Abstract
The gadolinium(III) complex of S-SSSS-NO2BnDOTMA exhibits water exchange kinetics that are optimal for use in high relaxivity or targeted contrast agents. However, the synthesis of this ligand is hampered by the steric encumbrance imparted upon the cyclen ring by the nitrobenzyl substituent. A relatively simple modification has been used to enable the synthesis of larger quantities of a bifunctional ligand that retains similar fast water exchange properties. The gadolinium complex of S-SSS-NO2BnDO3MA-1A is shown to retain the rapid water exchange kinetics characteristic of a twisted square antiprismatic (TSAP) coordination geometry (τM = 6 ± 0.4 ns).
Introduction
Magnetic resonance imaging (MRI) is now arguably one of the most important diagnostic techniques in clinical medicine. Contrast agents derived from low molecular weight complexes of paramagnetic metal ions are routinely administered prior to the acquisition of an image. These agents are most commonly complexes of the gadolinium(III) ion, which with its high spin state and long electron relaxation times, is highly effective at relaxing water protons during MRI scans. Although effective, the current generation of contrast agents is limited in terms of targeted contrast agent applications. A new generation of contrast agents that report changes in biological activity or that are targeted to specific sites are currently being developed.1,2 It is now readily accepted that in order to design this new generation of contrast agents the rate at which water molecules coordinated to the gadolinium ion exchange with the bulk is critical.3–5 Too fast and the protons are not effectively relaxed, too slow and a coordination site is needlessly occupied by a relaxed water molecule. Ideally a water molecule should reside in the inner coordination sphere of the gadolinium ion for a period averaging 10–40 ns in low field experiments;3 although, the optimal range is found to move to shorter τM values as the magnetic field strength is increased (ESI†).
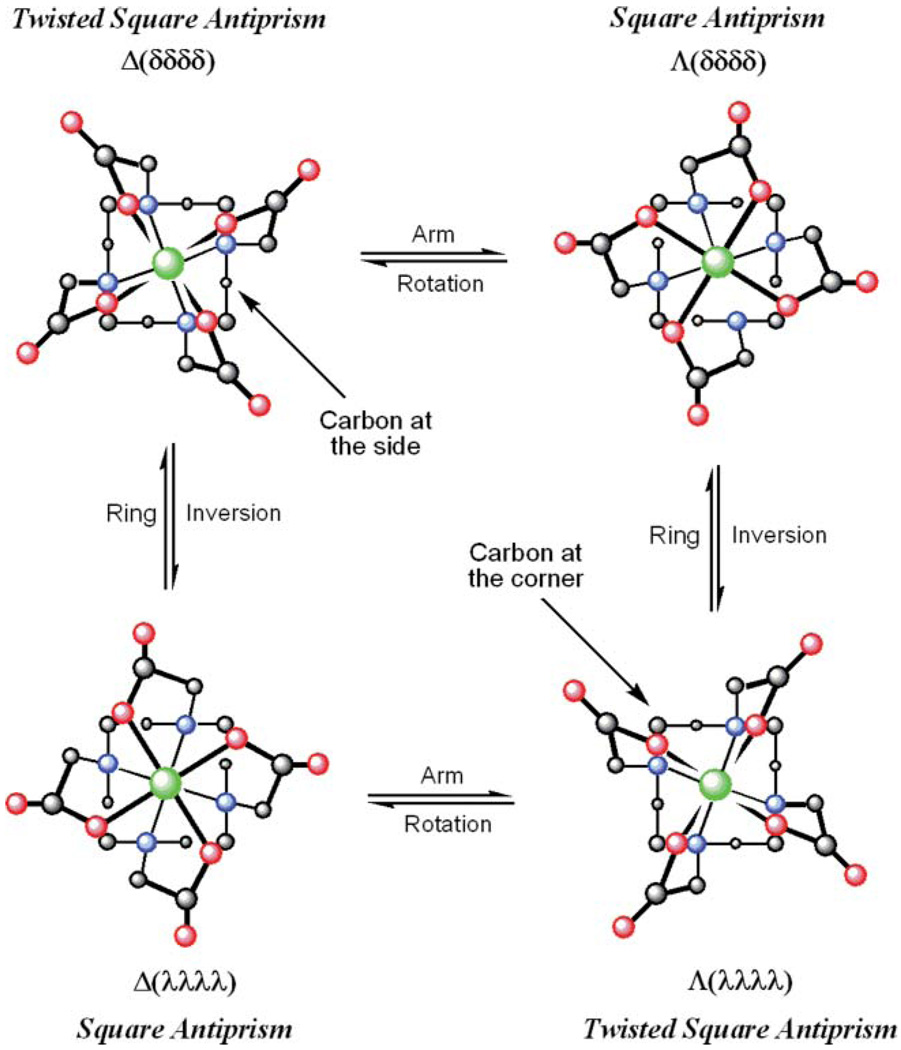
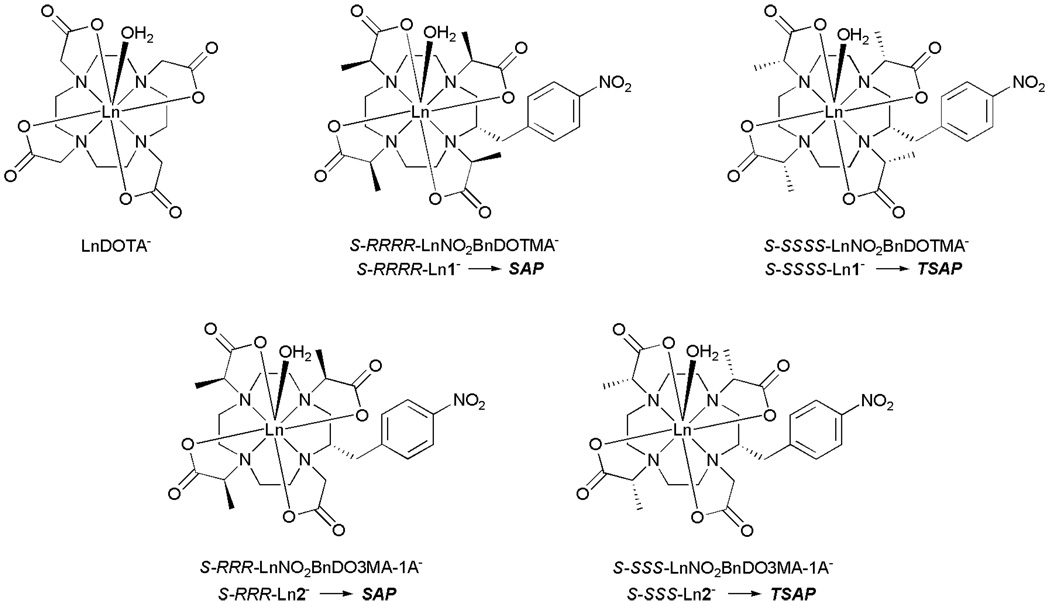
The factors that control water exchange are now comparatively well understood and have been manipulated in a number of ways to afford gadolinium complexes that exhibit water exchange in the optimal range.2,6–9 The approach we have taken to controlling water exchange is to influence the coordination geometry of a GdDOTA− derivative by selective substitution.10 LnDOTA− complexes exist as a mixture of two coordination isomers:11,12 a mono-capped square antiprismatic geometry (SAP) that exhibits slower water exchange and a mono-capped twisted square antiprismatic geometry (TSAP) that exhibits faster water exchange.10,13–17 These two coordination isomers interchange by one of two processes: ring inversion or arm rotation (Fig. 1).12 However, it has been shown that by appropriate substitution of the pendant arms, arm rotation can be halted.13,18–20 Likewise, appropriate substitution of the macrocyclic ring will halt ring inversion.19 We recently combined these two effects to halt all intramolecular exchange in this type of complex.10 Each coordination isomer is defined by the relative conformation of the macrocyclic ring (δδδδ or λλλλ) and the pendant arm orientation (Δ or Λ), and each of these is in turn defined by the configuration at the chiral carbon. One may therefore obtain either coordination isomer by careful selection of the chirality at each chiral center in the complex. Two stereoisomeric complexes of the ligand NO2BnDOTMA, S-RRRR-1 and S-SSSS-1, were previously shown to adopt a SAP and TSAP coordination geometry, respectively (Chart 1). They were also found to exhibit significantly different water residence lifetimes, τM = 120 ns (S-RRRR-1) and τM = 15 ns (S-SSSS-1),10 the latter value falling within the optimal range for use in high relaxivity or targeted contrast agents at 20 MHz.3,21
Fig. 1.
LnDOTA− complexes exist as a mixture of four stereoisomeric complexes, related as two enantiomeric pairs. These stereoisomers interconvert by ring inversion or arm rotation. Sequential ring inversion and arm rotation interconverts enantiomers.
Chart 1.
Results and discussion
Synthesis
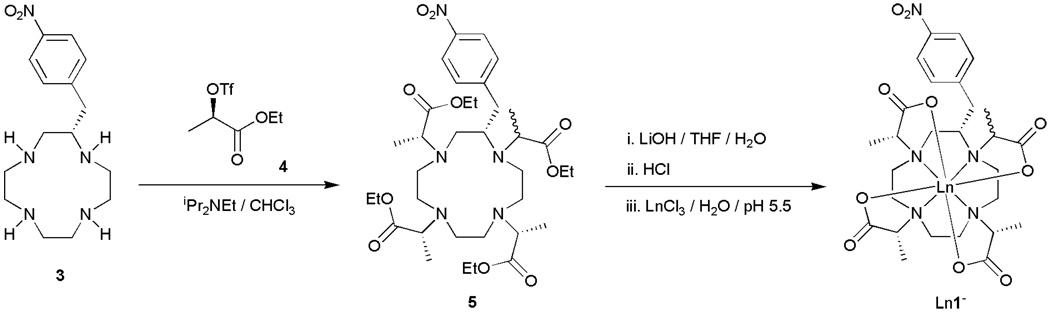
Although S-SSSS-1 exhibits near ideal water exchange kinetics for use as an MRI contrast agent and can readily be modified to facilitate conjugation to a targeting vector or macromolecule, the use of this complex is hampered by synthetic problems. NO2BnDOTMA was synthesized by alkylation of (S)-2-nitrobenzylcyclen 3 with a chiral alylkating agent 4 (Scheme 1). Providing that this alklyation reaction proceeds via a mechanism that is predominantly SN2 in nature then complete inversion of each chiral center will occur and a single stereoisomer will be obtained. However, the synthesis of both S-RRRR-1 or S-SSSS-1 produced substantial quantities of a stereoisomeric by-product during the alkylation reaction. These stereoisomers could not be separated using conventional chromatography at this stage in the synthetic scheme. Taking both stereoisomers through the ester hydrolysis and complexation steps allowed the two steroeoisomeric complexes to be separated by preparative HPLC. Clearly this is not practical if this complex is to be used in the large scale synthesis of an MRI contrast agent as a commercially viable product.
Scheme 1.
The synthesis of lanthanide(III) S-RRRR-LnNO2BnDOTMA− complexes, the corresponding S-SSSS complexes were synthesized in an analogous manner.
The most obvious explanation for the appearance of just two stereoisomers during the alkylation reaction is that one, and only one, of the nitrogen atoms of (S)-2-nitrobenzylcyclen 3 is unable to participate in an SN2 reaction. The four nitrogen atoms of (S)-2-nitrobenzylcyclen 3 are distinct and it is the nitrogen in the 1-position that is most likely to be the cause of this problem. The nitrogen atom closest to the nitrobenzyl substituent is inevitably more sterically encumbered than the others and this steric encumbrance will reduce the nucleophilicity of this nitrogen atom. The net result is that alkylation of this particular nitrogen atom is more likely to proceed via an SN1 mechanism; the more SN1 character in the reaction mechanism the greater the extent of racemisation at this site. This hypothesis is further supported by the observation that the extent of racemisation observed in this reaction is dependent upon the configuration of the alkylating agent (Table 1). The alkylation reaction appears to proceed with more SN1 character, and therefore greater racemisation, if the configuration of the alkylating agent is R. This is presumably the result of increased steric interactions between the alkylating agent and the (S)-nitrobenzyl substituent in comparison to the reaction with the S-isomer.
Table 1.
The distribution of stereoisomeric products obtained in the synthesis of Ln1− complexes
| Alkylating agent X | Product | Yielda(%) |
|---|---|---|
| R-4 | S-SSSS- | 55 |
| S-RSSS- | 45 | |
| S-4 | S-RRRR- | 63 |
| S-SRRR- | 37 |
Product yields are based upon the amount of complex obtained by HPLC separation of the final stereoisomeric complexes. Other trace stereoisomers produced as minor products that were not isolated were not taken into consideration in yield determination.
Although this racemisation provides extra complexes that are available for study, it also poses problems for scale-up. If S-SSSS- 1 is to be employed as the basis of a contrast agent it is first necessary to develop a viable synthesis that overcomes the problem of racemisation in the alkylation step. To do this it is necessary to promote an SN2 reaction involving the nitrogen in the 1-position. Since the quality of the nucleophile cannot be improved, three further approaches were taken for promoting an SN2 reaction: employing a poorer leaving group in the alkylating agent, altering the steric nature of the alkylating agent and reducing the polarity of the reaction solvent. However, neither using bromide as the leaving group, employing methyl or benzyl esters or methyl amides as protecting groups nor using acetonitrile, tetrahydrofuran, chloroform or dichloromethane as a reaction solvent significantly improved the ratio of stereoisomers obtained in the reaction. Thus, the determining factor appears to be the poor nucleophilicity of the 1-nitrogen and an alternative strategy to solving this problem had to be found.
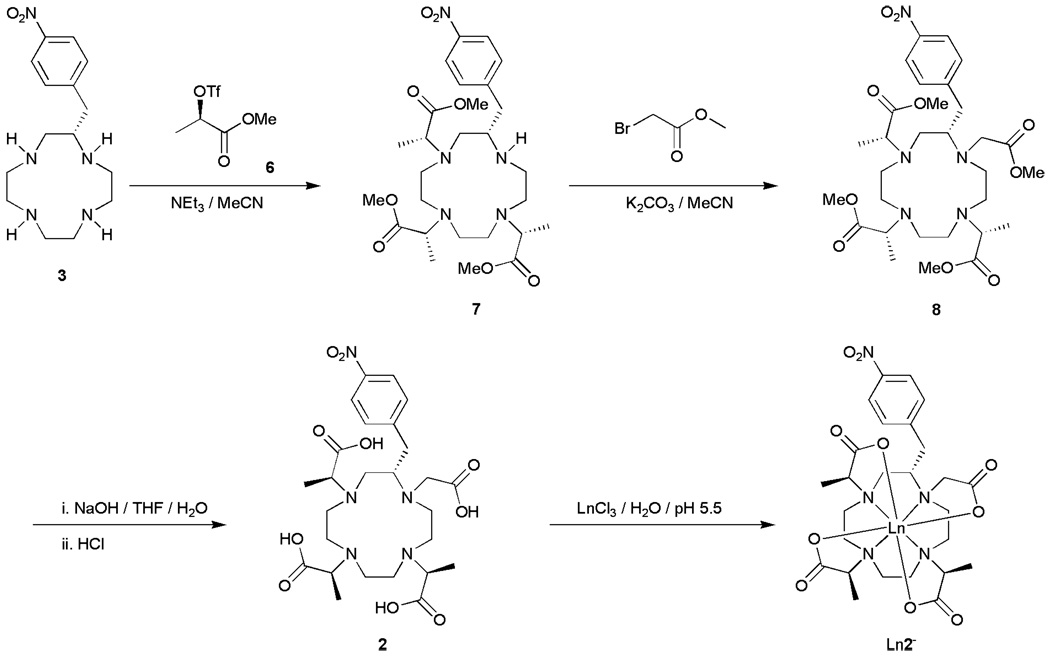
Since the 1-nitrogen cannot be alkylated stereoselectively, the target molecule was redesigned such that an SN1 reaction in this position would have not adversely affect the synthesis of the target molecule. Previous studies on the four stereoisomeric complexes of carboxyethyl-DOTA suggested that methyl substituents on just three of the pendant arms should be enough to control the orientation of the pendant arms.13 For this reason our attention turned from NO2BnDOTMA and focused instead upon NO2BnDO3MA-1A (Chart 1). In the synthesis of this new target, the poor nucleophilicity of the problematic 1-nitrogen could be turned to our advantage. During alkylation reactions with poorer alkylating agents such as bromides, it became clear that selective trialklyation of 2-nitrobenzylcyclen 3 is possible without any need for protecting groups. It was found that, even with a triflate as alkylating agent, complete and selective trialkylation can be achieved by employing 3.2 equivalents of a suitable alkylating agent and performing the reaction at room temperature instead of 55 °C.10 The 1-nitrogen was left unreacted by this procedure and so could be subsequently alkylated using an achiral alylkating agent. This afforded a simple synthetic pathway to NO2BnDO3MA-1A (Scheme 2). The triflate of methyl lactate 6 was synthesized using established procedures22 and used to trialkylate (S)-2-nitrobenzylcyclen 3 in acetonitrile at room temperature using triethylamine as a base. The trimethyl ester of NO2BnDO3MA 7 obtained from this reaction was then reacted with methyl bromoacetate in acetonitrile at 55 °C. Subsequent saponification of the esters of 8 with sodium hydroxide, followed by acidification, afforded the ligand 2. The europium complexes of both S-SSS-2 and S-RRR- 2 were synthesized from europium chloride in aqueous solution at pH 5.5. 1H NMR and HPLC were used to determine the stereoisomeric distribution of products. In each case the desired stereoisomer was found to constitute >92% of the complex obtained.
Scheme 2.
The synthesis of LnS-SSS-NO2BnDO3MA-1A. The corresponding S-RRR complexes were synthesized using the same approach.
NMR, Luminescence and relaxometric studies
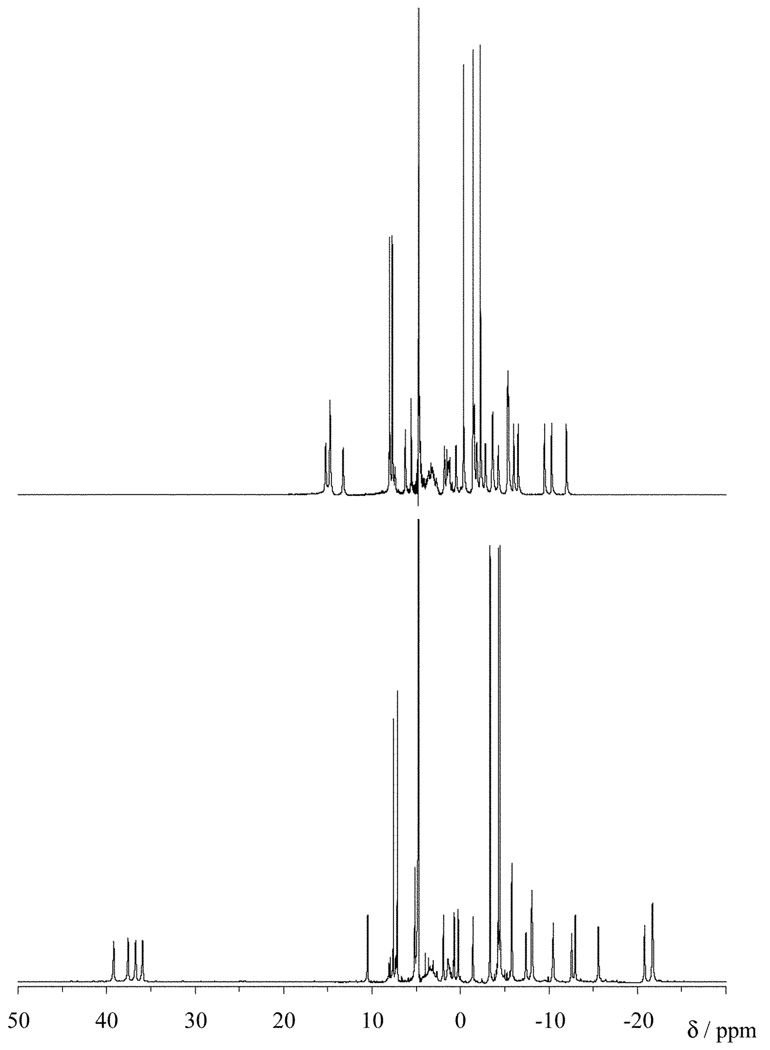
Since substitution of the DOTA framework renders each of the four structures shown in Fig. 1 diastereoisomers, rather than enantiomeric pairs, each structure of an Ln2− complex would have its own distinct NMR spectrum. Interchange of these complexes is slow on the NMR timescale12 so the absence of intramolecular motion in these complexes may be verified by examining the number of species present in the NMR spectrum.10 The high resolution extended sweep-width 1H NMR spectra of the europium complexes of both S-SSS-2 and S-RRR- 2 were recorded in D2O (Fig. 2). In each spectrum 27 resonances can be identified, indicating that a single coordination isomer is present in each case. This indicates that the three methyl substituted pendant arms are sufficient to “freeze-out” intramolecular motion. In addition the coordination geometry adopted by each stereoisomer is the same as those predicted by their NO2BnDOTMA analogues,10 and by theory.13,18,19,23 This is most easily seen by examining the resonances of one of the axial protons of the macrocycle. The resonances arising from the axial protons located on the carbon on the “sides” (rather than the corners) of the macrocycle are substantially shifted downfield, away from the other resonances of the complex.24 The resonances of these protons can be observed between 35 and 40 ppm for the S-RRR isomer and correspond to a SAP isomer (Λ(δδδδ)). In contrast, the resonances for the S-SSS isomer are much less shifted, appearing between 15 and 20 ppm and correspond to a TSAP isomer (Δ (δδδδ)).10 These assignments are further supported by the results of studies on the Yb complexes of S-RRRR- 1 and S-SSSS-1.25
Fig. 2.
The high resolution extended sweep 1H NMR spectra of EuS-SSS-NO2BnDO3MA-1A (top) and EuS-RRR-NO2- BnDO3MA-1A (bottom) recorded at 300 MHz and 298 K in D2O.
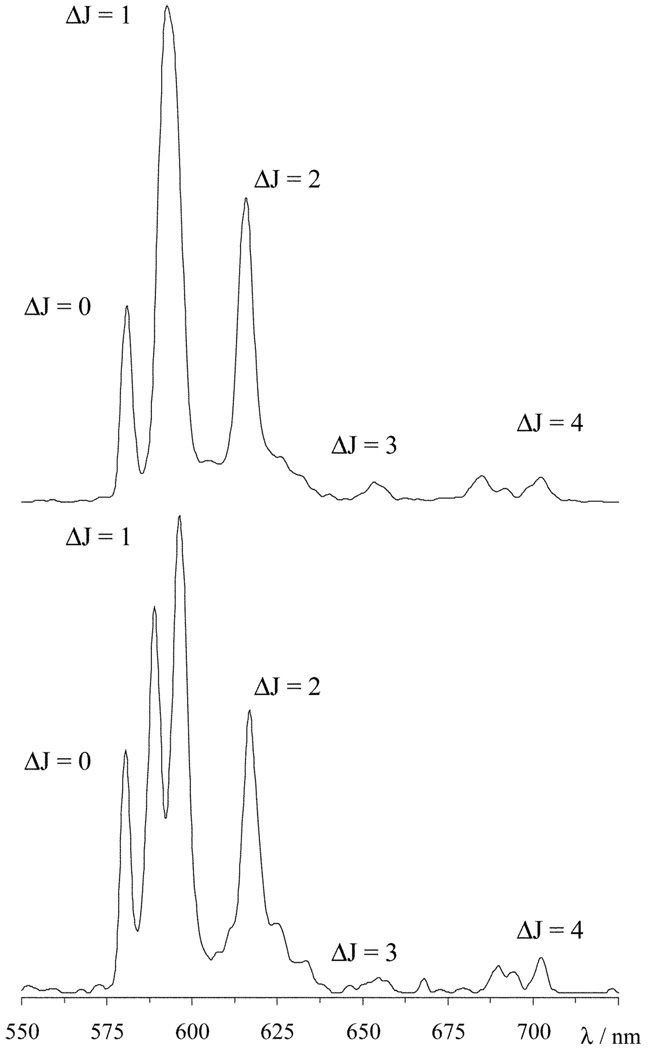
In addition to its NMR shift properties the europium(III) ion has attractive luminescence characteristics that can also aid in characterizing these complexes. As a general rule the presence of aromatic groups in close proximity to the europium ion serves to enhance the lanthanide based emission by acting as an antennae, harvesting light and transferring the energy to the europium ion.26 However, in our hands no substantial antennae effect from the nitrophenyl group has been observed for any europium complex of ligands derived from nitrobenzylcyclen.23 Thus, the europium ion was excited directly at 396 nm and the emission spectra of the two complexes recorded (Fig. 3). In the range 575–710 nm five emission bands, arising from the 5D0 → 7FJ (J = 0–4), may be observed. The transitions arising from ΔJ = 3 and 4 (at 659 and 700 nm, respectively) are usually weak27 and in both these cases unusually so. Considering the remaining three emission bands the emission spectra are dramatically different, even at the moderate resolution at which the spectra were obtained. The ΔJ = 2 band (615 nm) is considered to be hypersensitive to the coordination environment of the europium ion.27 However, despite the considerable difference in coordination polyhedron surrounding the europium ion in each complex, the form and intensity of the ΔJ = 2 band is more or less identical in the two spectra. We postulate therefore, that the ΔJ = 2 band is not significantly affected by comparitively small changes in the coordination geometry. Rather, it seems that the ΔJ = 2 band is influenced by the extent of electron donation around the europium ion in broader terms.28 In other words, because in each complex the europium ion is sandwiched between a plane of four amine nitrogen atoms and another of four carboxylate oxygen atoms, the overall distribution of electron density to the europium(III) ion is much the same in both complexes. As far as the ΔJ = 2 band is concerned the same situation prevails regardless of the precise location of the donor atoms in each of these complexes; in consequence the ΔJ = 2 band retains the same characteristics. Indeed the ΔJ = 2 band remains much the same in the emission spectra of EuDOTA and most other DOTA-type ligands.13
Fig. 3.
The emission spectra of EuS-SSS-NO2BnDO3MA-1A (top) and EuS-RRR-NO2BnDO3MA-1A (bottom) recorded in water by excitation at 396 nm.
The intensity of the ΔJ = 1 band (595 nm) on the other hand, is generally considered to be “insensitive” to changes in the coordination environment; but in complexes with an axially symmetric coordination polyhedron such as these, two transitions are allowed in this emission band. The separation between these transitions is known to be affected by the ligand field and is related to the axial crystal field coefficient, A0 2.13,29 In the emission spectrum of EuS-SSS-2 a single ΔJ = 1 band is observed, so presumably the separation between the these transitions is small and the resulting peaks lie too close to one another to be resolved. In contrast, the spectrum of EuS-RRR-2 has two clearly resolved peaks in the ΔJ = 1 band. Given that the size of the dipolar NMR shift is also related to A0 2 30–32 it is not surprising that these two transitions are better separated in this isomer, which also exhibits a greater lanthanide induced shifting of its NMR signals. So although the intensity of the ΔJ = 1 is unaffected by the change in coordination geometry, a dramatic change in its form is observed as a result of changes in the crystal field. This implies that the “insensitive” ΔJ = 1 emission band is more responsive to changes in coordination geometry than the “hypersensitive” ΔJ = 2 band. The non-degenerate ΔJ = 0 emission band (581 nm) is a good measure of the number of species in solution in high resolution emission spectra. However, the moderate resolution of the spectra in Fig. 3 does not allow this determination.
The 5D0 excited state of europium(III) is readily quenched by proximate OH oscillators. Because transfer of energy to OD oscillators is less efficient than that to OH oscillators, it is possible to measure the number of water molecules coordinated to europium by comparing the excited state lifetimes of europium in H2O and D2O by using the method developed by Horrocks and Sudnick.33,34 We used a version of this method, modified to take into account contributions from outer-sphere oscillators,35 to determine the number of coordinated water molecules (q) in each complex. Exciting at 396 nm and monitoring at 595 nm, values of q = 0.74 (EuS-SSS-2) and q = 0.97 (EuS-RRR-2) were obtained. Fractional q values are not uncommon when using Horrocks’ method, largely as a result of the strong distance dependence of energy transfer (ET α r−6). The S-SSS isomer, which adopts the more rapidly exchanging TSAP coordination geometry has a markedly lower value of q. A similar difference in q was also observed for the two isomers of Eu1.10 This difference can be rationalised if the Eu–OH2 bond distance in a TSAP isomer is longer and therefore weaker than that in the SAP isomer. Indeed if the Eu–OH2 bond distance is considered to be directly proportional to the distance from the europium ion to the OH oscillator then it is possible to calculate this difference. By taking the Eu–OH2 bond distance measured in the square antiprismatic crystal structure of EuDOTA−, 2.480 Å ,36 and the measured q value, 0.98,35 we can calculate the Eu–OH2 bond distance using a similar method to Parker and co-workers.35 The bond length calculated in this way for EuS-SSS-2 was 2.598 Å , 5% longer than the 2.485 Å calculated for EuS-RRR-2. Almost identical values (2.597 and 2.484 Å) were obtained if the values for tetracarboxyethylDOTA (2.447 Å and q = 1.06)13 were used in the calculation. While the Eu–OH2 distance calculated for the SAP isomer is almost identical to that observed in the crystal structure of EuDOTA, also a SAP isomer, the 2.598 Å calculated for the TSAP isomer is close to 2.590 Å , the Ce–OH2 distance measured in the crystal structure of CeDOTA, which adopts a TSAP coordination geometry.37 Such good agreement with the values observed in the crystal structure allows us to conclude that both complexes possess one coordinated water molecule, the difference in measured q values is the result of a 5% longer Eu–OH2 bond distance in the TSAP isomer.
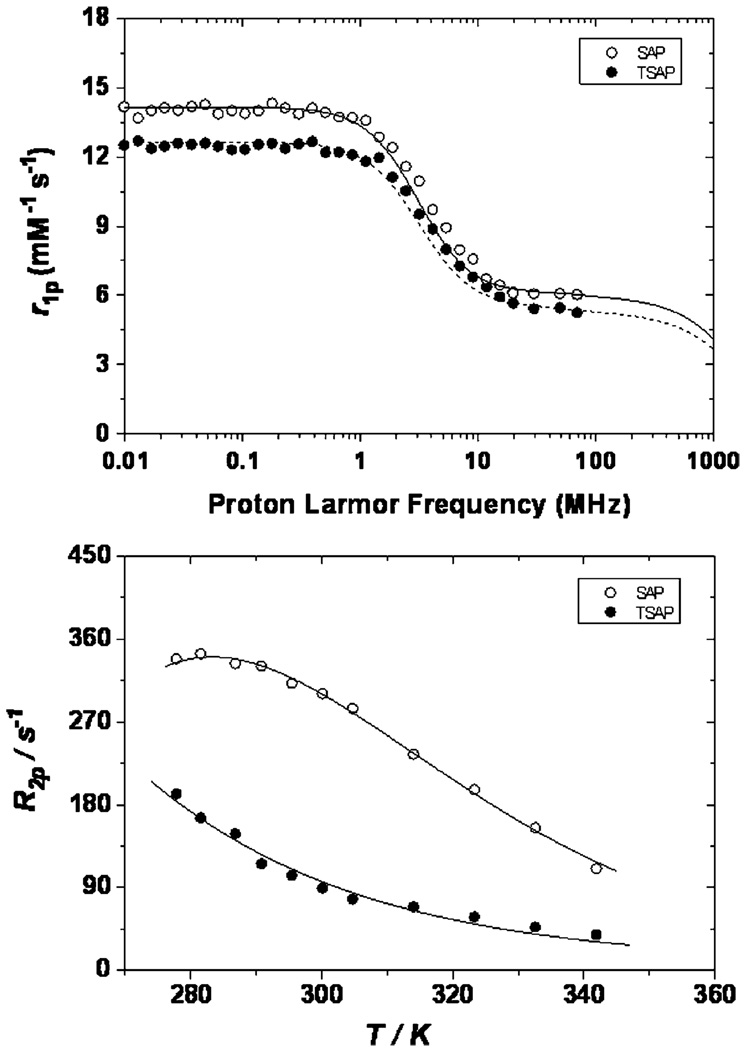
The effect of this elongated Ln–OH2 bond on the exchange kinetics of water can be gauged by measuring the transverse 17O relaxation rates of a solution of the gadolinium complex as a function of temperature.38 That the complexes exhibit different water exchange rates is apparent from inspection of the profiles obtained at 2.1 T (Fig. 4). The profile obtained for GdS-RRR-2 (SAP) exhibits the classic bell shaped curve which is observed to reach a maximum around 285 K. In contrast the profile of GdS-SSS- 2 (TSAP) continues to rise until further measurements become impossible, resulting in a shift of the bell curve toward lower temperature that is indicative of faster water exchange in this isomer. Fitting these profiles to established theory3,38 provides values of τM for each complex. Since several factors involved in this fitting procedure also influence proton relaxivity, this fitting procedure is improved when the 17O profiles are fitted simultaneously with the nuclear magnetic relaxation dispersion (NMRD) profiles. NMRD profiles measure the proton relaxivity of a complex as a function of the applied magnetic field; the profiles of the two complexes, recorded at 25 °C, are also shown (Fig. 4). Given the number of parameters involved in fitting these data it is helpful to constrain some parameters prior to commencing the fitting procedure. In light of the results obtained for the Eu–OH2 bond distances from luminescence measurements the gadolinium–water oxygen distance (rGd–O) and gadolinium–water proton distance (rGd–H) were fixed to typical values for the GdS-RRR-2 (SAP) isomer (Table 2). The values employed for GdS-SSS-2 (TSAP) were 0.1 Å longer in each case reflecting of an elongation of the Gd–OH2 bond by approximately 5%. Since both a, the distance of closest approach of an outer sphere water molecule to the paramagnetic metal ion, and D, the relative diffusion coefficient, should reflect the molecular radius of the complex, and this is the same in isomeric complexes, these values were constrained to be the same for both complexes.
Fig. 4.
The NMRD profiles at 25 °C (top) and transverse 17O relaxation temperature profiles at 2.1 T (bottom) of GdS-SSS-2 (filled circles) and GdS-RRR-2 (open circles). Concentrations of 16.5 and 16.2 mM were employed in the 17O experiments for the TSAP and SAP isomers, respectively.
Table 2.
Fitting parameters for the NMRD and 17O NMR profiles shown in Fig. 4
| Gd2− | S-RRR (SAP) | S-SSS (TSAP) |
|---|---|---|
| τMa/ns | 70 ± 5 | 6 ± 0.4 |
| ΔHMa/kJ mol−1 | 38 ± 2 | 23 ± 1 |
| τV/ps | 14 ± 1 | 15 ± 2 |
| ΔHVa/kJ mol−1 | 3.3 ± 0.7 | 3.6 ± 0.6 |
| τR/ps | 113 ± 3 | 113 ± 3 |
| rGd–Ob/Å | 2.50 | 2.60 |
| rGd–Hb/Å | 3.0 | 3.10 |
| ab/Å | 4.0 | 4.0 |
| 10−19 Δ2/s−2 | 2.2 ± 0.2 | 2.1 ± 0.2 |
| 10−5Db/cm2 s−1 | 2.24 | 2.24 |
| (A/h)a/MHz | −3.8 ± 0.2 | −3.6 ± 0.1 |
Obtained from 17O NMR profile.
Parameter fixed prior to fitting.
The results of the simultaneous fitting of the NMRD and 17O profiles are summarised in Table 2. As predicted the rate of water exchange in the two complexes is very different; the value of τM observed for GdS-SSS-2 (TSAP) is one order of magnitude shorter than that of the GdS-RRR-2 (SAP). These results are in close agreement with those observed for the two isomers of Gd1;10 although water exchange appears to be somewhat faster in these new complexes. This means that GdS-SSS-2, while retaining the more rapid water exchange kinetics associated with a TSAP isomer, exhibits a water exchange rate that falls marginally outside the optimal range of values predicted by theory at 20 MHz.3,4 Almost identical values of τR, the rotational correlation time, were obtained. The complexes have the same molecular weight and similar structures so any difference in hydrodynamic volume is expected to be negligable. Given that both complexes have relatively short values of τR it is not surprising that the NMRD profiles of the two complexes are remarkably similar. This is particularly true at higher fields (>10 MHz) where relaxivity is limited by τR for low molecular chelates such as these. The largest difference between the two NMRD profiles is observed at lower fields (<1 MHz) where relaxivity is primarily determined by electronic relaxation parameters. Despite the larger difference between the two profiles in this region the two complexes were found to have fairly similar electronic relaxation times, although both are rather short, τS0 = 0.28 ns and 0.26 ns for S-RRR-2 (SAP) and S-SSS-2 (TSAP), respectively. The parameters that influence τS0 are not yet completely understood nor is it clear whether ligand field has any influence upon electronic relaxation.
One further effect of a longer Gd–OH2 bond distance in the TSAP isomer may perhaps be seen in the relaxivity values of this complex. The dipole–dipole interaction that results in proton relaxation by the gadolinium ion has the same distance dependence as energy transfer to the OH oscillators in luminescence experiments. In consequence, in the absence of other mitigating factors (such as a shorter τM value), an increase in Gd–H distance of 0.1 Å would lead to a reduction in relaxivity of 20%.39 This may help to explain why the relaxivity of the SAP isomer, with its shorter Gd–OH2 bond distance, is slightly higher at all fields than that of its TSAP counterpart. It is noteworthy that this is a reversal of the situation that prevails in the complexes of NO2BnDOTMA, in which the TSAP isomer exhibits a slightly higher relaxivity than the SAP isomer at 20 MHz and 25 °C.10 Values of 6.05 and 5.65 mM−1 s−1 (20MHz, 25 °C) were obtained for the relaxivity of GdS-RRR-2 (SAP) and GdS-SSS-2 (TSAP), respectively. The relaxivity of both agents at higher fields are expected to rise sharply as the rate of molecular reorientation is slowed in each complex as a result of the rapid water exchange kinetics observed in each case.
Conclusions
We have devised a simple and effective synthetic route to a ligand that adopts the TSAP coordination geometry exclusively when coordinated to a lanthanide ion. The water molecule coordinated to the gadolinium(III) complex of this ligand, GdS-SSS-2 exchanges at a rate that is one order of magnitude more rapid than that of the water molecule in the SAP isomer. With a value of τM = 6 ± 0.4 ns, the water exchange kinetics in the complex GdS-SSS-2 are a shade faster than optimal for application in high relaxivity and targeted MRI contrast media at low fields. However, such rapid water exchange kinetics may prove beneficial from two standpoints: (1) new generation clinical scanners are moving to higher fields making more rapidly exchanging systems more attractive (ESI†); (2) if reports indicating that water exchange deccelerates upon binding of a targeted contrast agent to a protein40 are typical, then it may in fact prove beneficial if water exchange is a little too rapid in the free complex in solution. The more rapid water exchange observed in the TSAP isomer, versus the SAP isomer, is the result of an Ln–OH2 bond that is elongated by approximately 5%. The nitro group in this type of complex may be readily converted to an isothiocyanate, thereby affording a bifunctional chelator. In combination, these results indicate that GdS-SSS-2 represents the solid basis for the development of both targeted and high relaxivity contrast agents
Experimental
General remarks
All solvents and reagents were purchased from commercial sources and used as received. HPLC purifications were performed on Water δ-Prep 150 HPLC system using a Phenomenex Luna C-18 reversed-phase (50 × 250 mm) column. In all cases absorbance was monitored at 205 and 270 nm. The solvent system employed for the purification of complexes eluted with water (0.037% HCl) for 5 min and then with a linear gradient to 80% MeCN and 20% water (0.037% HCl) after 40 min, at a flow rate of 50 mL min−1. The solvent system employed for the purification of ligands eluted with water (0.037% HCl) for 5 min and then with a linear gradient to 40% MeCN and 60% water (0.037% HCl) after 35 min, at a flow rate of 50 mL min−1. 1H and 13C NMR spectra were recorded on a JEOL Eclipse 270 spectrometer at 270.17 and 67.93 MHz, respectively, or on a Varian Mercury 300 spectrometer operating at 300.01 and 75.47 MHz, respectively. The 1/T1 nuclear magnetic relaxation dispersion profiles of water protons were measured over a continuum of magnetic field strengths from 0.00024 to 0.5 T (corresponding to 0.01–20 MHz proton Larmor frequency) on a fast field-cycling Stelar Spinmaster FFC 2000 relaxometer equipped with a silver magnet. The relaxometer operates under complete computer control with an absolute uncertainty in the 1/T1 values of ± 1%. The typical field sequences used were the NP sequence between 40 and 8 MHz and PP sequence between 8 and 0.01 MHz. The observation field was set at 13 MHz. 16 experiments of 2 scans were used for the T1 determination for each field. Additional data at higher fields (40–80 MHz) were measured on a Stelar Spinmaster relaxometer equipped with a Bruker magnet operating in the range 20–80 MHz. Variable-temperature 17O NMR measurements were recorded on a JEOL EX-90 (2.1 T) spectrometer, equipped with a 5 mm probe, by using D2O as external lock. Experimental settings were: spectral width 10000 Hz, pulse width 7 µs, acquisition time 10 ms, 1000 scans and no sample spinning. Solutions containing 2.6% of 17O isotope (Yeda, Israel) were used. The observed transverse relaxation rates were calculated from the signal width at half height. Luminescence emission spectra were recorded on a Perkin Elmer LS50B spectrometer exciting at 396 nm. A gate time of 50 ms was used and excitation slits were set to 10 nm and the emission slits to 2.5 nm. Lifetimes were measured using the pHlemming programme supplied by Dr Andrew Beeby of the University of Durham using data points acquired every 50 ms over a 3 s period.
Tetraethyl(1S,4S,7S,10S)-α,α′,α″,α″′-tetramethyl[(S)-2-(nitro-benzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate (S-SSSS-5)
A solution of (S)-2-nitrobenzylcyclen 3 (1.49 g, 4.9 mmol) and N,N′-diisopropylethylamine (2.68 g, 20.7 mmol) in chloroform (25 mL) was cooled, under an argon atmosphere, to −78 °C. (R)-Ethyl O-trifluoromethanesulfonyl lactate 4 (5.12 g, 20.7 mmol) was added dropwise over a period of 10 min and the reaction stirred for 3 h at −78 °C. The reaction was allowed to warm to room temperature, stirred for a further 12 h before heating at 60 °C for 48 h. The reaction products were separated by column chromatography over silica gel eluting first with chloroform and then 20% tetrahydrofuran in chloroform. Removal of the solvents in vacuo afforded a pale yellow oil (1.5 g, 43%) which was found to contain a mixture of the S-SSSS and S-RRRR isomers which could not be separated at this stage. Both isomers were taken through the remainder of the synthesis as a mixture of stereoisomers and would be separated only once the complexes had been synthesized. Rf = 0.2 (5% THF in CHCl3, SiO2). 1H NMR (270 MHz, CDCl3), δ 8.11 (1H, d, 3JH–H = 8 Hz, Ar), 8.07 (1H, d, 3JH–H = 8 Hz, Ar), 7.45 (1H, d,3JH–H = 8 Hz, Ar), 7.42 (1H, d, 3JH–H 8 Hz, Ar), 4.25-1.75 (25H, m), 1.18 (24H, m, CHCH3 and CH2CH3). 13C NMR (67.9 MHz, CDCl3), δ 7.1, 7.7, 7.8, 11.4, 13.8, 15.2, 21.2, 25.6, 30.4, 34.3, 35.0, 36.6, 40.4. 43.8, 44.4, 44.6, 44.9, 46.8, 47.0, 49.2, 50.2, 53.7, 56.0, 56.1, 56.4, 57.7, 61.3, 61.8, 67.7, 123.4 (Ar), 123.9 (Ar), 125.2 (Ar), 128.1 (Ar), 129.8 (Ar), 130.2 (Ar), 135.9 (Ar), 146.2 (Ar), 146.4 (Ar), 147.2 (Ar), 148.6 (Ar), 175.5 (C=O), 175.8 (C=O), 176.0 (C=O), 176.1 (C=O), 176.2 (C=O), 176.3 (C=O), 177.1 (C=O). m/z (ESMS EI+): 708 (8%, [M + H+]), 730 (100%, [M + Na+]). νmax/cm−1: 2978, 2934, 2852, 1723 (C=O), 1516, 1456, 1372, 1173, 1138, 1094, 1020.
Tetraethyl(1R,4R,7R,10R)-α,α′,α″,α″′-tetramethyl[(S)-2-(nitro-benzyl)]-1,4,7,10 - tetraazacyclododecane-1,4,7,10 - tetraacetate (S-RRRR-5)
(S)-Ethyl O-trifluoromethanesulfonyl lactate 4 was used in the synthesis described above. The pale yellow oil obtained (0.56 g, 52%) was found to contain a mixture of the S-RRRR and S-SRRR isomers which could not be separated at this stage. Both isomers were taken through the remainder of the synthesis as a mixture of stereoisomers and would be separated only once the complexes had been synthesized. Rf = 0.2 (5% THF in CHCl3, SiO2 ). 1H NMR (270 MHz, CDCl3), δ 8.05 (2H, d, 3JH–H = 9 Hz, Ar), 7.28 (2H, d, 3JH–H = 9 Hz, Ar), 4.09 (8H, m, OCH2CH3), 3.90-2.15 (21H, m), 1.19 (24, m, CHCH3 and CH2 CH3). 13C NMR (67.9 MHz, CDCl3), δ 14.1, 14.2, 14.3, 14.4, 14.5, 15.8, 17.3, 23.8, 29.1, 35.9, 44.0, 48.2, 49.9, 50.1, 50.2, 50.5, 52.2, 54.7, 57.1, 57.7, 57.9, 59.3, 59.7, 59.9, 61.1, 60.4, 61.1, 123.4 (Ar), 130.2 (Ar), 146.2 (Ar), 149.1 (Ar), 173.6 (C=O), 174.0 (C=O), 175.3 (C=O). m/z (ESMS EI+): 708 (100%, [M+ H+]), 730 (18%, [M+ Na+]). νmax/cm−1: 2980, 2942, 1728 (C=O), 1698, 1650, 1597, 1518, 1446, 1345, 1022.
(1S,4S,7S,10S)-α,α′,α″,α″′-Tetramethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate europium(III) complex (H[Eu(S-SSSS-1)])
The tetraester 5 as a mixture of the S-SSSS and S-RSSS isomers (0.1 g, 0.14 mmol) was dissolved in THF (3 mL). A solution of lithium hydroxide monohydrate (30 mg, 0.71 mmol) in water (3 mL) was added and the reaction stirred at 60 °C for 24 h. After cooling, HCl (1 M, 6 mL) was added and the solvents removed on a freeze dryer. The ligand so obtained was used without purification. A 100 mg sample of the ligand was taken and dissolved in water (8 mL). A solution of europium chloride hexahydrate (0.1 g, 0.28 mmol) in water (8 mL) was added and the reaction stirred at 60 °C for 48 h. The solution was filtered through a 40 µm syringe filter and the solvents removed on a freeze dryer. The residue was purified by preparative HPLC. The complex was isolated after removal of the solvents by lyopholization to afford a pale yellow solid (13 mg, 12%). HPLC: RT = 28.61 min. 1H NMR (270 MHz, D2O), δ 21.6 (1H), 18.5 (1H), 18.1 (1H), 14.2 (1H), 8.3 (1H), 8.0 (2H), 7.6 (2H), 6.8 (1H), 1.9 (1H), 0.5 (3H), 0.0 (1H), −0.2 (1H), −0.5 (1H), −1.5 (3H), −1.9 (3H), −2.9 (3H), −4.0 (1H), −5.3 (1H), −5.9 (1H), −6.8 (1H), −7.4 (1H), −10.8 (1H), −11.3 (1H), −11.7 (1H), −12.0 (1H). m/z (ESMS EI−): 744 (100%, [M−]) an appropriate isotope pattern was observed.
(1R,4S,7S,10S)-α,α′,α″,α″′-Tetramethyl[(S)-2-(nitrobenzyl)]-1, 4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate europium(III) complex (H[Eu(S-RSSS-1)])
The title complex was isolated as a by-product from the synthesis of EuS-SSSS-1 (11 mg, 10%). HPLC: RT = 29.71 min. 1H NMR (270 MHz, D2O), δ 27.1, (1H), 21.8 (1H), 17.5 (1H), 16.8, (1H), 10.7 (2H), 9.7, (2H), 8.3 (1H), 5.2 (1H), 0.9 (1H), −0.3 (3H), −1.3 (1H), −1.6 (3H), −2.0 (3H), −2.8 (3H), −3.2 (1H), −5.4 (1H), −6.2 (1H), −6.4 (1H), −6.8 (1H), −6.9 (1H), −7.3 (1H), −7.6 (1H), −8.5 (1H), −10.1 (1H), −10.4 (1H), −12.6 (1H). m/z (ESMS EI−): 744 (100%, [M−]) an appropriate isotope pattern was observed.
(1S,4S,7S,10S)-α,α′,α″,α″′-Tetramethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate gadolinium(III) complex (H[Gd(S-SSSS-1)])
The title complex was prepared in an analogous manner to EuS-SSSS-1 using gadolinium chloride hexahydrate. The complex was obtained as a pale yellow solid (12 mg, 11%). HPLC: RT = 27.36 min. m/z (ESMS EI−): 749 (100%, [M−]) the appropriate isotope pattern was observed.
(1R,4S,7S,10S)-α,α′,α″,α″′ - Tetramethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate gadolinium(III) complex (H[Gd(S-RSSS-1)])
This complex was isolated as a by-product from the synthesis of GdS-SSSS-1 (10 mg, 9%). HPLC: RT = 29.96 min. m/z (ESMS EI−): 749 (100%, [M−]) the appropriate isotope pattern was observed.
(1S,4S,7S,10S)-α,α′,α″,α″′-Tetramethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate terbium(III) complex (H[Tb(S-SSSS-1)])
The title complex was prepared in an analogous manner to EuS-SSSS-1 using terbium chloride hexahydrate. The complex was obtained as a pale yellow solid (11 mg, 10%). HPLC: RT = 28.67 min. 1H NMR (270 MHz, D2O), δ 200.6 (1H), 143.2 (1H), 116.4 (1H), 108.5 (1H), 87.7 (3H), 81.7 (3H), 35.2 (3H), 17.2 (3H), 13.9 (2H), 11.3 (2H), 4.0 (1H), 1.3 (1H), 0.3 (1H), −32.3 (1H), −47.5 (1H), −54.4 (1H), −57.8 (1H), −61.2 (1H), −88.9 (1H), −92.2 (2H), −103.1 (1H), −151.1, (1H), −307.7 (1H), −321.6 (1H), −339.4 (1H), −369.1 (1H). m/z (ESMS EI−): 750 (100%, [M−]) an appropriate isotope pattern was observed.
(1R,4R,7R,10R)-α,α′,α″,α″′-Tetramethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate europium(III) complex (H[Eu(S-RRRR-1)])
The title complex was prepared from the a mixture of the S-RRRR and S-SRRR isomers of 5 according to the protocol used to prepare EuS-SSSS-1. The title complex as a pale yellow solid (16 mg, 28%). HPLC: RT = 27.14 min. 1H NMR (270 MHz, D2O), δ 44.6 (1H), 43.9 (1H), 39.4 (1H), 35.7 (1H), 12.8, 7.4, 6.8, 5.3, −0.2 (1H), −2.2 (3H), −2.5 (1H), −4.2 (3H), −4.5 (1H), −4.7 (3H), −4.8 (3H), −7.2 (1H), −7.9 (1H), −9.0 (1H), −13.3 (1H), −13.7 (1H), −17.5 (1H), −22.7 (1H), −23.8 (1H), −24.6 (1H). m/z (ESMS EI−): 744 (100%, [M−]) an appropriate isotope pattern was observed. Anal. Found: C, 36.5; H, 5.7; N, 8.1. C27H38N5O10Eu·8H2O requires C, 36.5; H, 6.1; N, 7.9%.
(1S,4R,7R,10R)-α,α′,α″,α″′-Tetramethyl[(S-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate europium(III) complex (H[Eu(S-SRRR-1)])
This complex was isolated as a by-product from the synthesis of EuS-RRRR-1 (9 mg, 15%). HPLC: RT = 28.32 min. m/z (ESMS EI−): 744 (100%, [M−]) an appropriate isotope pattern was observed.
(1R,4R,7R,10R)-α,α′,α″,α″′-Tetramethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate gadolinium(III) complex (H[Gd(S-RRRR-1)])
The title complex was prepared in an analogous manner to EuS-RRRR-1 using gadolinium chloride hexahydrate. The complex was obtained as a pale yellow solid (15 mg, 27%) HPLC RT = 28.35 min. m/z (ESMS EI−): 749 (100%, [M−]) appropriate isotope patterns were observed.
(1S,4R,7R,10R)-α,α′,α″,α″′-Tetramethyl[(S-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate gadolinium(III) complex (H[Gd(S-SRRR-1)])
This complex was isolated as a by-product from the synthesis of GdS-RRRR-1 (8 mg, 15%). HPLC: RT = 29.43 min. m/z (ESMS EI−): 749 (100%, [M−]) appropriate isotope patterns were observed.
(1R,4R,7R,10R)-α,α′,α″,α″′-Tetramethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate terbium(III) complex (H[Tb(S-RRRR-1)])
The title complex was prepared in an analogous manner to EuS-RRRR-1 using terbium chloride hexahydrate. The complex was obtained as a pale yellow solid (12 mg, 26%). HPLC: RT = 27.40 min. 1H NMR (270 MHz, D2O), δ 270.0 (1H), 216.5 (1H), 205.6 (1H), 168.9 (1H), 151.1 (1H), 121.4 (1H), 101.5 (1H), 82.5 (3H), 78.4 (3H), 63.2 (3H), 42.8 (3H), 12.2 (2H), −0.4 (2H), −31.6 (1H) −73.6 (1H), −80.8 (1H), −82.2 (1H), −83.7 (1H), −94.9 (1H), −114.6 (1H), −117.8 (2H), −142.1 (1H), −412.7 (1H), −438.5 (1H), −479.1 (1H), −500.1 (1H). m/z (ESMS EI−): 750 (100%, [M−]) the appropriate isotope pattern was observed.
Trimethyl-(4S,7S,10S)-α,α′,α″-trimethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-4,7,10-triacetate (S-SSS-7)
(S)-2-(Nitrobenzyl)cyclen 3 (1.0 g, 3.26 mmol) and triethylamine (1.27 g, 12.56 mmol) were dissolved in dry acetonitrile (10 mL), under an argon atmosphere and cooled to 0 °C. (R)-Methyl O-trifluoromethanesulfonyl lactate 6 (2.97 g, 12.56 mmol)22 was added dropwise over a period of 10 min. The reaction was then warmed to room temperature and stirred for 4 days at ambient temperature. The solvents were the removed under reduced pressure. The residue was taken up into dichloromethane (150 mL) and water (30 mL) and the two layers separated. The aqueous layer was extracted with dichloromethane (50 mL) and the organic extracted combined, dried (Na2SO4), and the solvents removed under reduced pressure. The orange–brown residue was purified by dry-flash column chromatography over silica gel eluting with 5% methanol in dichloromethane. After drying under high vacuum the title compound was obtained as a orange oil (1.78 g, 96%). Rf = 0.3 (5% MeOH in CH2Cl2, SiO2). 1H NMR (300 MHz, CDCl3), 8.08 (2H, d, 3JH–H = 8 Hz, Ar), 7.52 (2H, d, 3JH–H = 8 Hz, Ar), 3.72-2.31 (29H, m br), 1.34-1.04 (9H, m br, CHCH3). 13C NMR (75.5 MHz, CDCl3), δ 8.3 (Me), 8.5 (Me), 8.7 (Me), 34.8, 46.2 (br), 49.0 (br), 50.8, 51.0, 51.3, 53.7, 54.3, 56.4, 59.0 (br), 66.1, 123.8 (Ar), 130.3 (Ar), 143.8 (Ar), 147.1 (Ar), 167.9 (C=O), 173.6 (C=O), 173.8 (C=O). m/z (ESMS EI+): 566 (100%, [M + H+]), νmax/cm−1: 2952, 2852, 1729 (C=O), 1519, 1456, 1346, 1240, 1222, 1153, 1028.
Trimethyl-(4R,7R,10R)-α,α′,α″-trimethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-4,7,10-triacetate (S-RRR-7)
The title compound was synthesized using the same protocol as S-SSS-7 using S-methyl O-trifluoromethanesulfonyl lactate 6.22 The title compound was obtained as a orange oil (1.6 g, 89%). Rf = 0.3 (5% MeOH in CH2Cl2, SiO2). 1H NMR (300 MHz, CDCl3), δ 8.01 (2H, d, 3JH–H = 9 Hz, Ar), 7.53 (2H, d, 3JH–H = 9 Hz, Ar), 3.73-2.35 (29H, m), 1.26 (3H, d, 3JH–H = 8 Hz, CHCH3), 1.23 (3H, d, 3JH–H = 8 Hz, CHCH3), 1.16 (3H, d, 3JH–H = 7 Hz, CHCH3). 13C NMR (75.5 MHz, CDCl3), δ 9.9 (Me), 13.4 (Me), 15.7 (Me), 35.5, 45.4, 46.6, 47.6, 48.4, 51.3, 51.5, 51.7, 52.0, 53.6, 56.1, 57.8, 58.4, 58.5, 123.7 (Ar), 130.4 (Ar), 143.7 (Ar), 146.9 (Ar), 173.3 (C=O), 173.7 (C=O), 174.1 (C=O). m/z (ESMS EI+): 566 (100%, [M + H+]), 587 (5%, [M + Na+], νmax/cm−1: 2952, 2847, 1728 (C=O), 1596, 1519, 1356, 1204, 1131, 1030, 972.
Tetramethyl-(4S,7S,10S)-α,α′,α″-trimethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate (S-SSS-8)
The triester S-SSS-7 (1.65 g, 2.91 mmol) was dissolved in acetonitrile (10 mL) under argon. Potassium carbonate (0.44 g, 3.2 mmol) and methyl bromoacetate (0.49 g, 3.2 mmol) were added. The reaction mixture was stirred, under argon at 60 °C for 4 days. The solvents were then removed under reduced pressure and the residue taken up into dichloromethane (150 mL) and water (30 mL). The two layers were separated and the aqueous layer extracted with dichloromethane (50 mL). The organic layers were combined, dried (Na2SO4), and the solvents removed under reduced pressure. The residue was purified by dry flash column chromatography over silica gel eluting with 2% methanol in dichloromethane. After drying under vacuum the title compound was obtained as a brownish orange oil which was found to solidify on standing (0.74 g, 40%); mp 216–218 °C, Rf = 0.3 (2% MeOH in CH2Cl2, SiO2). 1H NMR (300 MHz, CDCl3), δ 8.18 (2H, dd, Ar), 7.48 (1H, d, 3JH–H = 8 Hz, Ar), 7.37 (1H, d,3JH–H = 8 Hz, Ar), 3.77-2.05 (34H, m br), 1.18 (9H, m, CHCH3). 13C NMR (75.5 MHz, CDCl3), δ 7.1 (Me), 7.8 (Me), 30.7, 31.1, 32.3, 41.6, 44.4, 44.7, 45.1, 46.4, 48.6, 50.4, 51.8, 52.1, 52.3, 53.3, 56.0, 56.3, 60.3, 61.2, 121.8 (br, Ar), 130.3 (br, Ar), 146.3 (br, Ar) 147.9 (br, Ar), 174.2 (C=O), 175.8 (C=O), 176.1 (C=O). m/z (ESMS EI+): 638 (5%, [M + H+]), 660 (100%, [M + Na+]), 674 (15%, [M + K+]), νmax/cm−1: 2953, 2844, 1728 (C=O), 1519, 1344, 1260, 1222, 1132, 1030. Anal. Found C, 47.1; H, 6.1; N, 8.6. C30H47N5O10·2CH2Cl2 requires C, 47.6; H, 6.4; N, 8.7%.
Tetramethyl-(4R,7R,10R) -α,α′,α″-trimethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate (S-RRR-8)
The title compound was synthesized from S-RRR-7 according to the protocol used for S-SSS-8. The title compound was obtained as a brownish orange solid (0.4 g, 28%); mp 129–131 °C, Rf = 0.3 (2% MeOH in CH2Cl2, SiO2 ). 1H NMR (300 MHz, CDCl3), δ 8.03 (2H, d, 3JH–H =8Hz, Ar), 7.38 (2H, d, 3JH–H = 8 Hz, Ar), 3.8-2.3 (34 H, m br), 1.17 (9H, m, CHCH3). 13C NMR(75.5MHz, CDCl3), δ 14.9 (Me), 15.5 (2 × Me), 33.5, 35.2, 44.9, 46.7, 47.3, 49.2, 49.9, 51.2, 51.6, 51.9, 52.5, 55.7, 56.4, 57.7, 60.4, 123.3 (Ar), 130.1 (Ar), 146.1 (Ar), 147.6 (Ar), 172.3 (C=O), 174.5 (2 × C=O), 175.6 (C=O). m/z (ESMS EI+): 638 (35%, [M+ H+]), 660 (100%, [M+ Na+], νmax/cm−1: 2949, 2845, 1727 (C=O), 1517, 1433, 1343, 1199, 1138, 1052.
(4S,7S,10S)-α,α′,α″-Trimethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (S-SSS-2)
The tetramethyl ester S-SSS-8 (130 mg, 0.36 mmol) was dissolved in THF (5 mL). A 1 M solution of sodium hydroxide (1.8 mL) and water (5 mL) was added and the reaction stirred at 55 °C for 24 h. After cooling, the solvents were removed under reduced pressure. The residue was dissolved in water (5 mL) and purified by HPLC (97 mg, 82%); mp: decomposes >180 °C, HPLC: RT = 27.98 min. 1H NMR (300 MHz, CDCl3), δ 8.08 (2H, d, 3JH–H = 8 Hz, Ar), 7.48 (2H, d, 3JH–H = 8 Hz, Ar), 4.6-2.5 (22H, m br), 1.57 (3H, d, 3JH–H = 6 Hz, CH3), 1.45 (3H, d, 3JH–H = 6 Hz, CH3), 1.25 (3H, d, 3JH–H = 6 Hz, CH3). 13C NMR (75.5 MHz, CDCl3), δ 10.6 (Me), 11.6 (Me), 11.9 (Me), 33.6, 34.3, 45.6, 47.1, 49.4, 50.0, 50.8, 51.0, 51.4, 52.0, 53.2, 53.3, 55.2, 57.9, 62.6, 61.9, 126.6 (Ar), 132.8 (Ar), 147.5 (Ar), 148.9 (Ar), 173.7 (2 × C=O), 176.9 (C=O), 179.1 (C=O). m/z (ESMS EI−): 309 (26%, [HL + K]2−), 618 (100%, [H2L + K]−), νmax/cm−1: 1716 (C=O), 1645, 1519, 1456, 1394, 1349, 1251, 1107. Anal. Found: C, 42.3; H, 7.1; N, 9.5. C27H39N5O10·4H2O·2.5HCl requires C, 41.9; H, 6.7; N, 9.4%.
(4R,7R,10R)-α,α′,α″-Trimethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (S-RRR-2)
The title compound was prepared from S-RRR-8 according to the procedure employed for S-SSS-2. The complex was isolated after removal of the solvents by lyopholization to afford a pale yellow solid (120 mg, 62%). HPLC: RT = 27.77 min, mp 223–225 °C. 1H NMR(300 MHz, CDCl3), δ 8.07 (2H, d, 3JH–H = 7 Hz, Ar), 7.46 (2H, d, 3JH–H = 7 Hz, Ar), 4.4-2.8 (22H, m br), 1.58 (3H, d, 3JH–H = 6 Hz, CH3), 1.48 (3H, d, 3JH–H = 6 Hz, CH3), 1.31 (3H, d, 3JH–H = 6 Hz, CH3). 13C NMR (75.5 MHz, CDCl3), δ 13.8 (Me), 15.3 (Me), 15.5 (Me), 35.0, 46.7, 48.6, 50.6, 51.1, 54.4, 57.8, 58.1, 60.7, 62.9, 63.5, 65.2, 125.5 (Ar), 131.8 (Ar), 147.4 (Ar), 149.0 (Ar), 173.6 (2 × C=O), 178.0 (C=O), 178.8 (C=O). m/z (ESMS EI−): 309 (100%, [HL + K]2−), 618 (18%, [H2L + K]−), νmax/cm−1: 3343, 2992,2863, 1720 (C=O), 1599, 1517, 1468, 1398, 1346, 1225, 1181, 1103.
(4S,7S,10S)-α,α′,α″-Trimethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate europium(III) complex (H[Eu(S-SSS-2)])
S-SSS–2 (121 mg, 0.18 mmol) was dissolved in water (3 mL). A solution of europium chloride hexahydrate (46 mg, 0.18 mmol) in water (3 mL) was added and the reaction stirred at 60 °C for 24 h. After cooling, the solution was filtered through a 40 µm syringe filter and then purified by HPLC. The complex was isolated after removal of the solvents by lyopholization to afford a pale yellow solid (100 mg, 77%). HPLC: RT = 27.00 min. 1H NMR (300 MHz, D2O), δ 15.24 (1H), 14.75 (2H), 13.27 (1H), 8.01 (2H, 3JH–H = 9 Hz), 7.70 (2H, 3JH–H = 9 Hz), 6.23 (1H), 5.58 (1H), 1.81 (1H), 0.51 (1H), −0.33 (3H), −1.40 (3H), −1.51 (1H), −1.55 (1H), −1.83 (1H), −2.24 (3H), −2.81 (1H), −3.63 (1H), −4.28 (1H), −5.34 (1H), −5.40 (1H), −5.44 (1H), −6.02 (1H), −6.49 (1H), −6.52 (1H), 9.50 (1H), −10.30 (1H), −11.97 (1H). m/z (ESMS EI−): 730 (100%, [M−]) appropriate isotope patterns were observed. Anal. Found: C, 38.5; H, 5.6; N, 8.6. C26H36N5O10Eu·4.5H2O requires C, 38.5; H, 5.5; N, 8.6%.
(4S,7S,10S)-α,α′,α″-Trimethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate gadolinium(III) complex (H[Gd(S-SSS-2)])
The title complex was prepared in an analogous manner to EuS-SSS-2 using gadolinium chloride hexahydrate. The complex was obtained as a pale yellow solid (31 mg, 72%). HPLC: RT = 26.98 min. m/z (ESMS EI−): 735 (100%, [M−]) appropriate isotope patterns were observed.
(4R,7R,10R) -α,α′,α″-Trimethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate europium(III) complex (H[Eu(S-RRR-2)])
The title complex was prepared from S-RRR-2 according to the protocol used to prepare EuS-SSS-2. The complex was purified by preparative HPLC and removal of the solvents by lyopholization afforded the title complex as a pale yellow solid (54 mg, 69%). HPLC: RT = 25.66 min. 1H NMR (300 MHz, D2O), δ 36.87 (1H), 35.44 (1H), 34.19 (1H), 33.21 (1H), 10.75 (1H), 7.55 (2H, 3JH–H = 7 Hz), 7.12 (2H, 3JH–H = 7 Hz), 5.21 (1H), 1.64 (1H), 0.18 (1H), −0.25 (1H), −2.18 (1H), −3.01 (3H), −4.11 (3H), −4.24 (3H), −6.35 (3H), −7.14 (1H),−8.88 (1H),−9.70 (1H),−12.09 (1H),−12.30 (1H), −13.79 (1H), −18.67 (1H), −19.62 (1H), −19.77 (1H). m/z (ESMS EI−): 730 (100%, [M−]) appropriate isotope patterns were observed.
(4R,7R,10R) -α,α′,α″-Trimethyl[(S)-2-(nitrobenzyl)]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate gadolinium(III) complex (H[Gd(S-RRR-2)])
The title complex was prepared in an analogous manner to EuS-RRR-2 using gadolinium chloride hexahydrate. The complex was obtained as a pale yellow solid (19 mg, 64%). HPLC: RT = 25.76 min. m/z (ESMS EI−): 735 (100%, [M−]) appropriate isotope patterns were observed.
Supplementary Material
Acknowledgements
The authors thank the National Institutes of Health (EB-04285, M.W.) and (CA-84697 and RR-02584, A. D. S.) and the Robert A. Welch Foundation (AT-584) for financial support for financial support of this work. We also thank Dr Dario Longo (Italy) for access to the simultaneous fitting program that uses genetic algorithm libraries implemented in C++. Prof. Silvio Aime (Torino, Italy) is gratefully acknowledged for access to his equipment.
Footnotes
Electronic supplementary information (ESI) available: Fig. S1: Simulation of the effect of altering τM on relaxivity at four different magnetic fields for a gadolinium chelate with retricted rotation. See http://dx.doi.org/10.1039/b510778d
References
- 1.Woods M, Kovacs Z, Sherry AD. J. Supramol. Chem. 2003;2:1. [Google Scholar]
- 2.Woods M, Zhang S, Sherry AD. Curr. Med. Chem. 2004;4:349. doi: 10.2174/1568013043357338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aime S, Botta M, Fasano M, Terreno E. Chem. Soc. Rev. 1998;27:19. [Google Scholar]
- 4.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem. Rev. 1999;99:2293. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 5.Aime S, Botta M, Fasano M, Terreno E. Acc. Chem. Res. 1999;32:941. [Google Scholar]
- 6.Laus S, Ruloff R, Toth E, Merbach AE. Chem. Eur. J. 2003;9:3555. doi: 10.1002/chem.200204612. [DOI] [PubMed] [Google Scholar]
- 7.Laus S, Sour A, Ruloff R, Toth E, Merbach AE. Chem. Eur. J. 2005;11:3064. doi: 10.1002/chem.200401326. [DOI] [PubMed] [Google Scholar]
- 8.Rudovsky J, Kotek J, Hermann P, Lukes I, Mainero V, Aime S. Org. Biomol. Chem. 2005;3:112. doi: 10.1039/b410103k. [DOI] [PubMed] [Google Scholar]
- 9.Congreve A, Parker D, Gianolio E, Botta M. Dalton Trans. 2004:1441. doi: 10.1039/b402230k. [DOI] [PubMed] [Google Scholar]
- 10.Woods M, Kovacs Z, Zhang S, Sherry AD. Angew. Chem., Int. Ed. 2003;42:5889. doi: 10.1002/anie.200352234. [DOI] [PubMed] [Google Scholar]
- 11.Aime S, Botta M, Ermondi G. Inorg. Chem. 1992;31:4291. [Google Scholar]
- 12.Hoeft S, Roth K. Chem. Ber. 1993;126:869. [Google Scholar]
- 13.Woods M, Aime S, Botta M, Howard JAK, Moloney JM, Navet M, Parker D, Port M, Rousseaux O. J. Am. Chem. Soc. 2000;122:9781. [Google Scholar]
- 14.Dunand FA, Aime S, Merbach AE. J. Am. Chem. Soc. 2000;122:1506. [Google Scholar]
- 15.Port M, Rousseaux O, Raynal I, Woods M, Parker D, Moreau J, Rimbault J, Pierrard J-C, Aplincourt M. Acad. Radiol. 2002;9 Suppl 2:S300. doi: 10.1016/s1076-6332(03)80209-x. [DOI] [PubMed] [Google Scholar]
- 16.Aime S, Barge A, Bruce JI, Botta M, Howard JAK, Moloney JM, Parker D, de Sousa AS, Woods M. J. Am. Chem. Soc. 1999;121:5762. [Google Scholar]
- 17.Aime S, Barge A, Botta M, De Sousa AS, Parker D. Angew. Chem., Int. Ed. 1998;37:2673. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2673::AID-ANIE2673>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Di Bari L, Pintacuda G, Salvadori P. Eur. J. Inorg. Chem. 2000:75. [Google Scholar]
- 19.Howard JAK, Kenwright AM, Moloney JM, Parker D, Woods M, Port M, Navet M, Rousseau O. Chem. Commun. 1998:1381. [Google Scholar]
- 20.Aime S, Botta M, Ermondi G, Terreno E, Anelli PL, Fedeli F, Uggeri F. Inorg. Chem. 1996;35:2726. [Google Scholar]
- 21.Aime S, Botta M, Terreno E. In: Advances in Inorganic Chemistry. ch 4. Eldik Rv., editor. vol. 57. Amsterdam: Elsevier; 2005. [Google Scholar]
- 22.Effenberger F, Burkard U, Willfart J. Liebigs. Ann. Chem. 1986:314. [Google Scholar]
- 23.Woods M, Kovacs Z, Kiraly R, Brücher E, Zhang S, Sherry AD. Inorg. Chem. 2004;43:2845. doi: 10.1021/ic0353007. [DOI] [PubMed] [Google Scholar]
- 24.Marques MPM, Geraldes CFGC, Sherry AD, Merbach AE, Powell H, Pubanz D, Aime S, Botta M. J. Alloys Compd. 1995;225:303. [Google Scholar]
- 25.Di Bari L, Pescitelli G, Sherry AD, Woods M. Inorg. Chem. 2005 doi: 10.1021/ic0511118. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker D, Dickins RS, Puschmann H, Crossland C, Howard JAK. Chem. Rev. 2002;102:1977. doi: 10.1021/cr010452+. [DOI] [PubMed] [Google Scholar]
- 27.Bunzli J-CG. In: Lanthanide Probes in Life, Chemical and Earth Sciences: Theory and Practice. Bunzli J-CG, Choppin GR, editors. Amsterdam: Elsevier; 1989. [Google Scholar]
- 28.Woods M, Sherry AD. Inorg. Chem. 2003;42:4401. doi: 10.1021/ic0300823. [DOI] [PubMed] [Google Scholar]
- 29.Judd BR. Mol. Phys. 1959;2:407. [Google Scholar]
- 30.Bleaney B, Dobson CM, Levine BA, Martin RB, Williams RJP, Xavier AV. J. Chem. Soc., Chem. Commun. 1972:791. [Google Scholar]
- 31.Bleaney B. J. Magn. Reson. 1972;8:91. [Google Scholar]
- 32.Golding RM, Pykko P. Mol. Phys. 1973;26:1389. [Google Scholar]
- 33.Horrocks WD, Jr, Sudnick DR. Acc. Chem. Res. 1981;14:384. [Google Scholar]
- 34.Horrocks WD, Jr, Sudnick DR. J. Am. Chem. Soc. 1979;101:334. [Google Scholar]
- 35.Beeby A, Clarkson IM, Dickins RS, Faulkner S, Parker D, Royle L, de Sousa AS, Williams JAG, Woods M. J. Chem. Soc., Perkin Trans.2. 1999:493. [Google Scholar]
- 36.Spirlet MR, Rebizant J, Desreux JF, Loncin MF. Inorg. Chem. 1984;23:359. [Google Scholar]
- 37.Benetollo F, Bombieri G, Calabi L, Aime S, Botta M. Inorg. Chem. 2003;42:148. doi: 10.1021/ic025790n. [DOI] [PubMed] [Google Scholar]
- 38.Powell DH, Ni Dhubhghaill OM, Pubanz D, Helm L, Lebedev YS, Schlaepfer W, Merbach AE. J. Am. Chem. Soc. 1996;118:9333. [Google Scholar]
- 39.Merbach AE, Toth E. In: The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging. Merbach AE, Toth E, editors. New York: J. Wiley and Son; 2001. [Google Scholar]
- 40.Aime S, Botta M, Fasano M, Crich SG, Terreno E. J. Biol. Inorg. Chem. 1996;1:312. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.