Abstract
In the present study, we demonstrate that elevated levels of the progesterone receptor (PR)-B isoform in breast cancer cells induces down-regulation of estrogen receptor (ER) α mRNA and protein content, causing concomitant repression of the estrogen-regulated genes insulin receptor substrate 1, cyclin D1, and pS2, addressing a specific effect of PR/PR-B on ERα gene transcription. ERα gene promoter activity was drastically inhibited by PR-B overexpression. Promoter analysis revealed a transcriptionally responsive region containing a half-progesterone response element (PRE) site located at −1757 bp to −1752 bp. Mutation of the half-PRE down-regulated the effect induced by PR/PR-B overexpression. Moreover chromatin immunoprecipitation analyses revealed an increase of PR bound to the ERα-regulatory region encompassing the half-PRE site, and the recruitment of a corepressor complex containing nuclear receptor corepressor (NCoR) but not silencing mediator of retinoid and thyroid hormone receptor and DAX1, concomitantly with hypoacetylation of histone H4 and displacement of RNA polymerase II. Furthermore, NCoR ablation studies demonstrated the crucial involvement of NCoR in the down-regulatory effects due to PR-B overexpression on ERα protein and mRNA. We also demonstrated that the ERα regulation observed in MCF-7 cells depended on PR-B expression because PR-B knockdown partially abrogates the feedback inhibition of ERα levels after estrogenic stimulus. Our study provides evidence for a mechanism by which overexpressed PR-B is able to actively repress ERα gene expression.
Estradiol induced up-regulation of progesterone receptor B plays a crucial role in the persistent decrease of estrogen receptor α in breast cancer cells.
The sex steroid hormones, estradiol and progesterone (Pg), play important roles in normal mammary gland development, and it is thought that breast cancer progression is influenced by them and their receptors (1,2). The level of these steroid hormone receptors is a prognostic factor for patients with breast cancer and has been used in clinical management as an indicator of endocrine responsiveness (3,4). Although it is well accepted that enhanced expression of estrogen receptor α (ERα) is an early event in breast carcinogenesis, the role of progesterone receptor (PR) has been more controversial. Recent studies published on the largest retrospective analysis of early breast cancer treated with tamoxifen found that patients with ER+/PR+ tumors derived more benefit from adjuvant tamoxifen therapy than those patients with ER+/PR− tumors (5). Importantly, in multivariate analyses including lymph node involvement, tumor size, and age, PR status was independently associated with disease-free and overall survival (6).
PRs belong to the subfamily of classical nuclear steroid receptors, and human PR proteins exist as two isoforms, termed PR-A and PR-B, that are transcribed from a single gene under the control of separate promoters (7). Despite structural similarities, PR-A and PR-B regulate different subsets of genes and, although PR-B is transcriptionally more active, there are genes, known to be involved in breast cancer progression, that are uniquely regulated by the PR-A isoform (8,9). In vivo the two PR isoforms are usually coexpressed at similar levels in normal cells, but their ratio varies dramatically in different tissues, in varying physiological states, and disease sites (10,11). With regard to the mammary gland, 3:1 overexpression of PR-A over PR-B in transgenic mice results in extensive epithelial cell hyperplasia, excessive ductual branching, and disorganized basement membrane, all features associated with neoplasia. In contrast, overexpression of PR-B leads to premature ductal growth arrest and inadequate lobulo-alveolar differentiation (12,13). Moreover the loss of coordinated PR-A and PR-B expression is thought to be an early event in carcinogenesis and is evident in premalignant breast lesions (14). A significant proportion of carcinomas express a predominance of the PR-A isoform, and elevated PR-A has been associated with poor clinical outcomes in endometrial cancer, indicating a direct association between PR-A isoform predominance and poor prognosis (15).
Although ER and PR are members of different steroid hormone receptor subfamilies and recognize distinct hormone response elements, there is considerable biological evidence for cross talk between their receptor-signaling pathways. For instance, progestins can suppress the stimulatory effects of estrogens in target cells; estrogen increases the expression of both c-fos and PR mRNA in uterine cells, and progestins block these effects (16,17). This blockade appears to be mediated via the PRs, but it is unclear whether ER or some other component of the estrogen-ER signaling pathway is the target for repression. It is also known that liganded PRs can suppress E2-stimulated ER activity, with the magnitude of repression dependent on the PR isoform, progestin ligand, promoter, and cell type (18,19). The exact molecular mechanisms regulating ERα expression in breast tumors are unclear, but studies suggest that they are partly at the level of transcription (20).
In the present study we examined whether alterations in the PR-B to PR-A ratio could affect response to E2 in ERα-positive breast cancer cells. We demonstrate that PR-B overexpression down-regulates ERα mRNA, protein content, and gene promoter activity, whereas PR-A isoform overexpression does not elicit these effects. Investigation of the ERα gene promoter nucleotide sequence, along with site-directed mutagenesis of a half-PRE region in the ERα promoter, and chromatin immunoprecipitation demonstrate that PR-B is able to negatively regulate expression of ERα by the recruitment of a corepressor transcriptional complex containing NCoR, but not silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) and DAX1, causing hypoacetylation of histone H4, resulting in displacement of RNA polymerase II. Furthermore NCoR knockdown completely abrogated the down-regulatory effects on ERα protein and mRNA levels due to PR-B overexpression. PR-B ablation studies using small interfering RNA further demonstrated that endogenous PR-B levels are determinant in regulating ERα.
Results
Pg acting through PR-B decreases E2-induced cell proliferation in breast cancer cells
Reports about the effects of progestins on cell proliferation are contradictory, and there is debate about their actions in human breast tissue (21). In many instances, progestins can either inhibit or stimulate the growth of breast cancer cells (22).
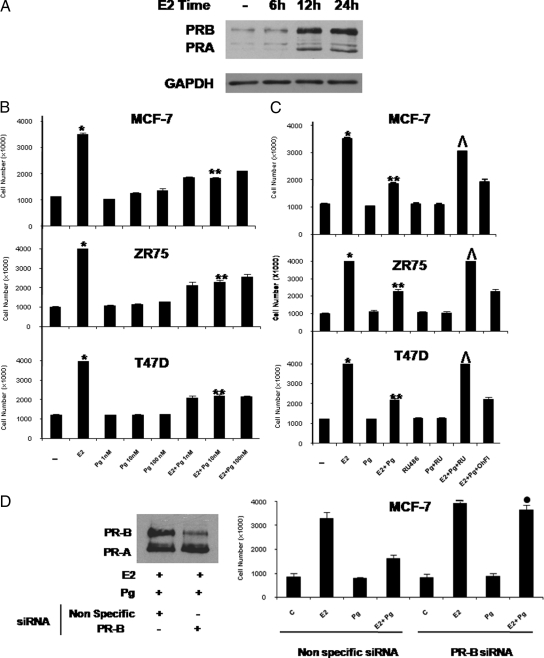
To examine whether progestins affect estrogen-stimulated cell proliferation, we evaluated the effects of prolonged exposure to different concentrations of Pg (1 nm, 10 nm, 100 nm) on E2-stimulated proliferation of breast cancer cells. As expected after 6 d of E2 treatment, the numbers of MCF-7 cells, which express low levels of PR (Fig. 1A) were significantly increased, and Pg alone at all concentrations tested had no significant effect compared with the control experimental conditions. However Pg treatment at 10 nm caused the maximal inhibitory effects (48%) on the E2-induced cell proliferation (Fig. 1B). Similar results were obtained in ZR75, which express low levels of PR, and in T47D cells, which are known to express lower levels of ER but elevated levels of endogenous PR (23).
Figure 1.
Pg decreases the E2-induced cell proliferation in breast cancer cells. A, Immunoblot analysis of PR. MCF-7 cells were treated with vehicle (−) or 10 nm E2 at different time as indicated; GAPDH was used as loading control. Results are representative of three independent experiments. B, MCF-7, ZR75, and T47D cells were treated with vehicle (−) or 10 nm E2 and/or increasing amount of Pg (1 nm, 10 nm, 100 nm) in medium containing 5% charcoal-stripped fetal bovine serum (medium was refreshed and treatments were renewed every 2 d) and counted on d 6. C, MCF-7, ZR75, and T47D cells cultured in the experimental conditions described in panel B were also treated with vehicle (−) or 10 nm E2 and/or 10 nm Pg in combination with 1 μm RU or 1 μm OHFl and counted on d 6. D, MCF-7 cells were transfected with nonspecific siRNA or targeted against PR-B and cultured in the experimental conditions described in panel B. Columns indicate mean of three independent experiments done in triplicate; bars represent sd; *, P < 0.001 compared with vehicle; **, P < 0.001 compared with E2; ^, P < 0.001 compared with E2+Pg; •, P < 0.001 vs. cells treated with E2+Pg transfected with nonspecific siRNA.
RU 486 is an antagonist of progestin action in human breast cancer cells, and it is known to bind with high affinity to PR (24). Under our control experimental conditions, 1 μm RU 486 did not affect the growth of MCF-7, ZR75, and T47D cells (Fig. 1C). However, the combination of 10 nm E2, 10 nm Pg, and 1 μm RU486 significantly reversed the inhibitory effects of Pg on E2-induced cell proliferation, suggesting that the inhibitory action was indeed mediated by PR.
It is largely documented that, in addition to their progestational effects, progestins, depending on dosage and tissue site, can also bind to the androgen receptor (AR) (25), which was also been described to antagonize ERα signaling in breast cancer (26). It is interesting to note that under our experimental conditions 1 μm OH-Fl, an AR antagonist (27), did not modify the down-regulatory response of Pg on E2 stimulated proliferation, indicating that AR was not involved in these effects.
To investigate the role of the endogenous PR isoforms in mediating the Pg-inhibitory effects, MCF-7 cells were transfected with an oligo PR-B small interfering RNA (siRNA). As shown in Fig. 1D, specific PR-B knockdown reversed the inhibitory effect on E2-induced cell proliferation produced by Pg cotreatment, demonstrating that this down-regulatory action is mediated by the endogenous PR-B isoform.
PR-B isoform overexpression represses the ERα transcriptional activity
The inhibitory action of Pg on breast cancer cell proliferation described in Fig. 1, required a priming treatment with E2 to induce elevated levels of both PR isoforms, which despite known structural similarities have markedly different transcriptional effects on progestin-responsive promoters (28,29,30).
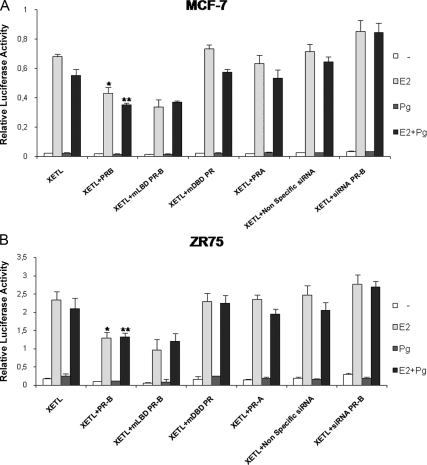
To study whether changes in the relative expression levels of PR-B and -A isoforms could affect E2-induced signaling, and to identify the specific PR isoform involved in such modulatory action, we analyzed the effects of PR-A or PR-B overexpression on genomic activity induced by E2 treatment. To this aim a luciferase reporter plasmid containing a consensus estrogen-responsive element (ERE) sequence from the Xenopus vitellogenin promoter (XETL) was transiently cotransfected into MCF-7 and ZR75 cells, in the presence or absence of expression plasmids encoding either full-length PR-A or PR-B isoforms. Cells were treated with 10 nm E2 and/or 10 nm Pg, as indicated (Fig. 2, A and B) for 18 h. Treatment with E2 alone resulted, as expected, in a substantial increase in luciferase activity which was partially inhibited in the presence of transient overexpression of PR-B. Similar inhibition was seen in the presence of PR-B with a truncated ligand-binding domain (LBD) (PR-B 1-675 called mLBD PR-B), demonstrating that the inhibitory action of PR-B does not require ligand binding, whereas estrogen-induced transcriptional activity was not substantially affected in the presence of PR-A overexpression. A PR mutant with a disrupted DNA-binding domain (DBD) (Cys587 to Ala, called mDBD PR) was unable to induce any inhibitory effects on E2-mediated signaling, suggesting that PR-B effects occurred at the transcriptional level.
Figure 2.
PR-B overexpression down-regulates the E2-induced signal. ERE luciferase reporter assay. MCF-7 (panel A) and ZR75 (panel B) cells were transiently cotransfected with XETL in the presence or absence of full-length PR-B or mLBD PR or mDBD PR or PR-A expression plasmid, nonspecific siRNA, or targeted against PR-B. After transfection cells were treated with vehicle (−) or 10 nm E2 and/or 10 nm Pg, harvested after 18 h, and then luciferase activities were determined. Columns indicate mean of luciferase activities observed in three independent experiments; bars represent sd; *, P < 0.001 compared with XETL+E2; **, P < 0.05 compared with XETL+E2+Pg.
To investigate the role of the endogenous PR-B isoform in mediating such inhibitory action, cells were also transfected with an oligo PR-B siRNA. As shown in Fig. 2, the specific PR-B knockdown caused a relative increase on E2-induced XETL reporter activity.
PR-B overexpression down-regulates ERα protein and mRNA
After demonstrating the inhibitory effects of PR-B overexpression on the ER-transcriptional activity, we next examined whether this action might be due to a decrease in the expression levels of ERα protein.
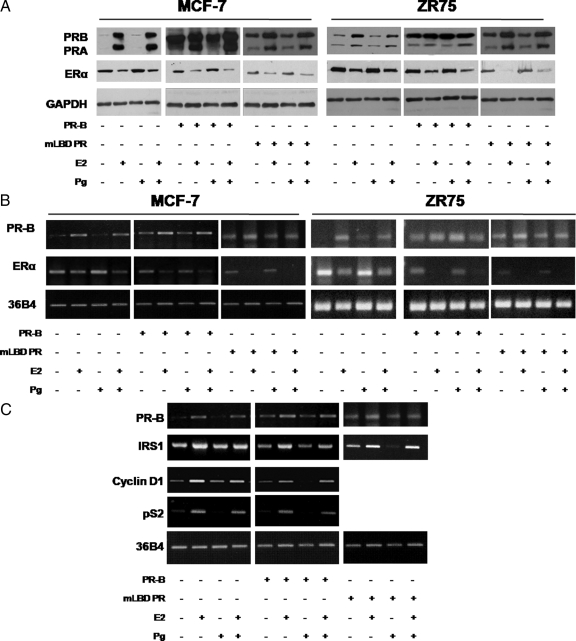
Previous studies provide strong evidence that ERα expression in breast cancer is regulated at both the transcriptional level by initially decreasing transcription of ERα and later via destabilization of ER mRNA (31,32), as well as by posttranslational mechanisms (19,20). Down-regulation of ERα expression in the presence of its own ligand is the feature of its transactivation; indeed, immunoblot analysis of lysates from MCF-7 and ZR75cells treated with 10 nm E2 and/or 10 nm Pg for 24 h showed that E2 treatment repressed the ERα expression levels whereas Pg alone had no effect (Fig. 3A). In contrast, however, PR-B over-expression caused a marked reduction in ERα content under control experimental conditions in the absence of treatment, as well as after ligand (E2 or Pg) exposures in both MCF-7 and ZR75 cells, and this inhibitory effect was still evident in cells transiently overexpressing mLBD PR-B.
Figure 3.
PR-B overexpression decreases ERα protein, ERα mRNA, and estrogen-regulated genes mRNA. A, Immunoblot analysis of PR and ERα. MCF-7, ZR75, and cells transient overexpressing PR-B or mLBD PR were treated with vehicle (−) or 10 nm E2 and/or 10 nm Pg for 24 h; GAPDH was used as a loading control. Columns, represent mean of three separate experiments in which the band intensities were evaluated in terms of optical density arbitrary units and expressed as the percentage of the control assumed as 100%; bars represent sd. B, RT-PCR assay. mRNA expression of PR-B and ERα in MCF-7, ZR75, and cells transiently overexpressing PR-B or mLBD PR treated with vehicle (−) or 10 nm E2 and/or 10 nm Pg for 24 h; the housekeeping gene 36B4 was determined as a control. Columns indicate mean of three separate experiments; bars, represent sd. C, RT-PCR assay. mRNA expression of PR-B, IRS-1, cyclin D1, and pS2 in MCF-7 cells and MCF-7 transiently overexpressing PR-B or mLBD PR treated with vehicle (−) or 10 nm E2 and/or 10 nm Pg for 24 h; the housekeeping gene 36B4 was determined as a control. Columns indicate mean of three separate experiments; bars, represent sd.
To further investigate the molecular basis of PR-B-mediated regulation of ERα expression, we also examined the effects of PR-B overexpression on ERα mRNA levels in MCF-7 and ZR75 cells treated for 24 h with 10 nm E2 and/or 10 nm Pg. As shown in Fig. 3B and consistent with previous studies (33), E2 caused a decrease in the steady state level of ERα mRNA. We also found that PR-B or mLBD PR-B overexpression further decreased ERα mRNA levels. These results suggest that PR-B overexpression affects ERα signaling through direct effects on ERα content in a ligand-independent manner.
PR-B overexpression decreases the levels of ERα-regulated genes in MCF-7 cells
The effects of E2 hormone are known to be mediated by ER through its gene-regulatory activities. To confirm whether Pg could inhibit the effect of E2 by modifying the transcriptional activity of ERα, the mRNA levels of known estrogen target genes, such as insulin receptor substrate 1 (IRS1), cyclin D1, and pS2, were studied (Fig. 3C). As expected, after 24 h treatment, 10 nm E2 markedly increased the mRNA levels of all three of these genes in MCF-7 cells after standardization with a housekeeping gene (ribosomal protein 36B4).
To further explore whether overexpression of PR-B could affect the expression of estrogen-regulated gene, plasmids encoding full-length PR-B were transiently transfected into MCF-7 cells. After 24 h of ligand treatment, cells were harvested and RT-PCR experiments were performed to compare mRNA levels for IRS1, cyclin D1, and pS2. As depicted in Fig. 3C, PR-B overexpression represses mRNA levels of all estrogen-regulated genes tested, in the control experimental conditions in absence of treatment as well as after exposure to ligands. The inhibitory action observed on IRS1 mRNA levels was still evident when we overexpressed a PR-B with a truncated LBD (mLBD PR-B).
Similar results were obtained when Western blotting analysis was performed in the same experimental conditions (data not shown).
Overexpressed PR-B mediates down-regulation of ERα via a region between −2769 bp to −1000 bp of its promoter
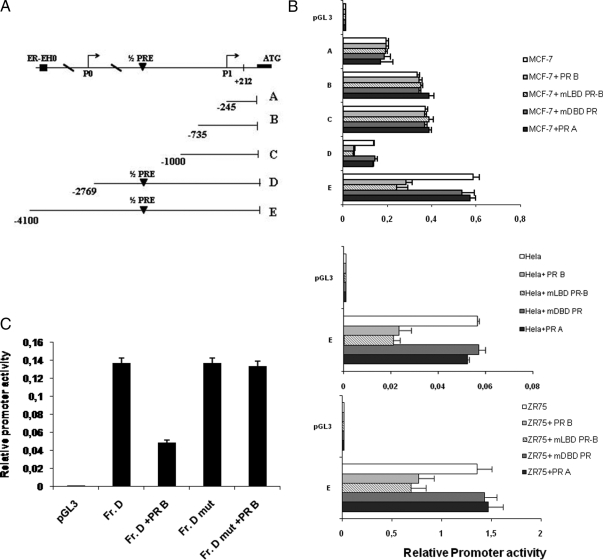
To analyze how PR-B interferes with ERα gene transcription, the ERα promoter was investigated first with a bioinformatics approach using the National Center for Biotechnology Information Genome data base (www.ncbi.nlm.nih.gov). The region examined in this study covered from −4100 bp to +212 bp, and a half-progesterone response element (PRE) (−1757 bp to −1752 bp) was identified as within this region.
To evaluate whether this region was a putative effector of PR action and involved in PR-mediated down-regulation of ERα expression, five overlapping ERα promoter deletion constructs, −245 bp to +212 bp (A), −735 bp to +212 bp (B), −1000 bp to +212 bp (C), −2769 bp to +212 bp (D), and −4100 bp to +212 bp (E), all relative to the first transcriptional ATG start site depicted in Fig. 4A, which had been subcloned into the luciferase reporter vector pGL3-basic, were analyzed in MCF-7 cells (34). Fragment A contains the region between −245 to −9 bp, known to posses low basal activity, as well as the two binding sites for AP2γ within the 5′-untranslated region (34). Fragment B extending to −735 bp includes a half-ERE at −420 bp. Fragment C includes two other half-EREs located at −860 and −888 bp, respectively. Fragments D and E include the P0 transcription start and the half-PRE that we identified in our NCBI genomic search; fragment E also contains the ER-EH0 enhancer (19). Plasmids containing these five ERα promoter fragments were transiently transfected into MCF-7 cells, and the data are shown as relative promoter activity in luciferase units.
Figure 4.
ERα promoter activity is down-regulated by PR-B overexpression, and mutation of the half-PRE abrogates this effect. A, Schematic representation of deletion fragments of the ERα gene promoter. Fragments coordinates are expressed relative to the primary transcription start site. B, Promoter activity of the ERα 5′-flanking region. Constructs depicted in panel A were transiently cotransfected in MCF-7, Hela, and ZR75 cells in the presence or absence of full-length PR-B or mLBD PR, mDBD PR, or PR-A expression plasmid. After 24 h, cells were harvested, and luciferase activities were determined. Columns indicate mean of luciferase activities observed in three independent experiments; bars represent sd. C, Site-directed mutagenesis of the half-PRE site present in the fragment D promoter construct. Fragment D and fragment D mut promoter constructs were cotransfected into MCF-7 cells, and promoter activity was assessed in the absence or presence of full-length PR-B expression plasmid after 24 h. Columns represent mean of luciferase activities observed in three independent experiments; bars indicate sd. Fr., Fragment.
Consistent with our previous studies (34), fragment A exhibited high levels of activity (Fig. 4B), the activities of fragments B and C were slightly increased relative to A, whereas the activity of fragment D decreased. The highest level of control promoter activity was seen with fragment E. We found that PR-B overexpression had no effect on the promoter activity of fragments A, B, and C, all which lack the half-PRE site. In contrast PR-B reduced the activity of fragments D and E by 64% and 50%, respectively, indicating that the region between −2769 bp to −1000 bp was responsible for PR-B-mediated down-regulation of ERα activity. Similar results were obtained cotransfecting equal amounts of expression plasmids encoding PR-B with a truncated LBD (mLBD PR-B) §while different results were observed after cotransfection of equal amounts of expression plasmids encoding either the PR-A isoform or the PR mutant with a disrupted DBD (mDBD PR, Fig. 4B). To confirm the findings obtained on activity of fragment E, the experiments were repeated in ZR75 and Hela cells, and similar results were obtained.
The presence of a functional half-PRE site within the ERα promoter has not been reported previously, and our results suggest a transcriptional cross talk mechanism between the two receptor networks at the level of the ERα promoter.
Site-directed mutagenesis reveals a role for the half-PRE in fragment D of the ERα promoter
To evaluate the role of the potential half-PRE site present within ERα gene promoter from −1757 bp to −1752 bp, we next used site-directed mutagenesis to alter this site. We changed 3 bp of the half-PRE to ensure that the altered binding site would not be recognizable by the PR (35). Transient transfections were performed in MCF-7 cells with multiple independent clones containing the desiderated mutation. Shown in a representative experiment, we found that the promoter activity of fragment D carrying the mutation in half-PRE site (Fr. D mut) was unaffected by PR-B overexpression (Fig. 4C). These results indicate that the half-PRE element in the ERα promoter is required for repression concomitant with PR-B overexpression.
The NCoR corepressor is recruited with PR-B to the ERα promoter
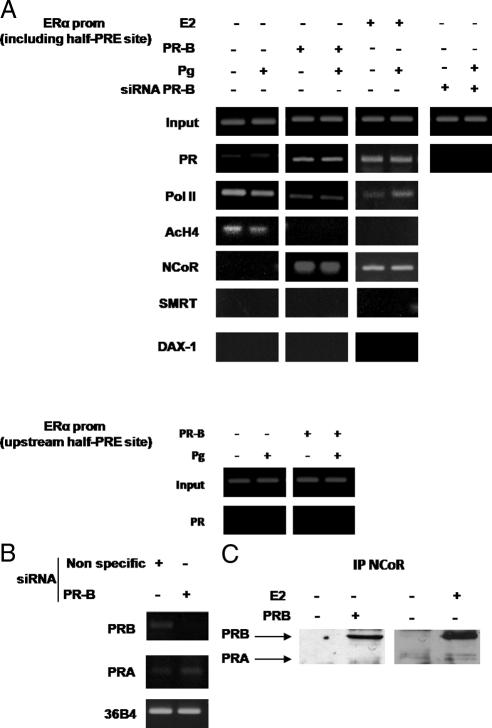
To demonstrate PR recruitment to the ERα gene promoter, we used ChIP assays. MCF-7 cells untreated or treated with E2 for 24 h to allow the expression of endogenous PR and MCF-7 cells ectopically overexpressing PR-B were exposed for 1 h with either control vehicle or 10 nm Pg, after which chromatin was cross-linked with formaldehyde, and protein-DNA complexes were immunoprecipitated with antibodies directed against PR, RNA polymerase II, acetyl histone H4-K16, or the corepressors NCoR, DAX-1, and SMRT. The PCR primers used in the ChIP assays encompass the half-PRE site we identified within the ERα promoter. Results obtained in MCF-7 cells overexpressing endogenous PR-B (treated with E2 for 24 h) or ectopic PR-B (Fig. 5A) demonstrate an enhanced recruitment of PR to the ERα promoter in the presence or absence of Pg treatment (upper panel); as a control we did not see recruitment to an unrelated ERα promoter region located upstream of the half-PRE site (lower panel).
Figure 5.
The NCoR corepressor is recruited with PR-B to the ERα promoter. A, ChIP was performed on the PR-responsive region of the ERα promoter, and various antibodies are used as indicated (upper panel). Nonspecific region upstream of the half-PRE site in the ERα promoter was tested as negative control (lower panel). MCF-7 transiently overexpressing PR-B cells or siRNA PR-B and MCF-7 cells treated for 24 h with vehicle (−) or 10 nm E2 were treated for 1 h with vehicle (−) or 10 nm Pg. Results are representative of three independent experiments. B, Knockdown of PR-B. MCF-7 cells were transfected with nonspecific siRNA or targeted against PR-B. RNA was isolated, and the expression of PR-B was analyzed by RT-PCR; the housekeeping gene 36B4 and PR-A were determined as controls. C, Coimmunoprecipitation analysis. Nuclear extracts were prepared from MCF-7 cells treated for 24 h with vehicle (−) or 10 nm E2 and from MCF-7 cells transiently overexpressing PR-B. Immunoprecipitation assay was performed using anti-NCoR antibody. Immunoprecipitated (IP) proteins were resolved and subjected to immunoblotting with anti-PR antibody. Results are representative of three independent experiments. prom, Promoter.
To determine which PR isoform interacts with this region of the ERα promoter, MCF-7 cells were also transfected with a specific oligo PR-B siRNA to achieve efficient knockdown of PR-B as shown in Fig. 5B. PR recruitment to the half-PRE site of the ERα promoter was strongly reduced by PR-B siRNA expression, confirming the specific recruitment of this isoform to the ERα promoter (Fig. 5A).
Concomitant with the increased recruitment of PR-B to the promoter, we saw that acetyl histone H4-K16 was not recruited and RNA polymerase II was displaced from the ERα promoter, indicating that the chromatin in this region is probably in a less permissive environment for gene transcription. Among the different PR corepressors previously described (36,37) and here tested, SMRT and DAX 1 were not detected under the examined experimental conditions. We only found NCoR to be recruited to the ERα promoter encompassing the half-PRE site, in a ligand-independent manner in MCF7 cells when endogenous or ectopic PR-B was overexpressed. These results highlight a possible new role of the NCoR corepressor in ERα regulation by PR-B.
To further assess whether endogenous NCoR could interact with PR, coimmunoprecipitation assays were performed. Nuclear extracts from control MCF-7 treated with E2 for 24 h, left untreated or transiently overexpressing PR-B, were coimmunoprecipitated with an anti-NCoR antibody and subjected to immunoblot analysis with an anti-PR antibody. As shown in Fig. 5C, a band of 114 kDa corresponding to PR-B was detected but only in MCF-7 cells overexpressing endogenous or ectopic PR-B.
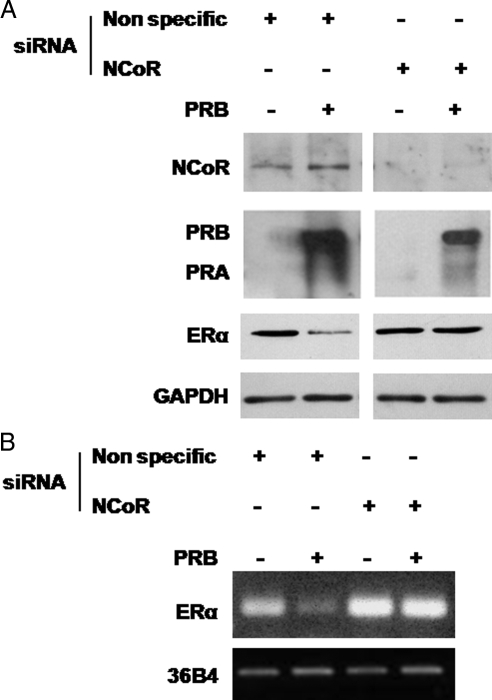
Because NCoR bound to PR-B may physically interfere with binding of RNA polymerase II to the ERα promoter resulting in the observed ERα down-regulation (38), we performed NCoR siRNA knockdown experiments in MCF-7 and in MCF-7 PR-B overexpressing cells in combination with immunoblot analysis. Figure 6A shows that NCoR protein levels were greatly decreased by specific siRNA treatment compared with a nonspecific siRNA; importantly, NCoR knockdown reversed the ERα protein down-regulation that is induced by PR-B overexpression. Similar results were obtained at mRNA levels (Fig. 6B).
Figure 6.
NCoR knockdown reverses the down-regulation of ERα protein levels and mRNA induced by PR-B overexpression. A, Immunoblot analysis of NCoR, PR, and ERα. MCF-7 and MCF-7 cells transient overexpressing PR-B were transfected with nonspecific siRNA or targeted against NCoR; GAPDH was used as control. Results are representative of three independent experiments. B, RT-PCR assay. mRNA expression of ERα in MCF-7 cells and MCF-7 cells transiently overexpressing PR-B, transfected with nonspecific siRNA, or targeted against NCoR; the housekeeping gene 36B4 was determined as a control. Results are representative of three independent experiments.
Endogenous PR-B modulates ERα protein levels in MCF-7 cells
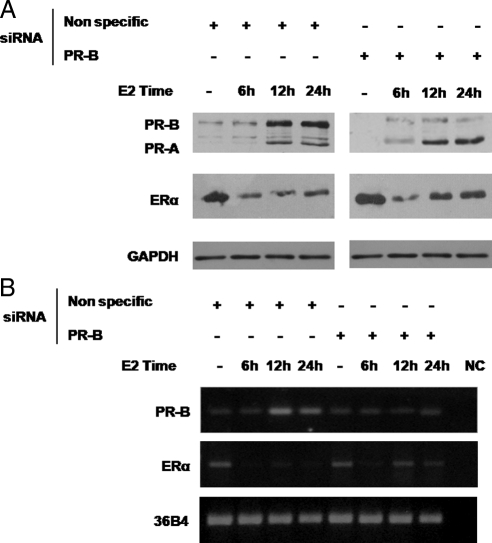
On the basis of all these findings we hypothesized that endogenous PR could be involved in ERα down-regulation upon E2 exposure. Therefore, we explored PR and ERα protein levels after 6 h, 12 h, and 24 h of E2 treatment in MCF-7 cells. As shown in Fig. 7A, ERα protein displayed a pattern of regulation consistent with previously published studies (20). Indeed, E2 caused a dramatic decrease in ERα levels starting from 6 h of treatment, and we observed an increase in PR expression after 12 h of treatment.
Figure 7.
PR-B knockdown partially reverses the down-regulation of ERα after E2 treatment. A, Immunoblot analysis of ERα and PR. MCF-7 cells were transfected with nonspecific siRNA or targeted against PR-B and treated with 10 nm E2 at different times as indicated; GAPDH was used as control. Results are representative of three independent experiments. B, RT-PCR assay. mRNA expression of PR-B and ERα. MCF-7 cells were transfected with nonspecific siRNA or targeted against PR-B and treated with 10 nm E2 at different times as indicated; the housekeeping gene 36B4 was determined as a control. Results are representative of three independent experiments. NC, Negative control.
To ascertain a specific role for PR-B enhancement by E2 on the maintenance of ERα down-regulation, we also performed PR-B siRNA knockdown experiments in MCF-7 cells following 6 h, 12 h, and 24 h of E2 treatment. PR-B knockdown partially reversed the down-regulation of ERα protein levels starting from 12 h until 24 h of E2 treatment whereas it appears not to affect the early down-regulatory effects. Similar results were obtained at mRNA levels (Fig. 7B). These results suggest that the early down-regulatory effects induced by E2 on its own receptor occur independently of PR-B action, whereas the latter plays a specific role after prolonged E2 exposure.
Discussion
Approximately 75% of primary breast cancers express ERα, and more than half of these cancers coexpress PR. ER and PR are considered independent prognostic factors for early breast cancer therapeutic management although both are weak and lose their prognostic value after long-term follow-up.
Increased expression of ERα is an early event in breast carcinogenesis; in contrast, a decrease of PR levels is associated with breast cancer progression (39). PR is able to inhibit the growth of ER+ breast cancer cells in ovariectomized nude mice despite Pg deficiency, addressing a specific inhibitory role of PR independent of its natural ligand (40). It emerges from experimental models that ER+/PR+ breast cancers are well differentiated, presenting as low-risk, well-defined lesions whereas ER+/PR− metastatic tumors display a much more aggressive course after loss of PR compared with tumors retaining PR (39).
In humans, PR exists as two isoforms, called PR-A and PR-B, which exhibit distinct roles in regulating the effects of Pg that at physiological concentration is able to bind and activate both isoforms (41), and enhanced PR-A/PR-B ratio is coincident with a major breast cancer growth and progression (6,42).
It is well documented that in hormone-dependent breast cancer cells, Pg may inhibit the induction of classical estrogen-regulated genes even though the molecular mechanisms underlying these effects remain to be fully elucidated (43,44). Our study undertook to examine these molecular mechanisms.
We demonstrated that PR-B overexpression repressed ERα levels in breast cancer cells in a ligand-independent manner as it emerges from data obtained on cells transiently overexpressing PR-B truncated in LBD. This inhibitory effect is mediated by the recruitment of NCoR bound to PR-B on ERα promoter. Furthermore we demonstrated that PR-B overexpression, but not PR-A, inhibited ERα transactivation together with the expression of estrogen-dependent genes such as pS2, cyclin D1, and IRS1. These effects correlate well with the inhibition of E2-induced cell proliferation by Pg through endogenous PR-B isoform.
Our findings corroborate previous clinical studies (42,45) illustrating that high PR-A/PR-B ratios in breast tumors predict shorter disease-free survival. The protective action of PR-B in breast cancer is further reinforced by studies showing that PR is inversely associated with HER-2/neu, the signaling of which is known to drive estrogen-independent breast cancer cell growth (46,47).
Also of note are the findings that the levels of Her-2 are significantly higher in ER+/PR-A+ xenografts, than in ER+/PR-B+ xenografts (42). Thus, it remains an intriguing question as to whether a loss or a dysregulation of PR-B isoform expression could exert a direct effect on Her2 and in such a way it could perturb ERα signaling.
To clarify the molecular mechanisms through which PR-B may interfere with ERα gene transcription, we analyzed the ERα promoter sequence and identified a PRE half-site located at −1757 bp to −1752 bp. Functional experiments using five deletion constructs of the ERα promoter showed that the down-regulatory effects induced by PR-B overexpression on ERα promoter activity were through the half-site and were not detected in the deletion constructs lacking the half-PRE site. Moreover site-directed mutagenesis of the above region completely reversed down-regulation of ERα promoter activity. ChIP assay results further confirmed the specific recruitment of the PR-B isoform to the half-PRE site within the ERα gene promoter because recruitment was prevented in PR-B knockdown experiments. The inhibitory effects on the ERα promoter transcriptional machinery addressed the ability of PR-B to recruit corepressors interfering with ERα gene transcription.
Corepressors function as counterparts to coactivators revealing that nuclear receptor-mediated transcription is subjected to both positive and negative regulation. It has been reported that unliganded nuclear receptors, such as TR and RXR, can repress basal transcription in the absence of their cognate ligands, and these functions are mediated, at least in part, by the NCoR and SMRT (48). These two corepressors interact with unliganded nuclear receptors, through an elongated helix of sequence LXX I/H IXXX I/L, alternatively referred to as the CoRNR-box (49,50,51). It has been recently documented that NCoR and SMRT are also recruited by both ER and PR in the presence of ligand antagonist to regulate transcription of different genes (52,53). For instance, our ChIP experiments showed that among potential corepressor molecules that are able to interact with PR, NCoR was the only one present on the PR-B/DNA complex regardless of its natural ligand. These results are consistent with evidence reporting that corepressors have different preferences and determinants for interactions with nuclear receptors and transcription factors at specific genes (54,55). Moreover we show that formation of this bipartite complex leads to hypoacetylation of histone H4, which causes stabilization of nucleosome structure, limiting accessibility to the basal transcriptional machinery and thus repressing ERα gene expression.
All these data support a model in which elevated expression levels of PR-B increase the interaction of the receptor with NCoR on the half-PRE site of the ERα promoter, an event incompatible with PR-coactivator interactions. The crucial role of NCoR emerges from our data showing that silencing of this corepressor was able to reverse the down-regulation of ERα expression induced by PR-B overexpression.
E2 is known to down-regulate the levels of ERα in breast cancer cell line through an increased turnover of E2-bound receptor and via reduced transcription of its own gene (33). This down-regulation represents a classical feature of ERα transactivation. Our novel findings show that PR-B is a determinant of these down-regulatory effects, because PR-B knockdown attenuated the feedback inhibition of ERα levels after estrogenic stimulus. Therefore we propose that ERα modulation by E2 could be due to early events independent from PR expression, and later transcriptional events leading to PR-B overexpression, which in turn can produce a decrease in ERα transcription, via recruitment of a corepressor complex containing NCoR and displacing RNA polymerase II (Pol II). Thus we here demonstrate that the E2 enhanced PR-B is crucial in determining the concomitant ERα down-regulation. These results highlight the importance of tightly regulated expression of PR-A and PR-B, and we have provided new insights into the molecular mechanisms through which PR-B overexpression antagonizes ERα in breast cancer cells.
In conclusion we suggest that inhibition of ERα by PR-B is a critical regulatory pathway in ER-positive cells, and we speculate that dysregulation of this repression mechanism in breast cancer may have dramatic effects such as breast tumor progression. In other words we propose that unliganded PR-B and its ratio with PR-A influences breast cancer cell biology modulating ERα states. Because a large percentage of breast tumors are ER+/PR+ and might fall into a potential low-risk group (excess of PR-B) the antiestrogenic action of PR-B signaling could be exploited for innovative adjuvant strategies in breast cancer treatment.
Materials and Methods
Materials
DMEM/F-12, l-glutamine, Eagle’s nonessential amino acids, penicillin, streptomycin, fetal calf serum, BSA, and PBS were purchased from Eurobio (Les Ullis Cedex, France). Triazol reagent was obtained from Invitrogen (Carlsbad, CA), and FuGENE 6 was from Roche Applied Science (Indianapolis, IN). Taq DNA polymerase, 100-bp DNA ladder, dual luciferase kit, thymidine kinase, and Renilla luciferase plasmid were provided by Promega Corp. (Madison, WI). Aprotinin, leupeptin, phenylmethylsulfonyl fluoride, and sodium orthovanadate were purchased from Sigma (Milan, Italy).
Antobodies used in this study, anti-PR (H-190), anti-ERα, anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH), anti-Pol II, anti-NCoR, anti-SMRT, anti DAX-1, were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); antiacetyl histone4-K16, from Upstate Biotechnology (Lake Placid, NY); anti PR, from NeoMarkers (Labvision, Freemont, CA). Salmon sperm DNA was from Santa Cruz Biotechnology. Biotinylated horse antigoat IgG and ABC complex/horseradish peroxidase were provided by VECTOR Laboratories (Burlingame, CA). Chromogen, 3-diaminobenzidine tetrachloride dihydrate, was purchased from Bio-Optica (Milan, Italy). ECL System was purchased from Amersham Pharmacia (Buckinghamshire, UK). VCX500 ultrasonic processor was provided by Sonics (Newtown, CT).
Cell culture
Human breast cancer MCF-7 cells were gifts from Dr. B. Van der Burg (Utrecht, The Netherlands); ZR75 cells were kindly provided from Dr. A. Weisz (Naples, Italy); and T47D and human uterine cervix adenocarcinoma (HeLa) cells were obtained from the American Type Culture Collection (Manassas, VA). MCF-7 and HeLa cells were maintained in DMEM/F-12 medium containing 5% fetal calf serum (5% FCS), 1% l-glutamine, 1% Eagle’s nonessential amino acids, and 1 mg/ml penicillin/streptomycin in a 5% CO2 humidified atmosphere. Cells were cultured in phenol red-free DMEM, 0.5% BSA (0.5% BSA), and 2 mm l-glutamine (serum-free medium), for 48 h before each experiment. Hormone stimulation was performed in DMEM/F12 containing 5% charcoal-treated fetal calf serum to reduce the endogenous steroid concentration (56). ZR75.1 were maintained in complete DMEM supplemented with 10% fetal bovine serum and 250 ng/ml amphotericin B (Sigma, Milan, Italy), 1 mg/ml penicillin/streptomycin. T47D cells were routinely maintained in RPMI 1640 supplemented with 5% FCS, 1 μg/ml insulin (Sigma), 1 mg/ml penicillin /streptomycin (Sigma).
Proliferation assays
For quantitative proliferation assays, 10,000 cells were seeded in 24-well plates in regular growth medium. Cells were washed once they had attached, serum starved for 48 h, and then incubated in medium containing 5% charcoal-treated FCS with the indicated treatments; medium was renewed every 2 d (with treatments), and cells were trypsinized and counted in a hemocytometer on d 6.
Plasmid
The following plasmids were used: XETL (57), the wild-type human ERα (HEGO) (58), the full-length PR-B consisting of the full-length PR-B cDNA fused with the SV40 early promoter and expressed in the pSG5 vector (a gift from Dr. D. Picard, University of Genève, Switzerland); the PR-B 1-675 including the N-terminal fragment of PR-B (mLBD PR-B) (59); and the PR DNA-binding mutant C587A (mDBD PR) previously described by Faivre et al. (60) (both gifts from Dr. C. Lange, University of Minnesota Cancer Center, Minneapolis, MN), the full-length PR-A (7); and the deletion fragments of the ERα gene promoter (34). The Renilla luciferase expression vector pRL-TK (Promega, Milan, Italy) was used as a transfection standard.
Total RNA extraction and RT-PCR assay
Total cellular RNA was extracted from MCF-7 cells using Triazol reagent (Invitrogen, Carlsbad, CA). Reverse transcription was done using RETROscript kit (Ambion, Austin, TX). Primer sequences include: ERα forward, 5′-GGAGACATGAGAGCTGCCA-3′, and reverse, 5′-CCAGCAGCATGTCGACGATC-3′; PR-B forward, 5′-TAGTGAGGGGGCAGTGGAAC-3′, and reverse, 5′-AGGAGGGGGTTTCGGGAATA-3′; pS2 forward, 5′-TTCTATCCTAATACCATCGACG-3′, and reverse, 5′-TTTGAGTAGTCAAAGTCAGAGC-3′; cyclin D1 forward, 5′-TCTAAGATGAAGGAGACCATC-3′, and reverse, 5′-GCGGTAGTAGGACAGGAAGTTGTT-3′; IRS1 forward, 5′-AGGATATTTAATTTGCCTCGG-3′ and reverse, 5′-AAGCGTTTGTGCATGCTCTTG 3; rRNA 36B4, forward, 5′-CTCAACATCTCCCCCTTCTC-3′, and reverse, 5′-CAAATCCCATATCCTCGTCC-3′. Equal amounts of PCR product were electrophoresed on 1% agarose gels and visualized by ethidium bromide staining. To check for the presence of DNA contamination, an RT-PCR was performed without Moloney murine leukemia virus-reverse transcriptase (negative control).
Western blotting and immunoprecipitation
Cytoplasmic and nuclear fractions of cellular protein extract were obtained as previously described (26,61). Proteins were resolved on an 8–10% sodium dodecyl sulfate (SDS)-polyacrylamide gel, transferred to a nitrocellulose membrane (Amersham Biosciences, Milan, Italy), and probed overnight at 4–C with the antibody indicated in the figure legends.
Transfections and luciferase assays
Cells (1 × 105) were plated into 24-well dishes with 500 μl of regular growth medium per well the day before transfection. The medium was replaced with that lacking serum on the day of transfection, which was done using Fugene 6 reagent as recommended by the manufacturer (Roche Diagnostics, Milan, Italy) with a mixture containing 0.5 μg of reporter plasmid, alone or in combination plasmids as indicated in the figure legends, and 5 ng of pRL-TK. Medium was renewed after which cells were treated for 24 h. Luciferase activity was measured with the Dual Luciferase kit (Promega) according to the manufacturer’s recommendations. Firefly luciferase values were normalized to the internal transfection control provided by the Renilla luciferase activity. Empty vector was used to ensure that DNA concentration were constant in each transfection.
For RNA preparation, whole and nuclear cell extractions, and ChIP assays, cells were serum starved for 48 h, plated in medium containing 5% charcoal-treated fetal calf serum, and then transfected using the FuGENE 6 reagent, with an appropriate amount of the various plasmids as indicated in the figure legends. Cells were changed with fresh medium containing 5% charcoal-treated fetal calf serum for 48 h and then treated as indicated.
Lipid-mediated transfection of siRNA duplexes
RNA oligonucleotides directed against PR-B or NCoR were purchased from Invitrogen (Carlsbad, CA), and transfection was performed as described previously (38,62). Cells were transfected using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions and then treated as indicated.
Site-directed mutagenesis
Mutagenesis was performed on Fragment D of the ERα promoter (34) using the QuikChange mutagenesis kit (Stratagene, La Jolla, CA) following the manufacturer’s instructions. The sequence for the sense primer was: 5′-AGCAGGGAGATGAGGATTGCTgaagTCCATGGGGGTATGT-3′. The plasmids were then sequenced to confirm the mutation of the desired site.
ChIP assays
ChIP methodology was performed as described by Morelli et al. (63).
Cells were grown in 100-mm plates 90% confluent cultures, were shifted to serum-free medium for 48 h, and then treated for 1 h before harvesting for the assay. Then cells were washed twice with PBS and cross-linked with 1% formaldehyde at 37 C for 10 min. Next they were washed twice, collected, and resuspended in 200 μl of lysis buffer (1% SDS; 10 mm EDTA; 50 mm Tris-Cl, pH 8.1) and left on ice for 10 min. Lysates were sonicated four times for 10 sec at 30% of maximal power and collected by centrifugation at 4 C for 10 min at 14,000 rpm. Supernatants were collected and diluted in 1.3 ml of immunoprecipitation buffer (0.01% SDS; 1.1% Triton X-100; 1.2 mm EDTA; 16.7 mm Tris-Cl, pH 8.1; 16.7 mm NaCl) followed by immunoclearing with 80 μl of sonicated salmon sperm DNA/protein A-agarose for 1 h at 4 C. The precleared chromatin was immunoprecipitated for 12 h either with PR, RNA Pol II, acetyl histone4-K16, NCoR, SMRT, and DAX-1 antibodies or with normal goat IgG as the negative control. After that, 60 μl of salmon sperm DNA/protein A-agarose was added, and precipitation was continued for 2 h at 4 C. Precipitates were washed sequentially for 5 min with the following buffers: Wash A (0.1% SDS; 1% Triton X-100; 2 mm EDTA; 20 mm Tris-Cl, pH 8.1; 150 mm NaCl); Wash B (0.1% SDS; 1% Triton X-100; 2 mm EDTA; 20 mm Tris-Cl, pH 8.1; 500 mm NaCl), and Wash C (0.25 m LiCl; 1% Nonidet P-40; 1% sodium deoxycholate; 1 mm EDTA; 10 mm Tris-Cl, pH 8.1), and then twice with TE buffer (10 mm Tris, 1 mm EDTA). The immune complexes were eluted with elution buffer (1% SDS, 0.1 m NaHCO3), and then they were reverse cross-linked by heating at 65 C for 12 h and digested with 0.5 mg/ml proteinase K at 45 C for 1 h. DNA was obtained by phenol and phenol/chloroform extractions. Two microliters of 10 mg/ml yeast tRNA was added to each sample, and DNA was precipitated with ethanol for 12 h at −20 C and resuspended in 20 μl of TE buffer. Five microliters of each sample was used for PCR with the following ERα promoter primers: forward, 5′-ACGTTCTTGATCCAGCAGGGTA-3′ and reverse, 5′-ACCTGCCAAATTATATGCAAATGGCAG-3′ containing the half-PRE site; and forward, 5′-GTGGCCATTGTTGACCTACAG-3′ and reverse, 5′-CTGTAGGTCAACAATGGCCAC-3′ upstream the half-PRE site. The amplification products, obtained in 30 cycles, were analyzed in a 2% agarose gel and visualized by ethidium bromide staining.
Statistical analysis
Each datum point represents the mean se of three different experiments. Data were analyzed by ANOVA test using the STATPAC computer program.
Acknowledgments
We thank Dr. D. Picard for the full-length PR-B plasmid and Dr. C. Lange for PR DNA-binding mutant plasmid and PR-B LBD truncated plasmid.
Footnotes
This work was supported by Associazone Italiana Ricerca sul Cancro-2007 and Grant RO1 CA72038 from the National Institutes of Health/National Cancer Institute (to S.A.W.F.).
Disclosure Summary: All authors have nothing to declare.
First Published Online January 15, 2009
Abbreviations: AR, Androgen receptor; ARE, androgen-responsive element; ChIP, chromatin immunoprecipitation; DBD, DNA-binding domain; E2, Estradiol; ERα, estrogen receptor α; ERE, estrogen-responsive element; FCS, fetal calf serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LBD, ligand-binding domain; Pg, progesterone; Pol II, polymerase II; PR-A, progesterone receptor-A; PR-B, progesterone receptor-B; PRE, progesterone responsive element; NCoR, nuclear receptor corepressor; SDS, sodium dodecyl sulfate; siRNA, small interfering RNA; SMRT, silencing mediator of retinoic acid and thyroid hormone receptor; XETL, luciferase reporter plasmid containing ERE consensus.
References
- Clarke CL, Sutherland RL 1990 Progestin regulation of cellular proliferation. Endocr Rev 11:266–330 [DOI] [PubMed] [Google Scholar]
- Dunnwald LK, Rossing MA, Li CI 2007 Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 9:R6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua SAW, Cui Y 2004 Estrogen and progesterone receptor isoforms: clinical significance in breast cancer. Breast Cancer Res Treat 87:S3–S10 [DOI] [PubMed] [Google Scholar]
- Knight III WA, Osborne CK, Yochmowitz MG, McGuire WL 1980 Steroid hormone receptors in management of human breast cancer. Ann Clin Res 12:202–207 [PubMed] [Google Scholar]
- Kim HJ, Cui X, Hilsenbeck SG, Lee AV 2006 Progesterone receptor loss correlates with human epidermal growth factor receptor 2 overexpression in estrogen receptor-positive breast cancer. Clin Cancer Res 12:1013s–1018s [DOI] [PubMed] [Google Scholar]
- Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM 2003 Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol 21:1973–1979 [DOI] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P 1990 Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus WL, Katzenellenbogen BS 1993 Regulation of progesterone receptor gene expression and growth in the rat uterus: modulation of estrogen actions by progesterone and sex steroid hormone antagonists. Endocrinology 132:2371–2379 [DOI] [PubMed] [Google Scholar]
- Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB 2002 Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem 277:5209–5218 [DOI] [PubMed] [Google Scholar]
- Boyd-Leinen PA, Fournier D, Spelsberg TC 1982 Nonfunctioning progesterone receptors in the developed oviducts from estrogen-withdrawn immature chicks and in aged nonlaying hens. Endocrinology 111:30–36 [DOI] [PubMed] [Google Scholar]
- Kato J, Hirata S, Nozawa A, Mouri N 1993 The ontogeny of gene expression of progestin receptors in the female rat brain. J Steroid Biochem Mol Biol 47:173–182 [DOI] [PubMed] [Google Scholar]
- Shyamala G, Yang X, Cardiff RD, Dale E 2000 Impact of progesterone receptor on cell-fate decisions during mammary gland development. Proc Natl Acad Sci USA 97:3044–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyamala G, Yang X, Silberstein G, Barcellos-Hoff MH, Dale E 1998 Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc Natl Acad Sci USA 20:696–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote PA, Bartow S, Tran N, Clarke CL 2002 Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat 72:163–172 [DOI] [PubMed] [Google Scholar]
- Arnett-Mansfield RL, de Fazio A, Wain GV, Jaworski RC, Byth K, Mote PA, Clarke CL 2001 Relative expression of progesterone receptors A and B in endometrioid cancers of the endometrium. Cancer Res 61:4576–4582 [PubMed] [Google Scholar]
- Loose-Mitchell DS, Chiappetta C, Stancel GM 1988 Estrogen regulation of c-fos messenger ribonucleic acid. Mol Endocrinol 2:946–951 [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Murthy L, Stancel GM 1992 Progesterone inhibits the estrogen-induced expression of c-fos messenger ribonucleic acid in the uterus. Endocrinology 130:3223–3230 [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS 2000 Mechanisms of action and cross-talk between estrogen receptor and progesterone receptor pathways. J Soc Gynecol Investig 7:S33–S37 [DOI] [PubMed] [Google Scholar]
- Weigel RJ, deConinck EC 1993 Transcriptional control of estrogen receptor in estrogen receptor negative breast carcinoma. Cancer Res 53:3472–3474 [PubMed] [Google Scholar]
- Martin MB, Saceda M, Garcia-Morales P, Gottardis MM 1994 Regulation of estrogen receptor expression. Breast Cancer Res Treat 31:183–189 [DOI] [PubMed] [Google Scholar]
- King RJ 1993 William L. McGuire Memorial Symposium. Estrogen and progestin effects in human breast carcinogenesis. Breast Cancer Res Treat 27:3–15 [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis P, Kuttenn F, Gompel A 1986 Estradiol/progesterone interaction in normal and pathologic breast cells. Ann NY Acad Sci 464:152–167 [DOI] [PubMed] [Google Scholar]
- Graham JD, Roman SD, McGowan E, Sutherland RL, Clarke CL 1995 Preferential stimulation of human progesterone receptor B expression by estrogen in T-47D human breast cancer cells. J Biol Chem 270:30693–30700 [DOI] [PubMed] [Google Scholar]
- Baulieu EE 1989 Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Science 245:1351–1357 [DOI] [PubMed] [Google Scholar]
- Ghatge RP, Jacobsen BM, Schittone SA, Horwitz KB 2005 The progestational and androgenic properties of medroxyprogesterone acetate: gene regulatory overlap with dihydrotestosterone in breast cancer cells. Breast Cancer Res 7:R1036–R1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzino M, De Amicis F, McPhaul MJ, Marsico S, Panno ML, Andò S 2005 Endogenous coactivator ARA70 interacts with estrogen receptor α (ERα) and modulates the functional ERα/androgen receptor interplay in MCF-7 cells. J Biol Chem 280:20421–20430 [DOI] [PubMed] [Google Scholar]
- Berrevoets CA, Umar A, Brinkmann AO 2002 Antiandrogens: selective androgen receptor modulators. Mol Cell Endocrinol 198:97–103 [DOI] [PubMed] [Google Scholar]
- Sartorius CA, Melville MY, Hovland AR, Tung L, Takimoto GS, Horwitz KB 1994 A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Mol Endocrinol 8:1347–1360 [DOI] [PubMed] [Google Scholar]
- Meyer ME, Quirin-Stricker C, Lerouge T, Bocquel MT, Gronemeyer H 1992 A limiting factor mediates the differential activation of promoters by the human progesterone receptor isoforms. J Biol Chem 267:10882–10887 [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP 1993 Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol 7:1241–1243 [DOI] [PubMed] [Google Scholar]
- Saceda M, Lippman ME, Chambon P, Lindsey RL, Ponglikitmongkol M, Puente M, Martin MB 1988 Regulation of the estrogen receptor in MCF-7 cells by estradiol. Mol Endocrinol 2:1157–1162 [DOI] [PubMed] [Google Scholar]
- Read LD, Greene GL, Katzenellenbogen BS 1989 Regulation of estrogen receptor messenger ribonucleic acid and protein levels in human breast cancer cell lines by sex steroid hormones, their antagonists, and growth factors. Mol Endocrinol 3:295–304 [DOI] [PubMed] [Google Scholar]
- Pink JJ, Jordan VC 1996 Models of estrogen receptor regulation by estrogens and antiestrogens in breast cancer cell lines. Cancer Res 56:2321–2330 [PubMed] [Google Scholar]
- deGraffenried LA, Hilsenbeck SG, Fuqua SAW 2002 Sp1 is essential for estrogen receptor α gene transcription. J Steroid Biochem Mol Biol 82:7–18 [DOI] [PubMed] [Google Scholar]
- Brayman MJ, Julian J, Mulac-Jericevic B, Conneely OM, Edwards DP, Carson DD 2006 Progesterone receptor isoforms A and B differentially regulate MUC1 expression in uterine epithelial cells. Mol Endocrinol 20:2278–2291 [DOI] [PubMed] [Google Scholar]
- Gao X, Loggie BW, Nawaz Z 2002 The roles of sex steroid receptor coregulators in cancer. Mol Cancer 14:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoulnik IU, Krause WC, Bingman III WE, Rahman HT, Amrikachi M, Ayala GE, Weigel NL 2003 Repressors of androgen and progesterone receptor action. J Biol Chem 278:31136–31148 [DOI] [PubMed] [Google Scholar]
- Dennis AP, Lonard DM, Nawaz Z, O'Malley BW 2005 Inhibition of the 26S proteasome blocks progesterone receptor-dependent transcription through failed recruitment of RNA polymerase II. J Steroid Biochem Mol Biol 94:337–346 [DOI] [PubMed] [Google Scholar]
- Gross GE, Clark GM, Chamness GC, McGuire WL 1984 Multiple progesterone receptor assays in human breast cancer. Cancer Res 44:836–840 [PubMed] [Google Scholar]
- Sartorius CA, Shen T, Horwitz KB 2003 Progesterone receptors A and B differentially affect the growth of estrogen-dependent human breast tumor xenografts. Breast Cancer Res Treat 79:287–299 [DOI] [PubMed] [Google Scholar]
- Nardulli AM, Katzenellenbogen BS 1988 Progesterone receptor regulation in T47D human breast cancer cells: analysis by density labeling of progesterone receptor synthesis and degradation and their modulation by progestin. Endocrinology 122:1532–1540 [DOI] [PubMed] [Google Scholar]
- Hopp TA, Weiss HL, Hilsenbeck SG, Cui Y, Allred DC, Horwitz KB, Fuqua SA 2004 Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res 10:2751–2760 [DOI] [PubMed] [Google Scholar]
- Eden J 2003 Progestins and breast cancer. Am J Obstet Gynecol 188:1123–1131 [DOI] [PubMed] [Google Scholar]
- Alexander IE, Shine J, Sutherland RL 1990 Progestin regulation of estrogen receptor messenger RNA in human breast cancer cells. Mol Endocrinol 4:821–828 [DOI] [PubMed] [Google Scholar]
- Graham JD, Yeates C, Balleine RL, Harvey SS, Milliken JS, Bilous AM, Clarke CL 1996 Progesterone receptor A and B protein expression in human breast cancer. J Steroid Biochem Mol Biol 56:93–98 [DOI] [PubMed] [Google Scholar]
- Huang HJ, Neven P, Drijkoningen M, Paridaens R, Wildiers H, Van Limbergen E, Berteloot P, Amant F, Christiaens MR, Vergote I 2005 Association between HER-2/neu and the progesterone receptor in oestrogen-dependent breast cancer is age-related. Breast Cancer Res Treat 91:81–87 [DOI] [PubMed] [Google Scholar]
- Bamberger AM, Milde-Langosch K, Schulte HM, Löning T 2000 Progesterone receptor isoforms, PR-B and PR-A, in breast cancer: correlations with clinicopathologic tumor parameters and expression of AP-1 factors. Horm Res 54:32–37 [DOI] [PubMed] [Google Scholar]
- Li X, Wong J, Tsai SY, Tsai MJ, O'Malley BW 2003 Progesterone and glucocorticoid receptors recruit distinct coactivator complexes and promote distinct patterns of local chromatin modification. Mol Cell Biol 23:3763–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L, Kao HY, Chakravarti D, Lin RJ, Hassig CA, Ayer DE Schreiber SL, Evans RM 1997 Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell 89:373–380 [DOI] [PubMed] [Google Scholar]
- Hu X, Lazar MA 1999 The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature 402:93–96 [DOI] [PubMed] [Google Scholar]
- Webb P, Anderson CM, Valentine C, Nguyen P, Marimuthu A, West BL, Baxter JD, Kushner PJ 2000 The nuclear receptor corepressor (N-CoR) contains three isoleucine motifs (I/LXXII) that serve as receptor interaction domains (IDs). Mol Endocrinol 14:1976–1985 [DOI] [PubMed] [Google Scholar]
- Giannoukos G, Szapary D, Smith CL, Meeker JE, Simons Jr SS 2001 New antiprogestins with partial agonist activity: potential selective progesterone receptor modulators (SPRMs) and probes for receptor- and coregulator-induced changes in progesterone receptor induction properties. Mol Endocrinol 15:255–270 [DOI] [PubMed] [Google Scholar]
- Heldring N, Nilsson M, Buehrer B, Treuter E, Gustafsson JA 2004 Identification of tamoxifen-induced coregulator interaction surfaces within the ligand-binding domain of estrogen receptors. Mol Cell Biol 24:3445–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li Y, Lazar MA 2001 Determinants of CoRNR-dependent repression complex assembly on nuclear hormone receptors. Mol Cell Biol 21:1747–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen K, Rosenfeld MG 2002 Biological roles and mechanistic actions of co-repressor complexes. J Cell Sci 115:689–698 [DOI] [PubMed] [Google Scholar]
- van der Burg B, Rutteman GR, Blankenstein MA, de Laat S W, van Zoelen EJ 1988 Mitogenic stimulation of human breast cancer cells in a growth factor-defined medium: synergistic action of insulin and estrogen. J Cell Physiol 34:101–108 [DOI] [PubMed] [Google Scholar]
- Bunone G, Briand PA, Miksicek RJ, Picard D 1996 Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J 15:2174–2183 [PMC free article] [PubMed] [Google Scholar]
- Tora L, Mullick A, Metzger D, Ponglikitmongkol M, Park I, Chambon P 1989 The cloned human oestrogen receptor contains a mutation which alters its hormone binding properties. EMBO J 8:1981–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung L, Abdel-Hafiz H, Shen T, Harvell DME, Nitao LK,. Richer JK, Sartorius CA, Takimoto G, Horwitz KB 2006 Progesterone receptors (PR)-B and -A regulate transcription by different mechanisms: AF-3 exerts regulatory control over coactivator binding to PR-B. Mol Endocrinol 20:2656–2667 [DOI] [PubMed] [Google Scholar]
- Faivre EJ, Lange CA 2007 Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol 27:466–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch M, Aluker M, Murdoch FE 1999 Identification of a unique liganded estrogen receptor complex released from the nucleus by decavanadate. Biochemistry 38:6987–6996 [DOI] [PubMed] [Google Scholar]
- Hardy DB, Janowski BA, Corey DR, Mendelson CR 2006 Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-κB activation of cyclooxygenase 2 expression. Mol Endocrinol 20:2724–2733 [DOI] [PubMed] [Google Scholar]
- Morelli C, Garofalo C, Sisci D, del Rincon S, Cascio S, Tu X, Vecchione A, Sauter ER, Miller Jr WH, Surmacz E 2004 Nuclear insulin receptor substrate 1 interacts with estrogen receptor α at ERE promoters. Oncogene 23:7517–7526 [DOI] [PubMed] [Google Scholar]