Abstract
Although the sartan family of angiotensin II type 1 (AT1) receptor blockers (ARBs), which includes valsartan, olmesartan, and losartan, have a common pharmacophore structure, their effectiveness in therapy differs. Although their efficacy may be related to their binding strength, this notion has changed with a better understanding of the molecular mechanism. Therefore, we hypothesized that each ARB differs with regard to its molecular interactions with AT1 receptor in inducing inverse agonism. Interactions between valsartan and residues Ser105, Ser109, and Lys199 were important for binding. Valsartan is a strong inverse agonist of constitutive inositol phosphate production by the wild-type and N111G mutant receptors. Substituted cysteine accessibility mapping studies indicated that valsartan, but not losartan, which has only weak inverse agonism, may stabilize the N111G receptor in an inactive state upon binding. In addition, the inverse agonism by valsatan was mostly abolished with S105A/S109A/K199Q substitutions in the N111G background. Molecular modeling suggested that Ser109 and Lys199 bind to phenyl and tetrazole groups of valsartan, respectively. Ser105 is a candidate for binding to the carboxyl group of valsartan. Thus, the most critical interaction for inducing inverse agonism involves transmembrane (TM) V (Lys199) of AT1 receptor although its inverse agonist potency is comparable to olmesartan, which bonds with TM III (Tyr113) and TM VI (His256). These results provide new insights into improving ARBs and development of new G protein-coupled receptor antagonists.
THE OCTAPEPTIDE HORMONE angiotensin II (Ang II) is the primary effector of the renin-angiotensin system. Ang II type 1 (AT1) receptor has a wide tissue distribution and mediates most known cardiovascular functions including vasoconstriction, cardiovascular hypertrophy, and hyperplasia (1,2).
Several naturally occurring and disease-causing G protein-coupled receptor (GPCR) mutants with increased constitutive activity relative to wild-type (WT) GPCRs have been identified (3). The most important pharmacological tools for analyzing constitutive activity are inverse agonists, which suppress basal activity upon binding to the receptors. Inverse agonists may be useful in the treatment of cardiovascular disease and disease caused by constitutively active GPCRs. Although such spontaneous mutations have not been reported for the AT1 receptor, slight constitutive activity of the AT1-WT receptor might be an important factor for consideration in treatment. In addition, mechanical stress has been shown to activate AT1 receptor independent of Ang II, and this activation can be blocked by an inverse agonist AT1 receptor blocker (ARB), candesartan (4). Although the most important effect of ARBs is their antagonism, which competitively inhibits Ang II-induced signaling, the inverse agonism of ARBs plays a more important role than initially thought.
In vitro mutagenesis studies (5,6,7,8) ARBs have shown that Exp 3174 (an active metabolite of losartan) (9) is an inverse agonist, but not the commonly prescribed drug, losartan, for inositol phosphate (IP) production against the constitutive activity of the AT1-N111G mutant receptor. The constitutive activity of the AT1-N111G receptor induced G1 arrest and hypertrophy, and this function was dependent on the amount of receptor present (2). There may be some mechanism by which the AT1 receptor overexpression causes a reversible transition between hyperplasia and hypertrophy. In addition, the ARB, olmesartan, was shown to have stronger inverse agonist activity using the superconstitutive activity of the AT1-F77A/N111G mutant receptor (10). Whether the AT1-WT receptor exhibited constitutive activity, to account for the therapeutic efficacy based on the molecular mechanisms of ARBs defined by using constitutively active AT1 mutant receptors, was unclear in these studies.
Although most ARBs share a common core structure, their effectiveness in therapy differs. It has been suggested that their efficacy may be related to binding strength, but this notion has changed with a better understanding of the molecular mechanism. Therefore, we hypothesized that each ARB differs in molecular interactions with AT1 receptor in inducing inverse agonism. In this study, we analyzed the molecular interactions between valsartan and AT1 receptor using mutagenesis, binding studies, inositol phosphate (IP) production assay, and computer modeling. We compared the molecular interactions of valsartan with those of olmesartan according to our previous report (10). We also confirmed that AT1-WT receptor had constitutive activity independent of Ang II stimulation and that valsartan stabilized the AT1 receptor in an inactive conformation.
RESULTS
AT1-WT Receptor Displays Constitutive Activity for IP Production in Native Vascular Smooth Muscle Cells (VSMCs) and Recombinant Overexpression Systems
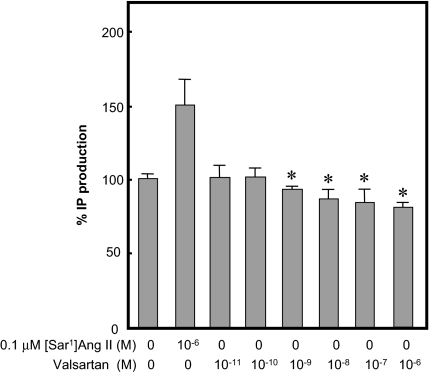
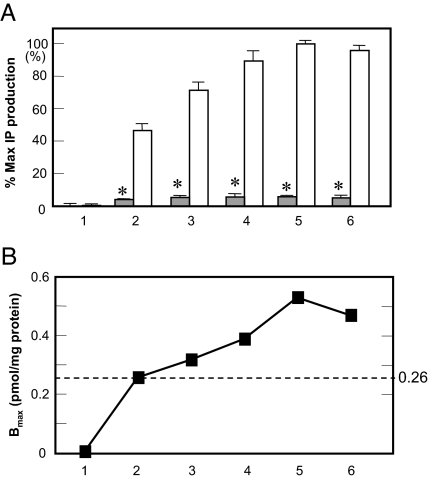
To confirm whether AT1-WT receptor has constitutive activity for IP production, we used endogenously AT1-WT receptor expressed in VSMCs (Bmax, 11.0 ± 0.6 fmol/mg protein). Valsartan significantly suppressed basal IP production in a dose-dependent manner (Fig. 1). Next, we transfected cells with different amounts of AT1-WT receptor and found different expression levels of AT1-WT receptor (Fig. 2). The Ang II-stimulated IP production gradually increased with an increase in AT1-WT receptor expression. At expression levels of more than 0.26 pmol/mg protein the WT receptor showed significantly higher basal activity when compared with mock-transfected cells.
Figure 1.
Percentage IP production with or without 0.1 μm [Sar1]Ang II or Indicated Concentration of Valsartan in the AT1-WT Receptor Expressing Native VSMCs
Percentage of IP production adjusted for basal IP production (570 cpm) as 100%. *, P < 0.05 vs. without [Sar1]Ang II.
Figure 2.
Percentage Maximum (% Max) IP Production and Expression Levels of AT1-WT Receptors
A, % Max IP production with (open bars) or without (gray bars) 0.1 μm [Sar1]Ang II in COS1 cells transiently expressing different levels of AT1-WT receptors (nos. 1–6). % Max IP production indicates [Sar1]Ang II-induced IP production (11,000 cpm) in WT AT1 receptor-transfected cells (100%) after adjusting for basal IP production (2,800 cpm) without treatment in mock-treated cells (0%). *, P < 0.05 vs. no treatment on No. 1. B, Bmax (pmol/mg protein) for the different expression levels of AT1-WT receptors (nos. 1–6).
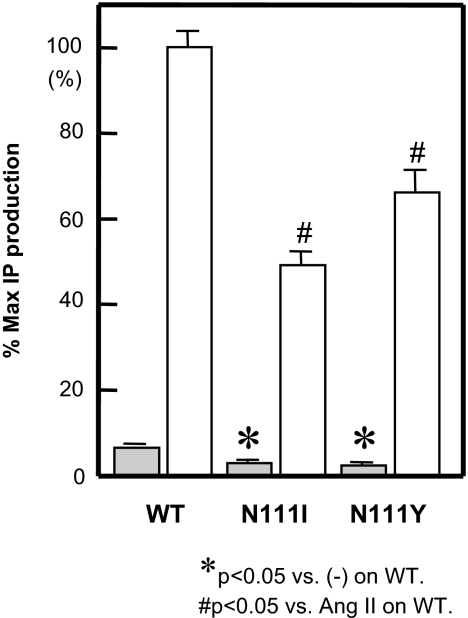
When the residue at Asn111 in the AT1-WT receptor was increased in size to Ile111 (N111I) or Tyr111 (N111Y), the mutant receptors showed a significant reduction in basal activity in IP production (Fig. 3). The expression levels of AT1-N111I and N111Y receptors were 0.33 ± 0.01 and 0.44 ± 0.08 pmol/mg protein, respectively (Table 1). Because these expression levels were greater than 0.26 pmol/mg protein in AT1-WT receptor (Fig. 2), AT1-N111I and N111Y receptors switched to an inactive state.
Figure 3.
Percentage Maximum (% Max) IP Production with (open bars) or without (gray bars) 0.1 μm [Sar1]Ang II in COS1 Cells Transiently Expressing AT1-WT, N111I, N111Y and N111G Receptors
*, P < 0.05 vs. no treatment on AT1-WT. #, P < 0.05 vs. [Sar1]Ang II on AT1-WT.
Table 1.
Maximal Binding Capacities (Bmax) and Binding Affinities (Kd) of [Sar1,Ile8]Ang II and Valsartan to AT1 WT and Mutant Receptors
| Receptor | Bmax (pmol/mg protein) | Kd (nm)
|
|
|---|---|---|---|
| [Sar1,Ile8]Ang II | Valsartan | ||
| WT | 0.58 ± 0.08 | 0.7 ± 0.3 (1.0) | 2.5 ± 0.5 (1.0) |
| N111I | 0.33 ± 0.01 | 0.4 ± 0.1 (0.6) | |
| N111Y | 0.44 ± 0.08 | 1.1 ± 0.2 (1.6) | |
| N111G | 0.40 ± 0.03 | 0.8 ± 0.2 (1.1) | 128 ± 40 (51) |
| F77A/N1111G | 0.64 ± 0.04 | 3.9 ± 0.5 (5.6) | 246 ± 44 (98) |
| S105A | 0.56 ± 0.10 | 1.6 ± 0.6 (2.3) | 26 ± 7 (10) |
| S107A | 0.53 ± 0.07 | 1.1 ± 0.4 (1.6) | 9 ± 4 (3.6) |
| S109A | 0.47 ± 0.04 | 1.4 ± 0.1 (2.0) | 27 ± 7 (11) |
| Y113F | 0.63 ± 0.11 | 2.1 ± 1.1 (3.0) | 9.7 ± 2.1 (3.9) |
| K199Q | 0.23 ± 0.03 | 2.5 ± 1.3 (3.6) | 118 ± 15 (47) |
| H256A | 0.33 ± 0.06 | 0.9 ± 0.6 (1.3) | 2.1 ± 1.1 (0.8) |
| Q257A | 0.20 ± 0.05 | 5.0 ± 2.2 (7.1) | 24 ± 8 (9.6) |
| S105A/N111G | 0.50 ± 0.02 | 1.1 ± 0.3 (1.6) | 167 ± 28 (67) |
| S109A/N111G | 0.66 ± 0.02 | 0.7 ± 0.3 (1.0) | 129 ± 41 (52) |
| N111G/K199Q | 0.31 ± 0.02 | 1.1 ± 0.8 (1.6) | 560 ± 172 (224) |
| S105A/S109A/N111G | 0.39 ± 0.02 | 0.5 ± 0.3 (0.7) | 173 ± 37 (69) |
| S105A/N111G/K199Q | 0.32 ± 0.02 | 1.0 ± 0.2 (1.4) | 371 ± 107 (148) |
| S109A/N111G/K199Q | 0.40 ± 0.03 | 0.9 ± 0.3 (1.3) | 357 ± 58 (143) |
| S105A/S109A/N111G/K199Q | 0.45 ± 0.05 | 1.0 ± 0.2 (1.4) | 337 ± 87 (135) |
Valsartan Exhibits Inverse Agonism and Stabilized the AT1-WT Receptor
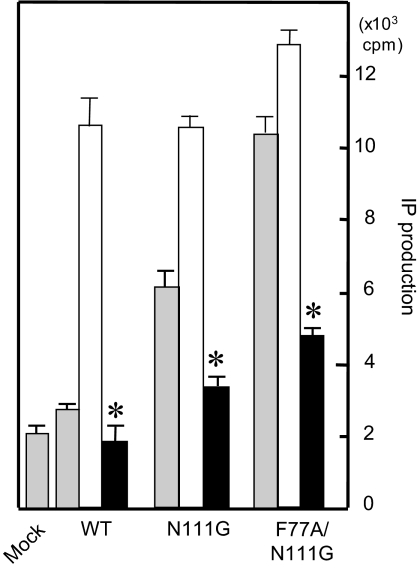
A recombinant AT1-WT receptor that was expressed at a high density showed constitutive activity. Valsartan significantly suppressed basal IP production (Fig. 4). However, the basal activity of the AT1-WT receptor is low for evaluating the molecular basis of inverse agonism by valsartan. To confirm the inverse agonistic ability of valsartan, we used AT1 mutant receptors. Asn111-mutated AT1 receptors (AT1-N111G) are thought to mimic the preactivated state of the AT1-WT receptor, and a comparison of the efficacy and affinity of ligands for such mutated receptors makes it easier to distinguish partial and inverse agonists from true antagonists (7). AT1-F77A/N111G mutant receptor, which behaves as a superconstitutive active receptor, is in the fully active state because IP production was comparable to that with the Ang II-induced fully active state as shown in Fig. 4. Valsartan clearly showed inverse agonism in the AT1-N111G mutant receptor as well as the full-activated state of AT1-F77A/N111G mutant receptor.
Figure 4.
Percentage Maximum (% Max) IP Production without (gray bar) or with 0.1 μm [Sar1]Ang II (open bar) and 1 μm Valsartan (solid bar) in COS1 Cells Transiently Expressing AT1-WT, N111G and F77A/N111G Receptors
*, P < 0.05 vs. no treatment on AT1-WT.
Valsartan Stabilizes the AT1-N111G Mutant Receptor
A substituted cysteine accessibility mapping (SCAM) study was performed to confirm that inverse agonist stabilized the AT1-N111G mutant receptor (Fig. 5). Because this method has previously been used to analyze inactive and constitutively active states of the AT1 receptor (9,12,13) and other GPCRs (11,12,13), a SCAM study should also be able to reveal relative changes in water accessibility. The CH3SO2-SCH2CH2NH3+ (MTSEA+) reagent employed is more than 100,000-fold reactive toward water-exposed Cys residues. This technique, combined with site-directed cysteine substitution and ligand binding analysis, has been successfully applied for mapping ligand pocket and helical movement in GPCRs without the difficulties of receptor purification.
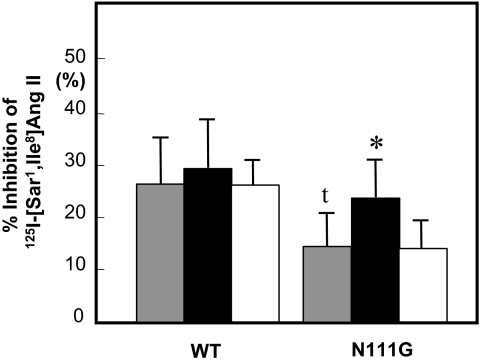
Figure 5.
Inhibition of 125I-[Sar1, Ile8]Ang II-Specific Binding without (gray bar) or with 1 μm Valsartan (solid bar) or Losartan (open bar) after Treatment with 2.5 mm MTSEA+
Experimental procedure is described in Materials and Methods. Maximal specific binding to each control sample without MTSEA+ reagent is taken to be 100%. *, P < 0.05 vs. without ARBs on AT1-N111G. t, P < 0.05 vs. without ARBs on AT1-WT.
First, we analyzed the percent inhibition of 125I-[Sar1, Ile8]Ang II binding in the AT1-WT receptor and determined that this value was 26 ± 9%. We previously reported that the % inhibition of 125I-[Sar1, Ile8]Ang II binding by MTSEA+ reagent is about 28% due to Cys76 in the AT1 receptor because Cys76 in the ligand pocket is accessible to water in the WT receptor (14). After pretreatment with valsartan or losartan, this inhibition did not change in the WT receptor. In the AT1-N111G receptor, the percent inhibition of 125I-[Sar1, Ile8]Ang II significantly decreased (15 ± 7%) because Cys76 was not accessible to water. Interestingly, after pretreatment with valsartan, inhibition increased to 24 ± 7%, which was comparable to that with WT receptor, whereas inhibition after pretreatment with losartan did not change (15 ± 6%). These results suggest that Cys76 moves back to a water-accessible area (receptor pocket) upon treatment with valsartan but not losartan, and the inverse agonist valsartan stabilizes the inactive state of AT1-N111G mutant receptor.
Ser105, Ser109, and Lys199 in the AT1 Receptor as Specific Binding Sites of Valsartan
Next, we selected candidate residues (Ser105, Ser107, Ser109, and Lys199) in the AT1 receptor as specific binding sites for valsartan according to the results of molecular modeling. As shown in Table 1, valsartan showed a more than 10-fold loss of binding affinity in AT1-S105A, AT1-S109A, and AT1-K199Q receptors but not AT1-S107A receptor compared with that in AT1-WT receptor, suggesting that valsartan may bind to Ser105, Ser109, and Lys199 in the AT1 receptor. Because Tyr113, His256, and Gln257 in the AT1 receptor were important sites for the binding of olmesartan to AT1 receptor (10), the dissociation constant (Kd) values of valsartan using Y113F, H256A, and Q257A were measured. The affinity of olmesartan was reduced by more than 10-fold in Y113F (24-fold reduction), H256A (16-fold reduction), and Q257A (103-fold reduction) (10), whereas valsartan showed reductions of less than 10-fold in Y113F (3.9-fold reduction), H256A (0.8-fold reduction), and Q257A (9.6-fold reduction) (Table 1). Therefore, we found clear differences in bonding interactions between valsartan and olmesartan.
Interactions between Valsartan and Ser105, Ser109, and Lys199 in the AT1 Receptor Were Also Critical for the Inverse Agonism of Valsartan
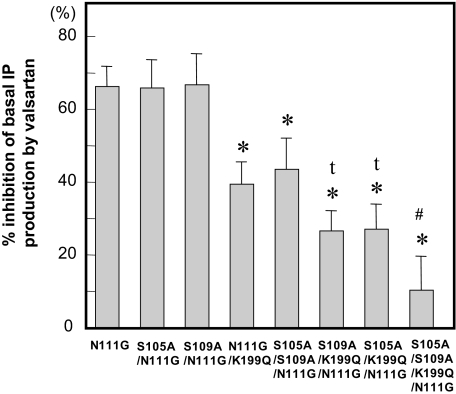
Although valsartan showed inverse agonism using AT1-S105A/N111G, AT1-S109A/N111G receptors, it significantly lost inverse agonism in the AT1-N111G/K199Q receptor (Fig. 6). The most important position of AT1 receptor for inducing inverse agonism by valsartan was Lys199. In addition, valsartan lost its inverse agonism in the AT1-S105A/S109S/N111G receptor. The greatest loss of inverse agonism of valsartan was toward AT1-S105A/S109S/N111G/K199Q receptor. Interactions between valsartan and Ser105 and Ser109 in addition to Lys199 in the AT1 receptor were also critical for inverse agonism.
Figure 6.
Percentage (%) Inhibition of the Basal Activity of IP production in Each Mutant by Valsartan
Percent inhibition of basal activity was calculated as described in Materials and Methods. Valsartan (10 μm) was added 18 h before the measurement of IP. *, P < 0.05 vs. AT1-N111G. t, P < 0.05 vs. AT1-N111G/K199Q. #, P < 0.05 vs. AT1-S105A/K199Q/N111G or AT1-S109A/K199Q/N111G.
Molecular Modeling of the Valsartan-AT1 Receptor Complex
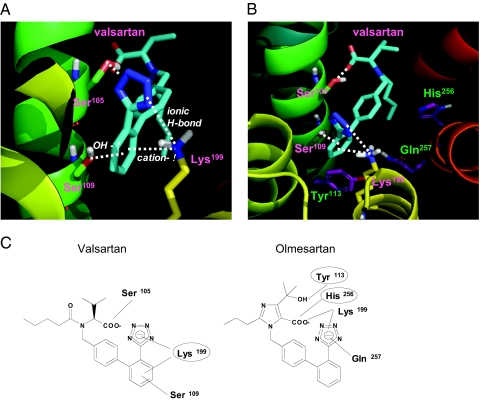
Because interactions between valsartan and Ser105, Ser109, and Lys199 in the AT1 receptor were critical for the inverse agonism of valsartan based on the results of binding studies and an IP production assay, molecular modeling was performed. The best docking pose obtained from the AutoDock docking runs in term of the calculated binding energy also showed the highest complementarity to the receptor and was used for further analyses. The estimated free energy of binding and estimated inhibition constant were −12.24 kcal/mol and 1.06*10−9, respectively. A representation of the valsartan-AT1 receptor complex is shown in Fig. 7, A and B. In this conformation, Ser109 and Lys199 in the AT1 receptor bind to the phenyl-tetrazol group of valsartan. Lys199 binds to the tetrazol group by an ionic H bond and to the phenyl group by cation-π interaction, and the distances were 3.0 Å and 3.4 Å, respectively. Ser109 binds to the phenyl group by OH-π interaction, and the distance was 3.2 Å In addition, Ser105 was a candidate for binding to the α-carboxyl group of valsartan, and the distance was 2.9 Å. Valsartan may not directly interact with the positions of Tyr113, His256, and Gln257 in the AT1 receptor (Fig. 7B).
Figure 7.
Molecular Modeling of the Valsartan-AT1 Receptor Complex
A, Molecular modeling of the interaction between valsartan and the AT1 receptor. The AT1 receptor is shown as a ribbon and Ser105, Ser109, Lys199, and valsartan are shown as stick models. Color notation in the helix is as follows: TM III, green; TM IV, yellow; TM V, light orange; TM VI, orange; and TM VII, red. Carbon atoms of valsartan are shown in blue. B, Close-up view of the interaction between valsartan and the AT1 receptor. C, Schematic drawing of chemical structures of the ARBs, valsartan, and olmesartan and binding sites of AT1 receptor in each ARB. Circles indicate binding sites that are important for inducing inverse agonism toward IP production by ARB in AT1 receptor.
DISCUSSION
Many basic and clinical studies have shown that ARBs are useful for preventing the development of various cardiovascular diseases. Based on the results presented here, it appears reasonable to conclude that the AT1-WT receptor spontaneously induces constitutive activity. For instance, valsartan significantly suppressed basal IP production in VSMCs. In addition, inactive AT1-N111I and N111Y mutant receptors display substantially lower intrinsic activity in coupling to IP production in recombinant high-expression systems. The results also show that inverse agonist valsartan binding not only blocks potential entry of Ang II into receptor pocket but also induces inactive conformation of the receptor. The conformation of the WT AT1 receptor in vivo must induce constitutive signaling and therefore, inverse agonist activity of ARBs is more important in the treatment of chronic diseases than initially thought.
The inverse agonist activity of valsartan was similar to that of olmesartan, which we defined previously (10). Because these two inverse agonists share a common molecular structure, which is biphenyl-tetrazole group, it is reasonable to think that their interaction with the receptor is similar. Surprisingly, the critical molecular interactions between valsartan and AT1 receptor established were different from those we found in the case of olmesartan (10). As depicted in Fig. 7, both these ARBs interacted with transmembrane (TM) III (although through different specific interactions) and TM V (Lys199). Movement of TM III is known to be responsible for an initial step in the activation of GPCRs including AT1 receptor (2,15,16). Hence, binding to TM III may be important for inhibition of TM III movement by inverse agonists. However, binding to TM VI via His256 and/or Gln257 is essential in most AT1 receptor inverse agonists studied until now, including olmesartan. Valsartan differs in this regard. Another residue on AT1 receptor essential for inducing inverse agonism by valsartan was Lys199. This residue is known to be important for binding the carboxyl group of Ang II in AT1 receptor activation (17) and for binding the acidic group on most biphenyl ARBs. Therefore, we propose that different ARBs bind to AT1 receptor primarily docking at Lys199 and through different rotations are able to interact with distinct sets of residues in the pocket and induce inverse agonism. A similar idea, network leaning model, was previously proposed for insurmountable antagonism in AT1 receptor (18). However, inverse agonism by rotational differences has not been seen until now.
Molecular modeling indicated that Lys199 in AT1 receptor interacts with the phenyl group of valsartan by cation-π interaction, and Ser109 interacts with the same phenyl group by OH-π interaction. The cation-π interaction as well as OH-π is an important contribution to small molecule recognition at a protein-binding site (19,20). In terms of energy, these interactions are comparable to or stronger than a typical hydrogen bond (21) or salt bridge (22). Inverse agonism by valsartan may require these π-interactions. Considering π-interactions in design and development of new GPCR inverse agonists seems very important.
There is no naturally occurring constitutively active mutation reported for AT1 receptor. However, reduction of basal activity by valsartan in the AT1-WT receptor expressing native VSMCs and lack of constitutive activity in certain mutant AT1 receptors suggest that AT1-WT receptor does have constitutive activity. Because the plasma concentration range of valsartan is 0.5–3.7 μmol/liter (23), valsartan-induced suppression of basal activity may be valuable for clinical use. These observations and the in vivo success of inverse agonists of the AT1 receptor suggest that ligand-independent signaling by AT1 receptor is not limited to overexpressing cell models and may be important under physiological conditions. A previous report indicated that losartan prevented Ang II-induced, but not mechanical stretch-induced, vascular endothelial growth factor secretion in human mesangial cells (24). Zou et al. (4) reported that mechanical stress activated the AT1 receptor independent of Ang II stimulation, and this activation was blocked by the inverse agonist candesartan. They also suggested that mechanical stress induces cardiac hypertrophy by activating the AT1 receptor independent of Ang II. In addition, tissue Ang II levels in the mouse heart and kidney are about 15- and 25-fold higher than plasma levels, respectively (25). Therefore, the inverse agonism of ARBs might also be important for their efficacy in the long-term treatment of heart and renal diseases independent of their ability to reduce blood pressure.
In conclusion, the molecular basis of inverse agonism by valsartan was found to be distinct from that of olmesartan and structurally analogous ARBs. Differences in the physical nature of interactions and orientation between inverse agonists within the receptor pocket may be responsible for vast differences in their efficacies in the treatment of cardiovascular disease. Existence of such variations should be the primer for development of new and improved ARBs as well as development novel GPCR targets.
MATERIALS AND METHODS
Mutagenesis and Expression of the AT1 Receptor and Membrane Preparation
The synthetic rat AT1 receptor gene, cloned in the shuttle expression vector pMT-2, was used for expression and mutagenesis, as described in our earlier studies (11). To express the AT1 receptor protein, 10 μg of purified plasmid DNA per 107 cells were used in transfection. COS cells (American Type Culture Collection, Manassas, VA) were grown in 5% CO2 at 37 C. The WT and mutant AT1 receptors were transfected into COS cells using the Lipofectamine 2000 liposomal reagent according to the manufacturer’s instructions (Roche Applied Science, Indianapolis, IN). Cell membranes were prepared by the nitrogen Parr bomb disruption method in the presence of protease inhibitors.
VSMCs Isolation
VSMCs were isolated from thoracic aorta of Wister-Kyoto rats by an explant method and maintained in DMEM in 5% CO2 at 37 C. Cells between passages 4 and 14 were used.
Competition Binding Studies
Kd and Bmax values of the receptor were estimated by binding experiments using 125I-[Sar1, Ile8]Ang II (Amersham Pharmacia Biotech, Piscataway, NJ) under equilibrium binding and a Scatchard plot analysis, as previously described (26).
IP Production Assay
Total soluble IP was measured by the perchloric acid extraction method, as described previously (26).
SCAM
The transfected cells were pretreated with or without valsartan (generously provided by Novartis Pharmaceuticals, Suffern, NY) or losartan (Toronto Research Chemicals, Inc., North York, Ontario, Canada) for 18 h in 5% CO2 at 37 C. The cells were harvested at 4 C, and cell membranes were prepared by the freeze/thaw method in the presence of protease inhibitors. The membranes were washed twice with 20 mm HEPES assay buffer (pH 7.4) at 4 C for washing out ARBs and subjected to a SCAM study. SCAM was performed as previously described (9,14,27). Aliquots of cell membranes (20 μl) were incubated with or without 2.5 mm sulfhydryl-specific alkylating reagent MTSEA+ (Toronto Research Chemicals Inc.) at 22 C for 2 min in assay buffer (pH 7.4). The reaction mix was then diluted 75-fold with cold buffer to stop the reaction, centrifuged for 10 min at 16,000 × g at 4 C, and resuspended in 200 μl of buffer. A 150-μl aliquot was used for 125I-[Sar1, Ile8]Ang II binding analysis. The percent inhibition of 125I-[Sar1, Ile8]Ang II binding was calculated as {1 − [(specific binding after MTS reagent)/(specific binding without reagent)]} × 100%. The experiment was performed four to eight times.
Molecular Modeling of the AT1 Receptor
A molecular model of the human AT1 receptor was built using the x-ray structure of bovine rhodopsin (Protein Data Bank code 1HZX) as a template. The primary sequence of the human AT1 receptor was aligned with that of bovine rhodopsin as well as other related GPCRs, using a multiple-sequence alignment technique. The modeling program Insight II/CHARMm was used for model building and energy minimization. During the entire modeling process, secondary structures were conserved by applying appropriate hydrogen bond constraints to the main chain atoms. The binding modes of the AT1 receptor blocker valsartan in the ligand-binding pocket of the human AT1 receptor were explored using the genetic algorithm docking program AutoDock 3.0. The most stable binding mode obtained from the docking process was further refined by energy minimization and a molecular dynamics simulation (28). During the whole modeling process, the tetrazole group of valsartan was treated as deprotonated, because the pKa value of the group reported in the literature (29) is 4.9.
Statistical Analysis
The results are expressed as the mean ± sd of three or more independent determinations. Significant differences in measured values were evaluated with an ANOVA using Fisher’s t test and an unpaired Student’s t test. Statistical significance was set at P < 0.05.
Acknowledgments
We thank S. Tomita for excellent technical assistance.
Footnotes
This work was supported in part by a Grant-in-Aid for Scientific Research (18590916) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and RO1 HL57470 (to S.S.K.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 27, 2007
Abbreviations: Ang II, Angiotensin II; ARB, AT1 receptor blocker; AT1, angiotensin II type 1; GPCR, G protein-coupled receptor; IP, inositol phosphate; MTSEA+, CH3SO2-SCH2CH2NH3+; SCAM, substituted cysteine accessibility mapping; TM, transmembrane; VSMCs, vascular smooth muscle cells; WT, wild type.
References
- De Gasparo M, Catt K, Inagami T, Wright JW, Unger T 2000 International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52:415–472 [PubMed] [Google Scholar]
- Miura S, Saku K, Karnik SS 2003 Molecular analysis of the structure and function of the angiotensin II type 1 receptor. Hypertens Res 26:937–943 [DOI] [PubMed] [Google Scholar]
- Bais C, Santomasso B, Coso O, Arvanitakis L, Geras-Raaka E, Gutkind JS, Asch AS, Cesarman E, Gershengorn MC, Mesri EA, Gershengorn MC 1998 G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86–89 [DOI] [PubMed] [Google Scholar]
- Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I 2004 Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol 6:499–506 [DOI] [PubMed] [Google Scholar]
- Feng YH, Noda K, Saad Y, Liu XP, Husain A, Karnik SS 1995 The docking of Arg2 of angiotensin II with Asp281 of AT1 receptor is essential for full agonism. J Biol Chem 270:12846–12850 [DOI] [PubMed] [Google Scholar]
- Noda K, Feng YH, Liu XP, Saad Y, Husain A, Karnik SS 1996 The active state of the AT1 angiotensin receptor is generated by angiotensin II induction. Biochemistry 35:16435–16442 [DOI] [PubMed] [Google Scholar]
- Feng YH, Miura S, Husain A, Karnik SS 1998 Mechanism of constitutive activation of the AT1 receptor: influence of the size of the agonist switch binding residue Asn(111). Biochemistry 37:15791–15798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Zhang J, Boros J, Karnik SS 2003 TM2-TM7 interaction in coupling movement of transmembrane helices to activation of the angiotensin II type-1 receptor. J Biol Chem 278:3720–3725 [DOI] [PubMed] [Google Scholar]
- Suzuki J, Ohta H, Hanada K, Kawai N, Ikeda T, Nakao M, Ikemoto F, Nishikibe M 2001 Acute effects of E-3174, a human active metabolite of losartan, on the cardiovascular system in tachycardia-induced canine heart failure. Hypertens Res 24:65–74 [DOI] [PubMed] [Google Scholar]
- Miura S, Fujino M, Hanzawa H, Kiya Y, Imaizumi S, Matsuo Y, Tomita S, Uehara Y, Karnik SS, Yanagisawa H, Koike H, Komuro I, Saku K 2006 Molecular mechanism underlying inverse agonist of angiotensin II type 1 receptor. J Biol Chem 281:19288–19295 [DOI] [PubMed] [Google Scholar]
- Jongejan A, Bruysters M, Ballesteros JA, Haaksma E, Bakker RA, Pardo L, Leurs R 2005 Linking agonist binding to histamine H1 receptor activation. Nat Chem Biol 1:98–103 [DOI] [PubMed] [Google Scholar]
- Lemaire K, Van de Velde S, Van Dijck P, Thevelein JM 2004 Glucose and sucrose act as agonist and mannose as antagonist ligands of the G protein-coupled receptor Gpr1 in the yeast Saccharomyces cerevisiae. Mol Cell 16:293–299 [DOI] [PubMed] [Google Scholar]
- Chen S, Lin F, Xu M, Graham RM 2002 Phe(303) in TMVI of the α(1B)-adrenergic receptor is a key residue coupling TM helical movements to G-protein activation. Biochemistry 41:588–596 [DOI] [PubMed] [Google Scholar]
- Miura S, Karnik SS 2002 Constitutive activation of angiotensin II type 1 receptor alters the orientation of transmembrane helix-2. J Biol Chem 277:24299–24305 [DOI] [PubMed] [Google Scholar]
- Farrens DL, Altenbach C, Yang K, Hubbell WL, Khorana HG 1996 Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science 274:768–770 [DOI] [PubMed] [Google Scholar]
- Gether U, Lin S, Ghanouni P, Ballesteros JA, Weinstein H, Kobilka BK 1997 Agonists induce conformational changes in transmembrane domains III and VI of the β2 adrenoceptor. EMBO J 16:6737–6747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K, Saad Y, Kinoshita A, Boyle TP, Graham RM, Husain A, Karnik SS 1995 Tetrazole and carboxylate groups of angiotensin receptor antagonists bind to the same subsite by different mechanisms. J Biol Chem 270:2284–2289 [DOI] [PubMed] [Google Scholar]
- Takezako T, Gogonea C, Saad Y, Noda K, Karnik SS 2004 “Network leaning” as a mechanism of insurmountable antagonism of the angiotensin II type 1 receptor by non-peptide antagonists. J Biol Chem 279:15248–15257 [DOI] [PubMed] [Google Scholar]
- Zacharias N, Dougherty DA 2002 Cation-π interactions in ligand recognition and catalysis. Trends Pharmacol Sci 23:281–287 [DOI] [PubMed] [Google Scholar]
- Sulpizi M, Carloni P 2000 Cation-pi versus OH-π interactions in proteins: a density functional study. J Phys Chem 104:10087–10091 [Google Scholar]
- Zhong W, Gallivan JP, Zhang Y, Li L, Lester HA, Dougherty DA 1998 From ab initio quantum mechanics to molecular neurobiology: a cation-π binding site in the nicotinic receptor. Proc Natl Acad Sci USA 95:12088–12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallivan JP, Dougherty DA 1999 Can lone pairs bind to a π system? The water hexafluorobenzene interaction. Org Lett 1:103–105 [DOI] [PubMed] [Google Scholar]
- Flesch G, Muller P, Lloyd P 1997 Absolute bioavailability and pharmacokinetics of valsartan, an angiotensin II receptor antagonist, in man. Eur J Clin Pharmacol 52:115–120 [DOI] [PubMed] [Google Scholar]
- Gruden G, Thomas S, Burt D, Zhou W, Chusney G, Gnudi L, Viberti G 1999 Interaction of angiotensin II and mechanical stretch on vascular endothelial growth factor production by human mesangial cells. J Am Soc Nephrol 10:730–73726 [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Alexiou T, Xiao HD, Fuchs S, McKinley MJ, Corvol P, Bernstein KE 2004 Effect of reduced angiotensin-converting enzyme gene expression and angiotensin-converting enzyme inhibition on angiotensin and bradykinin peptide levels in mice. Hypertension 43:854–859 [DOI] [PubMed] [Google Scholar]
- Miura S, Feng YH, Husain A, Karnik SS 1999 Role of aromaticity of agonist switches of angiotensin II in the activation of the AT1 receptor. J Biol Chem 274:7103–7110 [DOI] [PubMed] [Google Scholar]
- Miura S, Karnik SS, Saku K 2005 Constitutively active homo-oligomeric angiotensin II type 2 receptor induces cell signaling independent of receptor conformation and ligand stimulation. J Biol Chem 280:18237–18244 [DOI] [PubMed] [Google Scholar]
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ 1998 Distributed automated docking of flexible ligands to proteins: parallel applications of AutoDock 2.4. J Computational Chem 19:1639–1662 [DOI] [PubMed] [Google Scholar]
- Cagogal E, González L, Alonso RM, Jiménez RM 2001 pKa determination of angiotensin II receptor antagonists (ARA II) by spectrofluorimetry. Eur J BioMed Anal 26:477–486 [DOI] [PubMed] [Google Scholar]