Abstract
In most mammals, the sexually dimorphic development of embryos is typically achieved by the differentiation of the external genitalia. Hence, the sexual distinction of mammalian newborns is based on the external genital structure. Although it was shown in the 1940s and 1950s that androgen from the testes establishes the male sexual characteristics, the involvement of nongonadal and locally produced masculine effectors remains totally unknown. It is noteworthy that the disorders of fetal masculinization, including hypospadias, one of the most frequent birth defects, occur at a high frequency. Furthermore, their causative factors remain unclear. In this study, the involvement of the coordinated actions of androgen and the growth factor systems was genetically analyzed for the first time on mammalian reproductive organ formation. The results demonstrated that the Wnt/β-catenin pathway is indispensable masculine factor for the external genital development. The bilateral mesenchymal region adjacent to the urethral plate epithelium displayed a sexually dimorphic activity of Wnt/β-catenin signaling. Loss- and gain-of-function β-catenin mutants displayed altered sexual development of the external genitalia. These results indicate the novel functions of the Wnt/β-catenin pathway as a locally expressed masculine effector. This could be the first genetic study analyzing the roles of the genetic interactions between androgen and locally expressed growth factor signaling during the development of reproductive organs. These results also shed new insight on the reproductive genetics and the causative factors of genital disorders.
The Wnt/β-catenin pathway genetically interacts with the androgen signaling pathway and functions as a locally expressed masculine factor during male external genitalia formation.
The male sexual characteristics of embryos are generally established by androgens, which depend on testis development. Such hormonal control of sexual development has been shown since the beginning of the 1900s (1). However, the involvement of nongonadal and locally produced masculine factors in the sexually developing organs has not yet been elucidated. Sexual differentiation is a remarkably complex process, which depends on the orchestration of the signaling network. The presence of putative effectors that can potentially interact with hormonal signaling or can function parallel with the hormonal pathway remains completely unknown.
Disorders of sexual development are among the most common human birth defects. Of those, hypospadias is the most frequent malformation in which the urethral meatus is located at the ventral (lower) side of the penis. Males with severe hypospadias are born with ambiguous genitalia. The prevalence of hypospadias is increasing, occurring in 0.4–1% of boys at birth (2,3,4). Although any defects in the pathways along with androgen signaling can cause feminization and urogenital defects (5,6,7,8,9), the etiology of such disorders is obscure in most cases. Therefore, a better understanding of the principles of sexually dimorphic development will shed light on the causative mechanisms of the genital malformations.
The development of the fetal external genitalia is divided into two processes. The first phase corresponds to the initial outgrowth and early patterning of the genital primordia. The external genitalia of both sexes are derived from a common undifferentiated anlage, the genital tubercle (GT). In mice, it develops from the cloacal region starting at embryonic day (E) 10.5. The after GT outgrowth is a consequence of mesenchymal swelling around the cloaca, which is accompanied by the formation of the urethral plate epithelium (UPE), the future embryonic urethral epithelium, in the ventral midline. Androgen pathway-independent severe hypospadias with groove-like defects can therefore be affected at this phase of development (10,11).
The subsequent second phase is a hormonally regulated process. As an embryo develops, the prepuce elevates from the proximal to the distal region of the GT, which eventually engulfs the GT. The preputial elevation is accompanied by the thickening of the preputial mesenchyme and the enlarged future glans region. After the development of the prepuce, the urethral folds fuse in the midline of the GT, which eventually develops the tubular urethra (12). The mesenchyme in the glans gradually condenses and develops the various mesenchymal derivatives, such as corporal tissues and penile bones. On the other hand, the female external genitalia display few such characteristic processes. Hence, morphological sexual dimorphism is apparent at birth with larger external genitalia, a well-developed prepuce, tubular urethra, and the condensation of a bilaterally segmented prospective corporal body in males (Fig. 1A).
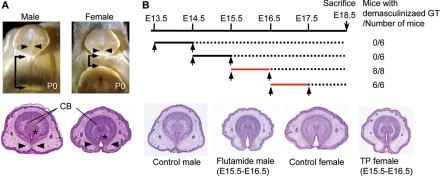
Figure 1.
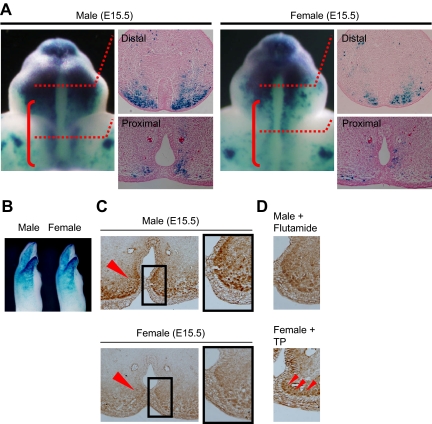
The time window for external genital masculinization. A, Sexual dimorphisms of the external genitalia in newborn mice (postnatal d 0). In males, the fusion of the urethral folds in the ventral midline results in canalization of the urethral epithelium (arrowheads) and bilateral fusion of the prepuce. The prospective corporal body (CB) condenses and bilaterally separates in the male but not in the female GTs. In the male, the anogenital distance, the distance from the external genitalia to the anus, is longer than that in the female (arrows). *, Urethral meatus. B, The timeline for flutamide treatment (upper panel). The GTs of all male embryos treated with flutamide on E15.5–16.5 and E16.5–17.5 (red lines) are demasculinized, displaying a morphology similar to those of the female GTs. The representative demasculinized and masculinized GTs and the GT of control mice with the vehicles are shown.
Sexual differentiation of the male external genitalia is under the influence of androgen. Fetal androgen production begins shortly after Leydig cell differentiation at around E13 in the mouse and reaches a peak shortly before birth (13). However, the timing of sexually dimorphic development has not yet been fully characterized. In mice, the obvious morphological sexual difference of the GTs appears at E16.5 (14). On the other hand, a recent study indicated that the androgen action commences several days earlier than the timing of the morphological differentiation in rat embryos (15). Not only the timing but also the spatially regulated androgen signaling for the masculinization remain poorly understood. Previous tissue-recombination experiments indicated that the GT develops through epithelial-mesenchymal interactions (16,17). However, the contribution of mesenchymally or epithelially expressed androgen receptor (AR) in the masculinization processes of the external genitalia is still not known.
Various mutant studies have shown that the initial GT outgrowth and patterning requires the coordinated output of several growth factors, including Hedgehog (Hh), Wnt, bone morphogenetic protein (Bmp), and fibroblast growth factor (Fgf) (18,19,20,21,22,23,24). However, virtually no studies have focused on these growth factor cascades during masculinization. This is partly because the mutants for such genes exhibit drastic defects often with lethal phenotypes before the masculinization processes. Recently, loss-of-function mutants for β-catenin display very early developmental arrest of the GTs (25). However, the role of canonical Wnt/β-catenin, with regard to the GT masculinization processes remains totally unknown. Wnt/β-catenin signaling also regulates the growth and development of the prostate around the perinatal stage, implying a possibility that they can affect urogenital organ development in later sexually dimorphic organogenesis (26). Wnt/β-catenin signaling is therefore an attractive candidate growth factor system to be involved in sexually dimorphic GT development.
Androgen has been thought to play a central role in such a network, but it is not even clear whether androgen is necessary or sufficient for male external genital development. Furthermore, the downstream pathway of androgen signaling has not been elucidated at all. The current study examined the contribution of growth factor signaling in the external genital development, especially focusing on the Wnt/β-catenin signaling. To this end, molecular genetic analyses using the spatially and temporally regulated inducible expression of Cre recombinase and several mice with the loxP-flanked gene alleles were applied. First, the critical time window of external genital masculinization was identified, and the indispensable function of mesenchymally expressed AR was clarified. Intriguingly, the degree of Wnt/β-catenin signaling activity was higher in the male GTs than that of the female GTs. Loss- and gain-of-function mutant studies revealed the function of Wnt/β-catenin signaling as a masculine effector in the GTs. In fact, the current study showed that excessive Wnt/β-catenin activity could yield adult male-type external genitalia in the female background. This study therefore indicates that Wnt/β-catenin signal is an essential masculine effector for the external genitalia. Because external genital development is a typical and sensitive organogenesis displaying embryonic sexual dimorphisms, this study could be the first to identify the candidate masculine effectors for embryonic organogenesis.
Results
Critical time window of androgen actions on GT masculinization
One of the characteristics of the development of the embryonic external genitalia, the GTs, is the prominent morphological sexual dimorphisms, which are already evident in the later stage of embryogenesis. This makes GTs ideal subjects to study the mechanisms of sexual development in mammals. Before elucidating the contribution of growth factor signaling in GT masculinization, it is necessary to clarify the spatially and temporally regulated androgen signaling. Therefore, this study initially attempted to define the critical time window for differentiation into the male sexual characteristics of the GTs.
To define the critical time window of androgen actions for GT masculinization, antiandrogenic chemical exposure experiments for mouse embryos were performed in utero. GT masculinization can be defined with morphological criteria, the formation of a tubular urethra with well-developed prepuce, and the condensation of a bilaterally segmented prospective corporal body (Fig. 1A). In contrast to such male characteristics, female GTs do not form a tubular urethra and exhibit an unclosed prepuce at the lower (ventral) midline of the GTs (arrowheads in Fig. 1A).
The intrauterine exposure of flutamide demasculinizes the external genitalia of male rodent offspring (14,15,27). To accurately examine the timing of GT masculinization, the modulation of the masculine processes was attempted by administering two successive injections of flutamide at various stages during pregnancy. The resultant morphologies of the embryonic GTs were then analyzed at E18.5 (see treatment timeline, Fig. 1B). The GTs of male mouse embryos treated with flutamide on E13.5–14.5 and E14.5–15.5 displayed normal male-type morphology (data not shown). In contrast, when the embryos were treated with flutamide on E15.5–16.5 and E16.5–17.5 (red lines in the timeline), the GTs were demasculinized and their morphologies were similar to those of the female GTs (Fig. 1B). In addition, female GTs were masculinized when the embryos were treated with testosterone propionate at E15.5–16.5 (Fig. 1B). Based on these observations, the critical time window of GT masculinization regulated by androgens is demonstrated as E15.5–16.5 in mice.
Spatially regulated androgen signals for masculinization processes of external genitalia
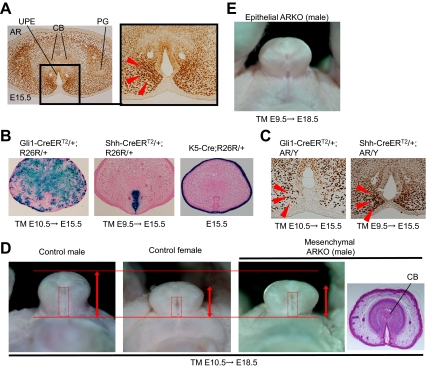
Epithelial-mesenchymal interactions between the UPE and its adjacent mesenchyme have been suggested to play an essential role during external genital formation. A tissue-recombination study suggested that the GT mesenchyme induces mature urethral epithelia formation (16). On the other hand, the UPE is thought to play an inductive role in mesenchymal differentiation (17,28). However, the region-specific androgen function mediated by each epithelially and mesenchymally expressed AR, if any, has never been analyzed. In fact, the expression of AR has been found in both the UPE and mesenchyme at E15.5, the currently identified time window for masculinization (Fig. 2A). A region-specific conditional AR knockout (KO) mouse study was therefore conducted to analyze the mesenchymally or epithelially expressed AR functions in GTs; the mesenchymal tissue-specific KO of AR was first examined using Gli1CreERT2 mice (hereafter referred to as mesenchymal ARKO). Gli1 is expressed in the mesenchyme around the cloacal epithelium (pericloacal mesenchyme) at E10.5 (29). The cells from the pericloacal mesenchyme contribute to the external genital mesenchyme in the later stages (29). The mice were shown to be an effective Cre line for targeted recombination in the urogenital organ mesenchyme [samples treated with tamoxifen (TM) at E10.5 are examined at E15.5; Fig. 2B]. AR is significantly expressed in the mesenchyme adjacent to the urethral epithelium (Fig. 2A). In fact, in the mesenchymal ARKO mice, the mesenchymal AR tends to be expressed in dispersed manner (arrowhead in Fig. 2C).
Figure 2.
Involvement of the mesenchymally expressed AR for external genital masculinization. A, AR is expressed in both the UPE and the mesenchyme of the male GT at E15.5. Note the prominent expression in the mesenchyme adjacent to the UPE in the male GTs (arrowheads). CB, Prospective corporal body; PG, preputial gland. B, Targeted recombination driven by Gli1CreERT2, ShhCreERT2, and K5-Cre driver mice was assessed with Rosa26 reporter (R26R) mice. The TM was administrred at E10.5 or E9.5, respectively, and the labeled tissue was assessed at E15.5. The sections are counterstained with eosin. C, AR expression in the GTs of the mesenchyme- and epithelium-specific ARKO mice. In the mesenchymal KO mice, the mesenchymal AR expression is observed as a dispersed manner (arrowheads). D, The phenotypes of tissue-specific conditional ARKO mice. The mesenchyme-specific ARKO male mice exhibit hypoplastic external genitalia (arrows) with an unclosed prepuce similar to the control female GTs (red boxes). Histological sections also show the female-like GTs in the male mutants (compare that with Fig. 1A). E, The GTs of the epithelium-specific ARKO male mice appear morphologically indistinguishable from those of the control males.
Mesenchymal ARKO mice exhibited hypoplastic external genitalia with an undeveloped prepuce, which are characteristics morphologically similar to the female GTs (six of six embryos; Fig. 2D). In the wild-type male GTs, a marked degree of bilaterally segmented mesenchymal condensations was notably observed in the developing male GTs at the late stage (Fig. 1A). However, the prospective corporal bodies, defined by the histological criteria as mesenchymal condensation, neither differentiated nor bilaterally segmented in the mesenchymal ARKO mice (Fig. 2D). On the other hand, the development of the internal reproductive organs of such mesenchymal ARKO mice was basically not affected in comparison with the development of the control male organs (data not shown).
To exclude the possibility of the phenotype elicited by a low level of Cre activity in the ectoderm of the Gli1CreERT2 line (Fig. 2B), the ectoderm-specific ARKO mice were also analyzed using a Cre-expressing line under a keratin 5 promoter (K5-Cre; Fig. 2B) (30). These mice did not display any defects in the GT masculinization (data not shown). This is consistent with the lack of AR expression in the GT ectoderm (Fig. 2A), excluding the possibility of ectodermally Cre-induced phenotypes.
Next, to elucidate the function of AR expressed in the UPE, AR-floxed mice were crossed with ShhCreERT2 mice (hereafter referred to as epithelial ARKO). The UPE is derived from the endodermal cloacal epithelium where Shh is expressed (31). For the recombination of urethral epithelial cells, ShhCreERT2 embryos were treated with TM at E9.5. This treatment procedure was sufficient for the targeted recombination in the UPE (Fig. 2B). In the epithelial ARKO mice, more than 80% of cells in the endoderm display recombination (the recombination frequency was estimated by assessing the ratio of AR-positive cells in the endoderm), whereas the prominent AR expression in the mesenchyme is still detected (Fig. 2C, right). The mutants did not exhibit any abnormal GT phenotypes (10 embryos were examined; Fig. 2E), also indicating the essential roles of the mesenchymally expressed AR gene function for GT masculinization.
Sexually dimorphic activity of Wnt/β-catenin signaling in the developing GTs
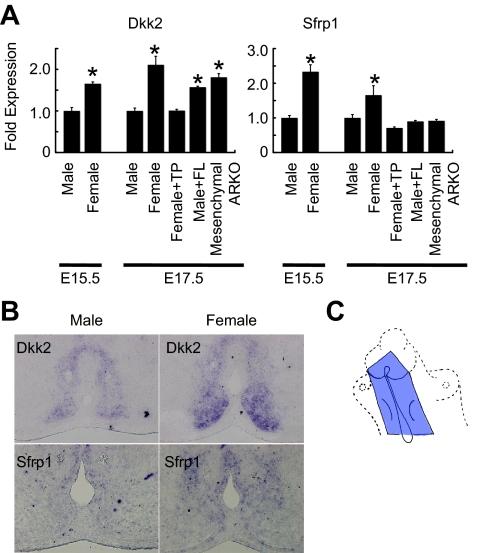
After determining the critical timing and establishing a conditional gene-targeting strategy, the effector signal pathways for masculinization, estimated downstream targets of androgen, were analyzed. The sexually dimorphic expressing genes have been analyzed using a DNA microarray (32). Those analyses identified the expression of several Wnt inhibitory genes that were increased in the female GT at E15.5. The increased expression of Dkk (dickkopf) 2 and Sfrp (secreted frizzled-related protein) 1 in the female GTs in comparison with the males was confirmed by quantitative RT-PCR and in situ hybridization analyses (Fig. 3, A and B). To our knowledge, this could be the first case to demonstrate the sexually dimorphic expression of the Wnt signal genes. The expression of several Wnt ligand and R-spondin (Rspo) genes was also analyzed with RT-PCR (Table 1). Although a series of in situ gene expression and RT-PCR analyses were conducted for these genes in the sexually dimorphic stage of the GTs, prominent sexual dimorphisms were not detected in the current experimental conditions (data not shown).
Figure 3.
Wnt/β-catenin signaling during GT development. A, A quantitative RT-PCR analysis reveals the expression of Dkk2 and Sfrp1 to increase in wild-type females in comparison with those of wild-type males at E15.5 and E17.5. Dkk2 expression is increased in the male GTs treated with flutamide (FL) and the GTs of mesenchymal ARKO mice. The tissue specimens are collected from the mesenchyme adjacent to the UPE of the GTs (blue area in C). TP, Testosterone propionate. *, P < 0.05 vs. control male. B, Dkk2 and Sfrp1 expression is abundantly detected in the wild-type female in comparison with those of the wild-type male at E15.5.
Table 1.
The expression pattern of the Wnt ligands in the wild-type GTs at E15.5
| Gene expression | Primer sets | |
|---|---|---|
| Wnt1 | − | AAATGGCAATTCCGAAACCG/CGAAGATGAACGCTGTTTCTCG |
| Wnt2 | + | AGATGTGATGCGTGCCATTG/CGATGCTGGCGGAACTG |
| Wnt2b | + | TACGGTGTTCGCTTTGCCA/TTCAGGAATCTCCGAACAGCC |
| Wnt3 | − | GCCGCAATTACATCGAGATCAT/CCAGGCTGTCATCTATGGTGGT |
| Wnt3a | − | ATGTGAGCTCGCATGGCATAG/GACGTAGCAGCACCAATGGAA |
| Wnt4 | + | CATCGAGGAGTGCCAATACCA/GACAGGGAGGGAGTCCAGTGT |
| Wnt5a | + | GCGTGGCTATGACCAGTTTAAGA/TTGACATAGCAGCACCAGTGAA |
| Wnt5b | + | TGTGGAGACAACGTGGAGTACG/TGTAGGTTCATGAGAGCTCGGC |
| Wnt6 | − | GGTTTACACCAGCCCACGAA/GGAACTAGCAAAGGGCCTTTC |
| Wnt7a | + | CGCAAGCCCATGGACACT/GCCTGTCACTGGGTCCTCTTC |
| Wnt7b | + | CAATGGTGGTCTGGTACCCAAT/AGTCTCATGGTCCCTTTGTGGTT |
| Wnt8a | − | TCCGGCAGATGGGAAATTAC/TGGCGCTTGTCCATCTCAA |
| Wnt8b | − | CCCTGCCTTTCTCCGAAGAC/GCGGTGAAGGACACAAGTGA |
| Wnt9a | − | CGTGGGTGTGAAGGTGATAAAG/GCAGGAGCCAGACACACCAT |
| Wnt9b | + | GCTCAACGAGACCCCTAATATGTATAAG/TCAACCCTAGTTATGTTTATCTGAGTCTCAC |
| Wnt10a | − | CGGAACAAAGTCCCCTACGA/TGCTATGGCGTAGGCGAAAG |
| Wnt10b | + | ATGAGAGGTTTTCGGTTGGAAA/CCTCCCAAGAGCCTGACAAG |
| Wnt11 | + | ATGTGCGGACAACCTCAGCTA/CGCATCAGTTTATTGGCTTGG |
| Wnt16 | − | TGTATGGTCGCCACTACCACTT/GGTGCCGCTACTCAGCTCAT |
| Rspo1 | − | TGTGAAATGAGCGAGTGGTCC/TCTCCCAGATGCTCCAGTTCT |
| Rspo2 | − | TTGCATAGAGGCCGCTGCTTT/CTGGTCAGAGGATCAGGAATG |
| Rspo3 | − | GTACACTGTGAGGCCAGTGAA/ATGGCTAGAACACCTGTCCTG |
| Rspo4 | + | CTGGAGTCCCTGCATACACAA/CACGGGGAGAAGGAAAGTTTC |
The expression of the Wnt ligands in the wild-type male GTs is assessed by RT-PCR. +, Presence of the corresponding gene expression; −, absence of an expression.
To investigate the hormone dependency of Wnt inhibitory gene expression, a quantitative RT-PCR analysis was performed for Dkk2 and Sfrp1 in the female GTs treated with testosterone propionate and male GTs treated with flutamide (testosterone propionate or flutamide was treated at E15.5 and E16.5). Testosterone propionate treatment decreased the Dkk2 and Sfrp1 expression in the female GTs (Fig. 3A). Dkk2, but not Sfrp1, gene expression was increased by flutamide treatment in the male GTs (Fig. 3A). These data suggest that Dkk2 may be a novel and reliable feminized marker for GT development. In fact, Dkk2 expression was increased in the GTs of the mesenchymal ARKO mice in comparison with those of control males (Fig. 3A, and see also Fig. 5C).
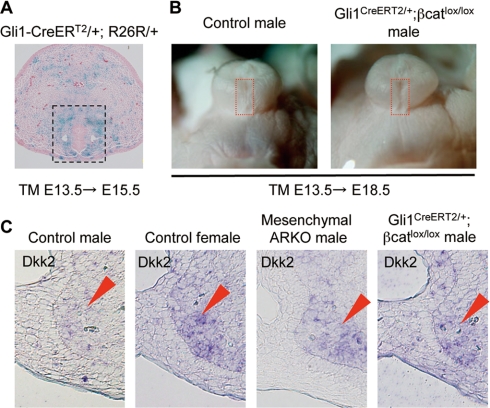
Figure 5.
Impaired GT development in the loss-of-function mutants of β-catenin. A, The Cre-mediated LacZ pattern in Gli1CreERT2 mice (crossed with R26R mice) treated with TM at E13.5 was examined at E15.5. The LacZ signal was detected in the mesenchyme adjacent to the UPE. B, The loss-of-function β-catenin mutants exhibit an unclosed prepuce at the midline of the male GT (red boxes). C, Dkk2 expression is increased in such genetic mutant male mice (arrowheads).
The decreased level of Wnt inhibitory genes expressed in the male GTs could be associated with the sexually dimorphic Wnt/β-catenin activity. To investigate the presence of its signaling, the BatGAL mouse line, the Wnt/β-catenin signaling indicator mice, was analyzed (33). The LacZ signal derived from the BatGAL allele was detected in the mesenchymal cells adjacent to the UPE (Fig. 4A). This bilateral mesenchyme has been suggested to be essential for GT masculinization as shown above. This led to the examination of the sexually dimorphic activity of Wnt/β-catenin signaling during the GT masculinization processes. Notably, the LacZ signal derived from the BatGAL line was enhanced in the wild-type male GTs in comparison with those of the females (Fig. 4A). The LacZ signal in the limb, whose development is sex independent, was not altered (Fig. 4B). Next, the expression level of β-catenin was examined in the mesenchyme adjacent to the UPE of the male and female GTs. Consistent with the results of the Wnt/β-catenin signaling indicator mice, immunohistochemical analyses revealed that β-catenin accumulated in the mesenchymal cells of the male GTs in comparison with those of the female GTs (Fig. 4C). Notably, β-catenin expression level was decreased in the male embryos treated with flutamide and was increased in the female embryos treated with testosterone propionate (Fig. 4D). The above data all suggest that Wnt/β-catenin may function as one of the effector signals for androgen signaling in the male GTs.
Figure 4.
Sexually dimorphic activity of Wnt/β-catenin signaling during GT development. A, The LacZ signal stained with the BatGAL line at E15.5. Note the enhanced LacZ signal in the male shown by the red brackets (shown in the high-magnitude pictures and in cross-sections for the distal and proximal region of the GT). B, In the limb, the sexually dimorphic pattern of the LacZ signal is not detected. C and D, Immunohistochemistry for anti-β-catenin antibodies in the GTs. β-Catenin is accumulated in the mesenchyme adjacent to the UPE in the male (arrowheads). Flutamide treatment represses β-catenin expression in the male embryos (D). In the GTs of female embryos treated with TP, β-catenin expression is increased in the mesenchyme when compared with those of control female (arrows in D).
Impaired sexual differentiation of external genitalia in loss- or gain-of-function mutants of β-catenin
Loss- or gain-of-function mutants of β-catenin were analyzed for the possible involvement of the Wnt/β-catenin activity for GT masculinization. To achieve the targeted gene recombination in the GT mesenchyme during the sexual developmental stage, the Gli1CreERT2 line was treated with TM at E13.5. Under these conditions, recombined cells were observed in the mesenchyme adjacent to the UPE at E15.5, which is monitored by the R26R reporter allele (boxed region in Fig. 5A). Notably, this region overlaps with the region with the Wnt/β-catenin signal as shown by the BatGAL activity and β-catenin protein localization. This Cre mouse line was thus considered to be a useful tool to investigate the roles of Wnt/β-catenin signals during GT sexual development.
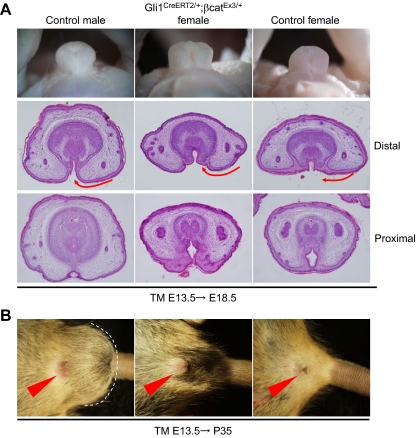
The β-catenin floxed mutant mice (β-cateninlox), which contains two loxP sites that flank exons 3–6 (34), was first analyzed, and its conditional mutation in the mesenchyme during the GT masculinization stage was generated. In such a conditional loss-of-function mutant line, approximately 40% of the male embryos failed to develop the proper prepuce and ventral (lower) midline formation (seven of 18 embryos; Fig. 5B). Perturbed male GT development associated with a mesenchymal ARKO mutation or by Gli1CreERT2/+;β-cateninlox/lox mutation was demonstrated by the increased Dkk2 expression, which is a feminized marker of the GTs (Fig. 5C). The GTs in the mutant females developed similarly to the control females (data not shown). These results suggest that β-catenin may be required for the ventral midline formation of the prepuce during male-type GT development.
Next, a constitutive active β-cateninloxEx3 mutant allele was analyzed to determine whether an enhanced Wnt/β-catenin signaling can masculinize the female GTs. Cre activity derived from the Gli1CreERT2 allele regulated by TM at E13.5 induces the constitutive active β-catenin that lacks the N-terminal domain phosphorylation sites essential for its degradation (35). The GTs of such compound mutant females showed well-developed prepuce in comparison with those of the control females at E18.5 (six of six embryos; Fig. 6A). This is similar to the phenotypes of the female embryos treated with androgens, which show prepuce hypertrophy adjacent to the midline seam sites (36). Some mutants can survive during the postnatal stage, and their adult external genital phenotypes (at postnatal d 35) were examined. Surprisingly, such adult mutant female mice exhibited a hyperplasic prepuce with enlarged external genitalia similar to the male GTs (Fig. 6B). The specificity of the phenotypes was confirmed by an analysis of the scrotum region. Indeed, such mutant mice still showed a prominent female-type perineum region in contrast to the male-type scrotum region (Fig. 6B).
Figure 6.
Prepuce hyperplasia in the female GTs of the gain-of-function mutants of β-catenin. A, In the female GT with activated β-catenin in the mesenchyme, the prepuce exhibits hyperplasia. The histological sections corresponding to the levels of the distal GT represent preputial development to be accelerated in the constitutive active β-catenin mutants (arrows). B, Well-developed external genitalia observed in the gain-of-function β-catenin mutant female mice in comparison with those of the control female and male mice. Note that scrotal/perineal regions were not affected, showing the specificity of the resultant phenotypes. The dotted line indicates the scrotum.
Discussion
Mesenchymally expressed AR is indispensable for GT masculinization
The male and female external genitalia enable highly efficient fertilization in mammals. During embryogenesis, sexually dimorphic organogenesis is achieved by hormones produced in the gonads. Among such organs, the external genitalia develop from a single primordium, the GT. This bipotential nature of embryonic external genital development is particularly unique and therefore is a suitable model system to study developmental and reproductive biology.
The morphological sexual difference of the external genitalia is established at E16.5 in mice, at E17.5 in rat, and at 11–13 wk in humans (14,37). An androgen-sensitive time window has been well documented by either antiandrogen or androgen exposure experiments in pregnant animals. According to such experiments, the androgen-sensitive window of sexual differentiation is indicated to be several days earlier than the morphologically dimorphic stages at E15-E19 in the rat and at 8–14 wk in humans (15). The current study identified the critical time point for the onset of sexually dimorphic development in mice as E15.5. The phenotype of the current genetically modified mice that were treated with TM at E13.5 showed sexual disorders.
A reciprocal interaction between the epithelium and mesenchyme is thought to be essential for urogenital organ development. For instance, mesenchymally expressed AR is required for the androgen-dependent epithelial cell differentiation in the prostate, another representative androgen target organ (38). In a reciprocal fashion, the epithelium is required for the differentiation and spatial organization of the smooth muscle in the prostate (39,40). Previous tissue graft experiments demonstrated the inductive effects of the epithelium on mesenchymal growth and differentiation in the rat and mouse GTs (17,28). However, the region-specific androgen function for GT masculinization has never been analyzed. In the current conditional ARKO mice study, the epithelially or mesenchymally specific functions of AR were genetically examined for the first time during GT development. AR was significantly expressed in the mesenchyme adjacent to the UPE, whereas such prominent AR expression was not detected in the mesenchymal ARKO mice. The current results demonstrate that the proper mesenchymal androgen signaling through its receptor is necessary for GT masculinization.
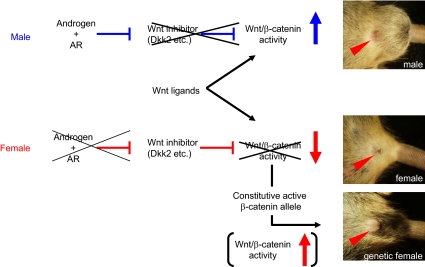
The androgen controls the GT masculinization hormonally during normal embryogenesis. In addition to androgen signaling through mesenchymally expressed AR, Wnt/β-catenin signaling in the mesenchyme is also essential for GT masculinization. Growth factor pathways have barely been identified as the effectors for reproductive organ masculinization including the GTs. Embryonic growth factor signaling has been generally suggested as the essential embryonic developmental program. Hence, mutations in such pathways have been considered to potentially induce an early embryonic lethality, masking their roles in the masculinization process. The current study identified several Wnt inhibitory genes that were decreasingly expressed in the male GTs in comparison with those in female GTs. Accompanying the decreased level of Wnt inhibitors, the β-catenin expression and BatGAL activity in the mesenchyme adjacent to the UPE was increased in the male GTs. Although sexually dimorphic expression of Wnt gene ligands and R-spondins was not detected in the GTs, it is possible that sexually dimorphic Wnt/β-catenin activity is elicited by combinational action of several Wnt gene ligands and their possible antagonists. In addition, loss- and gain-of-function studies for β-catenin demonstrated impaired sexual differentiation of the GTs. Of note is the fact that an activated β-catenin conditional mutant allele led to enlarged external genitalia in the XX female adult mice. These findings thus demonstrate the newly identified function of the Wnt/β-catenin signaling for embryonic masculinization, potentially functioning as a downstream effector of the androgen signaling.
It has been suggested that nuclear accumulation of β-catenin can be enhanced upon interacting with AR. Multiple findings have suggested an interaction between β-catenin and the AR, which is enhanced by the presence of an AR agonist (41,42). Upon interacting with AR, β-catenin can translocate to the nucleus and affect AR-mediated transcriptional activities. In addition to the canonical β-catenin/Tcf/Lef pathway, it is thus also possible that β-catenin functions as a coactivator for AR enhancing GT masculinization. Further experiments are necessary to investigate such possibilities of β-catenin functions.
Considering the high incidence and an increasing prevalence of disorders of external genital development at birth, an understanding of their causative mechanisms and normal developmental process is vital in medical genetics. Effectors that mediate androgen signaling have not been identified so far. Various KO mouse studies demonstrated that defects in the initial outgrowth and patterning during the early morphogenesis stage induce severe lower (ventral) urethral groove defects or agenesis of the external genitalia (12). Generally, these defects can occur before androgen signaling genes are expressed. The current results demonstrate that abnormalities in Wnt/β-catenin signaling can not only affect early GT morphogenesis but also induce the embryonic masculinization disorders. A loss- and gain-of-function mutant mice for β-catenin showed early developmental anomalies, demonstrating a requirement of β-catenin during the early phase of GT development (25). However, it was previously not possible to analyze the roles for sexual development of the GTs. The current study took advantage of the TM-inducible Cre system and clearly demonstrated an essential role of β-catenin as a masculine effector of the GTs for the first time. Intriguingly, in the gonad, activation of β-catenin results in male-to-female sex reversal, suggesting that Wnt/β-catenin signaling is involved in the female pathway (43). Mice lacking Wnt4 or R-spondin1 genes exhibit a female-to-male sex-reversal in the gonads (44,45,46). Thus, β-catenin locates in the downstream of these genes (43). Hence, Wnt/β-catenin signaling is involved in sexual differentiation in a developmental context-dependent manner with different activities for male and female pathways.
The goal of the current study was to obtain a better understanding of the orchestrated signal networks essential for external genital masculinization. Little information about the integration of the signaling networks has been available for external genital masculinization, to date. The study provided perspectives for normal reproductive biology and is also important for understanding the causative factors of the genital disorders. In summary, it is suggested that male GT development is regulated by Wnt/β-catenin signaling associated with the decreased expression levels of Wnt inhibitory genes, such as Dkk2 and Sfrp1. Wnt/β-catenin pathway exhibited thus sexually dimorphic activities, and their loss- and gain-of-function mutants displayed altered GT sexual development. These data suggest that the Wnt/β-catenin pathway can be a downstream effector of androgen signaling and an essential effector for GT masculinization (Fig. 7). The current results demonstrate that Wnt/β-catenin signaling is necessary for the orchestrated genital development and the masculinization of the external genitalia. To our knowledge, this could be the first genetic study analyzing the roles of the genetic interactions between androgen and locally expressed growth factor signaling during the development of reproductive organs.
Figure 7.
A schematic diagram of the possible signaling cross talk among androgen and Wnt/β-catenin signaling for GT masculinization.
Materials and Methods
Mouse and hormone treatments
The mutant alleles used herein were ARlox (7), β-cateninloxEx3 (35), β-cateninlox (34), K5-Cre (30), Gli1CreERT2 (47), ShhCreERT2 (48), R26R (49), and BatGAL (33). All experimental procedures and protocols were approved by the Committee on the Animal Research at the Kumamoto University. Embryos for each experiment were collected from more than three independent pregnant females. Statistical differences among the experimental groups were assessed by Fisher’s exact probability test, and P < 0.05 was considered as statistically significant differences. Noon on the day when a vaginal plug was detected was designated as E0.5.
The TM-inducible Cre recombinase system removes the floxed sequence of the target genome (50). TM (Sigma Chemical Co., St. Louis, MO) was dissolved in sesame oil (Kanto Chemical, Tokyo, Japan) at a final concentration of 10 mg/ml. Four milligrams of TM per 40 g body weight was administered (ip) to the pregnant mice. For the Gli1-CreERT2, β-cateninloxEx3 mice, 2 mg TM per 40 g body weight was administered. Under these conditions, no overt teratological effects or sexual disorders of the reproductive organs were observed (29). To determine the critical time point of sexual differentiation of the GTs, pregnant C57BL/6 mice (CLEA, Tokyo, Japan) were given (ip) two successive injections of 100 mg/kg body weight flutamide or testosterone propionate (Sigma) dissolved in sesame oil.
Histology, X-gal staining, and immunohistochemistry
Hematoxylin and eosin staining and X-gal staining were performed by standard procedures as previously described (29). For immunohistochemistry, mouse embryos were fixed in 4% paraformaldehyde and embedded in paraffin. Deparaffinized sections were treated for antigen retrieval (microwave treatment 10 min in citrate buffer, pH 6.0) and incubated with 0.3% H2O2 in methanol for 10 min to eliminate endogenous peroxidases. The sections were incubated at a 1:200 dilution of anti-AR antibody (N-20; Santa Cruz Biotechnology, Santa Cruz, CA) and a 1:1000 dilution of anti-β-catenin antibody (clone 14; BD Biosciences, Franklin Lakes, NJ). After washing with PBS, the sections were stained with the Vectastain ABC Kit (Vector Laboratories, Burlingame, CA).
In situ hybridization for gene expression analysis
For in situ hybridization, paraformaldehyde-fixed, paraffin-embedded sections were deparaffinized, rehydrated, incubated in 1 μg/ml proteinase K for 7 min at 37 C, and refixed with 4% paraformaldehyde for 10 min at room temperature. After washing in PBS containing 0.1% Tween 20, overnight hybridization was performed in a buffer (50% formamide, 5× saline sodium citrate, 50 μg/ml yeast tRNA, 1% sodium dodecyl sulfate, 50 μg/ml heparin) with 1 μg/ml probe at 68 C. The slides were washed in 5× saline sodium citrate and 50% formamide for 1 h at 68 C, TBST buffer (140 mm NaCl, 2.7 mm KCl, 0.1% Tween 20, and 25 mm Tris-HCl, pH 7.5) for 5 min at room temperature before incubating for 2 h with blocking solution (10% blocking reagent (Roche, Mannheim, Germany) in 100 mm maleate buffer and TBST). Anti-digoxigenin antibody (Roche) in a blocking solution was added to the slides and incubated for 1 h. After washing with TBST, the sections were equilibrated in NTMT buffer (100 mm NaCl; 50 mm MgCl2; 0.1% Tween 20; and 100 mm Tris-HCl, pH 9.5) including 2 mm levamisole (Sigma) and incubated in color solution containing 3.5 μg nitroblue tetrazolium (Roche) and 1.75 μg 5-bromo-4-chloro-3-indolyl phosphate (Roche) per milliliter of NTMT buffer.
The template used in this study was kindly provided from Dr. Christof Niehrs (Dkk2). The template of Sfrp1 was obtained by standard RT-PCR procedures. The primer sequences were TTC TAC ACC AAG CCC CCG CAG and GAT GGG CCC CAG CTT CAA GG. The preparation of the digoxigenin-labeled probes was performed according to the manufacturer’s instructions (Roche).
Quantitative RT-PCR
The changes in gene expression were confirmed and quantified using the 7500 real-time PCR system (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. One microgram of total RNA, isolated with ISOGEN (Nippongene, Tokyo, Japan), was used in RT-PCR carried out with SuperScript III (Invitrogen, Carlsbad, CA) and SYBR Green master mix (Applied Biosystems). RNA from each group was purified from the mesenchyme adjacent to the UPE of the GTs (blue area shown in Fig. 3C). The relative RNA equivalents for each sample were determined by standardization with the ribosomal protein L8 levels. More than three pools of samples per group were tested in triplicate, and the average relative RNA equivalents per sample were used for further analysis. Error bars represent se. The statistical comparisons among the experimental groups were assessed by ANOVA. When F ratios were significant (P < 0.05), Scheffe post hoc tests between two groups were done, and P < 0.05 was considered as statistically significant differences. The primer sequences were as follows: Dkk2, TGT CTG AAG CAC AGG CTG GAT and CTT CTG GAG CCT CTG ATG GC; Sfrp1, AAG GAG AGG CAG AAT CCT TTC A and TTT CCA AAC CGG CCA ACA; and ribosomal protein L8, ACA GAG CCG TTG TTG GTG TTG and CAG CAG TTC CTC TTT GCC TTG T.
Acknowledgments
We thank Drs. A. Joyner, P. Chambon, A. McMahon, W. Birchmeier, T. Yamaguchi, C. Niehrs, H. Westphal, M. Renfree, A. Moon, S. Oottanmasathien, C. Mendelsohn, J. Takeda, T. Tsukiyama, S. Ohta, and L. Ma for materials and suggestions. We also express our appreciation T. Tanaka, C. Inoue, A. Omori, A. Murashima, K. Tanaka, C. Nakahara, H. Nishida, and S. Kitagawa for their assistance.
Footnotes
This work was supported by Grant-in-Aid for Young Scientists B (19790153), Grant-in-Aid for Scientific Research B, Scientific Research on Priority Areas (Mechanisms of Sex Differentiation and General promotion of Cancer research in Japan), and the global COE program Cell Fate Regulation Research and Education Unit from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and a grant for Child Health and Development (17-2, 20-3) and Health Sciences Research Grant from the Ministry of Health, Labor, and Welfare, Japan. This work was also supported by National Institutes of Health Grant R01-ES016597-01A1.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 12, 2009
Abbreviations: AR, Androgen receptor; E, embryonic day; GT, genital tubercle; KO, knockout; TM, tamoxifen; UPE, urethral plate epithelium.
References
- Jost A 1953 Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Recent Prog Horm Res 8:379–418 [DOI] [PubMed] [Google Scholar]
- Baskin LS, Himes K, Colborn T 2001 Hypospadias and endocrine disruption: is there a connection? Environ Health Perspect 109:1175–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisen KA, Chellakooty M, Schmidt IM, Kai CM, Damgaard IN, Suomi AM, Toppari J, Skakkebaek NE, Main KM 2005 Hypospadias in a cohort of 1072 Danish newborn boys: prevalence and relationship to placental weight, anthropometrical measurements at birth, and reproductive hormone levels at three months of age. J Clin Endocrinol Metab 90:4041–4046 [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM 2001 Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 16:972–978 [DOI] [PubMed] [Google Scholar]
- Clark AM, Garland KK, Russell LD 2000 Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod 63:1825–1838 [DOI] [PubMed] [Google Scholar]
- Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, Metzger J, Schweigert FJ, Luppa PB, Nykjaer A, Willnow TE 2005 Role of endocytosis in cellular uptake of sex steroids. Cell 122:751–762 [DOI] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, Nakamura T, Yoshimura K, Yoshizawa T, Metzger D, Chambon P, Kato S 2004 Brain masculinization requires androgen receptor function. Proc Natl Acad Sci USA 101:1673–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada G, Satoh Y, Baskin LS, Cunha GR 2003 Cellular and molecular mechanisms of development of the external genitalia. Differentiation 71:445–460 [DOI] [PubMed] [Google Scholar]
- Yong W, Yang Z, Periyasamy S, Chen H, Yucel S, Li W, Lin LY, Wolf IM, Cohn MJ, Baskin LS, Sánchez ER, Shou W 2007 Essential role for co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J Biol Chem 282:5026–5036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JE, Leshin M, Wilson JD 1982 Androgen resistance syndromes. Am J Physiol 243:E81–E87 [DOI] [PubMed] [Google Scholar]
- Wilson JD 1992 Syndromes of androgen resistance. Biol Reprod 46:168–173 [DOI] [PubMed] [Google Scholar]
- Yamada G, Suzuki K, Haraguchi R, Miyagawa S, Satoh Y, Kamimura M, Nakagata N, Kataoka H, Kuroiwa A, Chen Y 2006 Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Dev Dyn 235:1738–1752 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Baker PJ, Johnston H 2006 The foetal Leydig cell: differentiation, function and regulation. Int J Androl 29:90–95; discussion 105–108 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Ogino Y, Murakami R, Satoh Y, Bachiller D, Yamada G 2002 Embryonic development of mouse external genitalia: insights into a unique mode of organogenesis. Evol Dev 4:133–141 [DOI] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM 2008 Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest 118:1479–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzrock EA, Baskin LS, Cunha GR 1999 Ontogeny of the male urethra: theory of endodermal differentiation. Differentiation 64:115–122 [DOI] [PubMed] [Google Scholar]
- Kurzrock EA, Baskin LS, Li Y, Cunha GR 1999 Epithelial-mesenchymal interactions in development of the mouse fetal genital tubercle. Cells Tissues Organs 164:125–130 [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G 2001 Unique functions of Sonic hedgehog signaling during external genitalia development. Development 128:4241–4250 [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Suzuki K, Murakami R, Sakai M, Kamikawa M, Kengaku M, Sekine K, Kawano H, Kato S, Ueno N, Yamada G 2000 Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development 127:2471–2479 [DOI] [PubMed] [Google Scholar]
- Morgan EA, Nguyen SB, Scott V, Stadler HS 2003 Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development 130:3095–3109 [DOI] [PubMed] [Google Scholar]
- Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ 2002 Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol 247:26–46 [DOI] [PubMed] [Google Scholar]
- Petiot A, Perriton CL, Dickson C, Cohn MJ 2005 Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development 132:2441–2450 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Bachiller D, Chen YP, Kamikawa M, Ogi H, Haraguchi R, Ogino Y, Minami Y, Mishina Y, Ahn K, Crenshaw 3rd EB, Yamada G 2003 Regulation of outgrowth and apoptosis for the terminal appendage: external genitalia development by concerted actions of BMP signaling. Development [Erratum (2003) 130:6643] 130:6209–6220 [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S 1999 A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126:1211–1223 [DOI] [PubMed] [Google Scholar]
- Lin C, Yin Y, Long F, Ma L 2008 Tissue-specific requirements of β-catenin in external genitalia development. Development 135:2815–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Putz O 2008 Molecular signaling pathways that regulate prostate gland development. Differentiation 76:641–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JD, Griffin JE, George FW, Leshin M 1983 The endocrine control of male phenotypic development. Aust J Biol Sci 36:101–128 [DOI] [PubMed] [Google Scholar]
- Murakami R, Mizuno T 1986 Proximal-distal sequence of development of the skeletal tissues in the penis of rat and the inductive effect of epithelium. J Embryol Exp Morphol 92:133–143 [PubMed] [Google Scholar]
- Haraguchi R, Motoyama J, Sasaki H, Satoh Y, Miyagawa S, Nakagata N, Moon A, Yamada G 2007 Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development 134:525–533 [DOI] [PubMed] [Google Scholar]
- Tarutani M, Itami S, Okabe M, Ikawa M, Tezuka T, Yoshikawa K, Kinoshita T, Takeda J 1997 Tissue-specific knockout of the mouse Pig-a gene reveals important roles for GPI-anchored proteins in skin development. Proc Natl Acad Sci USA 94:7400–7405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Harfe BD, Cohn MJ 2008 Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol 318:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H, Miyagawa S, Matsumaru D, Wada Y, Satoh Y, Ogino Y, Fukuda S, Iguchi T, Yamada G 2008 Gene expression analyses on embryonic external genitalia: identification of regulatory genes possibly involved in masculinization processes. Congenit Anom (Kyoto) 48:63–67 [DOI] [PubMed] [Google Scholar]
- Nakaya MA, Biris K, Tsukiyama T, Jaime S, Rawls JA, Yamaguchi TP 2005 Wnt3a links left-right determination with segmentation and anteroposterior axis elongation. Development 132:5425–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W 2001 β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105:533–545 [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM 1999 Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J 18:5931–5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel S, Cavalcanti AG, Desouza A, Wang Z, Baskin LS 2003 The effect of oestrogen and testosterone on the urethral seam of the developing male mouse genital tubercle. BJU Int 92:1016–1021 [DOI] [PubMed] [Google Scholar]
- Reyes FI, Winter JS, Faiman C 1973 Studies on human sexual development. I. Fetal gonadal and adrenal sex steroids. J Clin Endocrinol Metab 37:74–78 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Chung LW, Shannon JM, Reese BA 1980 Stromal-epithelial interactions in sex differentiation. Biol Reprod 22:19–42 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Battle E, Young P, Brody J, Donjacour A, Hayashi N, Kinbara H 1992 Role of epithelial-mesenchymal interactions in the differentiation and spatial organization of visceral smooth muscle. Epithelial Cell Biol 1:76–83 [PubMed] [Google Scholar]
- Hayward SW, Haughney PC, Rosen MA, Greulich KM, Weier HU, Dahiya R, Cunha GR 1998 Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation 63:131–140 [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC 2005 Interaction of nuclear receptors with the Wnt/β-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev 26:898–915 [DOI] [PubMed] [Google Scholar]
- Verras M, Sun Z 2006 Roles and regulation of Wnt signaling and β-catenin in prostate cancer. Cancer Lett 237:22–32 [DOI] [PubMed] [Google Scholar]
- Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B 2008 Stabilization of β-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet 17:2949–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC 2008 Activation of β-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet 17:1264–1277 [DOI] [PubMed] [Google Scholar]
- Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, Oshima T, Kakitani M 2008 R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet 17:1278–1291 [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP 1999 Female development in mammals is regulated by Wnt-4 signalling. Nature 397:405–409 [DOI] [PubMed] [Google Scholar]
- Ahn S, Joyner AL 2004 Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell 118:505–516 [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ 2004 Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118:517–528 [DOI] [PubMed] [Google Scholar]
- Soriano P 1999 Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71 [DOI] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P 1997 Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun 237:752–757 [DOI] [PubMed] [Google Scholar]