Abstract
Hypoxia has been previously linked to the development of both benign prostatic hyperplasia and prostate cancer. This study investigated the effect of maspin, an extracellular matrix (ECM) tumor suppressor, on the apoptotic response of prostate cancer cells to hypoxia. Gene expression profiling of human benign and malignant prostate epithelial cells after exposure to hypoxia or normoxia revealed dramatic changes in ECM regulators. Maspin was found to be overexpressed in response to hypoxia in prostate cancer cells, but not in benign prostate cells. To dissect the contribution of maspin to tumor cell responses within a hypoxic microenvironment, we used maspin-overexpressing DU-145 human prostate cancer cells. Exposure to hypoxic conditions (1% O2) led to a significant increase in apoptosis in the DU-145 maspin cells, compared to DU-145 neo-transfectants without a significant effect on cell migration. This enhanced sensitivity to hypoxia-induced apoptosis leads to a significant suppression of tumor growth and tumor vascularity in vivo by targeting Akt and focal adhesion kinase activation. Our findings implicate maspin in prostate cancer cell response to hypoxia via recruitment of intracellular signaling partners. This study may have significance in the identification of maspin-driven therapeutic targeting in advanced metastatic prostate cancer.
Keywords: hypoxia, maspin, prostate cancer, apoptosis, tumor suppression, angiogenesis
Introduction
Despite enhanced methods of detection and treatment, prostate cancer remains the most common malignancy and the second leading cause of cancer death in males (Jemal et al., 2006). Prostate cancer is a heterogeneous disease that often develops from prostatic intraepithelial neoplasia to locally invasive carcinoma and eventually to an aggressive, hormone-refractory metastatic carcinoma (Debes and Tindall, 2004; McKenzie and Kyprianou, 2006). Prostate growth characterized by a balance is maintained between proliferative and apoptotic factors; disruptions in this homeostasis lead to loss of apoptosis control and enhanced expression of cell survival factors, frequently detected in prostate tumors (Kyprianou et al., 2000; McKenzie and Kyprianou, 2006). Identification of the dysfunctional apoptotic mechanisms driving prostate cancer progression in the context of signaling contributions by the tumor micro-environment (Rennebeck et al., 2005) may lead to the development of novel effective therapies for metastatic disease.
The tumor microenvironment is characterized by a fluctuation of hypoxia and nutrient deprivation, leading to epigenetic and genetic adaptation of clones and increased invasiveness and metastasis. Hypoxia—the reduction of normal tissue levels of oxygen—is one of the hallmarks of tumor development (Semenza, 2002). In normal tissues, oxygen has the capacity to diffuse 180 µm from adjacent capillaries before it is metabolized (Welsh and Powis, 2003). Tumors undergoing rapid proliferation often outgrow the existing vasculature creating intratumoral hypoxia (Semenza, 2003). Tumor cells must survive by adapting to the low pO2 by increasing vascularization, inducing apoptosis and increasing glucose transporter-1 (GLUT-1; Kimbro and Simons, 2006). These hypoxic adaptations ultimately confer enhanced therapeutic resistance and promote tumor progression. The cellular response to hypoxia is mediated primarily by hypoxia-inducible factor-1α (HIF-1α; Semenza, 2002). HIF-1α is a highly conserved transcription factor that activates specific hypoxia response genes in response to low oxygen tension (Fu et al., 2005). Overexpression of HIF-1α correlates with an aggressive phenotype and poor disease prognosis in a variety of cancers including prostate cancer (Semenza, 2002; Ferrara et al., 2003). Key angiogenesis genes are regulated by HIF-1α including vascular endothelial growth factor (VEGF) and GLUT-1 (Kimbro and Simons, 2006).
Maspin is a 42 kDa tumor suppressor member of the serine protease inhibitor family, originally discovered in normal mammary epithelium (Zou et al., 1994). The protein is encoded by a gene on chromosome 18q21.3 and is found localized to the cytoplasm, the cell membrane and the extracellular matrix (ECM; Schaefer and Zhang, 2003). Maspin suppresses tumor growth and metastasis in vivo, inhibits basement membrane invasion in vitro (Domann et al., 2000) and is functionally linked to apoptosis of human breast and prostate cancer cells. Elegant in vivo studies demonstrated that maspin exerts a potent inhibitory effect on osteolysis occurring in prostate cancer bone metastases (Jiang et al., 2002; Cher et al., 2003). Moreover, evidence from prostate cancer xenograft models, documented a role for maspin as a critical regulator of angiogenesis, by its function to reduce tumor microvessel density and endothelial cell migration (Zhang et al., 2000). Unlike type I tumor suppressors, which become mutated during tumor progression, maspin becomes downregulated/silenced by hypermethylation, and as such is classified as a class II tumor suppressor (Domann et al., 2000).
Interactions between the reactive site loop of maspin and various components of the ECM, including collagens I and III, present a potential mechanism by which maspin may enhance prostate cancer cell adhesion (Abraham et al., 2003) and prevent basement membrane invasion and subsequent metastasis (Blacque and Worrall, 2002; Ngamkitidechakul et al., 2003). In addition, the proapoptotic protein bax was identified as a key mediator of the apoptotic effects of maspin in maspin-overexpressing human prostate cancer cells (Liu et al., 2004). We recently demonstrated the ability of maspin to alter the apoptotic threshold of androgen-independent DU-145 prostate cancer cells in response to the quinazoline-based compound doxazosin (Tahmatzopoulos et al., 2005). The present findings demonstrate that exposure of human prostate cancer cells to a hypoxic microenvironment leads to maspin transient upregulation, whereas maspin overexpression results in enhanced hypoxia-induced apoptosis.
Results
Hypoxic response of benign and malignant prostate epithelial cells
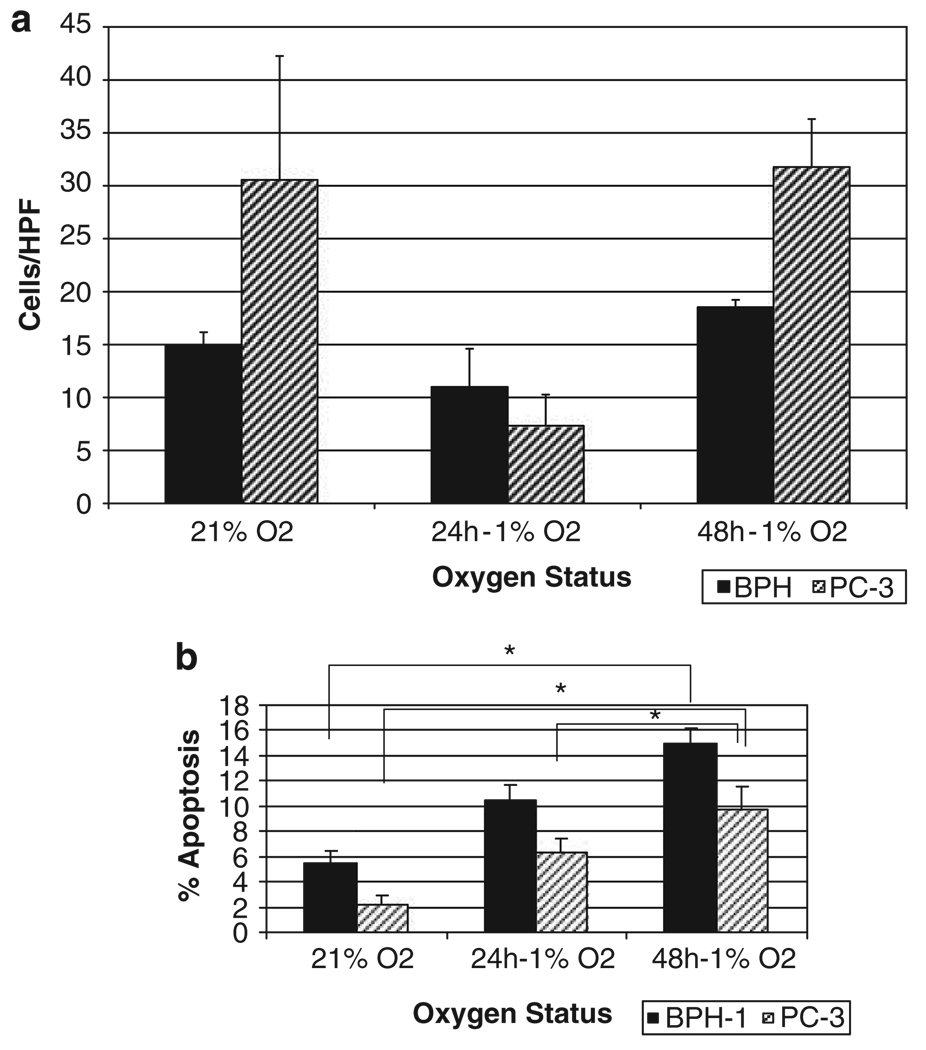
The response of human benign prostate epithelial (BPH)-1 cells to hypoxic conditions was comparatively analysed to prostate cancer cells, PC-3. As shown in Figure 1a, there is a differential response in terms of cell migration between the two cell lines. In normoxia, PC-3 cells show enhanced ability to migrate as compared to BPH-1 cells. Following exposure to hypoxia (24 h), both cell lines exhibited diminished ability to migrate across a high-powered field. This was a transient effect as after 48 h of hypoxic conditions, the ability to migrate is enhanced in both cell lines; in PC-3 prostate cancer cells, there was a significant increase in migration compared to benign BPH-1 cells. Increased exposure of both PC-3 cells and BPH-1 cells to hypoxia leads to increased apoptosis (Figure 1b); however, although BPH-1 cells show a linear apoptotic response to increased hypoxia exposure, PC-3 cells exhibited considerable apoptotic resistance between 24 and 48 h.
Figure 1.
Response of benign prostate epithelial cells benign prostate epithelial (BPH)-1 and malignant prostate cells PC-3 to hypoxia. (a) The effect of hypoxia on prostate cell migration. Wounding assays were performed on benign and malignant prostate epithelial cells and the number of migratory cells was quantified. Three random fields (× 400) were counted under light microscopy and the average values per field were determined. Under normoxia, PC-3 cells show enhanced migration potential compared to BPH-1 cells and following hypoxia exposure (24 h), both cell lines exhibited a diminished ability to migrate in a transient manner. (b) The apoptotic response of the two cell lines to hypoxia; exposure of PC-3 and BPH-1 cells to hypoxia leads to enhanced apoptosis. *P < 0.001.
We subsequently performed array analysis to characterize the differential response to hypoxia at the transcriptional level. The data summarized in Table 1 indicate that exposure of PC-3 prostate cancer cells to hypoxia, resulted in an increased transcription in key cell adhesion molecules, including tumor-necrosis factor (TNF)-α, urokinase and maspin, although none of these alterations were detected in BPH-1 cells. Conversely, BPH-1 cells exhibited upregulation of a critical apoptosis regulator transforming growth factor (TGF)-β (ligand), and its receptor, a change not detected in PC-3 cells. This gene profiling revealed induced expression of maspin in PC-3 cells after exposure to hypoxia with subsequent downregulation after longer-term hypoxic conditions (Table 1).
Table 1.
Gene expression profiling in response to hypoxia
| Protein | PC3–4 h hypoxia | PC3–8 h hypoxia | BPH-1 4 h hypoxia | BPH-1 4 h hypoxia |
|---|---|---|---|---|
| Platelet/endothelial cell adhesion molecule (CD31) | — | 2.30 | — | −2.23 |
| Platelet factor 4 | 3.24 | 3.10 | — | — |
| Collagen VIII (endostatin) | 2.94 | 2.05 | — | — |
| TGF-β | — | — | 2.89 | 3.23 |
| TGF-β receptor 2 | 3.89 | — | 2.43 | −2.54 |
| SMAD-1 | 2.06 | −2.67 | 2.48 | — |
| TNF-α | — | 3.58 | — | — |
| Thrombin | −13.73 | 2.57 | — | — |
| Urokinase | 2.44 | 2.39 | — | — |
| MMP-9 | — | — | 3.64 | — |
| Chemokine ligand 2 | 2.40 | 2.43 | −4.20 | — |
| Maspin | 10.2 | 4.22 | — | — |
Abbreviations: TGF, transforming growth factor; TNF, tumor necrosis factor; MMP-9, matrix metalloprotease-9.
BPH-1 and PC-3 prostate cell lines were cultured under normoxia and hypoxia conditions.
RNA was extracted and subjected to microarray analysis as described in ’Materials and methods’.
Maspin enhances apoptosis of prostate cancer cells in hypoxia
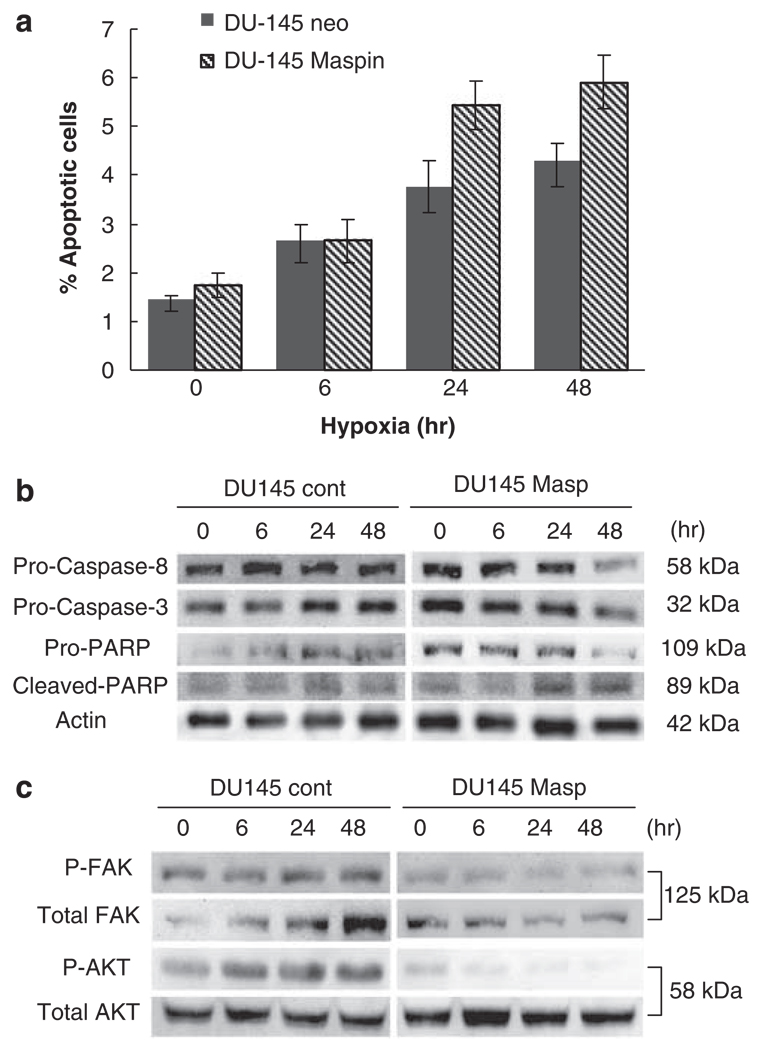
Driven by the above observations, subsequent experiments sought to examine whether maspin overexpression impacts the sensitivity of DU-145 human prostate cancer cells to hypoxia-induced apoptosis, migration and invasion. The basal expression levels of maspin under normoxia in prostate epithelial cells BPH-1 and malignant prostate cells PC-3, DU-145 and LNCaP are shown in the Supplementary Data (S1). DU-145 maspin-expressing transfectants and DU-145 neo-transfectants (control) were exposed to increasing periods of hypoxia (6–48 h). Western blot analysis verified that maspin transfectants maintained their overexpression for up to 48 h of hypoxia, whereas neo-transfectants showed no expression until 48 h of hypoxia was reached (data not shown.) Figure 2a illustrates the results of Annexin-V analysis of the cells undergoing apoptosis in response to hypoxia. As shown, hypoxia exposure (within 24 h) resulted in significantly increased apoptosis in DU-145 maspin cells compared to DU-145 neo cells. Western blot analysis confirmed that poly(ADP)-ribose polymerase cleavage occurred within 24 h of hypoxic conditions in maspin-expressing cells; additionally increased procaspase-3 and -8 cleavage was evident after 24 h of hypoxia in DU-145 maspin cells and was furthered after 48 h, compared to DU-145 neo-control transfectants (Figure 2b).
Figure 2.
Effect of maspin overexpression on apoptosis of prostate cancer cells under hypoxic conditions. The ability of prostate cancer cells DU-145 neo (controls) and DU-145 Maspin-overexpressing cells to undergo apoptosis was evaluated after exposure to hypoxia for 6, 24 and 48 h. (a) reveals the results of a Annexin-V analysis of hypoxia-induced apoptosis in DU-145 maspin and DU-145 neo-control cells. This experiment was performed three independent times; numerical data shown represent the average values ± s.e.m. (b) indicates caspase activation in prostate cancer cells in response to hypoxia. After exposure to hypoxia DU-145 neo and DU-145 maspin-expressing cells were subjected to western blotting. Increased cleavage for both procaspase 3 and caspase-8 was evident after 48hrs of hypoxia in DU-145 maspin-transfectants as compared to DU-145 neo controls. (c) indicates a representative western blot analysis of focal adhesion kinase (FAK) and AKT and their phosphorylated forms. Maspin inhibits hypoxia-induced FAK (invasion) and AKT (survival) activation in prostate cancer cells. DU-145 neo and DU-145 maspin-expressing cells were exposed to hypoxia for 6, 24 and 48 h and cell lysates were prepared and subjected to western blotting. Phopsphorylated forms of FAK and AKT were detected using the respective antibodies and normalized to total FAK and total AKT respectively. Membranes were incubated with SuperSignal West Dura Extended Substrate (Pierce Biotechnology) and visualized using chemiluminescent imaging in an EpiChemi-3 Darkroom (UVP Bioimaging System). In maspin-expressing prostate cancer cells, hypoxia resulted in a rapid reduction on in phoshorylation of both FAK and AKT within 24 h, compared to the DU-145 neo-control cells.
To gain a mechanistic insight into the effect of maspin on hypoxia-induced tumor cell survival and apoptotic resistance, we examined the activation status of the key intracellular survival pathway, AKT in DU-145 neo-control transfectants and DU-145 maspin-overexpressing cells. Figure 2c indicates that DU-145 cells constitutively express phosphorylated AKT that is enhanced within 6 h of hypoxic conditions. Maspin expression inhibited constitutive AKT phosphorylation, as well as hypoxia-driven AKT phosphorylation (activation; Figure 2c). Also shown in Figure 2c is that focal adhesion kinase (FAK) levels were elevated in response to hypoxia and maspin abrogated this upregulation.
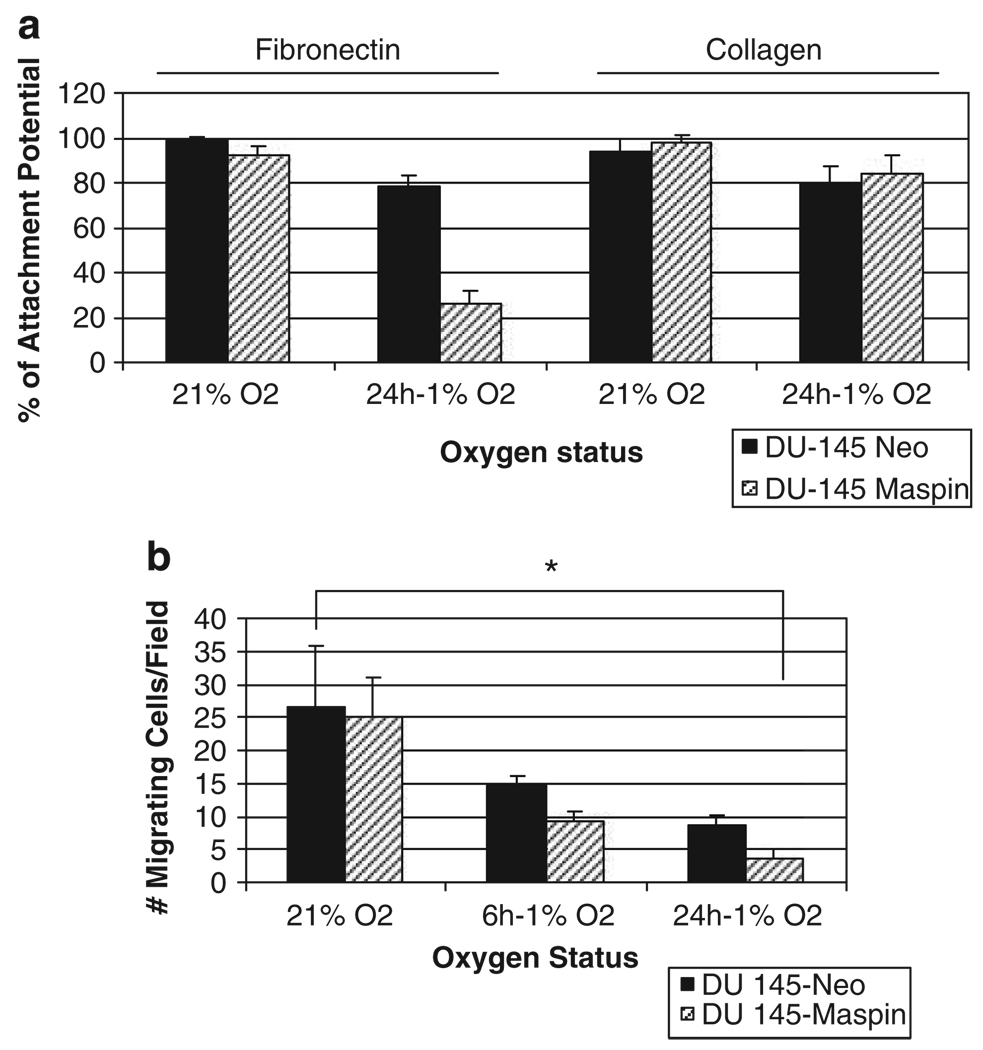
As maspin is a tumor suppressor serpin that inhibits cell invasion and migration (Denk et al., 2007), we subsequently examined the differential response of maspin-expressing prostate cancer cells in terms of adhesion and migration. The presence of maspin in DU-145 cells resulted in a significant suppression in the attachment potential to fibronectin-coated plates, under hypoxic conditions (24 h); maspin-expressing cells had an increased adhesion to collagen I; however, this difference was not statistically significant (Figure 3a). The results shown on Figure 3b reveal that in response to hypoxia DU-145 maspin-expressing cells exhibited a marked decrease in cellular migration compared to DU-145 neo-control cells within 6 h of hypoxic conditions; this suppression of cellular migration was further enhanced after 24 h of hypoxia.
Figure 3.
Effect of maspin on prostate cancer cell attachment and migration under hypoxia. (a) The ability of prostate cancer cells DU-145 neo and DU-145 maspin transfectants to adhere to extracellular matrix (ECM) protein components was evaluated after exposure to hypoxia for 24 h. Attached cells to fibronectin or collagen 1-coated plates dishes were determined as described in ’Materials and methods’. (b) The migration potential of human prostate cancer cells DU-145 neo- (controls) and DU-145 maspin-overexpressing cells after exposure to hypoxia for 6, 24 and 48 h (as described in Figure 1). The results on prostate cancer cell migration under hypoxic conditions indicate no significant differences in the migratory capacity of DU-145 neo-control cells vs the DU-145 maspin-expressing cells; *P < 0.001.
Effect of maspin on HIF-1α and VEGF expression in prostate cancer cells
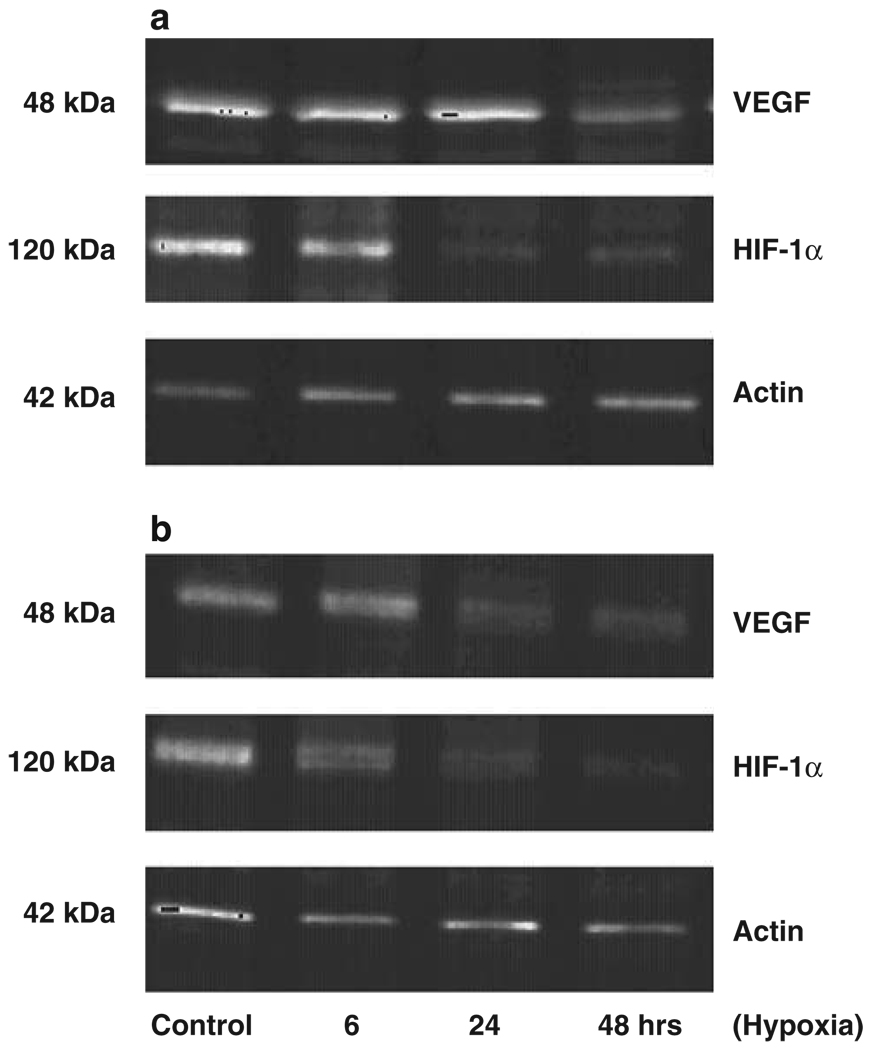
Under normoxia both DU-145 neo-control cells (Figure 4a) and DU-145 maspin-expressing cells (Figure 4b) had constitutive protein expression of VEGF and HIF-1α. Exposure to hypoxia led to a transient upregulation of VEGF and HIIF-1α protein levels (6–24 h) in the DU-145 neo cells and a subsequent decrease to control normoxia levels by 48 h. In the DU-145 maspin cells hypoxia resulted in an earlier downregulation of HIF-1α, within 6 h, followed by a marked reduction in VEGF levels by 24 h (Figure 4).
Figure 4.
Effect of hypoxia on angiogenesis modulators. DU-145 neo prostate cancer cells (a) and DU-145 maspin-expressing cells (b) were exposed to normoxia (control) and hypoxia (for 6, 24 and 48 h) and cell lysates (20 µg protein) were subjected to electrophoretic analysis on SDS–polyacrylamide gel electrophoresis (PAGE) and western blotting. Expression of HIF-1α and VEGF was determined using the corresponding antibodies as described in ’Materials and Methods’. Specific protein expression was normalized to α-actin expression, using the actin mouse monoclonal antibody. Hypoxia led to an earlier swift decrease in VEGF and HIF-1α protein levels in maspin-expressing cells compared to DU-145 neo prostate cancer cells.
Maspin decreases prostate tumor growth by increasing apoptosis and targeting vascularity in cancer xenografts
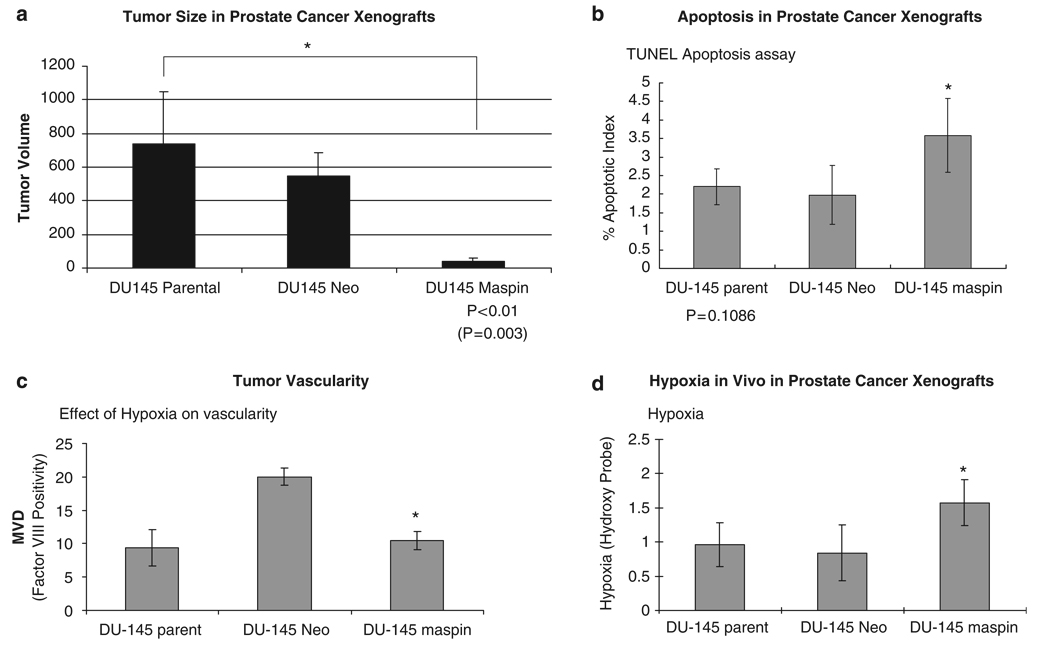
In vivo tumorigenicity studies demonstrated a significant decrease in tumor vessel density in maspin-overexpressing DU-145 xenografts compared to neo-control transfectants. We noted a significant decrease in tumor growth among maspin-transfectant DU-145 xenografts after at 6 weeks compared to controls (Figure 5a). To determine the processes are driving this antitumor effect, we subsequently performed immunohistochemical analysis of paraffin-embedded tissue serial sections from prostate cancer xenografts derived from the various lines. Apoptosis evaluation (using the terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) analysis) indicated that the apoptotic index was significantly higher in maspin-overexpressing tumor xenografts (Figure 5b). Tissue vascularity was assessed on the basis of factor VIII immunoreactivity; we found a marked reduction in tumor vascularity in the prostate cancer xenografts derived from the maspin transfectants compared to the DU-145 neo-control cells (Figure 5c). For the parental DU-145 control-derived tumors however, the large variation in the microvessel density values, did nor allow for a statistically significant difference with the DU-145 maspin-expressing cells (Figure 5c). Pimonidazole (hydoxy probe) was used to determine the intratumoral hypoxia status in these tumors. As shown in Figure 5d, the maspin transfectant-derived prostate tumors exhibited a significant increase in intratumoral tissue hypoxia (as expected from the in vitro data).
Figure 5.
Maspin suppresses tumor growth in vivo by enhancing apoptosis and suppressing vascularity. Following subcutaneous inoculation of nude mice (n = 6 per group) with DU-145 parental, DU-145 neo control and DU-145 maspin-overexpressing, tumor volume was measured as described in ’Materials and methods’. (a) Analysis of the tumor volume from the human prostate xenografts (established as described above), revealing that maspin cells exhibit a significant reduction in prostate tumor volume compared to the parental DU-145 control cell line, P <0.01. Prostate cancer xenografts were excised from hypoxia and control tumor-bearing mice, paraffin-embedded and tissue sections (6 µm) were subjected to terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) staining for apoptosis (b) and tumor vascularity (factor VIII for microvessel density; c). The numerical data (quantitative analysis) reveal that maspin expression in these prostate tumors results in enhanced apoptotic response and decreased tumor vascularity. (d) The results of intratumoral hypoxia evaluation (as determined on the basis of hydroxy-probe) in tumor sections from the prostate cancer xenografts. *P <0.05.
Discussion
The hypoxic microenvironment that characterizes most solid organ tumors toward metastatic phenotypes and apoptotic resistance has been well described in several models including prostate cancer (Semenza, 2003; Kimbro and Simons, 2006). Here we show that the aggressive androgen-independent human prostate cancer cells PC-3 and DU-145 develop apoptotic resistance during hypoxic exposure (not detected in benign prostate cells). This study identifies the functional involvement of the tumor suppressor protease inhibitor maspin in the regulation of prostate cancer cell response to a ‘hostile’ hypoxic microenvironment.
This hypoxic selection of more aggressive phenotypes in malignant PC-3 cells in the in vitro setting, led us to the characterization of the protein players that contribute to increased tumor aggressiveness in hypoxic prostate cancer cells. The observation that early hypoxia stimulates maspin expression in a transient fashion is mechanistically intriguing. Amir et al. (2005) described the ability of maspin to inhibit the hypoxia-mediated stimulation of the uPA system in metastatic breast cancer cells. One could argue that maintaining maspin overexpression throughout prolonged hypoxia exposure would mitigate the hypoxia-driven selection of aggressive prostate cancer cell clones. Using the DU-145 prostate cancer cells-overexpressing maspin (Sheng et al., 1996) as an experimental model, we demonstrated that maspin conferred considerable apoptotic sensitivity of prostate cancer cells to hypoxia, both in vitro and in vivo. These observations gain direct support from existing evidence by our group (Tahmatzopoulos et al., 2005), and others (Jiang et al., 2002; Liu et al., 2004), documenting the ability of maspin to sensitize prostate and breast cancer cells to apoptosis induction.
We found that maspin overexpression inhibited the adhesion potential of prostate cancer cells to fibronectin but not to collagen I, evidence that is partly in contrast with previous reports that maspin can promote prostate cancer cell adhesion via collagen connections (Abraham et al., 2003). The presence of maspin conferred an inhibitory effect on prostate cancer cell migration under hypoxia. Moreover, the major physiological significance of the present study stems from the in vivo observed dramatic reduction of tumor vascularity in prostate cancer xenografts derived from maspin-overexpressing cells. These results are in accordance with the established role of this player in inhibiting angiogenesis during prostate cancer metastasis (Zhang et al., 2000; Cher et al., 2003; Schaefer and Zhang, 2003). The present in vitro findings indicate that in response to hypoxia maspin-expressing prostate cancer cells exhibited a rapid downregulation of both angiogenesis effectors VEGF and HIF-1α. To understand the impact of maspin on prostate cancer cell response to hypoxia, one has to consider that while HIF-1α upregulation has been implicated in the development of apoptotic resistance in some tumor models (Welsh and Powis, 2003), in others, hypoxia-associated apoptotic resistance occurs independent of HIF-1α (Semenza, 2002; Rofstad et al., 2005). This evidence points to a maspin targeting of a survival pathway upstream from direct phenotypic effectors such as uPA, HIF-1α and VEGF.
The PTEN/PI3/AKT pathway has been implicated in promoting prostate cancer apoptotic resistance, invasive behavior and poor therapeutic response (McKenzie and Kyprianou, 2006). Tumor hypoxia leads to enhanced AKT phosphorylation in cancer models, ultimately resulting in apoptotic resistance and increased tumor angiogenesis (Corley et al., 2005; Lee et al., 2006; Lee et al., 2008). In cancers of the breast and prostate, it appears that the upregulation of uPA, HIF-1α, VEGF and other cell survival pathways such as the nuclear factor-κB/IkB system are initiated by AKT phosphorylation (Gao et al., 2004; Shi et al., 2005). Our findings suggest that maspin overexpression inhibits hypoxiainduced AKT activation thus sensitizing prostate tumor cells to apoptosis. These data establish a mechanistic link between maspin-regulated-tumor suppression and direct inhibition of the PI3K/AKT survival pathway toward reverting the apoptotic resistance to hypoxia. One could easily argue that such a link can (at least in part) provide a molecular basis for the diversity of roles maspin is implicated in from tumor suppression pathways to elevating the apoptotic sensitivity of prostate cancer cells (Tahmatzopoulos et al., 2005). Interestingly enough, although the tumor size was significantly smaller in prostate tumors derived from DU-145 maspin cells, the level of intratumoral hypoxia was significantly higher than the parental DU-145-derived tumors. The critical role of the tumor microenvironment and its subsequent targeting cannot be understated. The ability of maspin to create a more austere, hypoxic environment through angiogenesis inhibition coupled with it apoptosis induction under hypoxic conditions at the epithelial prostate cancer cell level suggests that maspin targeting should provide significant tumor suppression synergy in vivo.
In summary, this study indicates that tumor hypoxia leads to apoptotic resistance via inhibition of AKT (and possible its downstream effectors), which can be mitigated by persistent maspin expression. Our in vivo findings point to maspin as a regulator of tumor cell response to hypoxia and apoptotic sensitivity, highlighting the value of therapeutic targeting of the hypoxic microenvironment within prostate tumors. Our findings may have important implications in the development of maspin-targeted therapeutic intervention for advanced metastatic prostate cancer. The functional relationship between the hypoxia-driven AKT phosphorylation and its subsequent inhibition by maspin overexpression may dictate cell signaling-directed therapy in metastatic prostate tumors meriting further exploration.
Materials and methods
Cell culture
Human prostate cancer PC-3 and DU-145 cells are cultured routinely in our laboratory. The benign prostate epithelial cell line BPH-1 was a gift from Dr Simon Hayward (Vanderbilt University). The prostate cells are cultured in medium containing RPMI medium (Gibco, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (Gibco) and penicillin/streptomycin mixture with L-glutamine (BioWhittaker, Walkersville, MD, USA). Prostate cancer DU-145 neomycin-transfectant cells (control) and DU-145 maspin-transfectants were obtained through a collaboration with Dr Sheng (Wayne State University) and were cultured under selection medium containing G418 (500 µg/ml) (Tahmatzopoulos et al., 2005).
cDNA array analysis
BPH-1 and PC-3 cell lines were cultured in the normoxia (5% CO2 in room air) at 37 °C in an incubator (Thermo Electron Corp., Marietta, OH, USA); or hypoxia (1% O2, 5% CO2, 94% N2) in an oxygen-regulating incubator (Thermo Electron Corp.). RNA was extracted using the Trizol extraction and centrifugation (10 000 r.p.m.) method. RNA pellets were reconstituted in water and RNA (5 µg) samples were subjected to cDNA gene array analysis using GEArray as per the manufacturer’s protocol (Superarray Bioscience Corp., Frederick, MD, USA).
In vivo tumorigenicity studies
In vivo xenograft growth
Athymic nude mice from Harlan (Indianapolis, IN, USA) ages 3–6 weeks and virus free were purchased and were allowed to acclimate in animal facilities at the University of Kentucky. Mice were inoculated subcutaneously with (1.5 × 106) parental control DU-145 cells, neo-transfectants or maspin-transfectants DU-145 cells and tumor xenografts were allowed to grow for 6 weeks. Tumor xenografts were measured using a vernier caliper and tumor volumes were calculated (V = L × W2/2, where L is the longest diameter and W is the shortest diameter perpendicular to L). When tumors reached at least 150 mm3 in size, animals were killed with CO2 inhalation and tumors were surgically excised. Tissue specimens were subsequently fixed in 10% formalin (Sigma HT50-1-128, St Louis, MO, USA) for 2 days at 4 °C and paraffin embedded. Formalin-fixed paraffin-embedded specimens of prostate xenografts were sectioned (6 µm) using a Finesse microtome (Thermo Shandon Inc., Pittsburgh, PA, USA). Hematoxylin and eosin staining was used for the histopathologic evaluation of prostate tumors.
Hypoxia evaluation in vivo
Hypoxyprobe-1 (Chemicon International, Ternecula, CA, USA), the commercially available preparation of pimonidazole was injected into the peritoneum of tumor-bearing mice at a dose of 60 mg/kg per 100 µl sterile saline. Mice were killed after 1.5 h by CO2 overdose and tumor tissues and prostate glands were harvested, fixed in formalin, preserved in paraffin and subsequently embedded and sectioned as described above. Tissue sections (4 µm) were stained with Hypoxyprobe-1Mab1, according to the manufacturer’s protocol for detecting Hypoxyprobe-1 adducts.
Apoptosis evaluation
Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling staining was performed to evaluate the incidence of apoptosis in the tumor xenografts from the various treatment groups. Parental, maspin and neo-control-derived tumor xenografts were subjected to TUNEL analysis, using ApopTag Peroxidase In situ Apoptosis Detection Kit S7100 (Chemicon International), as previously described (Garrison et al., 2007). This method is based on the in situ detection using the TUNEL assay. Three fields per tissue section were evaluated under microscopic analysis. The apoptotic index was determined on the basis of percentage of apoptotic cells over the total cell number in each section (Garrison et al., 2007).
Immunostabling analysis
The tumor staining procedure was carried out as described in the product protocol kit from ABC Staining System: SC 2018 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), with some modifications. Tissue sections were deparaffinized in three washes of xylene and rehydrated in decreasing concentrations of ethanol. The parental, neo- and maspin-derived prostate tumor sections were exposed to different primary antibodies and incubated at 4 °C overnight. Tissue sections were washed with phosphate-buffered saline (PBS) and were subsequently exposed to the respective secondary antibody and diaminobenzidine staining for signal detection. The anti-maspin (human) antibody was purchased from Pharmingen (Franklin Lakes, NJ, USA). For evaluation of tissue vascularity the factor VIII antibody (von Willebrand Factor, DakoCytomation, Carpinteria, CA, USA) was used for determining the microvessel density.
In vitro efficacy studies
Hypoxia exposure
DU-145 prostate cancer cells, both neo-transfectants and maspin-overexpressing cells were cultured in normoxia (5% CO2 in room air) at 37 °C in an incubator (Thermo Electron Corp.); or hypoxia (1% O2, 5% CO2, 94% N2) in an oxygen-regulating incubator (Thermo Electron Corp.).
Apoptosis detection
Hoechst staining
Cells were treated at different time intervals of 6, 24 and 48 h in hypoxic conditions (1% O2). After treatment, cells were fixed in 4% paraformaldehyde for 10 min. After washing with 1 × PBS, Hoechst dye was added (10 µg/ml), using Triton X-100 to permeabilize the cells, and the plates were incubated overnight at 4°C. Apoptotic cells were counted in three random fields under fluorescence microscopy and apoptotic percentage was determined based on the amount of apoptotic cells within the total cell population of the field.
Annexin-V flowcytometric analysis
The Vybrant Apoptosis Assay kit (Molecular Probes, Eugene, OR, USA) was used to assess early apoptosis induction of prostate cells in response to hypoxia, according to instructions by the manufacturer. Briefly, after exposure to normoxia or hypoxia, cells were washed with cold PBS and then stained with Pacific Blue Annexin-V. Samples were subsequently subjected to flow cytometric analysis using the PARTEC instrument (Munster, Germany).
Attachment assay
Cells were treated for 24 h in hypoxic conditions. After 24 h, cells were seeded in three six-well plates (105 cells per well) coated with either type I collagen (1 µg/cm2, Sigma) or fibronectin. Following 10-min incubation, attached cells were fixed with methanol (5 min) and after methanol removal, the cells were placed in 1 × PBS. Three random fields (× 400) were counted under light microscopy and the average values per field were determined.
Cell migration assay
The wounding assay was applied to cells growing in six-well plates as previously described (Tahmatzopoulos et al., 2005). At 70% confluency, cell monolayers were wounded with a toothpick and placed in hypoxia for 24 hand 48 h. Cells migrating to the wounded areas were counted and the migration was defined as the average number of cells migrating in three random fields per well.
Western blot analysis
DU-145 neo and DU-145 maspin cells were exposed to hypoxia at intervals of 0, 6, 24 and 48 h. Cell lysates were then prepared from hypoxia-treated and from untreated control cultures. Cells were harvested and lysed with lysis buffer (50 mm Tris-HCl (pH 7.4), 1% Triton X-100, 150 mm NaCl, 5 mm MgCl2 and 1 mm phenylmethylsulphonyl fluoride). Cell lysates (20 µg protein) were subjected to electrophoretic analysis through 15% SDS/polyacrylamide gel electrophoresis and then transferred to Hybond C membranes (Amersham Biosciences, Piscataway, NJ, USA). Membranes were incubated with HIF-1α rabbit polyclonal antibodies (NOVUS Biologicals, Littleton, CO, USA) overnight at 4 °C and were then exposed to the secondary antibody, goat antirabbit immunoglobulin G (Jackson Immunoresearch, West Grove, PA, USA; 1 h at room temperature). Expression of VEGF, AKT, pAKT, FAK and pFAK was determined using the following antibodies: VEGF mouse monoclonal antibody (Santa Cruz Biotechnology), AKT mouse monoclonal antibody and phosphorylated pAKT mouse monoclonal antibody (Cell Signaling Technology, Beverly, MA, USA); FAK monoclonal antibody was obtained from Upstate Biotechnology (Millipore, Billerica, MA, USA); the antibody again pFAK was from Sigma. Specific protein expression was normalized to α-actin expression, as determined using the actin Ab-1 mouse monoclonal antibody (Oncogene Research Products, San Diego, CA, USA). For signal detection, membranes were incubated with SuperSignal West Dura Extended Substrate (Pierce Biotechnology, Rockford, IL, USA) and visualized using chemiluminescent imaging in an EpiChemi-3 Darkroom (UVP Bioimaging System, Upland, CA, USA).
Statistical analysis
Statistical analysis of significance was performed using a two-way analysis of variance (ANOVA) for non-parametric ANOVA or by the Student’s t-test. All numerical data are expressed as the mean value ± s.e.
Supplementary Material
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Acknowledgements
This work was supported by a grant from the National Institutes of Health, NIH CA107575-01 (NK). We thank Menglei Zhu for expert assistance with the preparation of the figures and Lorie Howard for help with the paper submission.
Abbreviations
- BPH-1
benign prostatic hyperplasia cells
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- HIF-1α
hypoxia-inducible factor-1α
- SDS-PAGE
SDS–polyacrylamide gel electrophoresis
- TGF-β
transforming growth factor-βl
- TUNEL
terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling
- VEGF
vascular endothelial growth factor.
References
- Abraham S, Zhang W, Greenberg N, Zhang M. Maspin functions as tumor suppressor by increasing cell adhesion to extracellular matrix in prostate tumor cells. J Urol. 2003;169:1157–1161. doi: 10.1097/01.ju.0000040245.70349.37. [DOI] [PubMed] [Google Scholar]
- Amir S, Margaryan NV, Odero-Marah V, Khalkhali-Ellis Z, Hendrix MJ. Maspin regulates hypoxia-mediated stimulation of uPA/uPAR complex in invasive breast cancer cells. Cancer Biol Ther. 2005;4:400–406. doi: 10.4161/cbt.4.4.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacque O, Worrall D. Evidence for a direct interaction between the tumor suppressor serpin, maspin, and types I and III collagen. J Biol Chem. 2002;211:10783–10788. doi: 10.1074/jbc.M110992200. [DOI] [PubMed] [Google Scholar]
- Cher M, Biliran H, Jr, Bhagat S, Meng Y, Che M, Lockett J, et al. Maspin expression inhibits osteolysis, tumor growth, and angiogenesis in a model of prostate cancer bone metastasis. Proc Natl Acad Sci USA. 2003;100:7847–7852. doi: 10.1073/pnas.1331360100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley KM, Taylor CJ, Lilly B. Hypoxia-inducible factor 1 alpha modulates adhesion, migration and FAK phosphorylation in vascular smooth cells. J Cell Biochem. 2005;96:971–985. doi: 10.1002/jcb.20559. [DOI] [PubMed] [Google Scholar]
- Denk AE, Bettstetter M, Wild PJ, Hoek K, Bataille F, Dietmaier W, et al. Loss of maspin expression contributes to a more invasive potential in malignant melanoma. Pigment Cell Res. 2007;20:112–119. doi: 10.1111/j.1600-0749.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. New Engl J Med. 2004;351:1488–1490. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- Domann F, Rice J, Hendrix M, Futscher B. Epigenetic silencing of maspin gene expression in human breast cancers. Int J Cancer. 2000;85:805–810. doi: 10.1002/(sici)1097-0215(20000315)85:6<805::aid-ijc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber H, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Fu X, Choi E, Bubley G, Balk S. Identification of hypoxia inducible factor-1α (HIF-1α) polymorphism as a mutation in prostate cancer that prevents normoxia-induced degradation. Prostate. 2005;63:215–221. doi: 10.1002/pros.20190. [DOI] [PubMed] [Google Scholar]
- Gao N, Shen L, Zhang Z, Leonard SS, He H, Zhang XG, et al. Arsenite induces HIF-1alpha and VEGF through PI3 K, Akt and reactive oxygen species in DU145 human prostate carcinoma cells. Mol Cell Biochem. 2004;255:33–45. doi: 10.1023/b:mcbi.0000007259.65742.16. [DOI] [PubMed] [Google Scholar]
- Garrison JB, Shaw Y-J, Chen C-S, Kyprianou N. Novel quinazoline-based compounds impair prostate tumorigenesis by targeting tumor vascularity. Cancer Res. 2007;67:11344–11352. doi: 10.1158/0008-5472.CAN-07-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer Statistics, 2006 CA. Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Jiang N, Meng Y, Zhang S, Mensah-Osman E, Sheng S. Maspin sensitizes breast carcinoma cells to induced apoptosis. Oncogene. 2002;21:4089–4098. doi: 10.1038/sj.onc.1205507. [DOI] [PubMed] [Google Scholar]
- Kimbro KS, Simons JW. Hypoxia-inducible factor-1 in human breast and prostate cancer. Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr Relat Cancer. 2006;13:739–749. doi: 10.1677/erc.1.00728. [DOI] [PubMed] [Google Scholar]
- Kyprianou N, Bruckheimer EM, Guo Y. Cell proliferation and apoptosis in prostate cancer: significance in disease progression and therapy. Histol Histopathol. 2000;15:1211–1223. doi: 10.14670/HH-15.1211. [DOI] [PubMed] [Google Scholar]
- Lee BL, Kim WH, Jung J, Cho SJ, Park JW, Kim J, et al. A hypoxia-independent up-regulation of hypoxia-inducible factor-1 by AKT contributes to angiogenesis in human gastric cancer. Carcinogenesis. 2008;29:44–451. doi: 10.1093/carcin/bgm232. [DOI] [PubMed] [Google Scholar]
- Lee SM, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. Hypoxia confers protection against apoptosis via PI3K/Akt and ERK pathways in lung cancer cells. Cancer Lett. 2006;242:231–238. doi: 10.1016/j.canlet.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Liu J, Yin S, Reddy N, Spencer C, Sheng S. Bax mediates the apoptosis-sensitizing effect of maspin. Cancer Res. 2004;64:1703–1711. doi: 10.1158/0008-5472.can-03-2568. [DOI] [PubMed] [Google Scholar]
- McKenzie S, Kyprianou N. Apoptosis evasion: the role of survival pathways in prostate cancer progression and therapeutic resistance. J Cell Biochem. 2006;97:18–32. doi: 10.1002/jcb.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngamkitidechakul C, Warejcka D, Burke J, O’Brien W, Twinning S. Sufficiency of the reactive site loop of maspin for induction of cell-matrix adhesion and inhibition of cell invasion. J Biol Chem. 2003;278:31796–31806. doi: 10.1074/jbc.M302408200. [DOI] [PubMed] [Google Scholar]
- Rennebeck G, Martelli M, Kyprianou N. Anoikis and survival connections in the tumor microenvironment: is there a role in prostate cancer metastasis. Cancer Res. 2005;65:11230–11235. doi: 10.1158/0008-5472.CAN-05-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofstad EK, Mathiesen B, Henriksen K, Kindern K, Galappathi K. The tumor bed-effect: increased metastatic dissemination from hypoxia-induced up-regulation of metastasis-promoting gene products. Cancer Res. 2005;65:2387–2396. doi: 10.1158/0008-5472.CAN-04-3039. [DOI] [PubMed] [Google Scholar]
- Schaefer J, Zhang M. Role of maspin in tumor metastasis and angiogenesis. Curr Mol Med. 2003;3:653–658. doi: 10.2174/1566524033479519. [DOI] [PubMed] [Google Scholar]
- Semenza G. HIF-1α and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:62–67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- Semenza G. Targeting HIF-1α for Cancer Therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Sheng S, Carey J, Seftor EA, Dias L, Hendrix MJ, Sager R. Maspin acts at the cell membrane to inhibit invasion and motility of mammary and prostatic cancer cells. Proc Natl Acad Sci USA. 1996;93:11669–11674. doi: 10.1073/pnas.93.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YH, Wang YX, Bingle L, Gong LH, Heng WJ, Li Y, et al. In vitro study of HIF-1 activation and VEGF release by bFGF in the T47D breast cancer cell line under normoxic conditions: involvement of PI-3K/Akt and MEK1/ERK pathways. J Pathol. 2005;205:530–536. doi: 10.1002/path.1734. [DOI] [PubMed] [Google Scholar]
- Tahmatzopoulos T, Sheng S, Kyprianou N. Maspin sensitizes prostate cancer cells to doxazosin-induced apoptosis. Oncogene. 2005;24:5375–5383. doi: 10.1038/sj.onc.1208684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh S, Powis G. Hypoxia inducible growth factors as a cancer drug target. Curr Cancer Drug Targets. 2003;3:391–405. doi: 10.2174/1568009033481732. [DOI] [PubMed] [Google Scholar]
- Zhang M, Volpert O, Shi Y, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196–199. doi: 10.1038/72303. [DOI] [PubMed] [Google Scholar]
- Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)