Abstract
Aromatase (product of CYP19 gene), the critical enzyme in estrogen biosynthesis, is up-regulated in 70% of all breast cancers and is highly correlated with cyclooxygenase 2 (COX-2), the rate-determining enzyme in prostanoid biosynthesis. Expression of COX-2 also is correlated with the oncogene HER-2/neu. The efficacy of current endocrine therapies for breast cancer is predicted only if the tumor contains significant amounts of estrogen receptor. Because the progesterone receptor (PR) is an estrogen-induced target gene, it has been suggested that its presence may serve as an indicator of estrogen receptor functional capacity and the differentiation state of the tumor. In the present study, we tested the hypothesis that PR serves a crucial protective role by antagonizing inflammatory response pathways in the breast. We observed that progesterone antagonized the stimulatory effects of cAMP and IL-1β on aromatase, COX-2, and HER-2/neu expression in T47D breast cancer cells. These actions of progesterone were associated with increased expression of the nuclear factor-κB inhibitor, IκBα. In 28 breast cancer cell lines, IκBα expression was positively correlated with PR mRNA levels; overexpression of a phosphorylation-defective mutant of IκBα inhibited expression of aromatase, COX-2, and HER-2/neu. Moreover, in breast cancer cell lines cultured in the absence of progesterone, up-regulation of endogenous PR caused decreased expression of aromatase, COX-2, and HER-2/neu expression, whereas down-regulation of endogenous PR resulted in a marked induction of aromatase and HER-2/neu mRNA. Collectively, these findings suggest that PR plays an important antiinflammatory role in breast cancer cells via ligand-dependent and ligand-independent mechanisms.
THERE IS COMPELLING evidence that estrogen acting through its receptors, estrogen receptor (ER)α and ERβ, plays a predominant role in development and growth of breast cancer. Estrogen synthesis from C19-steroids is catalyzed by aromatase, an enzyme complex that comprises aromatase P450 (product of the CYP19 gene) and reduced nicotinamide adenine dinucleotide-cytochrome P450 reductase (1). Aromatase, the key regulatory enzyme in estrogen biosynthesis (2), is up-regulated in 70% of all human breast cancers (3) and is expressed both in tumor and in adjacent adipose stromal and endothelial cells (3). Thus, induction of aromatase within and surrounding the breast tumor can result in high local levels of estrogen production that stimulate tumor growth.
CYP19 expression in human estrogen-producing tissues and in breast cancer is controlled by unique promoters that lie upstream of tissue-specific first exons (4). These first exons are alternatively spliced onto a common site just upstream of the translation initiation codon in exon II. Whereas the aromatase mRNAs in these different tissues have unique 5′-untranslated regions, the sequences encoding the aromatase protein are identical. In human breast tumors and surrounding adipose stromal cells, aromatase is up-regulated via switching from the weak adipose-specific promoter (I.4) to the strong ovarian (IIa) (5) and so-called “breast cancer” (I.3) (6) promoters. Promoter I.3 lies just upstream of promoter IIa; consequently, both share important regulatory elements. Switching from promoter I.4 to promoters IIa and I.3 is likely mediated by induction of cyclooxygenase 2 (COX-2) in the breast tumor (3,7) (see below), resulting in increased prostaglandin E2 (PGE2) synthesis by breast tumor epithelial cells and infiltrating macrophages (5).
Breast tumor progression is promoted by activation of proliferative and inflammatory response pathways. Notably, the inflammatory transcription factor nuclear factor κB (NF-κB) appears to play an important role in normal mammary gland development and in breast carcinogenesis. Increased levels of IκB kinase activity and nuclear NF-κB and NF-κB DNA-binding activity have been observed in human breast cancer cell lines and in breast cancer specimens (see Ref. 8 for review). NF-κB is a major transcriptional regulator of COX-2, the rate-determining and highly regulated enzyme in prostanoid biosynthesis, which is overexpressed in breast cancer (9). COX-2 up-regulation is associated with increased aromatase (3) and enhanced expression of the tumorigenic marker, HER-2/neu (10). Furthermore, overexpression of HER-2/neu in NIH-3T3 fibroblasts increased COX-2 and aromatase activity (10). The finding that COX-2 inhibition blocked HER-2/neu induction of aromatase suggests that prostanoids mediate this induction (10). HER-2/neu and COX-2 appear to exist in a positive feedback loop, whereby HER-2/neu increases COX-2 transcription, and elevated COX-2, via PGE2, induces HER-2/neu expression (11).
It has long been appreciated that the presence of progesterone receptor (PR) in a breast tumor is an independent predictor for benefit from adjuvant endocrine therapy and of disease-free survival (12,13,14). Breast tumors that are PR(−) have a much higher proliferation rate and are more likely to manifest increased expression of the tumorigenic prognostic indicators, HER-2/neu and epidermal growth factor receptor, than PR(+) tumors (15,16,17,18). Because PR is an estrogen-induced target gene, it has been suggested that its presence serves as an indicator of ER functional capacity and differentiation status of the tumor (19). However, we propose that PR may have independent protective actions that involve antagonism of inflammatory response pathways. In recent studies, we observed that small interfering RNA (siRNA)-mediated knockdown of PR-A and PR-B in T47D breast cancer cells caused activation of NF-κB and a 30-fold induction of COX-2 mRNA (20). This was observed in the absence of exogenous progesterone, suggesting that PR exerts a ligand-independent action to inhibit NF-κB activation. Interestingly, we observed in cultured human myometrial cells that PR also acts in a progesterone-dependent manner to mediate increased expression of the NF-κB inhibitor, IκBα (20).
In the present study, we tested the hypothesis that PR acts in a ligand-dependent and -independent manner to inhibit NF-κB activation, thereby blocking inflammatory response pathways, expression of oncogenic growth factor receptors, and aromatase induction in breast cancer cells. We observed that progesterone treatment of breast cancer cells antagonized the stimulatory effects of cAMP and IL-1β on aromatase, HER-2/neu, and COX-2 expression. These actions were associated with increased expression of the NF-κB inhibitor, IκBα. In an analysis of 28 breast cancer cell lines, IκBα expression was found to be positively correlated with PR mRNA levels. An important role of IκBα as a PR target gene that plays a protective role in the breast was indicated by the finding that overexpression in breast cancer cells of a stable phosphorylation-defective IκBα mutant (21) inhibited COX-2, aromatase, and HER-2/neu. Finally, in breast cancer cell lines cultured in the absence of progesterone, up-regulation of endogenous PR caused decreased expression of aromatase and COX-2 mRNA, whereas down-regulation of endogenous PR caused a marked induction of COX-2, aromatase, and HER-2/neu mRNA. These findings suggest that PR also acts in a ligand-independent manner to antagonize inflammatory response pathways and aromatase in the breast.
RESULTS
Aromatase and HER-2/neu Induction by IL-1β or cAMP in Breast Cancer Cells Is Mediated by the Cyclooxygenase Pathway
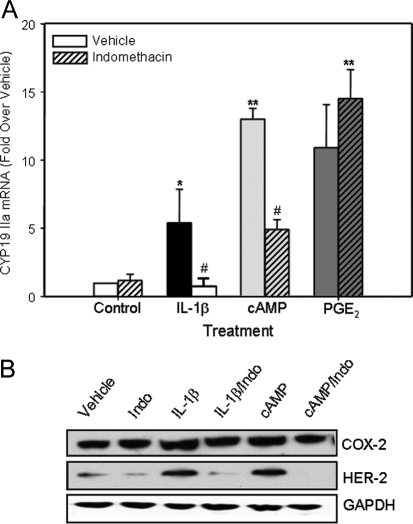
In this study, quantitative real-time RT-PCR (qRT-PCR) was used to analyze the effects of IL-1β (10 ng/ml) and dibutyryl cAMP (Bt2cAMP) (1 mm) and the role of the cyclooxygenase pathway on expression of aromatase and HER-2/neu in T47D cells after 24 h of culture in serum-free medium. As can be seen in Fig. 1A, both IL-1β and Bt2cAMP caused a marked induction of aromatase mRNA in the T47D cells (see Table 1 for primer sequences). As can be seen in the immunoblot in Fig. 1B, cAMP and IL-1β also had pronounced inductive effects on the levels of HER-2/neu protein (increased 3.5- and 3.1-fold, respectively, as compared with vehicle-treated cells). Because COX-2 inhibitors were previously reported to reduce basal levels of aromatase activity and expression in cultured breast cancer cells (22), we also determined whether the inductive effect of inflammatory cytokines on aromatase expression is mediated by stimulation of COX-2 and prostaglandin synthesis. To address this, T47D cells were cultured in the absence or presence of cAMP or IL-1β (10 ng/ml) with and without the cyclooxygenase inhibitor, indomethacin (10−6 m). As can be seen, whereas indomethacin alone had no effect on the basal levels of aromatase mRNA, cotreatment with indomethacin abrogated the stimulatory effect of IL-1β on aromatase expression (Fig. 1A). Similar inhibitory effects of indomethacin also were observed on cAMP (<0.1-fold relative to vehicle-treated cells) and IL-1β (0.3-fold relative to vehicle-treated cells) induction of HER-2/neu protein levels; however, no effect of indomethacin on COX-2 protein levels was observed (Fig. 1B). The supposition that the inductive effect of IL-1β is mediated by increased synthesis of prostaglandins was further supported by the finding that treatment with PGE2 (10−8 m), in the absence or presence of indomethacin, had a marked effect to stimulate aromatase expression (Fig. 1A).
Figure 1.
IL-1β Induction of Aromatase/CYP19Promoter IIa in T47D Cells Is Likely Mediated by Products of the Cyclooxygenase Pathway %T47D cells were cultured ± IL-1β (10 ng/ml), Bt2cAMP (1 mm) or PGE2 (10−8 m) ± indomethacin (10 mm) for 24 h. A, RNA was extracted and reverse transcribed, and levels of CYP19 mRNA transcripts containing exon IIa were assessed by qRT-PCR. Relative levels of CYP19 IIa mRNA transcripts were calculated by normalizing against ribosomal (r)RNA. Data are the mean ± sem of values from three independent experiments and are expressed as fold stimulation over vehicle. *, Significant difference (P < 0.05) between treatment and vehicle; **, significant difference (P < 0.01) between treatment and vehicle; # , significant difference (P < 0.05) between IL-1β and Indo + IL-1β. B, Cell lysate proteins were analyzed for levels of HER-2/neu, COX-2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Indo, Indomethacin.
Table 1.
qPCR Primers
| Gene | Primer (5′-3′) | Reference No. |
|---|---|---|
| H36B4 | Fwd, TGC ATC AGT ACC CCA TTC TAT CA | XR 017813 |
| Rev, AAG GTG TAA TCC GTC TCC ACA GA | XR 017813 | |
| CYP19 I.3 | Fwd, CAC TCT ACC CAC TCA AGG GCA | M74714 |
| Rev, TTG GCT TGA ATT GCA GCA TTT | M74714 | |
| CYP19 IIa | Fwd, CAG GAG CTA TAG ATG AAC CTT TTA GGG | S85356 |
| Rev, CTT GTG TTC CTT GAC CTC AGA GG | S85356 | |
| COX-2 | Fwd, TTC CAG ATC CAG AGC TCA TTA AA | AY462100 |
| Rev, CCG GAG CGG GAA GAA CT | AY462100 | |
| IκBα | Fwd, TTG GGT GCT GAT GTC AAT GC | AY496422 |
| Rev, AGG TCC ACT GCG AGG TGA AG | AY496422 | |
| PR | Fwd, TCA GTG GGC AGA TGC TGT ATT T | NM000926 |
| Rev, GCC ACA TGG TAA GGC ATA ATG A | NM000926 | |
| GR | Fwd, TCC CTG GTC GAA CAG TTT TTT | NM000176 |
| Rev, AGC TGG ATG GAG GAG AGC TT | NM000176 | |
| HER-2/neu | Fwd, AGC CGC GAG CAC CCA AGT | NM004448 |
| Rev, TTG GTG GGC AGG TAG GTG AGT T | NM004448 |
Fwd, forward; Rev, reverse.
Progesterone Causes a Coordinate Repression of IL-1β and cAMP Induction of COX-2, Aromatase, and HER-2/neu Expression in T47D Cells
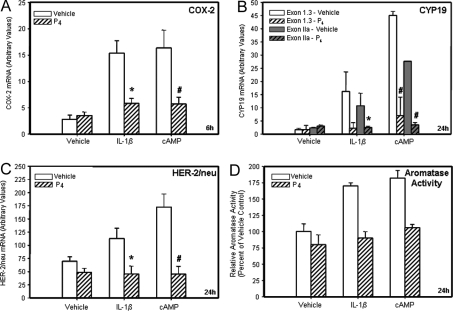
Given that products of the cyclooxygenase pathway are involved in the IL-1β induction of aromatase expression, that COX-2 and HER-2/neu expression levels are correlated in breast tumors (11), and that progesterone/PR impairs COX-2 expression in T47D cells (20), the effects of progesterone on cAMP and IL-1β induction of aromatase and HER-2/neu mRNA also were examined. T47D cells were cultured in the absence or presence of IL-1β (10 ng/ml) or cAMP (1 mm) with or without progesterone (100 nm) for 24 h. qRT-PCR demonstrated that IL-1β and cAMP each caused a marked up-regulation of COX-2 expression whereas cotreatment with progesterone abrogated this effect (Fig. 2A). Similar inhibitory effects of progesterone on IL-1β and cAMP-induced expression of ovarian (IIa) and breast cancer (I.3) CYP19 mRNA transcripts (Fig. 2B), and on HER-2/neu (Fig. 2C) mRNA expression, also were observed. CYP19 mRNA transcripts containing exons I.1 and I.4 were not detectable (data not shown). It is noteworthy that cAMP caused a greater stimulation of CYP19 IIa and I.3 expression than did IL-1β. In studies using MCF-7 breast cancer cells, we observed that cAMP and IL-1β stimulated aromatase activity and this was blocked by cotreatment with progesterone (Fig. 2D).
Figure 2.
Progesterone Suppresses IL-1β and cAMP Induction of COX-2 mRNA, CYP19 mRNA Containing Exons IIa and 1.3, and HER-2/neu mRNA in T47D Cells and IL-1β and cAMP Stimulation of Aromatase Activity in MCF-7 Cells %T47D breast cancer cells were cultured for 6 or 24 h either with vehicle, IL-1β (10 ng/ml) or Bt2cAMP (1 mm) in the absence or presence of progesterone (P4, 10−7 m). RNA was extracted, reverse transcribed to cDNA, and assessed by qRT-PCR for the levels of human COX-2 mRNA (panel A, 6-h treatment), exon-specific human CYP19 mRNA (panel B, 24-h treatment). Data shown only for exon I.3 and IIa-containing mRNA transcripts; exons 1.1- and 1.4-containing CYP19 mRNA transcripts were undetectable in all treatment groups), and HER-2/neu (panel C, 24-h treatment) mRNA transcripts. The relative levels of each transcript were assessed by normalizing against rRNA. The mRNA data are expressed as the mean ± sem of values from three independent experiments and are expressed as arbitrary units. *, Significant difference (P < 0.05) between IL-1β and IL-1β + P4; #, significant difference (P < 0.05) between cAMP and cAMP + P4. Aromatase activity (panel D) (fmol of andostenedione metabolized to estrogen/mg protein/h) was analyzed in MCF-7 cells after 24 h of treatment by assaying the incorporation of tritium from [1β-3H]androstenedione into water. Shown are the means ± sd of data from triplicate dishes of cells expressed relative to the vehicle-treated control from a representative experiment. The experiment was repeated several times with similar results.
Progesterone Inhibition of the IL-1β Induction of COX-2 Expression Is Associated with Recruitment of NF-κB p65 to the Proximal NF-κB Response Element within the COX-2 Promoter in T47D Cells
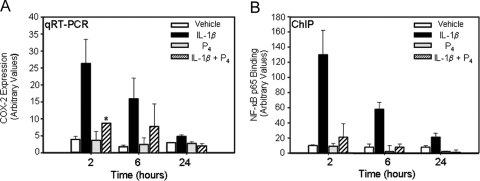
To begin to define the molecular mechanisms underlying the progesterone-induced impairment of COX-2 expression, qRT-PCR and chromatin immunoprecipitation (ChIP) were used to examine the time-dependent effects of progesterone and IL-1β on COX-2 expression and in vivo binding of NF-κB p65 to the proximal (−223 bp) NF-κB element of the COX-2 promoter, respectively. T47D cells were cultured in the absence or presence of IL-1β with or without progesterone (100 nm) for 2, 6, or 24 h. qRT-PCR revealed that IL-1β alone caused a marked up-regulation of COX-2 expression whereas cotreatment with progesterone abrogated this effect (Fig. 3A). Progesterone alone had no effect on the basal levels of COX-2 expression at all time points examined. Moreover, ChIP revealed that IL-1β stimulated recruitment of p65 to the proximal NF-κB response element, with maximal binding after 2 h of incubation (Fig. 3B). Notably, treatment with progesterone, which had little or no effect when added alone, markedly decreased the recruitment of p65 to the proximal NF-κB response element at all time points examined. Similar effects of IL-1β and progesterone on in vivo NF-κB p65 binding to the distal (−447 bp) NF-κB response element of the COX-2 promoter were also observed (data not shown). As reported previously (20), ChIP using nonimmune IgG or blank controls in place of p65 IgG, yielded essentially undetectable (Ct value >34 cycles) PCR products at all time points examined (data not shown). In addition to impairing the in vivo binding of p65 to the COX-2 promoter, progesterone inhibited (∼50%) IL-1β stimulation of nuclear p65 levels after 2 h and 6 h of incubation (Hardy, D. B., C.-C. Chien, and C. R. Mendelson, unpublished observations). These findings suggest that progesterone/PR antagonizes IL-1β induction of COX-2 expression, in part, by inhibiting NF-κB activation and nuclear translocation.
Figure 3.
Progesterone Impairs IL-1β Induction of COX-2 mRNA and NF-κB p65 Binding to the hCOX-2 Promoter in T47D Breast Cancer Cells %T47D cells were treated with vehicle, IL-1β (10 ng/ml), progesterone (100 nm), or IL-1β + progesterone, for 2–24 h. A, RNA was extracted and reverse transcribed, and levels of human COX-2 mRNA were assessed by qRT-PCR. Relative levels of COX-2 mRNA were calculated by normalizing against rRNA. Data are the mean ± sem of values from three independent experiments. *, Significant difference (P < 0.05) between IL-1β and IL-1β + P4. B, ChIP was carried out using antibodies for p65. In vivo p65 binding was quantified using qPCR with primers that amplify a region of the hCOX-2 promoter containing the proximal NF-κB binding site. Data are the mean ± sem of values from three sets of culture dishes. Shown is a representative of three independent experiments with comparable results.
The NF-κB Inhibitor, IκBα, May Serve as a PR Target Gene that Partially Mediates the Progesterone/PR Antiinflammatory Effects in Breast Cancer Cells
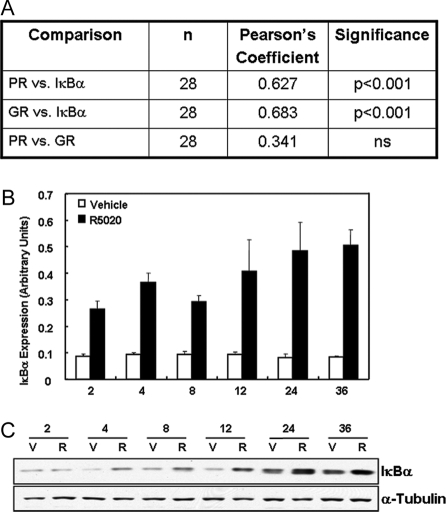
To begin to decipher the mechanisms for the progesterone-mediated decrease in NF-κB p65 binding to the NF-κB response elements of the COX-2 promoter in breast cancer cells, we analyzed the relationship of expression levels of the NF-κB inhibitor, IκBα, with expression of PR mRNA in human breast cancer cell lines. IκBα expression is induced by glucocorticoids (23,24,25,26) and by progestins (20,27,28) in a cell type- and promoter-specific manner. As can be seen in Fig. 4A, when we compared the relative expression levels of IκBα mRNA with expression of PR and glucocorticoid receptor (GR) mRNA in 28 breast cancer cell lines, there was a significant correlation between the expression of both nuclear receptors and the NF-κB inhibitor. There was no relationship between the relative expression of GR and PR in these breast cancer cell lines. Moreover, as shown in Fig. 4, B and C, levels of IκBα mRNA and protein, respectively, were induced by progesterone after 2–24 h in cultured T47D cells, a GR-deficient cell line (29).
Figure 4.
Expression of PR and IκBα Are Positively Correlated in 28 Breast Cancer Cell Lines %IκBα mRNA is up-regulated by progesterone in T47D cells. A, RNA from 28 breast cancer cell lines was extracted and reverse transcribed, and levels of PR, GR, and IκBα were assessed by qRT-PCR. The relative levels of all three mRNA transcripts were calculated by normalizing against rRNA, and statistical analyses were employed to determine the Pearson’s Coefficient of Correlation. B, T47D cells were treated either with vehicle or progesterone (10−7 m) for 2–36 h; the RNA was extracted and reverse transcribed, and levels of IκBα mRNA were assessed by qRT-PCR. Relative levels of IκBα mRNA were calculated by normalizing against rRNA. Data are the mean ± sem of values from four independent experiments and are expressed as fold progesterone stimulation over vehicle control. C, Cell lysates were analyzed for levels of IκBα protein over the same time course. V, Vehicle; R, R5020.
The data presented thus far suggest that PR impairs NF-κB transactivation of COX-2 via induction of IκBα; this, in turn, inhibits aromatase and HER-2/neu expression. To further define the role of endogenous NF-κB in the IL-1β and cAMP induction of COX-2, aromatase, and HER-2/neu, T47D cells were infected with a recombinant adenovirus expressing a phosphorylation-defective form of IκBα in which the IκB kinase serine phosphorylation sites (S32 and S36) were mutated (21), or with a recombinant adenovirus containing cytomegalovirus (CMV):βgal, as a control. This mutant form of IκBα, which is resistant to degradation by the ubiquitin/proteasome pathway, sequesters p65/p50 complexes within the cytoplasm, blocking NF-κB activation. Overexpression of the IκBα mutant (∼10-fold) was confirmed by qRT-PCR (data not shown). As can be seen in Fig. 5, overexpression of the IκBα mutant inhibited cAMP and IL-1β induction of COX-2, aromatase, and HER-2/neu mRNA, as compared with cells infected with the recombinant adenovirus containing CMV:βgal. It should be noted that the effect of the IκBα mutant on COX-2 expression was examined after 6 h of IL-1β and cAMP treatment, whereas effects on aromatase and HER-2/neu were studied after 24 h because these are the times at which maximal hormonal stimulation of each of these gene products was observed. Together, these findings suggest that IκBα plays an important mediatory role in the progesterone-induced inhibition of COX-2, aromatase, and HER-2/neu expression.
Figure 5.
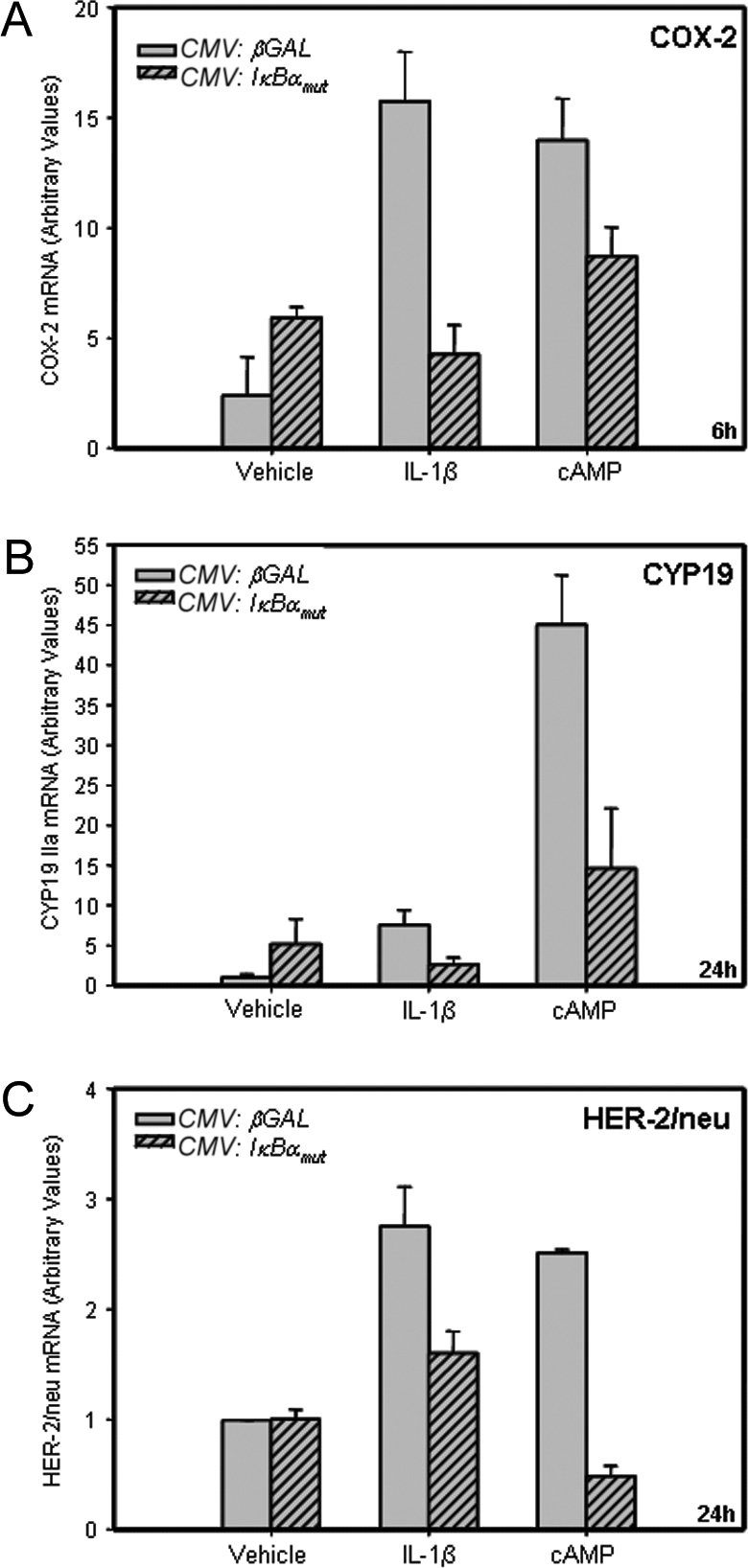
Effects of Overexpression of a Phosphorylation-Defective IκBα Mutant on IL-1β and cAMP-Induced Expression of COX-2, Aromatase, and HER-2/neu in T47D Cells %Cultured T47D breast cancer cells were infected with recombinant adenoviruses containing an expression vector for a phosphorylation-defective mutant form of IκBα (CMV:IκBαmut) (m.o.i. = 5), or with a control adenovirus expressing CMV:βgal (m.o.i. = 5) for 3 h. The cells were washed and placed in serum-free culture medium containing either no addition (Control), or in medium containing cAMP (1 mm) or IL-1β (10 ng/ml). After 6–24 h of incubation, the RNA was extracted and reverse transcribed, and levels of COX-2 (A), CYP19 (B), and HER-2/neu (C) mRNA were assessed by qRT-PCR. The relative levels of each mRNA transcript were calculated by normalizing against rRNA. Data are the mean ± sem of values from three sets of culture wells. Shown is a representative of three independent experiments with comparable results.
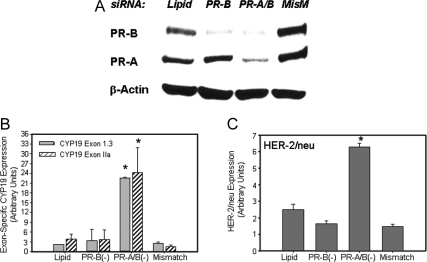
Ablation of PR Isoforms A and B using PR siRNA Markedly Induces Basal Expression of Aromatase and HER-2/neu in T47D Breast Cancer Cells
Previously, utilizing siRNA technology, we demonstrated that PR acts in a ligand-independent manner to inhibit COX-2 expression in T47D cells (20). To examine whether PR also exerts a ligand-independent suppressive effect on aromatase and HER-2/neu expression, in the present study, T47D cells were transfected with a siRNA specific for full-length PR (PR-B), with siRNAs for both PR-B and the truncated PR-A, or with an PR-B siRNA mismatch, as control (Fig. 6A and Table 2). Cells incubated with the transfection reagent in the absence of siRNA were also studied, as control (Lipid). After 4 d of culture, the levels of aromatase mRNA transcripts containing exons IIa and I.3 (Fig. 6B) and HER-2/neu mRNA (Fig. 6C) were analyzed by qRT-PCR. In control cells transfected with PR-B mismatch siRNA or with transfection reagent alone, expression of aromatase was unaltered (Fig. 6A). Reduction of PR-B protein levels alone also failed to alter basal aromatase mRNA levels. By contrast, ablation of PR-A plus PR-B using siRNA directed against both PR isoforms greatly enhanced expression of CYP19 IIa and 1.3 mRNA (Fig. 6B), as well as HER-2/neu (Fig. 6C) mRNA transcripts. Expression of CYP19 mRNA transcripts containing exons I.1 and I.4 were not altered by PR-A and -B knockdown (data not shown).
Figure 6.
Knockdown of PR-A and PR-B Enhances Expression of COX-2, HER-2/neu, and Exon-Specific CYP19 mRNA Transcripts in T47D Cells %T47D cells were transfected with siRNA targeted against PR-B alone or PR-B + PR-A; the cells were harvested 5 d after transfection for immunoblotting or qRT-PCR. Cell lysate proteins were analyzed for levels of PR, or β-actin by immunoblotting (A). mRNA was isolated from the cells and reverse transcribed to cDNA, and qRT-PCR was used to analyze levels of hCYP19 mRNA transcripts containing exons 1.3 and IIa at their 5′-ends (B) and HER-2/neu mRNA (C). Data are the means ± sem of values from four independent experiments and are expressed as arbitrary units. *, Significant difference (P < 0.05) between mismatch control and siRNA treatment. MisM, Mismatch.
Table 2.
Duplex siRNAs
| S1 | 5′-AUG ACU GAG CUG AAG GCA ATT-3′ | hPR-B only |
| S2 | 5′-GGG GAG UCC AGU CGU CAU GTT-3′ | hPR-B only |
| S2 mismatch | 5′-GCG GAG UGC AGU GGU CAAGTT-3′ | hPR-B only |
| S3 | 5′-GGU GUU GUC CCC GCU CAU GTT-3′ | hPR-A/B |
Only one strand (5′ to 3′) of each siRNA is shown. Mismatched bases are underlined.
Increasing PR Expression in MCF-7 Cells by a PR-Activating RNA (PR actRNA) Inhibits Expression of COX-2 and Aromatase
To test whether up-regulation of endogenous PR could affect COX-2 and aromatase expression in a ligand-independent manner, we used MCF-7 cells, which contain more than 30-fold lower levels of PR than the T47D cell line (30). Previously, it was observed that when MCF-7 cells were transfected with an RNA duplex (RNA PR11) complementary to the PR promoter sequence from −11 to +8 (Table 3), the expression of PR protein was greatly increased, whereas IL-1β induction of COX-2 expression was inhibited (31). To examine whether increased endogenous PR expression could also alter aromatase expression, MCF-7 cells were transfected either with the duplex RNA PR11 (Table 3), or with a duplex RNA mismatch, as control (Table 3). After 4 d of culture in serum-free medium, PR-A and PR-B protein levels were greatly increased in the PR11-transfected MCF-7 cells, as compared with cells transfected with the mismatch control (Fig. 7A). This up-regulation of PR-A and PR-B expression was associated with a pronounced decrease in aromatase mRNA transcripts containing exon IIa (Fig. 7B) and in COX-2 protein (∼40% by densitometry analysis relative to β-actin; Fig. 7A) and mRNA transcripts (Fig. 7C). Furthermore, the levels of HER-2/neu protein also were decreased (∼60% by densitometric analysis; Fig. 7A); however no change in HER-2/neu mRNA expression was observed (data not shown).
Table 3.
Duplex RNAs (actPR) Directed against PR Promoter
| PR11 | 5′-GCU GUC UGG CCA GUC CAC ATT | ActPR |
| PR11 mismatch | 5′-GCU GUC UGG CCA CUC GAC UTT | actPR |
Only one strand (5′ to 3′) of each siRNA is shown. Mismatched bases are underlined.
Figure 7.
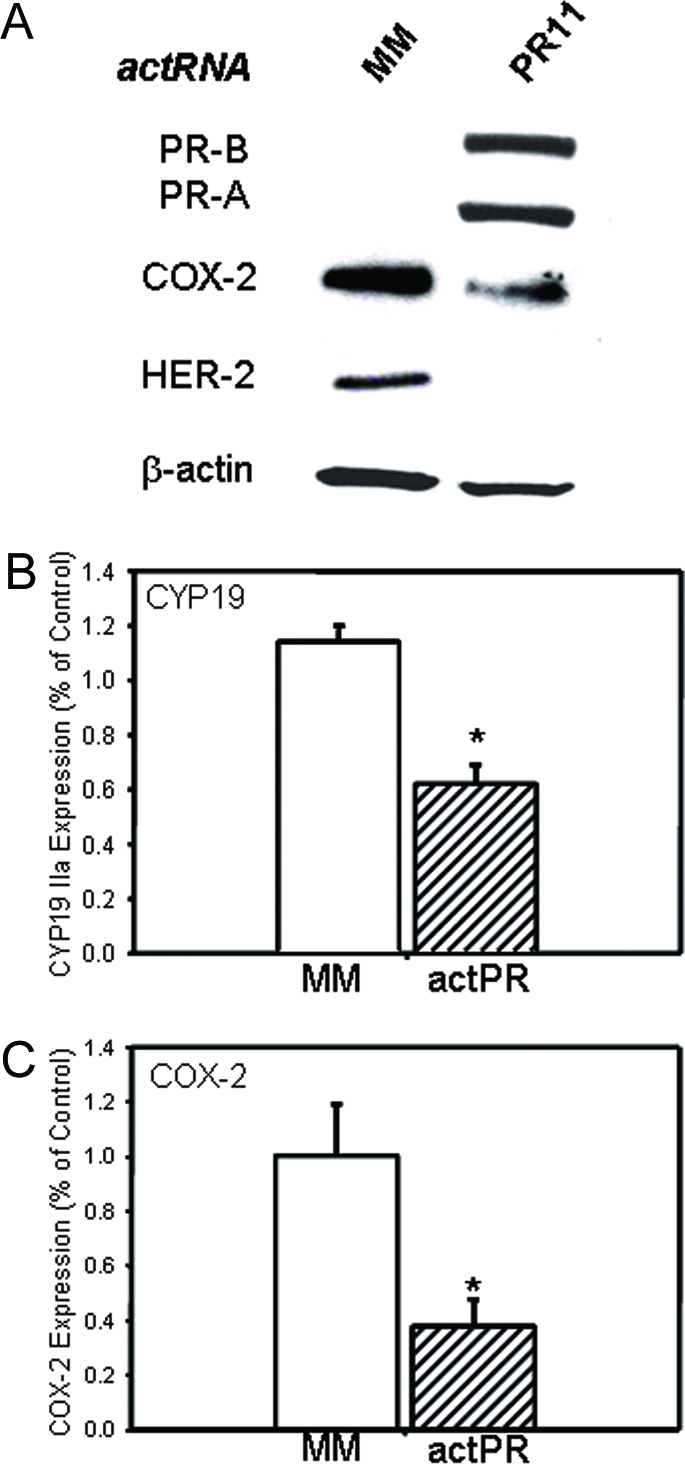
Increased Expression of PR by Activating PR RNAs (actRNA) Inhibits Basal Expression of COX-2 and CYP19 mRNA in MCF-7 Cells %MCF-7 cells were transfected with PR actRNAs targeted against the PR-B promoter and cultured for 5 d. Cell lysate proteins were analyzed for levels of PR, COX-2, HER-2/neu, and β-actin by immunoblotting (A). RNA was isolated and reverse transcribed to cDNA, and the levels of CYP19 mRNA transcripts containing exon IIa(B) and COX-2 (C) were analyzed by qRT-PCR. The relative levels of each transcript were assessed by normalizing against rRNA. Data are the means ± sem of values from three independent experiments and are expressed as % of values from PR11 mismatch control transfected samples. *, Significant difference (P < 0.05) between both groups. MM, Mismatch.
DISCUSSION
Increased expression of aromatase in the breast (32) and in surrounding adipose stromal cells (33) is considered to play a role in the pathogenesis of breast cancer. Because increased aromatase in breast cancer is associated with up-regulation of COX-2 (3), in the present study, we used breast cancer cells in culture to examine the effects of inflammatory mediators on aromatase expression. It was observed that IL-1β and cAMP both markedly increased expression of aromatase mRNA transcripts containing exons IIa and 1.3 at their 5′-ends, suggesting activation of the ovarian and breast cancer promoters, respectively. This was associated with an induction of COX-2 mRNA.
IL-1β stimulation of aromatase in breast cancer cells may be mediated by products of the cyclooxygenase pathway that activate adenylyl cyclase and increase cAMP (e.g. PGE2). In previous studies using human breast adipose stromal cells, PGE2 (which acts through cAMP and other second messengers depending upon the PGE2 receptor subtypes that are present) was found to cause a pronounced stimulation of aromatase activity, whereas, PGF2α (acting through Ca2+ and protein kinase C) did not (5). In the present study, we observed that IL-1β or cAMP stimulation of aromatase and HER-2/neu expression in T47D cells was blocked by the cyclooxygenase inhibitor, indomethacin. Moreover, PGE2 markedly stimulated aromatase expression in the T47D cells. These findings suggest that the stimulatory effects of IL-1β or cAMP on aromatase and HER-2/neu expression are mediated, in part, by the induction of COX-2 and prostaglandin production. IL-1β stimulation of aromatase also may be mediated directly by NF-κB, which has been reported to bind to the CYP19 IIa promoter to activate aromatase expression in ovarian granulosa cells (34). The IL-1β- and cAMP-induced effects on HER-2/neu expression in T47D cells provides further support for the positive feedback relationship between COX-2, aromatase, and HER-2/neu in breast cancer (10,11,35).
The role of progesterone and PR in the development and pathogenesis of breast cancer is unclear. On the one hand, progesterone/PR treatment of human breast cancer cell lines has been found to cause a rapid and transient increase in cell proliferation through up-regulation of MAPK signaling and cell cycle progression (36). Furthermore, clinical findings of the Women’s Health Initiative (WHI) indicated that hormone replacement therapy using estrogen + progesterone significantly increased breast cancer risk, as compared with use of estrogen alone (37). However, it should be emphasized, that the design of the WHI has been criticized because the majority of women in the study were in their 60s and 70s without ever having received prior hormone replacement therapy. Moreover, the progestin was administered in a continuous, rather than a cyclic, manner (38).
On the other hand, as mentioned, the presence of PR in a breast tumor has been found to serve as an independent predictor for benefit from adjuvant endocrine therapy and of disease-free survival (12,13,14). Breast tumors that are PR(−) have a higher proliferation rate and are more likely to manifest increased expression of the tumorigenic prognostic indicators, HER-2/neu and epidermal growth factor receptor, than PR(+) tumors (15,16,17,18). In postmenopausal women with advanced or metastatic breast cancer that had progressed during tamoxifen therapy, medroxyprogesterone acetate decreased disease progression (39,40,41,42). In addition, in postmenopausal women with early stage (I–IIB) breast cancer, medroxyprogesterone acetate significantly decreased the recurrence rate after a median follow-up of 37 months (43). Moreover, using clinical data obtained from breast tumor specimens at University of Texas Southwestern, we recently observed that PR expression levels serve as an important predictor for a lower stage of breast cancer at diagnosis, a measure of breast tumor progression (44).
In light of our recent findings that PR acts in a ligand-dependent and -independent manner to inhibit NF-κB activation and COX-2 expression in breast cancer and myometrial cells (20), it was our objective in the present study to further examine the molecular mechanisms underlying the antiinflammatory properties of PR on NF-κB and to characterize downstream effects on two genes previously linked to inflammation and the pathogenesis of breast cancer, aromatase and HER-2/neu. We observed that, similar to its effects on COX-2, progesterone treatment caused a pronounced inhibition of aromatase and HER-2/neu expression in T47D cells. It is likely that progesterone/PR exerts these effects through its action to inhibit expression of COX-2 and the synthesis of PGE2 and other prostanoids that mediate increased cAMP formation. It also is possible that progesterone/PR may prevent activation and/or binding of stimulatory transcription factors to the promoters of the CYP19 (34) and HER-2/neu genes.
To begin to elucidate the mechanisms for the inhibitory effects of progesterone on COX-2, we evaluated temporal effects on COX-2 mRNA expression and on the in vivo binding of NF-κB to the COX-2 promoter in T47D cells. Whereas progesterone alone had no effect, it acutely inhibited IL-1β induction of in vivo binding of NF-κB to the COX-2 promoter and of COX-2 expression. A similar inhibitory effect of progesterone on COX-2 expression and p65 binding to the COX-2 promoter was previously observed in cultured human myometrial cells (20). The effect of progesterone to inhibit p65 DNA binding is likely mediated by ligand-dependent and -independent mechanisms.
In support of previous studies of human myometrial (20), breast cancer (27), and macrophage (28) cell lines, we observed in the present study that PR acted in a ligand-dependent manner to inhibit NF-κB activation by increasing expression of IκBα. As a consequence of increased IκBα expression, more NF-κB is likely sequestered in an inactive state in the cytoplasm. In this regard, we observed that IL-1β increased nuclear levels of p65 in T47D cells after 6 h of treatment; this stimulatory effect of IL-1β was antagonized by coincubation with progesterone (Hardy, D. B., C.-C. Chien, and C. R. Mendelson, unpublished observations).
However, given that the greatest induction of IκBα protein by progesterone occurred approximately 4 h after its marked inhibitory effect on COX-2 mRNA and NF-κB binding were observed, progesterone may also cause acute impairment of NF-κB activation (i.e. via decreases in phosphorylation or direct interaction of PR with NF-κB) to decrease inflammatory processes. On the other hand, long-term antiinflammatory effects are likely sustained by increases in IκBα. To begin to evaluate the relationship between PR and IκBα in human breast tumors, we analyzed PR and IκBα mRNA levels in 28 human breast cancer cell lines using qRT-PCR. The analysis revealed a strong correlation between PR and IκBα mRNA expression in the cell lines, indicating that IκBα may be an important PR-target gene that prevents local inflammatory processes in breast tumors. Whereas the expression levels of GR were not associated with PR and were lower overall than PR in these breast cancer cells [based on high cycle time (Ct) values in qRT-PCR], there also was a striking correlation between GR and IκBα, suggesting that the presence of either of these nuclear receptors could play a protective, antiinflammatory role by increasing IκBα levels.
To directly test the potential inhibitory action of IκBα on expression of COX-2, aromatase, and HER-2/neu, we examined the effects of overexpressing a phosphorylation-defective, stable mutant form of IκBα (21) in cAMP- and IL-1-treated breast cancer cells. We observed that overexpression of this stable IκBα mutant suppressed both cAMP and IL-1β induction of all three genes, suggesting that NF-κB mediates the stimulatory effects of cAMP and IL-1β on COX-2, aromatase, and HER-2/neu expression. Interestingly, the protein kinase A catalytic subunit is maintained in an inactive state in the cytoplasm as a complex with IκB and NF-κB. Signals that cause degradation of IκB result in the activation of protein kinase A catalytic subunit, which in turn catalyzes phosphorylation and transcriptional activation of NF-κB (45).
PR can also exert its antiinflammatory actions in a ligand-independent manner by interacting directly with p65 to block its transcriptional activation and DNA binding (46). Upon knockdown of PR-A and PR-B in T47D cells using siRNA technology, we previously observed that COX-2 mRNA levels were induced more than 30-fold as compared with control cells; this effect was evident in the absence of added progesterone (20). In light of the inhibitory effects of progesterone/PR on IL-1β and cAMP induction of aromatase and HER-2/neu in breast cancer cells, we also wanted to examine ligand-independent actions of PR on expression of these target genes. siRNAs specific for PR-B and for PR-A plus PR-B were used to determine which PR subtype(s) mediates inhibition of aromatase and HER-2/neu in T47D cells. Whereas siRNA directed against PR-B alone had no apparent effect on basal expression of aromatase and HER-2/neu, complete knockdown of both PR-A plus PR-B markedly enhanced the expression of both aromatase and HER-2/neu genes. These effects were associated with increased NF-κB p65 nuclear translocation and COX-2 expression (20). The finding that siRNA-induced knockdown of PR-B alone had no effect to alter COX-2, CYP19, and HER-2/neu expression suggests either that the inhibitory action is specifically mediated by PR-A or that ablation of both PR isoforms is required. It is important to note that these effects were observed in the absence of progesterone, suggesting that PR exerts a hormone-independent effect to inhibit COX-2, CYP19, and HER-2/neu expression. A similar pronounced induction of COX-2 mRNA was observed in T47D cells transfected with an antigene nucleic acid that blocked PR transcription (47).
Conversely, when PR expression was up-regulated in MCF-7 cells [which normally express relatively low levels of PR (30)] by transfection of RNA duplexes complementary to −11 to +8 bp of the PR promoter (31), a reduction of CYP19 mRNA was observed, along with a decrease HER-2/neu and COX-2 expression. The decrease in COX-2 mRNA by overexpression of PR is consistent with previous studies (31). In addition to its important antiinflammatory properties, our recent findings suggest that PR may play an important role in the control of tumor malignancy; ablation of PR expression in T47D cells caused increased expression of the cytoskeletal protein, ezrin (47), which has been found to be associated with tumor metastasis (48).
In conclusion, our findings suggest that the PR may play an important antiinflammatory role in breast cancer cells as an inhibitor of COX-2, aromatase, and the oncogenic growth factor HER-2/neu. In the normal premenopausal breast, we propose that PR may exert these actions via ligand-dependent mechanisms involving up-regulation of the NF-κB inhibitor IκBα, resulting in decreased NF-κB activation and binding to the COX-2 promoter. This results in decreased prostaglandin production and intracellular cAMP and reduced aromatase and HER-2/neu expression. PR also may inhibit NF-κB transcriptional activity in a ligand-independent manner through its physical interaction with NF-κB p65 (46). It is tempting to speculate that this may provide an important protective mechanism in premenopausal women during phases of the menstrual cycle when circulating progesterone levels are low, as well as postmenopausally when circulating levels of progesterone are negligible. Thus, in breast cancer, when PR expression and/or function may be diminished, NF-κB activity increases, culminating in enhanced expression of these proinflammatory genes and, ultimately, in increased local estrogen production with enhanced tumor growth and progression. Our findings further suggest that measures to up-regulate endogenous PR expression in the absence of ligand may serve as an important therapeutic modality for preventing breast tumor progression.
MATERIALS AND METHODS
Cell Lines
Human breast cancer T47D and MCF-7 cells were maintained in RPMI medium with 10% fetal bovine serum (FBS) and 100 μm insulin. For experiments, cells were switched to serum-free medium and treated either with IL-1β (10 ng/ml; Sigma Chemical Co., St. Louis, MO), Bt2cAMP (1 mm; Sigma), or progesterone (100 nm; Sigma), added alone or in various combinations, for 0–24 h. In some experiments, indomethacin (10 μm, Sigma) or PGE2 (10 nm, Cayman Chemicals, Ann Arbor, MI) was added to the cells for 24 h. Total RNA or cell lysates were prepared and analyzed by qRT-PCR and immunoblotting, respectively, as described below.
Tritiated Water Assay of Aromatase Activity in MCF-7 Cells
MCF-7 cells were coincubated for 24 h with 2 μCi of [1β-3H]androst-4-ene-3,17-dione (39.5 nm; NEN Life Science Products, Boston, MA) in the absence or presence of IL-1β (10 ng/ml) or Bt2cAMP (1 mm) ± progesterone (100 nm). Aromatase activity was assayed by quantifying the release of tritium from [1β-3H]androstenedione and its incorporation into water, as described in detail previously (49). The cells were washed, scraped from the dishes, and analyzed for protein (50). Aromatase activity was expressed as femtomoles of [1β-3H]androstenedione metabolized to estrogen/h/mg protein (fmol/h/mg). Aromatase activity of MCF-7 cells cultured in serum-free medium in the absence of hormones was approximately 6.25 fmol/h/mg of protein. Effects of hormonal treatment on aromatase activity are presented relative to untreated control values.
qRT-PCR
Total RNA from T47D, MCF-7, and 26 other breast cancer cell lines was extracted by the one-step method of Chomczynski and Sacchi (51) (TRIzol; Invitrogen, Carlsbad, CA). The 26 breast cancer cell lines were derived from primary breast cancer biopsies at the Harold C. Simmons Comprehensive Cancer Center and were kindly donated by Dr. John Minna at University of Texas Southwestern Medical Center. RNA was treated with deoxyribonuclease to remove any contaminating DNA, and 4 μg were reverse transcribed using random primers and Superscript II ribonuclease H-reverse transcriptase (Invitrogen). Primer sets directed against human tissue-specific CYP19 mRNA transcripts, along with COX-2, PR, GR, IκBα, HER-2/neu, and rRNA (h36B4) were generated utilizing Primer Express software (PE Applied Biosystems, Foster City, CA) based on published sequences (Table 1).
The relative abundance of each RNA transcript was determined by qRT-PCR using the ABI Prism 7700 Detection System (Applied Biosystems, Foster City, CA) and the DNA-binding dye SYBR Green (PE Applied Biosystems) for the detection of PCR products as described in detail previously (20,52). Relative fold changes were calculated using the comparative cycle times (Ct) method with rRNA (h36B4) as the reference guide. Over a wide range of known cDNA concentrations, all primer sets were demonstrated to have good linear correlation (slope = −3.4) and equal priming efficiency for the different dilutions compared with their Ct values (data not shown). The relative abundance of each primer set compared with calibrator was determined by the formula, 2ΔΔCt, whereby ΔΔCt is the calibrated Ct value (primer − internal control).
ChIP
T47D breast cancer cells were cultured for 2–24 h in serum-free medium, in the absence or presence of IL-1β (10 ng/ml), progesterone (100 nm), or the two hormones in combination. ChIP was performed using antibody to NF-κB p65 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) to assess hormonal effects on its in vivo binding to the proximal and distal NF-κB response elements of the human COX-2 gene, as described previously (20). Briefly, T47D cells (3 × 107 cells per treatment) were washed once with PBS and incubated with 1% formaldehyde (in control medium) for 10 min at room temperature to cross-link proteins and DNA. Cross-linking was terminated by the addition of glycine (0.125 m, final concentration). The cells were washed twice with cold PBS and placed in 500 μl of lysis buffer [50 mm Tris (pH 8.1), 150 mm NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), protease inhibitor cocktail (Roche, Indianapolis, IN), and 5 mm EDTA]. The lysates were sonicated on ice to produce sheared, soluble chromatin. The soluble chromatin was precleared with Protein A/G Plus agarose beads (60 μl) at 4 C for 1 h. The samples were microfuged at 14,000 rpm to pellet the beads, and the supernatant containing the sheared chromatin was placed in new tubes. The precleared chromatin was aliquoted into 300-μl amounts and incubated with antibodies for p65 (Santa Cruz) at 4 C overnight. Two aliquots were reserved as controls: one incubated without antibody and the other with nonimmune IgG. Protein A/G Plus agarose beads (60 μl) were added to each tube, the mixtures were incubated for 2 h at 4 C, and the immune complexes were collected by centrifugation. The beads containing the immunoprecipitated complexes were washed sequentially for 5–10 min in wash buffer I (20 mm Tris-HCl, pH 8.1; 2 mm EDTA; 0.1% SDS; 1% Triton X-100; 150 mm NaCl), wash buffer II (same as I, except containing 500 mm NaCl), wash buffer III (10 mm Tris-HCl, pH 8.1; 1 mm EDTA; 1% Nonidet P-40; 1% deoxycholate; 0.25 m LiCl), and in 2× Tris-EDTA buffer. The beads were eluted with 250 μl elution buffer (1% SDS, 0.1 mm NaHCO3 + 20 μg salmon sperm DNA (Sigma) at room temperature. This was repeated once and eluates were combined. Cross-linking of the immunoprecipitated chromatin complexes and input controls (10% of the total soluble chromatin) was reversed by heating the samples at 65 C for 4 h. Proteinase K (15 μg, Invitrogen) was added to each sample in buffer (50 mm Tris-HCl, pH 8.5; 1% SDS; 10 mm EDTA) and incubated for 1 h at 45 C. The DNA was purified by phenol-chloroform extraction and precipitated in EtOH overnight at −20 C. Samples and input controls were diluted in 10–100 μl Tris-EDTA buffer just before PCR. Real-time PCR was employed using forward (5′-TAAAACATGTCAGCCTTT-CTTAACCTT-3′) and reverse (5′-CGGCCCTGAGGTCCG-3′) primers that amplify an approximately 100-bp region surrounding the proximal NF-κB response element, and forward (5′-GGAGGAGAGGGAGGGATC-AG-3′) and reverse (5′-TGCCCCAATTTGG-GAGC-3′) primers surrounding the distal NF-κB response element of the COX-2 promoter. Using serial dilutions of human chromosomal DNA, these primers were demonstrated to have equal efficiency in priming their target sequences.
Infection of Type II Cells with Recombinant Adenoviruses
T47D cells were infected with recombinant adenoviruses expressing a phosphorylation-defective form of IκBα in which the IκB kinase serine phosphorylation sites (S32 and S36) were mutated (21), or with a recombinant adenovirus containing CMV:β-Gal, as a control. Adenoviral infection was carried out as described previously (53). Briefly, the cells were incubated for 3 h with recombinant adenoviruses expressing either the mutant IκBα (CMV:IκBαmut) [multiplicity of infection (m.o.i.) = 5] or with CMV:β-Gal (m.o.i. = 5). The cells were then washed and incubated in the absence or presence of IL-1β (10 ng/ml) or cAMP (1 mm) for either 6 or 24 h.
Double-Stranded siRNAs and Antigene RNAs (agRNAs)
In previous studies, we observed that transfection of T47D breast cancer cells with siRNAs specific for PR-A and PR-B, which abrogated expression of these PR isoforms, caused a greater than 30-fold increase in COX-2 mRNA levels (20). Furthermore, it was found that a duplex agRNA complementary to the PR promoter sequence from −11 to +8 (PR11) caused a marked increase in PR-A and PR-B mRNA in MCF-7 breast cancer cells, whereas a mismatch duplex agRNA had no effect (31). These technologies were used in the present study to analyze the effects of altering endogenous PR on expression of aromatase, COX-2, and HER-2/neu. Twenty-one nucleotide siRNAs were synthesized by Integrated DNA Technologies (Coralville, IA). Oligonucleotides contained two 2′-deoxythymidine on the 3′-end were deprotected and desalted. Sequences are listed in Table 1 and identified relative to the transcription start site as described (PR: GenBank entry NM_000926). Duplex RNAs were made and melting temperatures of the duplexes measured as previously described (47).
Lipid-Mediated Transfection of RNA Duplexes
RNA duplexes were introduced into cells as previously described (20). Cells were plated 2 d before transfection at 200,000 cells per well in six-well plates (Costar, Cambridge, MA) without antibiotics. Transfections with agRNA or siRNAs were performed using Oligofectamine (Invitrogen) following the manufacturer’s instructions. Briefly, duplex RNA was mixed with oligofectamine in Optimem (Invitrogen) and added to the cells to a final concentration of 100 nm. Media were changed 24 h later, and cells were harvested 5 d after transfection for Western analysis or qRT-PCR.
Preparation of Cell Lysates and Nuclear Extracts and Immunoblot Analysis
T47D and MCF-7 cell cytoplasmic and nuclear extracts were prepared as described previously (52). Nuclear and cytoplasmic proteins were fractionated in gradient polyacrylamide gels (Invitrogen) and transferred onto Hybond-P (Amersham Pharmacia Biotech, Piscataway, NJ). Blots were probed using rabbit antibodies for PR (Cell Signaling Technology, Danvers, MA), which recognize PR-A and PR-B, diluted at 1:500 in 5% milk-Tris-buffered saline-Tween 20 buffer and with horseradish peroxidase-conjugated goat antirabbit IgG (1:10,000) diluted in 5% milk-Tris-buffered saline-Tween 20 buffer (Amersham Pharmacia) as secondary antibody. Rabbit antibodies raised against human HER-2/neu (Cell Signaling Technology, Inc., Danvers, MA; 1:1000), COX-2 (Cayman Chemicals, 1:500), and IκBα (Santa Cruz Biotechnology, 1:1000) were also employed for analysis of HER-2/neu, COX-2, and IκBα protein, respectively. Immunoreactive bands were visualized using an enhanced chemiluminescence detection system (Amersham Pharmacia); the relative abundance of each protein was analyzed by densitometry and compared with the corresponding levels of glyceraldehyde-3-phosphate, tubulin, or β-actin.
Statistics
For the majority of the experiments using qRT-PCR and ChIP, the data are expressed as the mean of arbitrary values ± sem. The significance of differences (P < 0.05) between mean values was evaluated using the unpaired Student’s t test.
Acknowledgments
We thank Dr. Richard B. Gaynor (Lilly Research Laboratories, Indianapolis, IN) for providing recombinant adenovirus expressing IκBα with mutations in the IκB kinase α phosphorylation sites.
Footnotes
This work was supported by National Institutes of Health Grant 5 R01 DK031206 (to C.R.M.). Daniel Hardy was supported, in part, by a postdoctoral fellowship from the Susan G. Komen Foundation (PDF0600877).
Disclosure Statement: None of the authors has anything to declare regarding potential conflicts of interest.
First Published Online May 15, 2008
Abbreviations: agRNA, Antigene RNA; Bt2cAMP, dibutyryl cAMP; ChIP, chromatin immunoprecipitation; CMV, cytomegalovirus; COX-2, cyclooxygenase 2; Ct, cycle time; ER, estrogen receptor; GR, glucocorticoid receptor; m.o.i., multiplicity of infection; NF-κB, nuclear factor κB; PGE2, prostaglandin E2; PR, progesterone receptor; qRT-PCR, quantitative real-time RT-PCR; SDS, sodium dodecyl sulfate; siRNA, small interfering RNA.
References
- Thompson Jr EA, Siiteri PK 1974 The involvement of human placental microsomal cytochrome P-450 in aromatization. J Biol Chem 249:5373–5378 [PubMed] [Google Scholar]
- Simpson ER, Zhao Y, Agarwal VR, Michael MD, Bulun SE, Hinshelwood MM, Graham-Lorence S, Sun T, Fisher CR, Qin K, Mendelson CR 1997 Aromatase expression in health and disease. Recent Prog Horm Res 52:185–213 [PubMed] [Google Scholar]
- Brodie AM, Lu Q, Long BJ, Fulton A, Chen T, Macpherson N, DeJong PC, Blankenstein MA, Nortier JW, Slee PH, van de Ven J, van Gorp JM, Elbers JR, Schipper ME, Blijham GH, Thijssen JH 2001 Aromatase and COX-2 expression in human breast cancers. J Steroid Biochem Mol Biol 79:41–47 [DOI] [PubMed] [Google Scholar]
- Kamat A, Hinshelwood MM, Murry BA, Mendelson CR 2002 Mechanisms in tissue-specific regulation of estrogen biosynthesis in humans. Trends Endocrinol Metab 13:122–128 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Agarwal VR, Mendelson CR, Simpson ER 1996 Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology 137:5739–5742 [DOI] [PubMed] [Google Scholar]
- Zhou C, Zhou D, Esteban J, Murai J, Siiteri PK, Wilczynski S, Chen S 1996 Aromatase gene expression and its exon I usage in human breast tumors. Detection of aromatase messenger RNA by reverse transcription-polymerase chain reaction. J Steroid Biochem Mol Biol 59:163–171 [DOI] [PubMed] [Google Scholar]
- Brueggemeier RW, Richards JA, Petrel TA 2003 Aromatase and cyclooxygenases: enzymes in breast cancer. J Steroid Biochem Mol Biol 86:501–507 [DOI] [PubMed] [Google Scholar]
- Cao Y, Karin M 2003 NF-κB in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia 8:215–223 [DOI] [PubMed] [Google Scholar]
- Ristimaki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J 2002 Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res 62:632–635 [PubMed] [Google Scholar]
- Subbaramaiah K, Howe LR, Port ER, Brogi E, Fishman J, Liu CH, Hla T, Hudis C, Dannenberg AJ 2006 HER-2/neu status is a determinant of mammary aromatase activity in vivo: evidence for a cyclooxygenase-2-dependent mechanism. Cancer Res 66:5504–5511 [DOI] [PubMed] [Google Scholar]
- Benoit V, Relic B, Leval Xd X, Chariot A, Merville MP, Bours V 2004 Regulation of HER-2 oncogene expression by cyclooxygenase-2 and prostaglandin E2. Oncogene 23:1631–1635 [DOI] [PubMed] [Google Scholar]
- Osborne CK, Yochmowitz MG, Knight III WA, McGuire WL 1980 The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer 46:2884–2888 [DOI] [PubMed] [Google Scholar]
- Ravdin PM, Green S, Dorr TM, McGuire WL, Fabian C, Pugh RP, Carter RD, Rivkin SE, Borst JR, Belt RJ 1992 Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest Oncology Group study. J Clin Oncol 10:1284–1291 [DOI] [PubMed] [Google Scholar]
- Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM 2003 Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol 21:1973–1979 [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL 1987 Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182 [DOI] [PubMed] [Google Scholar]
- Tandon AK, Clark GM, Chamness GC, Ullrich A, McGuire WL 1989 HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol 7:1120–1128 [DOI] [PubMed] [Google Scholar]
- Lebeau A, Unholzer A, Amann G, Kronawitter M, Bauerfeind I, Sendelhofert A, Iff A, Lohrs U 2003 EGFR, HER-2/neu, cyclin D1, p21 and p53 in correlation to cell proliferation and steroid hormone receptor status in ductal carcinoma in situ of the breast. Breast Cancer Res Treat 79:187–198 [DOI] [PubMed] [Google Scholar]
- Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, Elledge RM 2005 Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst 97:1254–1261 [DOI] [PubMed] [Google Scholar]
- Horwitz KB, Koseki Y, McGuire WL 1978 Estrogen control of progesterone receptor in human breast cancer: role of estradiol and antiestrogen. Endocrinology 103:1742–1751 [DOI] [PubMed] [Google Scholar]
- Hardy DB, Janowski BA, Corey DR, Mendelson CR 2006 Progesterone receptor (PR) plays a major anti-inflammatory role in human myometrial cells by antagonism of NF-κB activation of cyclooxygenase 2 (COX-2) expression. Mol Endocrinol 20:2724–2733 [DOI] [PubMed] [Google Scholar]
- Liu L, Kwak YT, Bex F, Garcia-Martinez LF, Li XH, Meek K, Lane WS, Gaynor RB 1998 DNA-dependent protein kinase phosphorylation of IκBα and IκBβ regulates NF-κB DNA binding properties. Mol Cell Biol 18:4221–4234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Cruz ES, Shapiro CL, Brueggemeier RW 2005 Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells. J Clin Endocrinol Metab 90:2563–2570 [DOI] [PubMed] [Google Scholar]
- Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS 1995 Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids. Science 270:283–286 [DOI] [PubMed] [Google Scholar]
- Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M 1995 Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science 270:286–290 [DOI] [PubMed] [Google Scholar]
- Dumont A, Hehner SP, Schmitz ML, Gustafsson JA, Liden J, Okret S, van der Saag PT, Wissink S, van der Burg B., Herrlich P, Haegeman G, De Bosscher K, Fiers W 1998 Cross-talk between steroids and NF-κB: what language? Trends Biochem Sci 23:233–235 [DOI] [PubMed] [Google Scholar]
- Alcorn JL, Islam KN, Young PP, Mendelson CR 2004 Glucocorticoid inhibition of SP-A gene expression in lung type II cells is mediated via the TTF-1-binding element. Am J Physiol Lung Cell Mol Physiol 286:L767–L776 [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Archer TK 2002 Differential activation of the IκBα and mouse mammary tumor virus promoters by progesterone and glucocorticoid receptors. J Steroid Biochem Mol Biol 81:309–317 [DOI] [PubMed] [Google Scholar]
- Miller L, Hunt JS 1998 Regulation of TNF-α production in activated mouse macrophages by progesterone. J Immunol 160:5098–5104 [PubMed] [Google Scholar]
- Deroo BJ, Archer TK 2001 Glucocorticoid receptor activation of the IκBα promoter within chromatin. Mol Biol Cell 12:3365–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski BA, Huffman KE, Schwartz JC, Ram R, Nordsell R, Shames DS, Minna JD, Corey DR 2006 Involvement of AGO1 and AGO2 in mammalian transcriptional silencing. Nat Struct Mol Biol 13:787–792 [DOI] [PubMed] [Google Scholar]
- Janowski BA, Younger ST, Hardy DB, Ram R, Huffman KE, Corey DR 2007 Activating gene expression in mammalian cells with promoter-targeted duplex RNAs. Nat Chem Biol 3:166–173 [DOI] [PubMed] [Google Scholar]
- Lu Q, Nakmura J, Savinov A, Yue W, Weisz J, Dabbs DJ, Wolz G, Brodie A 1996 Expression of aromatase protein and messenger ribonucleic acid in tumor epithelial cells and evidence of functional significance of locally produced estrogen in human breast cancers. Endocrinology 137:3061–3068 [DOI] [PubMed] [Google Scholar]
- O'Neill JS, Elton RA, Miller WR 1988 Aromatase activity in adipose tissue from breast quadrants: a link with tumour site. Br Med J (Clin Res Ed) 296:741–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan WQ, Yanase T, Morinaga H, Mu YM, Nomura M, Okabe T, Goto K, Harada N, Nawata H 2005 Activation of peroxisome proliferator-activated receptor-γ and retinoid X receptor inhibits aromatase transcription via nuclear factor-κB. Endocrinology 146:85–92 [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ 2002 Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer. Evidence for involvement of AP-1 and PEA3. J Biol Chem 277:18649–18657 [DOI] [PubMed] [Google Scholar]
- Lange CA, Gioeli D, Hammes SR, Marker PC 2006 Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Annu Rev Physiol 69:17.1–17.29 [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, Khandekar J, Petrovitch H, McTiernan A 2003 Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA 289:3243–3253 [DOI] [PubMed] [Google Scholar]
- Klaiber EL, Vogel W, Rako S 2005 A critique of the Women’s Health Initiative hormone therapy study. Fertil Steril 84:1589–1601 [DOI] [PubMed] [Google Scholar]
- Hultborn R, Johansson-Terje I, Bergh J, Glas U, Hallsten L, Hatschek T, Holmberg E, Idestrom K, Norberg B, Ranstam J, Soderberg M, Wallgren UB 1996 Second-line endocrine treatment of advanced breast cancer— a randomized cross-over study of medroxy-progesterone acetate and aminoglutethimide. Acta Oncol 35(Suppl 5):75 [DOI] [PubMed] [Google Scholar]
- Parazzini F, Colli E, Scatigna M, Tozzi L 1993 Treatment with tamoxifen and progestins for metastatic breast cancer in postmenopausal women: a quantitative review of published randomized clinical trials. Oncology 50:483–489 [DOI] [PubMed] [Google Scholar]
- Byrne MJ, Gebski V, Forbes J, Tattersall MH, Simes RJ, Coates AS, Dewar J, Lunn M, Flower C, Gill PG, Stewart J 1997 Medroxyprogesterone acetate addition or substitution for tamoxifen in advanced tamoxifen-resistant breast cancer: a phase III randomized trial. Australian-New Zealand Breast Cancer Trials Group. J Clin Oncol 15:3141–3148 [DOI] [PubMed] [Google Scholar]
- Buzdar A, Jonat W, Howell A, Jones SE, Blomqvist C, Vogel CL, Eiermann W, Wolter JM, Azab M, Webster A, Plourde PV 1996 Anastrozole, a potent and selective aromatase inhibitor, versus megestrol acetate in postmenopausal women with advanced breast cancer: results of overview analysis of two phase III trials. Arimidex Study Group. J Clin Oncol 14:2000–2011 [DOI] [PubMed] [Google Scholar]
- Pannuti F, Martoni A, Cilenti G, Camaggi CM, Fruet F 1988 Adjuvant therapy for operable breast cancer with medroxyprogesterone acetate alone in postmenopausal patients or in combination with CMF in premenopausal patients. Eur J Cancer Clin Oncol 24:423–429 [DOI] [PubMed] [Google Scholar]
- Coyle YM, Xie XJ, Hardy DB, Ashfaq R, Mendelson CR 2007 Progesterone receptor and not estrogen receptor expression is a marker for early stage breast cancer: Implications for progesterone receptor reducing breast cancer metastatic potential. Cancer Lett 258:253–261 [DOI] [PubMed] [Google Scholar]
- Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S 1997 The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89:413–424 [DOI] [PubMed] [Google Scholar]
- Kalkhoven E, Wissink S, van der Saag PT, van der Burg B 1996 Negative interaction between the RelA(p65) subunit of NF-κB and the progesterone receptor. J Biol Chem 271:6217–6224 [DOI] [PubMed] [Google Scholar]
- Janowski BA, Kaihatsu K, Huffman KE, Schwartz JC, Ram R, Hardy D, Mendelson CR, Corey DR 2005 Inhibiting transcription of chromosomal DNA with antigene peptide nucleic acids. Nat Chem Biol 1:210–215 [DOI] [PubMed] [Google Scholar]
- Hunter KW 2004 Ezrin, a key component in tumor metastasis. Trends Mol Med 10:201–204 [DOI] [PubMed] [Google Scholar]
- Ackerman GE, Smith ME, Mendelson CR, MacDonald PC, Simpson ER 1981 Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J Clin Endocrinol Metab 53:412–417 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ 1951 Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275 [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N 1987 Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
- Condon JC, Hardy DB, Kovaric K, Mendelson CR 2006 Upregulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of NF-κB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol 20:764–775 [DOI] [PubMed] [Google Scholar]
- Alcorn JL, Gao E, Chen Q, Smith ME, Gerard RD, Mendelson CR 1993 Genomic elements involved in transcriptional regulation of the rabbit surfactant protein-A gene. Mol Endocrinol 7:1072–1085 [DOI] [PubMed] [Google Scholar]