMs) induces formation of multinucleated osteoclasts as well as the expression of fusion-mediating molecules such as the d2 isoform of vacuolar ATPase Vo domain (Atp6v0d2) and the dendritic cell-specific transmembrane protein (DC-STAMP). Moreover, inactivation of NFATc1 by cyclosporin A treatment attenuates expression of Atp6v0d2 and DC-STAMP and subsequent fusion process of osteoclasts. We show that NFATc1 binds to the promoter regions of Atp6v0d2 and DC-STAMP in osteoclasts and directly induces their expression. Furthermore, overexpression of Atp6v0d2 and DC-STAMP rescues cell-cell fusion of preosteoclasts despite reduced NFATc1 activity. Our data indicate for the first time that the NFATc1/Atp6v0d2 and DC-STAMP signaling axis plays a key role in the osteoclast multinucleation process, which is essential for efficient bone resorption.
BONE IS CONTINUOUSLY remodeled by two coupled processes: bone resorption by osteoclasts (giant multinucleated cells that resorb bone) and matrix formation by osteoblasts (1,2,3,4,5). Both processes are responsible for normal skeletal formation and maintenance, and mineral homeostasis. Any imbalance in one of these processes can lead to bone diseases such as osteoporosis. In response to various bone resorbing hormones such as 1,25-(OH)2D3 and PTH, osteoblasts/stromal cells produce the essential cytokines macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor κB ligand (RANKL; also known as TRANCE, OPGL, and ODF), thereby supporting osteoclast formation from monocyte/macrophage precursors. RANKL, a TNF family member, supports osteoclast differentiation, survival, fusion, and activation (1). Binding of RANKL to its receptor, of nuclear factor κB ligand (RANK), activates and/or induces expression of key transcription factors such as nuclear factor-κB, c-Fos, Mitf, PU.1, and NFATc1, which are important for osteoclastogenesis in vitro and in vivo (6,7,8).
There are three stages of development by which monocyte/macrophage precursors develop into functionally active, mature osteoclasts (1): first, monocyte/macrophage precursors become preosteoclasts expressing tartrate-resistant acid phosphatase (TRAP) and calcitonin receptor; second, mononuclear preosteoclasts fuse together to become nonfunctional multinucleated osteoclasts that are polykaryons lacking ruffled borders and thus cannot resorb bone; third, nonfunctional multinucleated osteoclasts are activated into functional osteoclasts by various factors such as RANKL, TNF-α, and lipopolysaccharide (LPS) (1,2). These fully activated, mature osteoclasts have the capacity to resorb bone and die quickly after the resorption process, a point that underscores the necessity for rapid production and balance in this delicate system.
Cell-cell fusion is an essential process for the development of multinucleated cells such as giant cell macrophages and osteoclasts. Fusion-mediated giant cell formation is critical for osteoclast maturation; and without these cells, bone resorption is inefficient (7,9). Mononuclear cells appear to recognize each other and fuse to form multinuclear cells and cell-surface molecules are thought to play a fusion-mediating role. In vitro experiments using neutralizing antibodies and antisense RNAs have demonstrated that fusion process might be mediated by various molecules including, but not limited to: CD9, CD44, E-cadherin, meltrin-α, and macrophage fusion receptor (MFR) (10,11,12,13,14). Recently, it has been shown that gene-targeted mice null for the dendritic cell-specific transmembrane protein (DC-STAMP) and the d2 isoform of vacuolar (H+) ATPase (v-ATPase) Vo domain (Atp6v0d2) develop osteopetrosis due to defects in fusion process during osteoclastogenesis (15,16), suggesting that these molecules are important for osteoclast cell-cell fusion.
NFATc1, a member of the NFAT family of transcription factors, is up-regulated during RANKL-induced osteoclastogenesis (17). Recently, it was reported that ectopic expression of NFATc1 causes precursors to undergo efficient osteoclast differentiation in the absence of RANKL, and that NFATc1-deficient embryonic stem cells fail to differentiate into osteoclasts in response to RANKL (17,18), suggesting that NFATc1 acts as a master regulator of osteoclastogenesis. In addition to the important role for NFATc1 in osteoclast differentiation, recent data indicate that NFATc1 appears to be involved in osteoclast functions such as bone resorption (19). Indeed, previous reports have shown that NFATc1 regulates TRAP, cathepsin K, c-Src, and β3 integrin (17,20,21,22), which are known to be involved in resorption activity. Although NFATc1 appears to regulate osteoclast differentiation and the genetic program required for resorptive activity, a role for NFATc1 in osteoclast fusion has yet to be revealed.
We report here that NFATc1 binds directly to the promoter regions of the fusion-mediating molecules, Atp6v0d2 and DC-STAMP, and induces expression of these genes, thereby regulating osteoclast multinucleation. In addition, NFATc1 inactivation by cyclosporin A (CsA) attenuates expression of Atp6vod2 and DC-STAMP as well as osteoclast fusion. Furthermore, overexpression of Atp6vod2 and DC-STAMP rescues osteoclast fusion in CsA-treated cells. Thus, this study shows that NFATc1 plays a crucial role in RANKL-induced osteoclast fusion via direct up-regulation of the key fusion-mediating molecules: Atp6v0d2 and DC-STAMP.
RESULTS
RANKL Induces the Expression of Atp6v0d2 and DC-STAMP during Osteoclastogenesis
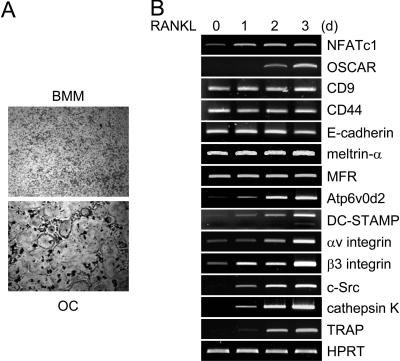
We analyzed the gene expression profiles of various candidate genes involved in cell-cell fusion during RANKL-mediated osteoclastogenesis. When M-CSF-dependent BMM precursors were cultured with M-CSF and RANKL for 3 d, we were able to generate TRAP-positive multinucleated osteoclasts [TRAP(+) MNCs] (Fig. 1A). Consistent with previous results (17,23,24), the expression of NFATc1 was up-regulated 1 d after RANKL stimulation (Fig. 1B). The induction of NFATc1 expression was followed by up-regulation of osteoclast-associated receptor (OSCAR), which is a member of the Ig-like surface receptor family and known to activate NFATc1 via association with the Fc receptor common γ chain (FcRγ) chain thus being a key costimulatory receptor for osteoclast differentiation (25,26,27).
Figure 1.
Gene Expression Profiles in RANKL-Induced Osteoclastogenesis
A, BMMs were cultured with M-CSF for 3 d in the absence or presence of RANKL. BMM and osteoclast (OC) cells were stained for TRAP, a marker for osteoclasts. B, BMMs were cultured with M-CSF and RANKL for the indicated times. Total RNA was collected from each time point. RT-PCR was performed to detect expression of the indicated genes.
In addition, various genes related to osteoclast function, including integrin αv/β3, c-Src, cathepsin K, and TRAP, were found to be expressed at later time points than NFATc1, suggesting that NFATc1 might induce the expression of these function-related genes. We examined the expression pattern of candidate genes known as fusion-mediating molecules both in vitro and in vivo. RANKL up-regulated the expression of Atp6v0d2 and DC-STAMP, whereas the expression of other molecules, including CD9, CD44, E-cadherin, meltrin-α, MFR, was not strongly affected by RANKL stimulation. These results suggest that NFATc1 regulates expression of Atp6v0d2 and DC-STAMP during RANKL-mediated osteoclastogenesis.
The Exogenous Expression of NFATc1 Induces Expression of Atp6v0d2 and DC-STAMP
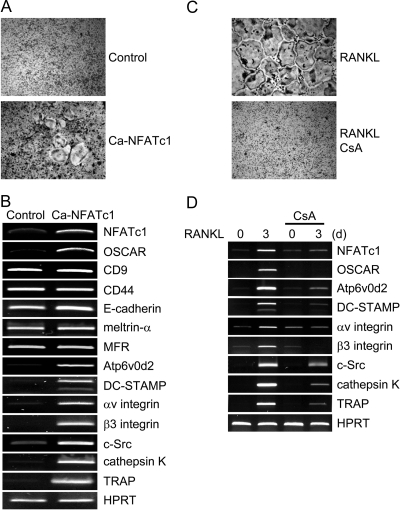
Next, we investigated whether overexpression of NFATc1 alone in BMMs could directly induce expression of Atp6v0d2 and DC-STAMP. When a constitutively active form of NFATc1 (Ca-NFATc1) was retrovirally overexpressed in BMMs, TRAP(+) MNCs were detected in the absence of RANKL (Fig. 2A), consistent with published results (17,23). Overexpression of Ca-NFAT induced OSCAR, integrin αv/β3, c-Src, cathepsin K, and TRAP, which are known as downstream target genes of NFATc1 (Fig. 2B). The expression of Atp6v0d2 and DC-STAMP was detected in Ca-NFATc1-transduced BMMs, but not in control-transduced BMMs. However, the expression of other candidate fusion-mediating molecules, including CD9, CD44, E-cadherin, meltrin-α, MFR, was not affected by NFATc1 overexpression. These data imply that ectopic expression of NFATc1 in osteoclast precursors induces expression of the fusion-mediating molecules Atp6v0d2 and DC-STAMP.
Figure 2.
The Effect of NFATc1 on Expression of Various Genes in Osteoclasts
A and B, BMMs were transduced with pMX-IRES-EGFP (control) or Ca-NFATc1 retroviruses and cultured for 6 d with M-CSF alone. A, Cultured cells were fixed and stained for TRAP. B, RT-PCR was performed to detect expression of the indicated genes. C and D, BMMs were cultured for 3 d with M-CSF and RANKL in the absence or presence of CsA (1 μg/ml). C, Cultured cells were fixed and stained for TRAP. D, Total RNA was collected at the indicated time points. RT-PCR was performed to detect the indicated genes.
NFATc1 Inactivation Attenuates RANKL-Mediated Expression of Atp6v0d2 and DC-STAMP
CsA has been shown to inhibit calcineurin activity, and inhibit RANKL-mediated osteoclastogenesis by suppressing NFATc1 gene expression (17,18). As expected, treatment with CsA (1 μg/ml) inhibited osteoclast formation mediated by M-CSF and RANKL in vitro (Fig. 2C). To investigate whether down-regulation of NFATc1 expression by CsA would affect the expression of Atp6v0d2 and DC-STAMP, we performed RT-PCR using cDNA from cells prepared in the absence or presence of CsA. Induction of NFATc1 mediated by RANKL was attenuated by CsA treatment (Fig. 2D). Compared with control, CsA inhibited the expression of OSCAR, integrin αv/β3, c-Src, cathepsin K, and TRAP: genes that are normally up-regulated by RANKL stimulation. Moreover, RANKL-mediated expression of Atp6v0d2 and DC-STAMP was attenuated by CsA treatment, indicating that NFATc1 is a key modulator of expression of the fusion-mediating molecules Atp6v0d2 and DC-STAMP during RANKL-induced osteoclastogenesis.
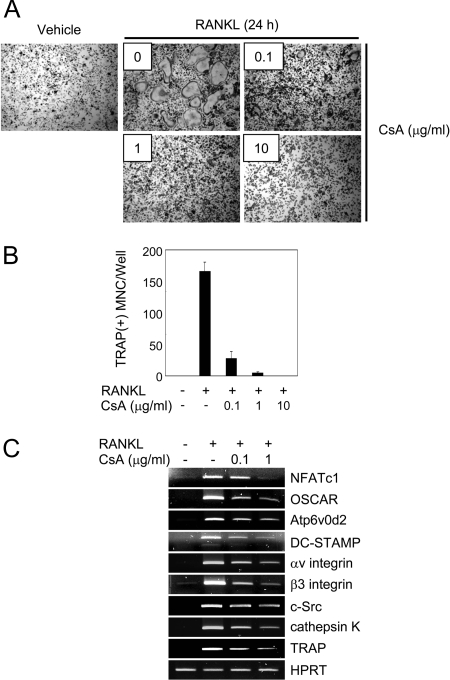
NFATc1 Is Involved in Cell-Cell Fusion Mediated by RANKL
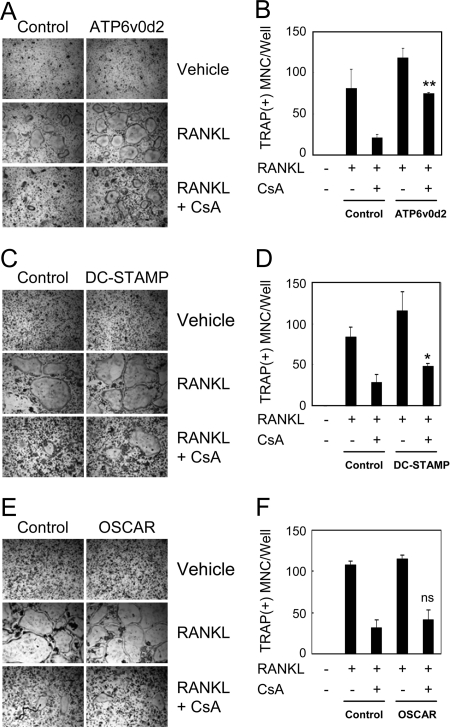
When BMMs were cultured for 48 h in the presence of M-CSF and RANKL, they differentiated into TRAP(+) mononuclear cells, designated as preosteoclasts. To investigate the role of NFATc1 in osteoclast fusion, we used preosteoclasts for fusion assays. When preosteoclasts were cultured for an additional 24 h in the presence of the fusion-inducing cytokine RANKL, TRAP(+) MNCs were formed, but multinuclear osteoclasts were undetectable in the absence (Vehicle) of RANKL (Fig. 3A). The number of TRAP(+) MNCs induced by RANKL was decreased in the presence of CsA in a dose-dependent manner (Fig. 3, A and B). RT-PCR analysis demonstrates that CsA treatment attenuates induction of NFATc1 expression mediated by RANKL, as well as induction of known NFATc1 target genes, including Atp6v0d2 and DC-STAMP (Fig. 3C). These results indicate that NFATc1 is critical for RANKL-induced fusion by means of up-regulating the fusion-mediating molecules, Atp6v0d2 and DC-STAMP.
Figure 3.
The Effect of NFATc1 on Expression of Various Genes during RANKL-Induced Osteoclast Fusion
A–C, To generate preosteoclasts, BMMs were cultured for 48 h in the presence of M-CSF and RANKL. Preosteoclasts were incubated for an additional 24 h with M-CSF, in the absence (Vehicle) or presence of RANKL with the various indicated concentrations of CsA. A, Cultured cells were fixed and stained for TRAP. B, TRAP(+) MNCs having more than three nuclei were counted as osteoclasts. Data represent means ± sd of triplicate samples. C, RT-PCR was performed to detect expression of the indicated genes.
NFATc1 Is Involved in Cell-Cell Fusion Mediated by Other Known Fusion-Inducing Factors
Inflammatory factors, such as TNF-α and LPS, have been shown to induce bone loss via activation of osteoclast differentiation and function (28,29). Therefore, we tested whether these factors could induce cell-cell fusion of preosteoclasts. TNF-α and LPS induced the fusion process, causing preosteoclasts to become TRAP(+) MNCs in a manner similarly associated with RANKL (Fig. 4A). To rule out the possibility that these fusion-inducing factors are merely up-regulating RANKL, we blocked RANKL activity by adding RANK-Fc into the cultures at a concentration (3 μg/ml) known to completely block RANKL-induced fusion process (Fig. 4, A and B). Even in the absence of RANKL-fusion activity, TNF-α and LPS induced formation of multinuclear osteoclasts from preosteoclasts (Fig. 4, A and B), suggesting that these factors can induce osteoclast fusion by a mechanism independent of RANKL-RANK.
Figure 4.
The Effect of NFATc1 on Expression of Various Genes during Osteoclast Fusion Mediated by Other Known Fusion-Inducing Factors
A and B, To generate preosteoclasts, BMMs were cultured for 48 h with M-CSF and RANKL. Preosteoclasts were incubated for an additional 24 h with M-CSF, in the absence (Vehicle) or presence of RANKL, TNF-α, or LPS, with or without RANK-Fc (3 μg/ml) as indicated. A, Cultured cells were fixed and stained for TRAP. B, TRAP(+) MNCs having more than three nuclei were counted as osteoclasts. Data represent means ± sd of triplicate samples. C–E, Preosteoclasts were incubated for 24 h with M-CSF, in the absence (Vehicle) or presence of RANKL, TNF-α, or LPS, with or without CsA (1 μg/ml) as indicated. C, Cultured cells were fixed and stained for TRAP. TRAP(+) MNCs having more than three nuclei were counted as osteoclasts. Data represent means ± sd of triplicate samples. D, RT-PCR was performed to detect expression of the indicated genes. E, Cytoplasmic and nuclear fractions were harvested from cultured cells and subjected to SDS-PAGE and Western blot analysis for detection of NFATc1. Antibodies for actin and histone H3 were used for the normalization of cytoplasmic and nuclear extracts, respectively.
Next, we examined the role of NFATc1 in cell-cell fusion induced by TNF-α and LPS. CsA treatment markedly inhibited fusion as well as NFATc1 expression (Fig. 4, C and D). The expression of NFATc1 target genes, including Atp6v0d2 and DC-STAMP, was also markedly down-regulated by CsA (Fig. 4D). We confirmed that NFATc1 nuclear translocation induced by fusion-inducing factors was also blocked by CsA treatment (Fig. 4E). These results demonstrate that NFATc1 is involved in fusion process induced by TNF-α and LPS.
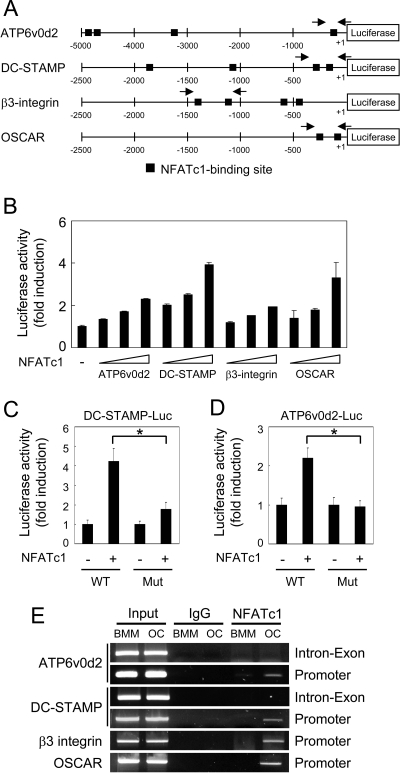
NFATc1 Directly Regulates the Expression of Atp6v0d2 and DC-STAMP
Because ectopic NFATc1 expression in BMMs induces the expression of Atp6v0d2 and DC-STAMP (Fig. 2B), we examined whether the promoter regions of these genes contain NFATc1-binding sites. We found four potential NFATc1-binding sites in the 5-kb promoter region of Atp6v0d2 and the 1.9-kb promoter region of DC-STAMP, respectively (Fig. 5A). To examine whether NFATc1 directly regulates the expression of these genes, we used a reporter assay involving transient transfections into RAW264.7 cells, which is a monocyte/macrophage lineage-derived cell line capable of differentiating into multinuclear osteoclasts. We demonstrate that NFATc1 increased luciferase activity of Atp6v0d2 and DC-STAMP in a dose-dependent manner (Fig. 5B). As a control, we also confirmed the regulatory role of NFATc1 in transactivation of OSCAR and β3 integrin, consistent with previous results (20,23). To examine whether potential NFATc1-binding sites are critical for NFATc1 binding, we used site-directed mutagenesis to engineer constructs containing point mutations in four NFATc1-binding sites in the promoter region of Atp6v0d2 and DC-STAMP, respectively. Mutation of four NFATc1-binding sites abolished efficient activation by ectopic expression of NFATc1 (Fig. 5, C and D).
Figure 5.
NFATc1 Transactivates Atp6v0d2 and DC-STAMP
A, The putative promoter regions of the indicated genes are shown. Closed boxes indicate putative NFATc1-binding sites. Arrows show the location of primers used for the ChIP assay. B, RAW264.7 cells were cotransfected with various luciferase reporter vectors with increasing concentrations (50, 100, and 300 ng) of NFATc1. C and D, DC-STAMP or ATP6v0d2 promoter luciferase reporters containing wild-type (WT) or point mutations (Mut) within the NFATc1-binding sites were transfected into RAW264.7 cells in the presence of control or NFATc1 expression plasmid. *, P < 0.005 vs. control vector. B–D, Luciferase activity was measured using a dual-luciferase reporter assay system. Data represent means ± sd of triplicate samples and the results shown are representative of at least three independent sets of similar experiments. E, ChIP assay of NFATc1 binding to the promoter regions of the indicated genes. BMMs were cultured with M-CSF alone (BMM) or M-CSF and RANKL [osteoclast (OC)] for 3 d before cross-linking. Samples were immunoprecipitated with control IgG or anti-NFATc1 antibodies and subjected to PCR amplification with primers specific for NFATc1-binding sites in the promoter regions (Promoter) of the indicated genes. As a negative control, PCR was performed with primers of 3′-intron/exon regions (Intron-Exon) of ATP6vod2 and DC-STAMP, respectively.
To determine whether NFATc1 binds to the promoter regions of Atp6v0d2 and DC-STAMP endogenously in osteoclasts, we performed a chromatin immunoprecipitation (ChIP) assay. The cross-linked protein/chromatin complex from BMMs or osteoclasts was precipitated with anti-NFATc1 or control antibody, respectively. The precipitated DNA was subjected to PCR amplification with primers specific for promoter regions of genes containing NFATc1-binding sites (Fig. 5A). A single PCR product of Atp6v0d2 and DC-STAMP was obtained using the DNA from osteoclasts after immunoprecipitation with anti-NFATc1 antibody, whereas no PCR product was observed using DNA immunoprecipitated by control antibody (Fig. 5E). In addition to the promoter regions of Atp6v0d2 and DC-STAMP, a 3′ region corresponding to internal intron/exon regions was used as a negative control. As an additional control, we confirmed the endogenous binding of NFATc1 to the promoter regions of OSCAR and β3 integrin. These results provide direct evidence that NFATc1 forms a complex with the Atp6v0d2 and DC-STAMP promoter regions in vivo. Taken together, these results clearly demonstrate that Atp6v0d2 and DC-STAMP are direct targets of the transcription factor NFATc1.
Overexpression of Atp6v0d2 and DC-STAMP Rescues Osteoclast Fusion in Spite of Reduced NFATc1 Activity
We have demonstrated that CsA treatment inactivates NFATc1 and abolishes RANKL-induced fusion of preosteoclasts as well as expression of the fusion-mediating molecules Atp6v0d2 and DC-STAMP (Fig. 3). Therefore, we examined whether overexpression of these genes could rescue cell-cell fusion in CsA-treated cells. CsA treatment reduced the formation of TRAP(+) MNCs from preosteoclasts in empty vector-infected cells (Fig. 6, A and B). Retroviral overexpression of Atp6v0d2 significantly increased the number of TRAP(+) MNCs even in the presence of CsA to similar levels observed in RANKL-treated control cultures. Overexpression of DC-STAMP also significantly increased cell-cell fusion as compared with control cultures in the presence of CsA (Fig. 6, C and D). It is worth noting, however, that DC-STAMP overexpression was less efficient than overexpression of Atp6v0d2 with respect to rescuing fusion in CsA-treated cells. As expected, retroviral overexpression of OSCAR (negative control) did not rescue osteoclast fusion in the presence of CsA (Fig. 6, E and F). These data indicate that Atp6v0d2 and DC-STAMP play a key role in NFATc1-mediated cell-cell fusion during RANKL-induced osteoclastogenesis.
Figure 6.
Overexpression of Atp6v0d2 or DC-STAMP Rescue Osteoclast Fusion Despite Reduced NFATc1 Activity
A and B, BMMs were infected with pMX-puro (control) or Atp6v0d2 retrovirus and selected with puromycin (1 μg/ml) for 48 h. C and D, BMMs were transduced with pMX-IRES-EGFP (control) or DC-STAMP retrovirus. E and F, BMMs were transduced with pMX-IRES-EGFP (control) or OSCAR retrovirus. A–F, To generate preosteoclasts, transduced cells were cultured with M-CSF and RANKL for 48 h. Preosteoclasts were incubated for an additional 24 h with M-CSF alone (Vehicle) or M-CSF/RANKL in the absence or presence of CsA (0.1 μg/ml). A, C, and E, Cultured cells were fixed and stained for TRAP. B, D, and F, TRAP(+) MNCs having more than three nuclei were counted as osteoclasts. Data represent means ± sd of triplicate samples. *, P < 0.05 vs. control with CsA; **, P < 0.0001 vs. control with CsA. ns, Not significant vs. control with CsA.
DISCUSSION
Giant, multinuclear osteoclasts, derived from mononuclear precursors of the monocyte/macrophage lineage, are unique cells that resorb bone. RANKL induces the formation of mature, active osteoclasts by regulating various transcription factors such as nuclear factor-κB, Mitf, c-Fos, and NFATc1 (6,8). In this study, we clearly demonstrate that NFATc1 plays an important role in the fusion of preosteoclasts which yields giant, multinuclear osteoclasts. Ectopic expression of NFATc1 in BMMs induces the formation of multinuclear osteoclasts in the absence of known fusion-inducing factors such as RANKL. In addition, down-regulation of NFATc1 activity by treatment with CsA in preosteoclasts markedly attenuates cell-cell fusion even in the presence of fusion-inducing factors such as RANKL, TNF-α, and LPS. Furthermore, we clearly demonstrate that NFATc1 induces expression of the key fusion-mediating molecules Atp6v0d2 and DC-STAMP, and that overexpression of these genes rescues the formation of multinuclear osteoclasts in CsA-treated cultures of mononuclear osteoclast precursors. These results indicate that NFATc1 is a key mediator of osteoclast fusion via up-regulation of Atp6v0d2 and DC-STAMP.
Many reports have provided strong evidence for NFATc1 as a critical transcription factor for RANKL-induced osteoclast differentiation. RANKL stimulation of BMMs, osteoclast precursors, induces expression of NFATc1 through c-Fos and auto-amplification by NFATc1 (17,30). NFATc1-deficient embryonic stem cells fail to differentiate into mature osteoclasts in response to RANKL (17). In addition, exogenous overexpression of ca-NFATc1 in BMMs efficiently induces differentiation of these cells into osteoclasts even in the absence of RANKL (17,23). These results indicate that NFATc1 is a master regulator of osteoclast differentiation.
In addition to its essential role in osteoclast differentiation, NFATc1 is most likely involved in regulating osteoclast function. NFATc1 induces expression of TRAP, cathepsin K, c-Src, and β3 integrin, which are responsible for bone resorption activity. Recently, Komarova et al. (19) demonstrated that acidification and RANKL stimulate resorption through the Ca2+/calcineurin/NFATc1 signaling axis in rodent osteoclasts. Fuller et al. (31) have shown that CsA, which down-regulates NFATc1 activity, causes substantial inhibition of bone resorption mediated by RANKL and IL-1. These data indicate that NFATc1 plays a key role in osteoclast function. In contrast, Kim et al. (32) have shown that bone resorption activity is not affected by a small interfering RNA knock-down of NFATc1 in mature human osteoclasts. This discrepancy might be due to differences in NFATc1 expression levels necessary for inducing optimal resorption activity in different cell types, or the compensatory effects of other NFAT family members in human osteoclasts. It remains to be elucidated whether NFATc1 is involved in osteoclast function.
NFATc1 induces many target genes responsible for osteoclast differentiation and function, including OSCAR, TRAP, cathepsin K, c-Src, integrin αv/β3. Our data clearly demonstrate that overexpression of Ca-NFATc1 induces osteoclast fusion (Fig. 2A) and that down-regulation of NFATc1 by CsA attenuates multinucleation of preosteoclasts (Figs. 2C; 3, A and B; and 4C). In addition, induction of NFATc1 and NFATc1 nuclear translocation are requisite for RANKL-mediated osteoclast cell-cell fusion as well as fusion mediated by TNF-α and LPS (Fig. 4). These data indicate that NFATc1-induced expression of fusion-mediating gene(s) is crucial for osteoclast fusion. Among the candidate fusion-mediating genes tested, only Atp6v0d2 and DC-STAMP were up-regulated by NFATc1. Notably, other candidate fusion-mediating molecules such as CD9, CD44, E-cadherin, meltrin-α, and MFR were expressed at similar levels in both BMMs and osteoclasts, whereas the expression of NFATc1 as well as Atp6v0d2 and DC-STAMP were expressed at relatively high levels in osteoclasts, but not in BMMs. Another key finding was that NFATc1 binds directly to the Atp6v0d2 and DC-STAMP promoter regions and induces the expression of these key genes. Interestingly, overexpression of Atp6v0d2 or DC-STAMP rescues fusion in the presence of lower concentrations (0.1 μg/ml) of CsA (Fig. 6), but not at higher concentrations (more than 1 μg/ml) of CsA (data not shown). These data imply that certain levels of both molecules, Atp6v0d2 and DC-STAMP, might be needed for osteoclast fusion. Indeed, mice deficient for Atp6v0d2 or DC-STAMP have defects in multinucleation of TRAP(+) mononuclear preosteoclasts, indicating that induction of both Atp6v0d2 and DC-STAMP might be necessary for preosteoclast cell-cell fusion.
Multinucleated giant cells are formed by the fusion of macrophages during chronic inflammation. IL-4 induces formation of foreign body giant cells (FBGC) from human monocytes/macrophages and murine bone marrow macrophages cultured in the presence IL-3 in vitro (33,34). To investigate whether Atp6v0d2 and DC-STAMP are also involved in macrophage cell-cell fusion, we examined the expression pattern of both genes in BMMs cultured in the presence of IL-3 and IL-4. We found that the expression of both genes was markedly up-regulated by treatment with IL-3 and IL-4 during the formation of FBGC (data not shown), suggesting that expression of Atp6v0d2 and DC-STAMP might be important for cell-cell fusion of macrophages. Indeed, macrophages from Atp6v0d2 or DC-STAMP knockout mice have marked defects in FBGC formation induced by IL-3 and -4 (15,16). Collectively, these data indicate that Atp6v0d2 and DC-STAMP expression plays an important role in osteoclast as well as macrophage cell-cell fusion. However, further studies are needed to elucidate the mechanism by which Atp6v0d2 and DC-STAMP induce osteoclast and macrophage fusion.
In this study, we demonstrate a novel role for NFATc1 as a positive regulator of RANKL-mediated osteoclast fusion. We provide strong evidence that NFATc1 promotes multinucleation of TRAP(+) mononuclear preosteoclasts through up-regulation of Atp6v0d2 and DC-STAMP. Therefore, further investigation of the detailed mechanism of the RANKL/NFATc1/Atp6v0d2 and DC-STAMP signaling axis will provide beneficial strategies for alternative therapies for the treatment of bone diseases such as osteoporosis and rheumatoid arthritis.
MATERIALS AND METHODS
Reagents
All cell culture media and supplements were obtained from Invitrogen Life Technologies (Carlsbad, CA). Soluble recombinant mouse RANKL and RANK-Fc were purified from insect cells as described previously (35). M-CSF and TNF-α were purchased from R&D Systems (Minneapolis, MN). LPS was purchased from Sigma-Aldrich (St. Louis, MO). CsA was purchased from Calbiochem (La Jolla, CA). Actin-specific antibodies were purchased from Sigma-Aldrich; anti-NFATc1 antibodies from BD Biosciences (Franklin Lakes, NJ); anti-histone H3 antibodies from Upstate Biotechnologies (Lake Placid, NY).
Constructs
The promoter regions of Atp6v0d2 (5.0 kb), DC-STAMP (1.9 kb), and β3-integrin (1.6 kb) were amplified by PCR using mouse genomic DNA. The amplified PCR fragments were digested with KpnI and XhoI and cloned into the pGL3 basic luciferase vector (Promega, Madison, WI). The oligonucleotide sequences for promoter luciferase constructs are shown in Supplemental Table 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org. The OSCAR reporter vector and retroviral vectors containing ca-NFATc1, Atp6v0d2, and DC-STAMP, OSCAR, respectively, were previously described (15,23,25,36,37). Four putative NFATc1-binding sites (GGAAAA) in the promoter region of Atp6v0d2 and DC-STAMP were changed to GGACCA by site-directed mutagenesis.
Osteoclast Formation
Murine osteoclasts were prepared from bone marrow cells as previously described (24). In brief, bone marrow cells were cultured in α-MEM containing 10% fetal bovine serum with M-CSF (5 ng/ml) for 16 h. Nonadherent cells were harvested and cultured for 3 d in the presence of M-CSF (30 ng/ml). Floating cells were removed and adherent cells were used as osteoclast precursors (BMMs). To generate osteoclasts, BMMs were cultured with M-CSF (30 ng/ml) and RANKL (100 ng/ml) for 3 d. Cultured cells were fixed and stained for TRAP as previously described (37). TRAP-positive multinuclear cells [TRAP(+) MNCs], containing more than three nuclei, were counted as osteoclasts.
Fusion Assay
For generation of preosteoclasts, BMMs were cultured for 48 h in the presence of M-CSF (30 ng/ml) and RANKL (100 ng/ml). Preosteoclasts were cultured for an additional 24 h with M-CSF (30 ng/ml) in the absence (vehicle) or presence of RANKL (150 ng/ml), TNF-α (20 ng/ml), or LPS (1 μg/ml). In some experiments, RANK-Fc (3 μg/ml) was added to the cultures. Cultured cells were subsequently fixed and stained for TRAP.
Fractionation and Western Blot Analysis
BMMs were cultured for 48 h in the presence of M-CSF (30 ng/ml) and RANKL (100 ng/ml). Preosteoclasts were incubated for an additional 24 h with M-CSF (30 ng/ml) in the absence (Vehicle) or presence of RANKL (150 ng/ml), TNF-α (20 ng/ml), or LPS (1 μg/ml). Cultured cells were fractionated using Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, IL) according to the manufacturer’s protocol. Cytoplasmic and nuclear extracts were subjected to SDS-PAGE and Western blotting. Signals were detected and analyzed by LAS3000 luminescent image analyzer (Fuji Co., Tokyo, Japan).
Retroviral Infection
To generate retroviral stocks, retroviral vectors were transfected into the packaging cell line Plat E using FuGENE 6 (Roche Applied Sciences, Indianapolis, IN). Viral supernatant was collected from culture media 24–48 h after transfection. BMMs were incubated with viral supernatant for 8 h in the presence of polybrene (10 μg/ml). After removing the viral supernatant, BMMs were cultured with M-CSF (30 ng/ml) and RANKL (100 ng/ml) for 48 h for fusion assay.
Semiquantitative RT-PCR
RT-PCR was performed as previously described (35). Primer sequences are shown in Supplemental Table 2.
Transfection and Luciferase Assay
For transfection of reporter plasmids, RAW264.7 cells were plated into 24-well plates at a density of 2 × 104 cells/well 24 h before transfection. Plasmid DNA was mixed with FuGENE 6 and transfected into the cells following the manufacturer’s protocol. After 48 h of transfection, the cells were washed twice with PBS and then lysed in passive lysis buffer (Promega). Luciferase activity was measured using a dual-luciferase reporter assay system (Promega) according to the manufacturer’s instructions.
ChIP Assay
A ChIP assay was performed with a ChIP kit (Upstate Biotechnologies) according to the manufacturer’s instructions, using antibodies against NFATc1 or IgG (control) (Santa Cruz Biotechnology, Santa Cruz, CA). The precipitated DNA was subjected to PCR amplification with primers specific for the promoter regions of genes containing NFATc1-binding sites. The primer sequences are shown in Supplemental Table 3.
Supplementary Material
Acknowledgments
We thank T. Kitamura and T. Miyamoto for reagents, H. M. Jin and A. R. Ko for excellent technical assistance, and J. P. Miller for editorial assistance.
Footnotes
This work was supported by Korea Research Foundation (KRF-2006-311-E00357), National Research Laboratory (R0A-2007-000-20025-0), Grant R13-2002-013-03001-0 from the Korea Science & Engineering Foundation through the Medical Research Center for Gene Regulation at Chonnam National University (to N.K.), and the U.S. National Institutes of Health (to Y.C.).
Disclosure Statement: The authors have nothing to declare.
First Published Online September 20, 2007
* K.K. and S.-H.L. contributed equally to this work.
Abbreviations: Atp6v0d2, v-ATPase Vo domain; BMMs, bone marrow-derived monocyte/macrophage cells; Ca-, constitutively active form; ChIP, chromatin immunoprecipitation; CsA, cyclosporin A; DC-STAMP, dendritic cell-specific transmembrane protein; FBGC, foreign body giant cells; LPS, lipopolysaccharide; M-CSF, macrophage colony-stimulating factor; MFR, macrophage fusion receptor; OSCAR, osteoclast-associated receptor; RANK, nuclear factor κB ligand; RANKL, RANK ligand; TRAP, tartrate-resistant acid phosphatase; v-ATPase, d2 isoform of vacuolar (H+) ATPase.
References
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ 1999 Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20:345–357 [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ 1998 Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176 [DOI] [PubMed] [Google Scholar]
- Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, Choi Y 1997 TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med 186:2075–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T 1998 Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L 1997 A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390:175–179 [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL 2003 Osteoclast differentiation and activation. Nature 423:337–342 [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL 2000 Bone resorption by osteoclasts. Science 289:1504–1508 [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Ross FP 2003 Genetic regulation of osteoclast development and function. Nat Rev Genet 4:638–649 [DOI] [PubMed] [Google Scholar]
- Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y 2006 Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol 24:33–63 [DOI] [PubMed] [Google Scholar]
- Abe E, Mocharla H, Yamate T, Taguchi Y, Manolagas SC 1999 Meltrin-α, a fusion protein involved in multinucleated giant cell and osteoclast formation. Calcif Tissue Int 64:508–515 [DOI] [PubMed] [Google Scholar]
- de Vries TJ, Schoenmaker T, Beertsen W, van der Neut R, Everts V 2005 Effect of CD44 deficiency on in vitro and in vivo osteoclast formation. J Cell Biochem 94:954–966 [DOI] [PubMed] [Google Scholar]
- Mbalaviele G, Chen H, Boyce BF, Mundy GR, Yoneda T 1995 The role of cadherin in the generation of multinucleated osteoclasts from mononuclear precursors in murine marrow. J Clin Invest 95:2757–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saginario C, Sterling H, Beckers C, Kobayashi R, Solimena M, Ullu E, Vignery A 1998 MFR, a putative receptor mediating the fusion of macrophages. Mol Cell Biol 18:6213–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Tachibana I, Miyado K, Kobayashi M, Miyazaki T, Funakoshi T, Kimura H, Yamane H, Saito Y, Goto H, Yoneda T, Yoshida M, Kumagai T, Osaki T, Hayashi S, Kawase I, Mekada E 2003 Tetraspanins CD9 and CD81 function to prevent the fusion of mononuclear phagocytes. J Cell Biol 161:945–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N, Kang JS, Miyamoto T, Suda T, Lee SK, Pignolo RJ, Koczon-Jaremko B, Lorenzo J, Choi Y 2006 v-ATPase V(0) subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med 12:1403–1409 [DOI] [PubMed] [Google Scholar]
- Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T 2005 DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med 202:345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T 2002 Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3:889–901 [DOI] [PubMed] [Google Scholar]
- Hirotani H, Tuohy NA, Woo JT, Stern PH, Clipstone NA 2004 The calcineurin/nuclear factor of activated T cells signaling pathway regulates osteoclastogenesis in RAW264.7 cells. J Biol Chem 279:13984–13992 [DOI] [PubMed] [Google Scholar]
- Komarova SV, Pereverzev A, Shum JW, Sims SM, Dixon SJ 2005 Convergent signaling by acidosis and receptor activator of NF-κB ligand (RANKL) on the calcium/calcineurin/NFAT pathway in osteoclasts. Proc Natl Acad Sci USA 102:2643–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotti TN, Flannery M, Walsh NC, Fleming JD, Goldring SR, McHugh KP 2006 NFATc1 regulation of the human β3 integrin promoter in osteoclast differentiation. Gene 372:92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda F, Nishimura R, Matsubara T, Hata K, Reddy SV, Yoneda T 2006 Activation of NFAT signal in vivo leads to osteopenia associated with increased osteoclastogenesis and bone-resorbing activity. J Immunol 177:2384–2390 [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Kogawa M, Wada S, Takayanagi H, Tsujimoto M, Katayama S, Hisatake K, Nogi Y 2004 Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU. 1. J Biol Chem 279:45969–45979 [DOI] [PubMed] [Google Scholar]
- Kim K, Kim JH, Lee J, Jin HM, Lee SH, Fisher DE, Kook H, Kim KK, Choi Y, Kim N 2005 Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J Biol Chem 280:35209–35216 [DOI] [PubMed] [Google Scholar]
- Lee J, Kim K, Kim JH, Jin HM, Choi HK, Lee SH, Kook H, Kim KK, Yokota Y, Lee SY, Choi Y, Kim N 2006 Id helix-loop-helix proteins negatively regulate TRANCE-mediated osteoclast differentiation. Blood 107:2686–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Takami M, Rho J, Josien R, Choi Y 2002 A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med 195:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T 2004 Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428:758–763 [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Arase N, Suenaga T, Saita Y, Noda M, Kuriyama T, Arase H, Saito T 2004 Involvement of FcRγ in signal transduction of osteoclast-associated receptor (OSCAR). Int Immunol 16:1019–1025 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T 2000 Tumor necrosis factor α stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med 191:275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda K, Woo JT, Takami M, Sexton PM, Nagai K 2002 Lipopolysaccharide supports survival and fusion of preosteoclasts independent of TNF-α, IL-1, and RANKL. J Cell Physiol 190:101–108 [DOI] [PubMed] [Google Scholar]
- Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H 2005 Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med 202:1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller K, Kirstein B, Chambers TJ 2006 Murine osteoclast formation and function: differential regulation by humoral agents. Endocrinology 147:1979–1985 [DOI] [PubMed] [Google Scholar]
- Kim MS, Day CJ, Selinger CI, Magno CL, Stephens SR, Morrison NA 2006 MCP-1-induced human osteoclast-like cells are tartrate-resistant acid phosphatase, NFATc1, and calcitonin receptor-positive but require receptor activator of NFκB ligand for bone resorption. J Biol Chem 281:1274–1285 [DOI] [PubMed] [Google Scholar]
- McInnes A, Rennick DM 1988 Interleukin 4 induces cultured monocytes/macrophages to form giant multinucleated cells. J Exp Med 167:598–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally AK, Anderson JM 1995 Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am J Pathol 147:1487–1499 [PMC free article] [PubMed] [Google Scholar]
- Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, Kim JH, Kobayashi T, Odgren PR, Nakano H, Yeh WC, Lee SK, Lorenzo JA, Choi Y 2005 Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med 202:589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukita T, Wada N, Kukita A, Kakimoto T, Sandra F, Toh K, Nagata K, Iijima T, Horiuchi M, Matsusaki H, Hieshima K, Yoshie O, Nomiyama H 2004 RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med 200:941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- So H, Rho J, Jeong D, Park R, Fisher DE, Ostrowski MC, Choi Y, Kim N 2003 Microphthalmia transcription factor and PU. 1 synergistically induce the leukocyte receptor osteoclast-associated receptor gene expression. J Biol Chem 278:24209–24216 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.