Abstract
Activation of the protein kinase A (PKA) signaling system is necessary for FSH-induced granulosa cell differentiation, but it is not known whether activation of PKA is sufficient to account for the complex pattern of gene expression that occurs during this process. We addressed this question by infecting granulosa cells with a lentiviral vector that directs the expression of a constitutively active mutant of PKA (PKA-CQR) and compared the cellular responses to PKA-CQR with cells stimulated by FSH. Expression of PKA-CQR in undifferentiated granulosa cells resulted in the induction of both estrogen and progesterone production in the absence of cAMP. The stimulatory effects of both PKA-CQR and FSH on estrogen and progesterone production were suppressed by the PKA inhibitor H-89 and were mimicked by PKA-selective cAMP agonists. mRNA levels for P450scc and 3β-HSD were induced to a similar extent by FSH and PKA-CQR, whereas mRNA levels for P450arom and the LHr were induced to a greater extent by FSH. Microarray analysis of gene expression profiles revealed that the majority of genes appeared to be comparably regulated by FSH and PKA-CQR but that some genes appear to be induced to a greater extent by FSH than by PKA-CQR. These results indicate that the PKA signaling pathway is sufficient to account for the induction of most genes (as identified by microarray analysis), including those of the progesterone biosynthetic pathway during granulosa cell differentiation. However, optimal induction of aromatase, the LHr, and other genes by FSH appears to require activation of additional signaling pathways.
THE TRANSITION OF an immature preantral follicle to a mature preovulatory follicle is associated with the acquisition of steroidogenic capacity as well as the acquisition of the ability of the mature follicle to ovulate and luteinize in response to the midcycle LH surge. It is indisputable that the primary trigger of this critical event in the female reproductive cycle is stimulation of granulosa cells by FSH (1,2). In response to FSH stimulation, the developing follicle gains the ability to produce large quantities of estradiol via the induction of aromatase (3,4) and gains the ability to respond to the midcycle LH surge by acquiring LH receptors on its granulosa cells (5). In addition, the maturing follicle acquires the ability to produce progesterone, which is also mediated by FSH through the induction of 3β-hydroxysteroid dehydrogenase (3β-HSD) and cholesterol side-chain cleavage enzyme (P450scc), both of which are required for the conversion of cholesterol to progesterone (6,7,8).
It is well known that FSH signaling in granulosa cells involves the activation of adenylyl cyclase and stimulation of protein kinase A (PKA) activity (9,10,11). However, it is uncertain as to whether the activation of PKA is sufficient to account for the complex pattern of gene expression that is observed during granulosa cell differentiation. This uncertainty arises because a number of other intracellular signaling cascades including the MAPK, the ERK, and the phosphatidylinositol-3 kinase (PI3K) pathway as well as the activation of cAMP-dependent guanine nucleotide exchange factors are also stimulated by FSH in granulosa cells (12,13,14,15).
To address the question whether the activation of PKA is sufficient to trigger granulosa cell differentiation, we created a lentiviral vector that directs the expression of a constitutively active mutant of PKA (PKA-CQR) in which a mutation of the catalytic subunit of PKA reduces its ability to bind to the regulatory subunit and therefore confers catalytic activity in the absence of cAMP (16). We compared the abilities of PKA-CQR and FSH to stimulate estrogen and progesterone production as well as the expression of differentiation-associated mRNAs in immature granulosa cells.
RESULTS
Effect of FSH, a Constitutively Active PKA Lentiviral Vector or a Kinase-Inactive PKA Lentiviral Vector on Estradiol, Progesterone, and cAMP Production by Undifferentiated Granulosa Cells
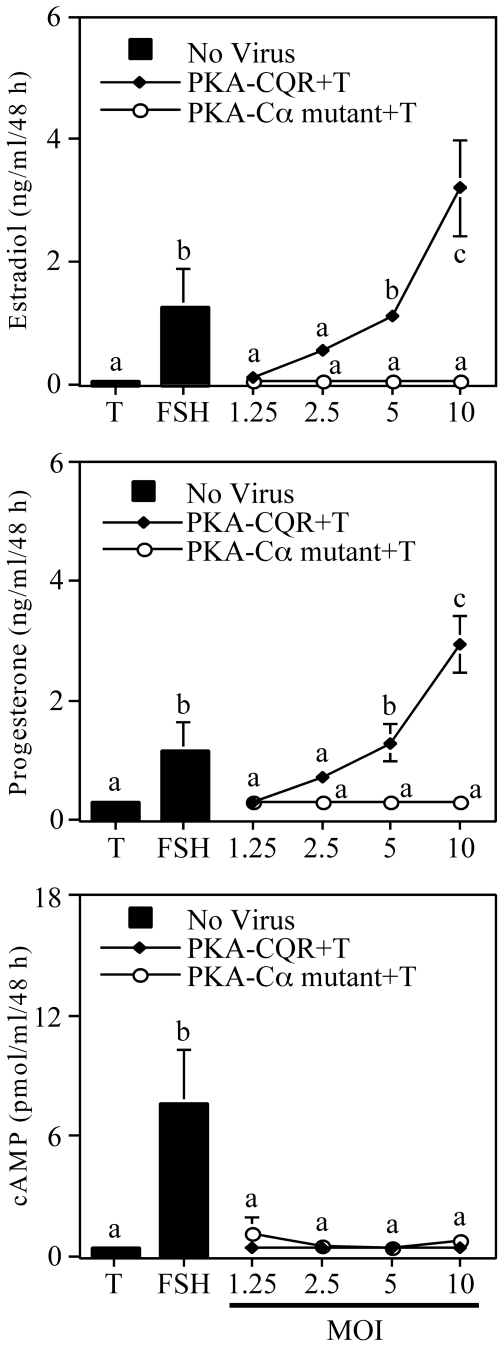
Primary cultures of granulosa cells collected from sexually immature female rats were stimulated by FSH or infected with increasing amounts of lentiviral vectors that direct the expression of either a constitutively active PKA (PKA-CQR) (16) or a kinase-inactive mutant of PKA (Cα-mutant) (17) as described in the legend (Fig. 1). Figure 1 (top panel) illustrates that, as expected, FSH plus testosterone stimulated estradiol production by these cells. Increasing amounts of a lentiviral vector that directs the expression of a constitutively active PKA-CQR also resulted in a dose-dependent increase in estradiol production, whereas increasing amounts of a lentiviral vector that directs the expression of a kinase-inactive mutant of PKA (Cα-mut) had no effect on estradiol production. The center panel of Fig. 1 shows that the constitutively active PKA, but not the kinase-inactive PKA mutant, also stimulated progesterone production. The lower panel of Fig. 1 shows that although FSH stimulated cAMP production, there was no increase in cAMP levels in medium from cells infected with constitutively active PKA-CQR. These findings indicate that activation of PKA is sufficient to induce both the estradiol and progesterone biosynthetic pathways and that this occurred independently of cAMP signaling. Figure 2 shows that whole-cell lysates of granulosa cells infected with either the PKA-CQR or Cα-mutant lentiviral vectors displayed increased amounts of immunoreactive catalytic subunit of PKA when compared with uninfected cells. When standardized to the GAPDH signal, levels of immunoreactive PKA catalytic subunit were 2.47 ± 0.7-fold (mean ± 1 sem, n = 3) greater in PKA-CQR-infected cells when compared with noninfected cells.
Figure 1.
Estradiol, Progesterone, and cAMP Production by Undifferentiated Granulosa Cells Infected with the PKA-CQR or PKA-Cα Mutant Lentiviral Vectors
Suspension of undifferentiated rat granulosa cells in M199 containing 6 μg/ml polybrene were infected with PKA-CQR or PKA-Cα mutant lentivirus at MOI ranging from 1.25–10. Control incubations were performed in M199 plus polybrene. Two hours later, the cell and virus suspensions were plated in 48-well plates with M199 containing a final concentration of 10% CS. The next morning, medium, unattached cells, and free lentivirus were removed and replaced with M199. After 24 h, medium was removed and replaced with fresh medium containing 30 ng/ml testosterone with or without FSH (100 ng/ml). Forty-eight hours later, medium was collected and analyzed for estradiol (top), progesterone (middle), and cAMP (bottom) content by RIA. Results show mean ± 1 sem of three groups of granulosa cells. Significant differences were considered when P < 0.05 and are indicated by different letters in the figure.
Figure 2.
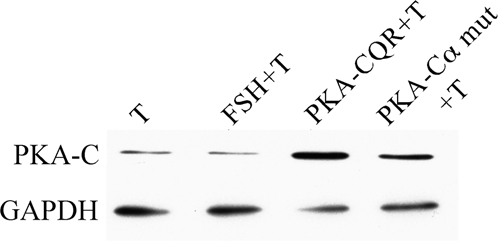
Immunoblot Analysis of Granulosa Cells Infected with Either the PKA-CQR or PKA-Cα Mutant Lentiviral Vectors
Granulosa cells were infected with either the PKA-CQR or PKA-Cα mutant lentiviral vectors (5 MOI) as described in Fig. 1. The next morning, unattached cells and free lentivirus were removed and replaced with M199. After 24 h, medium was removed and replaced with M199 containing 30 ng/ml testosterone with or without FSH (100 ng/ml). Cells infected with lentiviral vectors were cultured in M199 plus 30 mg/ml testosterone. Forty-eight hours later, cells were collected and processed for Western immunoblotting with anti-PKA-C followed by anti-GAPDH to verify equivalent loading of samples as described in Materials and Methods. Results are representative of three separate groups of granulosa cells.
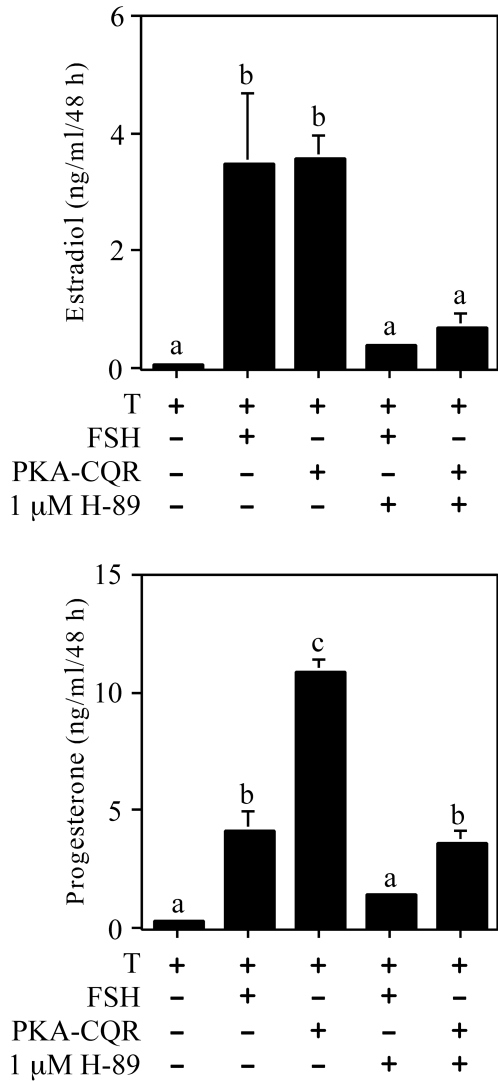
Figure 3 illustrates a time course of estradiol (top panel) and progesterone (center panel) production by undifferentiated granulosa cells that were stimulated by FSH, PKA-CQR, or the inactive PKA Cα-mutant. The temporal responses to FSH and PKA-CQR stimulation both showed a lag period before a significant stimulation of estradiol and progesterone production was observed at the 48-h time point. The inactive PKA Cα-mutant did not stimulate either estradiol or progesterone production. As shown in the lower panel, FSH, but not PKA-CQR or the inactive PKA Cα-mutant, stimulated cAMP production by these cells. Figure 4 shows that the PKA inhibitor H-89 suppressed both estradiol (top panel) and progesterone (lower panel) production by undifferentiated granulosa cells stimulated by either FSH or PKA-CQR over a 48-h culture interval.
Figure 3.
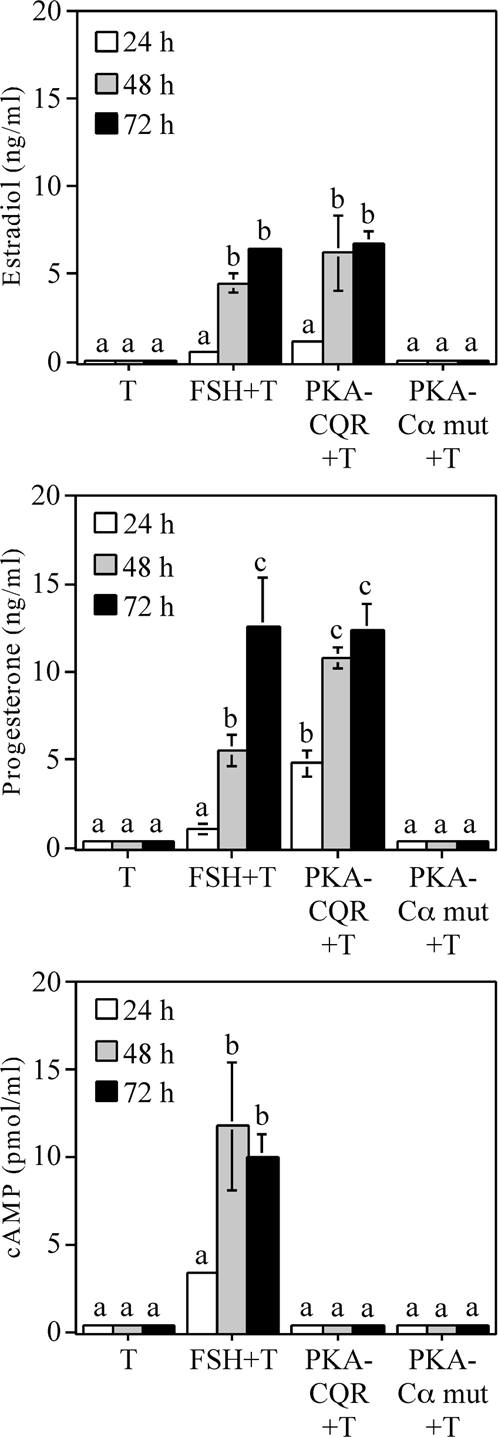
Time Course of Estradiol, Progesterone, and cAMP Production by Undifferentiated Granulosa Cells Infected with PKA-CQR or PKA-Cα Mutant Lentiviral Vectors
Granulosa cells were infected with either the PKA-CQR or PKA-Cα mutant lentiviral vectors (10 MOI) as described in Fig. 1. The next morning, unattached cells and free lentivirus were removed and replaced with fresh medium containing 30 ng/ml testosterone with or without FSH (100 ng/ml). After 24, 48, and 72 h, medium was collected and analyzed for estradiol (top), progesterone (middle), and cAMP (bottom) content by RIA. Results show mean ± 1 sem of three groups of granulosa cells. Significant differences were considered when P < 0.05 and are indicated by different letters in the figure.
Figure 4.
Effect of the PKA Inhibitor H-89 on Estradiol and Progesterone Production by Undifferentiated Granulosa Cells Infected with the PKA-CQR or PKA-Cα Mutant Lentiviral Vectors
Granulosa cells were infected with either the PKA-CQR or PKA-Cα mutant lentiviral vectors (10 MOI) as described in Fig. 1. The next morning, unattached cells and free lentivirus were removed and replaced with M199 with or without the PKA inhibitor H-89 at 1 μm. One hour later, 30 ng/ml testosterone with or without FSH (100 ng/ml) was added as indicated in the figure. Forty-eight hours later, medium was collected and analyzed for estradiol (top) and progesterone (bottom) content by RIA. Results show mean ± 1 sem of three groups of granulosa cells. Significant differences were considered when P < 0.05 and are indicated by different letters in the figure.
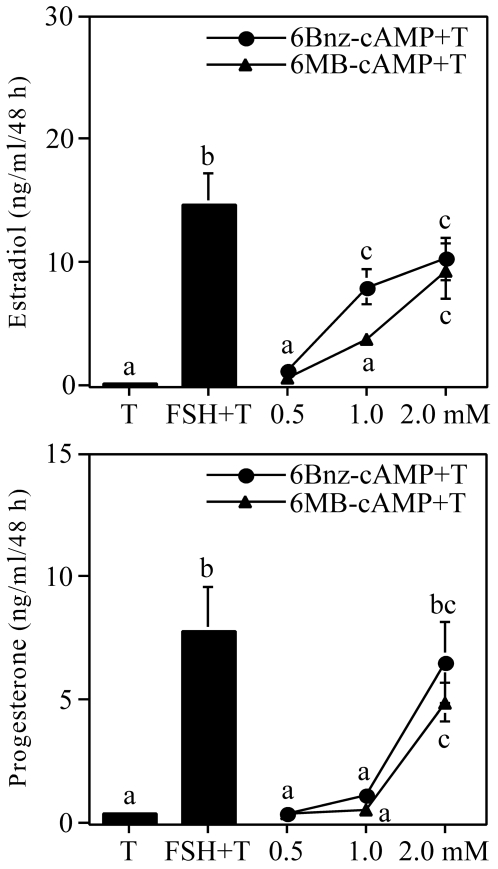
PKA-Selective cAMP Analogs Stimulate Estradiol and Progesterone Production by Undifferentiated Granulosa Cells
Collectively, the results presented in Figs. 1–4 indicate that constitutively active PKA, in the absence of any apparent cAMP production, is able to induce both the estradiol and progesterone biosynthetic pathways in undifferentiated granulosa cells. As an independent confirmation of these observations, we investigated the effects of two PKA-selective analogs of cAMP, 6-Bnz-cAMP and 6-MB-cAMP (18), upon estradiol and progesterone production by undifferentiated granulosa cells. Results presented in Fig. 5 show that both PKA-selective analogs were able to induce both estradiol (top panel) and progesterone (lower panel) synthesis in these cells.
Figure 5.
Estradiol and Progesterone Production by Undifferentiated Granulosa Cells Stimulated with FSH or cAMP Analogs
Undifferentiated rat granulosa cells were plated overnight in 48-well plates of M199 containing 10% CS. The next morning, media and unattached cells were removed and replaced with M199. After 24 h, medium was removed and replaced with fresh medium containing 30 ng/ml testosterone with or without FSH at 100 ng/ml and 6-Bnz-cAMP or 6-MB-cAMP at concentrations of 0.5, 1, and 2 mm. Forty-eight hours later, medium was collected and analyzed for estradiol (top) and progesterone (bottom) content by RIA. Results show mean ± 1 sem of four groups of granulosa cells. Significant differences were considered when P < 0.05 and are indicated by different letters in the figure.
Effects of FSH and Constitutively Active PKA-CQR on the Expression of Differentiation-Associated mRNAs
We performed ribonuclease (RNase) protection assays to compare the effects of FSH and constitutively active PKA-CQR on the expression of selected mRNAs associated with granulosa cell differentiation. As shown in Fig. 6, both FSH and PKA-CQR led to a comparable stimulation of mRNAs for P450scc, 3β-HSD, and the α-subunit of inhibin, whereas FSH stimulation led to a greater increase in mRNAs for LHr and P450arom. This finding extends our earlier observations that activation of the FSH receptor is more effective in inducing the LH receptor and aromatase than other activators of the cAMP/PKA signaling system (19,20).
Figure 6.
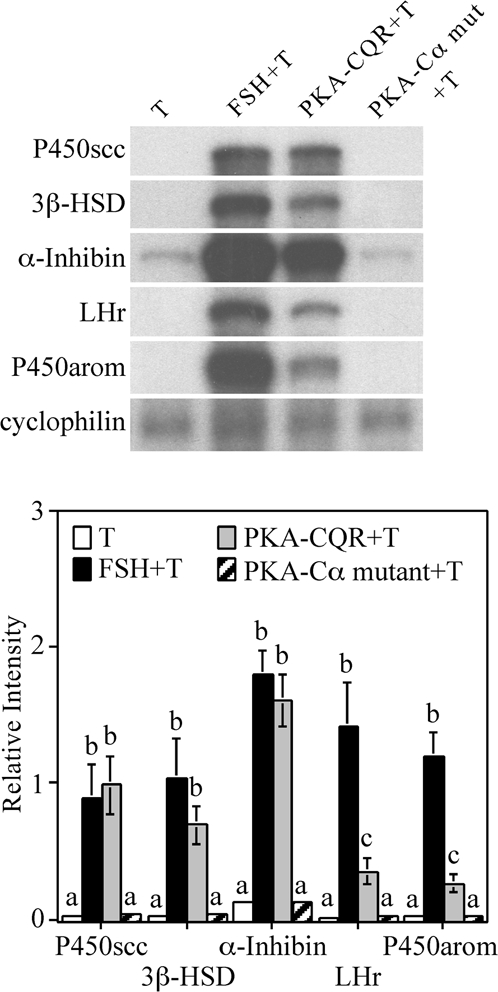
RNase Protection Assay of mRNAs Associated with Granulosa Cell Differentiation in Granulosa Cells Infected with PKA-CQR or PKA-Cα Mutant Lentiviral Vectors
Granulosa cells were infected with either the PKA-CQR or PKA-Cα mutant lentiviral vectors (5 MOI) as described in Fig. 1. The next morning, unattached cells and free lentivirus were removed and replaced with M199. Twenty-four hours later, medium was replaced with fresh medium containing 30 ng/ml testosterone with or without FSH (100 ng/ml). After 48 h, total RNA was extracted from monolayers and analyzed for mRNA by RNase protection assay. The housekeeping gene cyclophilin was included as a loading control. Results shown are representative of three separate groups of granulosa cells (top). Densitometry analysis of the RNase protection assay was performed using the NIH Image software (bottom). The signal of each mRNA was normalized to the housekeeping gene cyclophilin (relative intensity). Results show mean ± 1 sem of three groups of granulosa cells. Significant differences were considered when P < 0.05 and are indicated by different letters in the figure.
We further explored possible explanations for the aforementioned differences between FSH and PKA-CQR in the induction of mRNAs for aromatase and the LHr. On one hand, the preferential induction of aromatase and the LHr by FSH could be due to the possibility that, in addition to activation of PKA, FSH may also stimulate an additional pathway or pathways to optimally induce these genes. On the other hand, it is possible that stimulation of granulosa cells with PKA-CQR, because of its constitutive activity, generates an intracellular signal that differs from that of FSH, which, in turn, might result in suboptimal induction of aromatase and the LHr. We reasoned that we could discriminate between these two possibilities by stimulating granulosa cells with both FSH and PKA-CQR. If FSH activates an additional pathway, we would expect that combined stimulation of granulosa cells by FSH and PKA-CQR would lead to an induction of mRNAs for aromatase and LHr that is greater than that of PKA-CQR alone. Conversely, if the stimulus generated by PKA-CQR is unfavorable for the induction of aromatase and the LHr, we would expect that combined stimulation of granulosa cells by FSH and PKA-CQR would lead to a lower induction of these genes when compared with FSH stimulation alone. Results presented in Fig. 7 show that costimulation of granulosa cells with FSH plus PKA-CQR resulted in significantly greater levels of mRNA for aromatase and the LHr compared with PKA-CQR alone, whereas there was no interaction between PKA-CQR and FSH in the induction of mRNAs for P450scc or 3β-HSD. These findings are consistent with the hypothesis that FSH activates an additional pathway or pathways that interacts with PKA in the induction of aromatase and the LHr.
Figure 7.
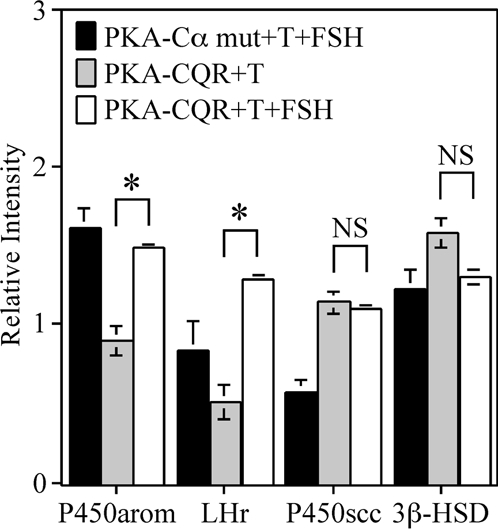
Interaction between FSH and PKA-CQR on mRNA Levels Associated with Granulosa Cell Differentiation
Granulosa cells were infected with either the PKA-CQR or PKA-Cα mutant lentiviral vectors (10 MOI) as described in Fig. 1. The next morning, unattached cells and free lentivirus were removed and replaced with M199 containing 30 ng/ml testosterone with or without FSH (100 ng/ml). After 48 h, total RNA was extracted from monolayers and analyzed for mRNA by RNase protection assay. Densitometry was performed using the NIH Image software. The signal of each mRNA was normalized to the housekeeping gene cyclophilin (relative intensity). Results show mean ± 1 sem of three groups of granulosa cells. Significant differences were considered when P < 0.01 and are indicated by asterisks in the figure.
Effects of FSH and Constitutively Active PKA-CQR on Global Gene Expression as Determined by Microarray Analysis
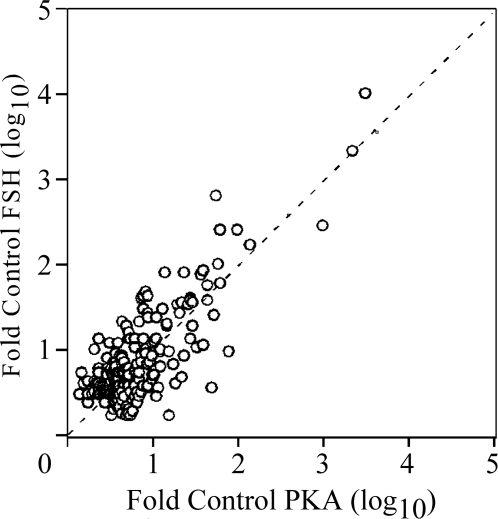
In view of the RNase protection data (Fig. 6), indicating that some mRNAs were equally induced by FSH and PKA-CQR whereas others (aromatase and the LH receptor) exhibited preferential stimulation by FSH, we sought to determine whether there may be other genes differentially regulated by FSH and PKA during granulosa cell differentiation. For this purpose, we conducted an Illumina microarray analysis of gene expression patterns using total RNA samples from granulosa cells that were stimulated by FSH or by constitutively active PKA-CQR as described in Fig. 6. Of the more than 22,000 genes included on the Illumina platform, 8047 passed a threshold cutoff (for details, see Materials and Methods) and were considered expressed genes. As shown in Fig. 8, of these 8047 expressed genes, FSH stimulated 156 genes to a level of at least 3-fold over control, whereas PKA-CQR stimulated 169 genes to a level of at least 3-fold over control (t test P < 0.005). Of these genes, 108 were stimulated to the same extent (≥3-fold over control) by both FSH and PKA-CQR, whereas there were subsets of genes that were stimulated at least 3-fold by FSH but not by PKA-CQR (48 genes) and subsets of genes that were stimulated at least 3-fold by PKA-CQR but not by FSH (62 genes).
Figure 8.
Microarray Analysis of FSH- and PKA-CQR-Stimulated Gene Expression
Total RNA samples obtained from the experiment presented in Fig. 6 were analyzed with the Illumina Rat-Ref-12 Expression BeadChip array as described in Materials and Methods. Output data from the Illumina microarray readout were analyzed by the ArrayAssist software at a P < 0.005 level of significance. Each data point represents the relative stimulation (fold over control) of individual transcripts that were increased to levels of at least 3-fold over control after FSH (y-axis) and PKA-CQR (x-axis) stimulation for 48 h based upon triplicate samples from cells infected with the PKA-CQR mutant lentivirus at MOI of 5 or stimulated by FSH (100 ng/ml) compared with unstimulated cells as described in Materials and Methods. All medium contained 30 ng/ml testosterone. The dashed line indicates equivalent stimulation by FSH and PKA-CQR. Note that the x- and y-axes are log10 scale.
Table 1 presents examples of three subsets of genes that appeared from the microarray analysis to be differentially regulated by FSH and PKA-CQR. Shown by the first two entries, P450arom and LHr transcripts were preferentially increased by FSH stimulation, which is similar to that observed from the results of the RNase protection assays (Fig. 6). Serum glucocorticoid kinase (SGK) and the βA subunit of inhibin were two other transcripts that were preferentially up-regulated by FSH stimulation. P450scc, 3β-HSD, and the α-subunit of inhibin were three transcripts that were comparably increased by FSH and PKA-CQR stimulation. Again, this is in agreement with the RNase protection assay results shown in Fig. 6. In addition to these, StAR mRNA was another example of a transcript that was regulated comparably by FSH and PKA-CQR. There were a number of transcripts that were preferentially increased by PKA-CQR, examples of which are the final four transcripts listed in Table 1. Importantly, as shown by the relative stimulation values (fold over control) in Table 1, differential regulation of gene expression by FSH or PKA-CQR is not absolute. Using P450arom as an example, FSH stimulated the expression of this transcript 260-fold over control, whereas PKA-CQR stimulated its expression 58-fold over control. Thus although FSH stimulation of aromatase was approximately 4-fold greater than that of PKA-CQR, there was still a substantial induction of aromatase by PKA-CQR.
Table 1.
Microarray Analysis of Gene Expression Patterns in Undifferentiated Granulosa Cells Stimulated by FSH and PKA-CQR
| Accession No. | Name | FSHa | PKA-CQRb | Maximal Expressionc |
|---|---|---|---|---|
| FSH > PKA-CQR | ||||
| NM_017085 | P450arom | 260 | 58.0 | 4,680 |
| NM_012978 | LH/CG receptor | 7.5 | 2.1 | 131 |
| NM_017128 | Inhibin-βA | 5.6 | 1.4 | 3,631 |
| NM_019232 | SGK | 12.4 | 3.0 | 13,812 |
| FSH ∼ PKA-CQR | ||||
| NM_017286 | P450scc | 2,186 | 2,131 | 25,870 |
| NM_017265 | 3β-HSD | 172 | 135 | 29,220 |
| NM_031558 | StAR | 35.6 | 27.7 | 7,997 |
| NM_012590 | α-Inhibin | 6.8 | 6.3 | 34,059 |
| PKA-CQR > FSH | ||||
| NM_017123 | Amphiregulin | 3.9 | 8.4 | 2,706 |
| NM_017031 | Phosphodiesterase 4B | 6.7 | 16.4 | 424 |
| NM_030991 | Snap 25 | 10.6 | 30.4 | 532 |
| NM_019291 | Carbonic anhydrase-2 | 2.8 | 10.4 | 1,504 |
Fold increase by FSH stimulation. Data are the means of Illumina expression units from cells stimulated by FSH divided by the means of Illumina expression units of control incubations for each transcript (n = 3 per group).
Fold increase by PKA-CQR stimulation. Data are the means of Illumina expression units from cells stimulated by PKA-CQR divided by the means of Illumina expression units of control incubations for each transcript (n = 3 per group).
Data present the mean value (n = 3) of the maximum Illumina expression units from cells stimulated by either FSH or PKA-CQR. This reflects the abundance of each transcript.
Finally, there were a number of transcripts down-regulated by FSH and PKA-CQR. Notably, three transcripts that encode for proteins that are present in lipid raft membrane domains were reduced by both FSH and PKA-CQR. Caveolin-1 mRNA levels were reduced 70% by FSH and 68% by PKA-CQR, caveolin-2 mRNA levels were reduced 69% by FSH and 62% by PKA-CQR, and AKAP12 mRNA levels were reduced 77% by FSH and 80% by PKA-CQR. Figure 9 presents an immunoblot analysis that confirms the down-regulation of caveolin-1 at the protein level.
Figure 9.

FSH and PKA-CQR Down-Regulate Caveolin-1 Expression
Granulosa cells were infected with either the PKA-CQR or PKA-Cα mutant lentiviral vectors (5 MOI) as described in Fig. 1. The next morning, unattached cells and free lentivirus were removed and replaced with M199. Twenty-four hours later, medium was replaced with fresh medium containing 30 ng/ml testosterone with or without FSH (100 ng/ml). After 48 h, whole-cell protein extracts were collected and used for immunoblots of caveolin-1 (cav-1) and then stripped and reprobed with anti-GAPDH. Results are representative of three separate groups of granulosa cells.
A supplemental data file (supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend. endojournals.org) presents raw data from each of the 8047 transcripts with respect to the extent of regulation (up and down) by FSH and PKA-CQR.
DISCUSSION
Although stimulation of PKA is necessary for granulosa cell differentiation, it is uncertain whether activation of PKA is sufficient to trigger the differentiation program in these cells. We tested this by constructing a replication-deficient lentiviral vector that directs the expression of a constitutively active mutant of PKA that has catalytic activity in the absence of cAMP (16). We show that infection of undifferentiated granulosa cells with this vector, but not a vector that directs the expression of a kinase-inactive mutant of the catalytic subunit of PKA (17), results in the induction of both the estrogen and progesterone biosynthetic pathways. Moreover, we demonstrate that two PKA-selective cAMP agonists, 6-Bnz-cAMP and 6-MB-cAMP, also stimulated estrogen and progesterone production by undifferentiated granulosa cells, an observation that is consistent with the results observed with the PKA-CQR mutant. Collectively, these results indicate that activation of PKA is sufficient to initiate the induction of both the estradiol and progesterone biosynthetic pathways during granulosa cell differentiation. This conclusion differs from a recently published study by Wayne et al. (13) that failed to observe increased mRNA levels for P450scc and P450-arom in granulosa cells stimulated by 6-Bnz-cAMP. A likely explanation for the lack of effect of 6-Bnz-cAMP in the Wayne et al. study was that the concentration of agonist used (100 μm) may have been insufficient because, as shown in Fig. 4, higher concentrations of this agonist (1–2 mm) were required to stimulate estrogen and progesterone production by granulosa cells.
To further identify the responses of undifferentiated granulosa cells to FSH and PKA-CQR stimulation, we measured mRNA levels by RNase protection assay for a panel of differentiation-associated proteins that have been shown previously to be increased by FSH stimulation. Results demonstrated that mRNAs for 3β-HSD, P450scc, and the α-subunit of inhibin were comparably induced by FSH and PKA-CQR stimulation. The similar increases in mRNAs for 3β-HSD and P450scc seen in FSH and PKA-CQR-stimulated cells are consistent with the equivalent stimulation of progesterone production and allow us to conclude that activation of the PKA signaling system is sufficient to account for the induction of the progesterone biosynthetic pathway during granulosa cell differentiation. Although granulosa cells infected with the PKA-CQR vector produced comparable amounts of estrogen as granulosa cells stimulated by FSH, we observed that the relative level of expression of mRNA for P450 aromatase as well as the LHr was greater in cells stimulated by FSH than cells stimulated by PKA-CQR. One possible explanation for the discrepancy between mRNA levels for aromatase and the actual amounts of estradiol produced is that our in vitro culture system, which used a fixed amount of testosterone, could be substrate limited. The observation that FSH stimulation resulted in a greater stimulation of mRNAs for aromatase and the LH receptor than did PKA-CQR is consistent with our previous findings that the expression of a constitutively active LH receptor, a constitutively active GαS signaling protein, or direct stimulation of undifferentiated granulosa cells by forskolin resulted in comparable increases in mRNAs for 3β-HSD, P450scc, and the α-subunit of inhibin as those seen in FSH-stimulated cells but did not increase the levels of mRNAs for P450 aromatase or the LH receptor to the same extent as FSH (19,20).
We extended our analysis of the effects FSH and PKA on granulosa cell differentiation by examining the global pattern of gene expression in response to FSH and PKA-CQR stimulation by microarray analysis. As shown in Table 1 and the supplemental data file, the results of the microarray analysis were entirely consistent with the results of our RNase protection assays. PKA-CQR was equally as effective as FSH in inducing mRNAs for 3β-HSD, P450scc, and the α-subunit of inhibin, whereas FSH was more effective than PKA-CQR in stimulating mRNA for aromatase and the LHr. Interestingly, another mRNA revealed by the array analysis that was equally stimulated by FSH and PKA-CQR was that for steroidogenic acute regulatory protein (StAR), a protein required for progesterone synthesis (21). This observation reinforces our conclusion that the induction of progesterone biosynthetic pathway by FSH can be accounted for entirely by the activation of the PKA signaling system. Furthermore, as indicated in Fig. 8 as well as supplemental Table 1, there were a number of other mRNAs that were regulated similarly by FSH and PKA-CQR and a number of mRNAs that were regulated differently. Some genes appeared to be activated to a greater extent by PKA-CQR than by FSH. One possible reason for this may be that the extent of PKA stimulation by FSH could be limited by FSH receptor saturation, whereas activated PKA in PKA-CQR-infected cells would not. It is also possible that PKA dosage could differentially influence the expression of some genes more than others.
Our microarray analysis also revealed a striking down-regulation of caveolin-1, caveolin-2, and AKAP12 mRNAs in undifferentiated granulosa cells after stimulation by FSH or PKA-CQR. Each of these proteins is targeted to the plasma membrane and interacts with G protein-coupled receptor signaling components (22). Interestingly, it has recently been demonstrated that TSH, via a cAMP- and PKA-dependent mechanism, down-regulates caveolin-1 mRNA in thyrocytes similar to what we have shown and that this is associated with increased proliferation of these cells (23). Whether FSH-mediated down-regulation of caveolin-1 and caveolin-2 is stimulatory or inhibitory to granulosa cell function is not known. On one hand, it is possible that compartmentalization of FSH receptors into caveolin-containing membrane rafts facilitates coupling of FSH receptors to G proteins and adenylyl cyclase. In this case, we would expect that the down-regulation of caveolins 1 and 2 would inhibit FSH signaling. On the other hand, because caveolin may interfere with G protein-coupled receptor signaling by inhibiting adenylyl cyclase activity and PKA activity (24,25), it is possible that the down-regulation of caveolins 1 and 2 by FSH would actually facilitate FSH signaling.
An obvious question is what could account for the apparent differential regulation of some genes by FSH and PKA-CQR that we observed in this current study. In this regard, it is important to note that our previous results demonstrated that the responses of granulosa cells to other activators of the cAMP/PKA signaling system including a constitutively active LH receptor, a constitutively active GαS and forskolin were similar to those observed in response to the constitutively active PKA-CQR (17,18). This would seem to indicate that there could be additional FSH-stimulated, cAMP- and PKA-independent pathways that interact with the PKA pathway to optimally induce aromatase and the LH receptor. This hypothesis is supported by data presented in Fig. 7 that show costimulation of granulosa cells with PKA-CQR and FSH led to greater levels of mRNAs for P450arom and the LHr when compared with cells stimulated by PKA-CQR alone. An attractive candidate for this additional signaling pathway is PI3K/AKT because it has been shown by others (26,27) that FSH stimulates phosphorylation of AKT in an H-89-independent manner and that expression of a constitutively active AKT in granulosa cells amplifies the ability of agents that stimulate cAMP production to induce aromatase and the LH receptor (20). We have evidence from preliminary studies that expression of constitutively active AKT also amplifies the ability of PKA-CQR to induce estrogen production by undifferentiated granulosa cells (data not shown). The mechanism by which FSH activates the PI3K/AKT pathway may involve activation of Rous sarcoma oncogene family tyrosine kinase (RSK) because pharmacological inhibition of this kinase reduced FSH-stimulated AKT phosphorylation (13). An alternate explanation for the differences in responses between FSH and PKA-CQR could be that because of its reduced ability to bind R-subunits (16), PKA-CQR may not tether effectively to AKAPs. Therefore, potential intracellular compartmentalization of a cAMP/PKA signaling network may not occur in PKA-CQR-stimulated cells. This could also explain why, as shown in Fig. 2, high intracellular levels of PKA-CQR seem to be necessary to induce the expression of differentiation-associated genes in these cells.
FSH signaling in undifferentiated granulosa cells also requires androgen receptor signaling because costimulation with testosterone or other androgens is required for FSH to induce aromatase and the LH receptor in these cells (28,29). In preliminary studies presented as a supplement (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend. endojournals.org), we show that the effect of testosterone on the induction of mRNAs for differentiation-associated genes differs between cells stimulated by FSH and PKA-CQR. In the absence of testosterone, there was no increase in mRNA levels for P450scc, 3β-HSD, aromatase, or the LHr in response to FSH stimulation, whereas granulosa cells stimulated by PKA-CQR did not require costimulation with testosterone for the induction of these mRNAs. Although elucidation of the mechanism of action of androgens is beyond the scope of the current study, if it is assumed that PKA activity is not limiting in cells expressing PKA-CQR, then these preliminary findings would seem to indicate that androgens amplify FSH signaling downstream of PKA.
One significant limitation of the current study with the use of the constitutively active PKA-CQR is that it is not possible to do the rapid time-course studies that are required to identify the acute and transient activation of signaling pathways in granulosa cells (12). Thus, there is no true time zero point for virus-infected cells because the presence of active PKA-CQR depends upon uptake of virus, the initiation of the transcription of the cDNA, and its translation into protein, coupled with the fact that the absolute PKA activity would increase in parallel with the amount of the constitutively active protein that is synthesized. Accordingly, we were unable to assess the extent to which potential downstream targets of PKA such as AKT, ERK, and CREB were transiently activated. Furthermore, we were not able to detect phosphorylated CREB or AKT in cells stimulated by FSH or PKA-CQR for 24 or 48 h. However, our finding that the PKA-selective cAMP agonists 6-Bnz-cAMP and 6-MB-cAMP mirrored the effects of PKA-CQR should allow for the performance of detailed time-course studies to elucidate the downstream targets of PKA in future studies.
MATERIALS AND METHODS
Chemicals and Reagents
Unless otherwise noted, all reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO). Human FSH (AFP-4161-B; 3205 IU Second International Reference Preparation of FSH/mg, 225 IU Second International Reference Preparation of LH/mg) and antiserum to cAMP (lot CV-27) were provided by the National Hormone and Pituitary Program (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD). The cAMP analogs, 6-Bnz-cAMP (N6-benzoyladenosine-3′,5′-cyclic monophosphate) and 6-MB-cAMP (N6-monobutyryladenosine-3′,5′-cyclic monophosphate) were purchased from BIOLOG Life Science Institute (Bremen, Germany). The following antibodies were used for Western immunoblot analyses: anti-PKAc (no. 610980) from BD Bioscience (Franklin Lakes, NJ) and anti-caveolin-1 (N-20, no. sc-894) and anti-GAPDH (no. sc-32233) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Lentivirus Vectors
The PKA-CQR (14) and PKA-Cα-mutant (15) transgenes were subcloned into the pFUGW lentiviral backbone (30) under the control of the ubiquitin C (UBC) promoter to generate lenti-UBC-PKA-CQR and lenti-UBC-PKA-Cα-mutant. Replication-defective lentiviral vectors were produced by transfecting the recombinant lentiviral shuttle vectors and the ViraPower lentiviral packaging mix [containing pLP1 (gag/pol), pLP2 (rev), and pLP/VSVG; Invitrogen, Grand Island, NY] into 293FT cells according to manufacturer guidelines. Viral supernatants were filtered (0.45 μm) to remove contaminating virus producer cells and concentrated by ultracentrifugation. Virus titer was determined by P24 ELISA (QuickTiter Lentivirus Titer Kit; Cell Bioloabs, Inc., San Diego, CA). Concentrated virus supernatant (approximately 109 PFU/ml for each virus) was added to target cells to achieve a multiplicity of infection (MOI) of one to 10 particles per cell.
Granulosa Cell Culture
All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Immature female rats (24 d old) were purchased from Hilltop Lab Animals (Scottdale, PA). Animals were euthanized with an ip injection of sodium pentobarbital (150 mg/kg body weight), and ovaries were collected into Medium 199 (M199; GIBCO-Invitrogen Corp., Gaithersburg, MD). Whole ovaries were incubated in a CO2 incubator at 37 C for 5 min in 6 mm EGTA in M199 followed by 0.5 m sucrose in M199 for 15 min. Ovaries were then transferred into fresh M199, and granulosa cells were collected by puncturing follicles with a 25-gauge hypodermic needle and expressing the cells into M199. Cells were then centrifuged at 100 × g for 10 min and resuspended into M199 containing 6 μg/ml polybrene (hexadimethrin bromide, catalog item 52495; Fluka/Sigma-Aldrich Corp., St. Louis, MO). Cell suspensions were infected with the PKA-CQR or PKA-Cα mutant lentiviral vectors for 2 h. Controls were performed with M199 plus 6 μg/ml polybrene for 2 h. After the 2-h infection, granulosa cell and virus suspensions were plated into 12-well (∼5 × 106 cells per well) or 24-well (∼2 × 105 cells per well) or 48-well (∼1 × 105 cells per well) tissue culture plates containing M199 plus 10% calf serum (CS; Mediatech, Inc., Manassas, VA), and the cells were allowed to attach overnight. The next morning, unattached cells and medium were removed and replaced with fresh serum-free M199. Cells were exposed to stimulatory agents, as described in the figure legends, and maintained in serum-free M199 containing testosterone at a final concentration of 30 ng/ml. At the end of the experiment, tissue culture medium was collected, boiled for 10 min to inactivate phosphodiesterases, and stored at −20 C for subsequent RIAs. Where indicated, total RNA was prepared from the cell monolayers using RNA-Bee (Tel-Test, Inc., Friendswood, TX) according to the manufacturer’s directions.
RIA
Estradiol and progesterone content in culture medium were determined by RIAs as described previously (31). cAMP concentration in culture medium was analyzed by RIA using [125I]cAMP-2-0′ monosuccinlyl cAMP tyrosine methyl ester (32) and anti-cAMP in accordance with the instructions provided by the National Hormone and Pituitary Program.
Western Immunoblot Analysis
Granulosa cells were cultured as above. After stimulation described in the respective figure legends, cell monolayers were washed twice with PBS, and then 150 μl 1× sodium dodecyl sulfate-sample buffer [62.5 mm Tris-HCl (pH 6.8), 2% wt/vol sodium dodecyl sulfate, 10% glycerol, 50 mm dithiothreitol, 0.01% wt/vol pyronin Y] was added to each well. Whole-cell extracts were transferred to Eppendorf tubes and sonicated in an ice bath with a sonic dismembrator (Model 100; Fisher Scientific Co. LLC, Pittsburgh, PA) for 10 sec at a power setting of 5. Whole-cell extracts were separated on a 12% SDS-PAGE, and the resolved proteins were electrophoretically transferred to nitrocellulose membranes, washed with Tris-buffered saline (TBS), and blocked with either 0.5% blocking reagent (Roche Diagnostics Corp., Indianapolis, IN) in TBS or 3% nonfat dry milk in TBS. Chemiluminescent detection was accomplished using the BM chemiluminescence Western blotting kit (Roche) with the antirabbit or antimouse horseradish peroxidase-conjugated secondary antibody diluted according to the manufacturer’s directions. Densitometric analysis of immunoblot signal was performed using the National Institutes of Health (NIH) Image program (version 1.61).
mRNA Analysis
Samples of total RNA (0.5–1 μg) were analyzed for P450scc, 3β-HSD, the α-subunit of inhibin (α-inhibin), aromatase (P450arom), and LHr mRNAs by RNase protection assay according to the instructions provided by the supplier (Ambion, Inc., Austin, TX). Antisense RNA probes were prepared using [α-32P]CTP (PerkinElmer Life Sciences, Boston, MA) from the following cDNA inserts: P450scc (bp 816-1614) (33), 3β-HSD (bp 453–932) (34), α-inhibin (bp 695-1095) (35), P450arom (bp 1411–1714) (36), rat LHr (bp 1–622) (37), and cyclophylin (bp 34–142) (38). After electrophoresis (5% acrylamide containing 8 m urea), gels were dried and exposed to x-ray film for 16–96 h. Densitometric analysis of protected RNA fragments was performed using NIH Image (version 1.61).
DNA Microarray Study
Three separate groups of granulosa cells were collected from sexually immature 24-d-old female rats, and granulosa cells were placed into primary culture as described above. For each of the three groups of cells, the following four treatments were applied: 1) testosterone alone (30 ng/ml), 2) FSH (100 ng/ml) plus testosterone, 3) PKA-CQR (5 MOI, delivered by lentiviral vector) plus testosterone, or 4) catalytically inactive PKA-Cα mutant (5 MOI) plus testosterone. Forty-eight hours later, total RNA was harvested from the cells, frozen at −80 C, and transported to the Genomics and Proteomics Core Laboratory of the University of Pittsburgh. Samples were prepared using the Illumina (San Diego, CA) Totalprep RNA Amplification Kit from Ambion according to the manufacturer’s instructions. Briefly, 500 ng total RNA was used to prepare double-stranded cDNA using T7-oligo(deoxythymidine) primer. Reverse transcription was followed by in vitro transcription in the presence of biotinylated nucleotides. Biotinylated cRNA samples were hybridized to the Illumina Rat-Ref-12 Expression BeadChip array in a proprietary hybridization buffer overnight at 58 C on a platform rocker. After the 18-h hybridization, arrays were washed in proprietary buffers to remove excess labeled cRNA. Florescent tagging was achieved by incubation with Cy3-streptavidin for 10 min at room temperature. Excess dye solution was removed with additional buffer washes. Slides were dried by low-speed centrifugation and scanned in the Illumina BeadScanner. Resulting image files were normalized, and background was subtracted using Bead Studio version 3.2.3.
Normalized and background subtracted data were transferred into ArrayAssist (Stratagene, La Jolla, CA) for further analysis. A threshold level of 125 Illumina units was set by eliminating all rows (individual genes) whose maximum expression level did not exceed 125 Illumina units. Of the more than 22,000 genes included on the Illumina platform, 8047 fell above the threshold cutoff and were considered expressed genes. Statistical analyses were performed using the ArrayAssist software by unpaired t tests that compared, for each of the expressed transcripts, the signal intensity of transcript in cells stimulated by FSH or PKA-CQR with the intensities of the respective control incubations (n = 3 per group). An appended supplemental data file presents raw data from each of the 8047 filtered transcripts.
Statistics
Where indicated, steroid and cAMP levels in culture medium and mRNA levels measured by RNase protection assays were assessed for statistical significance by ANOVA followed by comparison of group means with Fisher’s least significant difference analysis (StatView, version 4.5; Abacus Concepts, Berkeley, CA). Microarray results were analyzed for statistical significance at a level of P < 0.005 using the ArrayAssist software by unpaired t tests as described above.
Supplementary Material
Acknowledgments
We thank Dr. G. Stanley McKnight of the University of Washington for permission to use the PKA-CQR cDNA, Dr. Richard A. Maurer of the Oregon Health & Sciences University for the PKA-Cα mutant cDNA, Dr. Carlos Lois of the Massachusetts Institute of Technology for the FUGW lentiviral backbone vector, and Dr. William H. Walker of the University of Pittsburgh for suggestions on the preparation of the manuscript.
Footnotes
This work was supported by National Institutes of Health Grant HD 47260.
Disclosure Statement: The authors of this manuscript have nothing to disclose.
First Published Online June 5, 2008
Abbreviations: CS, Calf serum; 3β-HSD, 3β-hydroxysteroid dehydrogenase; M199, medium 199; MOI, multiplicity of infection; PI3K, phosphatidylinositol-3 kinase; PKA, protein kinase A; P450scc, cholesterol side-chain cleavage enzyme; RNAse, ribonuclease.
References
- Greep RO, Van Dyke HB, Chow BF 1942 Gonadotropins of the swine pituitary: various biological effects of purified thylakentrin (FSH) and pure metakentrin (ICSH). Endocrinology 30:635–649 [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM 1997 Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 15:201–204 [DOI] [PubMed] [Google Scholar]
- Erickson GF, Hsueh AJ 1978 Stimulation of aromatase activity by follicle stimulating hormone in rat granulosa cells in vivo and in vitro. Endocrinology 10:1275–1282 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SL, Richards JS 1991 Regulation of cytochrome P450 aromatase messenger ribonucleic acid and activity by steroids and gonadotropins in rat granulosa cells. Endocrinology 129:1452–1462 [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ, Midgley Jr AR, Reichert Jr LE 1974 Granulosa cell maturation in the rat: increased binding of human chorionic gonadotropin following treatment with follicle-stimulating hormone in vivo. Endocrinology 9:818–825 [DOI] [PubMed] [Google Scholar]
- Jones PB, Hsueh AJ 1982 Pregnenolone biosynthesis by cultured rat granulosa cells: modulation by follicle-stimulating hormone and gonadotropin-releasing hormone. Endocrinology 111:713–721 [DOI] [PubMed] [Google Scholar]
- Jones PB, Hsueh AJ 1982 Regulation of ovarian 3β-hydroxysteroid dehydrogenase activity by gonadotropin-releasing hormone and follicle-stimulating hormone in cultured rat granulosa cells. Endocrinology 110:1663–1671 [DOI] [PubMed] [Google Scholar]
- Goldring NB, Durica JM, Lifka J, Hedin L, Ratoosh SL, Miller WL, Orly J, Richards JS 1987 Cholesterol side-chain cleavage P450 messenger ribonucleic acid: evidence for hormonal regulation in rat ovarian follicles and constitutive expression in corpora lutea. Endocrinology 120:1942–1950 [DOI] [PubMed] [Google Scholar]
- Hillier SG, Zeleznik AJ, Ross GT 1978 Independence of steroidogenic capacity and luteinizing hormone receptor induction in developing granulosa cells. Endocrinology 102:937–946 [DOI] [PubMed] [Google Scholar]
- Beebe SJ, Segaloff DL, Burks D, Beasley-Leach A, Limbird LE, Corbin JD 1989 Evidence that cyclic adenosine 3′,5′-monophosphate-dependent protein kinase activation causes pig ovarian granulosa cell differentiation, including increases in two type II subclasses of this kinase. Biol Reprod 41:295–307 [DOI] [PubMed] [Google Scholar]
- DeManno DA, Cottom JE, Kline MP, Peters CA, Maizels ET, Hunzicker-Dunn M 1999 Follicle-stimulating hormone promotes histone H3 phosphorylation on serine-10. Mol Endocrinol 13:91–105 [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Maizels ET 2006 FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal 18:1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne CM, Fan HY, Cheng X, Richards JS 2007 Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol 21:1940–1957 [DOI] [PubMed] [Google Scholar]
- Cottom J, Salvador LM, Maizels ET, Reierstad S, Park Y, Carr DW, Davare MA, Hell JW, Palmer SS, Dent P, Kawakatsu H, Ogata M, Hunzicker-Dunn M 2003 Follicle-stimulating hormone activates extracellular signal-regulated kinase but not extracellular signal-regulated kinase kinase through a 100-kDa phosphotyrosine phosphatase. J Biol Chem 278:7167–7179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels ET, Cottom J, Jones JC, Hunzicker-Dunn M 1998 Follicle stimulating hormone (FSH) activates the p38 mitogen-activated protein kinase pathway, inducing small heat shock protein phosphorylation and cell rounding in immature rat ovarian granulosa cells. Endocrinology 139:3353–3356 [DOI] [PubMed] [Google Scholar]
- Orellana SA, McKnight GS 1992 Mutations in the catalytic subunit of cAMP-dependent protein kinase result in unregulated biological activity. Proc Natl Acad Sci USA 89:4726–4730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer RA 1989 Both isoforms of the cAMP-dependent protein kinase catalytic subunit can activate transcription of the prolactin gene. J Biol Chem 264:6870–6873 [PubMed] [Google Scholar]
- Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG, Døskeland SO 2003 cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem 278:35394–35402 [DOI] [PubMed] [Google Scholar]
- Bebia Z, Somers JP, Liu G, Ihrig L, Shenker A, Zeleznik AJ 2001 Adenovirus-directed expression of functional luteinizing hormone (LH) receptors in undifferentiated rat granulosa cells: evidence for differential signaling through follicle-stimulating hormone and LH receptors. Endocrinology 142:2252–2259 [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ, Saxena D, Little-Ihrig L 2003 Protein kinase B is obligatory for follicle-stimulating hormone-induced granulosa cell differentiation. Endocrinology 144:3985–3994 [DOI] [PubMed] [Google Scholar]
- Clark BJ, Wells J, King SR, Stocco DM 1994 The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269:28314–28322 [PubMed] [Google Scholar]
- Tao J, Wang HY, Malbon CC 2007 Src docks to A-kinase anchoring protein gravin, regulating β2-adrenergic receptor resensitization and recycling. J Biol Chem 282:6597–6608 [DOI] [PubMed] [Google Scholar]
- Costa MJ, Senou M, Van Rode F, Ruf J, Capello M, Dequanter D, Lothaire P, DessyC, Dumont JE, Many MC, Van Sande J 2007 Reciprocal negative regulation between thyrotropin/3′,5′-cyclic adenosine monophosphate-mediated proliferation and caveolin expression in human and murine thyrocytes. Mol Endocrinol 21:921–932 [DOI] [PubMed] [Google Scholar]
- Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA 2006 Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem 281:26391–26399 [DOI] [PubMed] [Google Scholar]
- Razani B, Lisanti MP 2001 Two distinct caveolin-1 domains mediate the functional interaction of caveolin-1 with protein kinase A. Am J Physiol Cell Physiol 281:C1241–C1250 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS 2000 Follicle-stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-induced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol 14:1283–1300 [DOI] [PubMed] [Google Scholar]
- Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, Chandel NS, Hunzicker-Dunn M 2004 Follicle-stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary for induction of select protein markers of follicular differentiation. J Biol Chem 279:19431–19440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier SG, De Zwart FA 1981 Evidence that granulosa cell aromatase induction/activation by follicle-stimulating hormone is an androgen receptor-regulated process in-vitro. Endocrinology 109:1303–1305 [DOI] [PubMed] [Google Scholar]
- El-Hefnawy T, Zeleznik AJ 2001 Synergism between FSH and activin in the regulation of proliferating cell nuclear antigen (PCNA) and cyclin D2 expression in rat granulosa cells. Endocrinology 142:4357–4362 [DOI] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D 2002 Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295:868–872 [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ, Resko JA 1980 Progesterone does not inhibit gonadotropin-induced follicular maturation in the female rhesus monkey. Endocrinology 106:1820–1826 [DOI] [PubMed] [Google Scholar]
- Steiner AL, Parker CW, Kipnis DM 1972 Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem 247:1106–1113 [PubMed] [Google Scholar]
- John ME, John MC, Ashley P, MacDonald RJ, Rutter WJ 1984 Identification and characterization of cDNA clones specific for cholesterol side-chain cleavage cytochrome P-450. Proc Natl Acad Sci USA 81:5628–5632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorence MC, Naville D, Graham-Lorence SE, Mack SO, Murry BA, Trant JM, Mason JI 1991 3β-Hydroxysteroid dehydrogenase/Δ5–4-isomerase expression in rat and characterization of the testis isoform. Mol Cell Endocrinol 80:21–31 [DOI] [PubMed] [Google Scholar]
- Esch FS, Shimasaki S, Cooksey K, Mercado M, Mason AJ, Ying SY, Ueno N, Ling N 1997 Complementary deoxyribonucleic acid (cDNA) cloning and DNA sequence analysis of rat ovarian inhibins. Mol Endocrinol 1:388–396 [DOI] [PubMed] [Google Scholar]
- Hickey GJ, Krasnow JS, Beattie WG, Richards JS 1990 Aromatase cytochrome P450 in rat ovarian granulosa cells before and after luteinization: adenosine 3′,5′-monophosphate-dependent and independent regulation. Cloning and sequencing of rat aromatase cDNA and 5′ genomic DNA. Mol Endocrinol 4:3–12 [DOI] [PubMed] [Google Scholar]
- McFarland KC, Sprengel R, Phillips HS, Kohler M, Rosemblit N, Nikolics K, Segaloff DL, Seeburg PH 1989 Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science 245:494–499 [DOI] [PubMed] [Google Scholar]
- Danielson PE, Forss-Petter S, Brow MA, Calavetta L, Douglass J, Milner RJ, Sutcliffe JG 1988 p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA 7:261–267 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.