Abstract
Aims
Clinically, Chlamydia pneumoniae infection and its heat shock protein 60 (cHSP60) may contribute to atherogenesis; however, its underlying mechanisms are largely unknown. The objective of this study was to determine whether cHSP60 could cause endothelial dysfunction in human coronary artery endothelial cells (HCAECs) and porcine coronary arteries.
Methods and results
When HCAECs were treated with recombinant cHSP60, endothelial nitric oxide synthase (eNOS) mRNA and protein levels, enzyme activities, cellular NO levels, mRNA stability, and promoter activities were significantly decreased. Superoxide anion production was significantly increased due to the inhibition of mitochondrial membrane potential and catalase and superoxide dismutase (SOD) activities as well as activation of NADPH oxidase. Antioxidant seleno-l-methionine (SeMet) or SOD mimetic MnTBAP effectively blocked cHSP60-induced eNOS downregulation. In addition, cHSP60 activated mitogen-activated protein kinases (MAPKs) including p38, c-Jun-N-terminal kinase/stress-activated protein kinase, and extracellular signal-regulated kinases. Specific chemical inhibitors or their dominant-negative mutant forms of these MAPKs effectively blocked cHSP60-induced eNOS downregulation. cHSP60-induced eNOS downregulation and oxidative stress were also demonstrated in porcine coronary artery rings in vitro. Functionally, endothelium-dependent vasorelaxation was significantly reduced in cHSP60-treated vessels.
Conclusion
cHSP60 directly induces eNOS downregulation through oxidative stress and MAPK activation in both HCAECs and porcine coronary arteries, thereby causing endothelial dysfunction.
Keywords: Chlamydia pneumonia, Heat shock protein 60, Endothelial nitric oxide synthase, Superoxide anion, MAPK, Oxidative stress, Antioxidant, SeMet, MnTBAP, Atherosclerosis
1. Introduction
Chlamydia pneumoniae, a ubiquitous Gram-negative bacterium, is a common respiratory pathogen in children and adults.1,2 Recently, chronic C. pneumoniae infection has been associated with cardiovascular disease by seroepidemiologic data and direct detection of the organism within atherosclerotic plaques.3,4 Animal models and antibiotic treatment also support these findings.5,6 Furthermore, growing evidence indicates that Chlamydia heat shock protein 60 (cHSP60) may serve as a possible link between C. pneumoniae infection and atherosclerosis.7,8 Since cHSP60 and human HSP60 are genetically conserved and share an immunological cross-reactivity, it has been hypothesized that autoimmune reactions against human HSP60 may play an important role in atherogenesis.9
It has become evident that decreased bioavailability of nitric oxide (NO) produced from endothelial nitric oxide synthase (eNOS), referred to as endothelial dysfunction, plays a crucial role in the development and progression of atherosclerosis.10 In the endothelium, eNOS converts l-arginine to l-citrulline and NO. Active NO levels are largely regulated by eNOS gene expression or its activity.11 A decrease in the relative bioavailability of NO not only impairs endothelium-dependent vasodilation but also activates other mechanisms that play an important role in atherogenesis.12 In addition, various modulators for eNOS also alter cellular redox state, suggesting that reactive oxygen species (ROS) might affect eNOS expression in the vascular system.13,14 However, it is not clear whether cHSP60 could regulate eNOS expression and ROS production in endothelial cells.
In the present study, we hypothesized that cHSP60 may affect eNOS expression and functions through oxidative stress and mitogen-activated protein kinase (MAPK) activation. Specifically, the effects of cHSP60 on eNOS mRNA, protein levels, and activities were determined in human coronary artery endothelial cells (HCAECs). Superoxide anion production and its potential sources as well as MAPK activation were investigated. Vasomotor function and molecular events were also studied in porcine coronary artery rings. This study may uncover new mechanisms of C. pneumoniae pathogenesis for cardiovascular disease.
2. Methods
A detailed description of the methods used can be found in the Supplementary material online.
2.1. Cell cultures
HCAECs (Cambrex BioWhittaker Inc., Walkersville, MD, USA) were cultured and treated with different concentrations (0.2, 2, or 10 µg/mL) of cHSP60 or heat-inactivated recombinant cHSP60 (HI-cHSP60, 10 µg/mL) for different times (6, 24, or 48 h). Recombinant cHSP60 was obtained as a gift from Dr Grant N. Pierce at the St Boniface General Hospital Research Centre, Manitoba, Canada.7 Actinomycin D was used for studying eNOS mRNA stability. Antibodies against human HSP 60 (Abcam, Cambridge, MA, USA), cHSP60 (Affinity BioReagents, Golden, CO, USA), toll-like receptor (TLR)-2, or TLR-4 were used to block the effects of cHSP60 in HCAECs. Antioxidants seleno-l-methionine (SeMet), superoxide dismutase (SOD) mimetic MnTBAP, p38 MAPK inhibitor (SB239063), JNK inhibitor (SP600125), and ERK1/2 inhibitor (PD098059) were also used to block the effects of cHSP60 on HCAECs.
2.2. Myograph tension system
The porcine right coronary arteries obtained from a local slaughterhouse were carefully dissected and cut into multiple 5 mm rings for myograph analysis as described previously.14,15
2.3. Real-time PCR
eNOS, GAPDH, NADPH oxidase subunit (NOX) 1, NOX4, p22phox, catalase (CAT), SOD1, glutathione peroxidase (GPX) 1, GPX7, TLR-2, and TLR-4 and porcine eNOS and GAPDH primers (see Supplementary material online, Table S1) and their mRNA levels were determined by real-time PCR.
2.4. Western blot
eNOS protein was detected using a mouse anti-human eNOS monoclonal antibody diluted 1:1000 (BD Biosciences, San Jose, CA, USA), and β-actin was detected using a mouse anti-human β-actin monoclonal antibody diluted 1:10 000.
2.5. eNOS enzyme activity assay
An eNOS fluorimetric assay kit (Sigma, St Louis, MO, USA) was used to determine eNOS activity.
2.6. eNOS promoter activity assay
eNOS promoter plasmid constructs were kindly provided by Dr Philip A. Marsden (University of Toronto, Toronto, Ontario, Canada).16 HCAECs were co-transfected with pGL2 construct and pRL-SV40 vector carrying Renilla luciferase (as an internal control reporter) by using Lipofectin reagent (Invitrogen Co., Carlsbad, CA, USA).
2.7. Flow cytometry
For eNOS staining, cells were pre-treated with 500 µL of cytofix/cytoperm buffer for 20 min, followed by primary antibody and secondary antibody for 30 min each. For O−2 and NO staining, dihydroethidium (DHE) and 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM DA) were, respectively, added to the cells and incubated for 30 min.
2.8. Lucigenin-enhanced chemiluminescence
Levels of the superoxide anion produced by porcine endothelial cells were detected by using the lucigenin-enhanced chemiluminescence method.14,15
2.9. Assessment of mitochondrial membrane potential (Δψm)
The Δψm was assessed using flow cytometry analysis of cells stained with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazole-carbocyanide iodine (JC-1) (MitoScreen kit, BD Biosciences).
2.10. Measurement of ATP content
Cells were treated with or without cHSP60 (2 µg/mL) for 24 h. ATP levels were measured with an ATPLite kit (PerkinElmer, Wellesley, MA, USA).
2.11. Measurement of activities of NADPH oxidase, CAT, and SOD activity
NADPH oxidase activity was determined with enhanced lucigenin chemiluminescence assay. CAT and SOD enzyme activities were measured with commercial enzyme assay kits (Cayman Chemical, Ann Arbor, MI, USA).
2.12. Bio-Plex immunoassay
Phosphorylation of MAPKs (p38, JNK, and ERK2) was detected by Bio-Plex luminex immunoassay (Bio-Rad Laboratories, Hercules, CA, USA).
3. Results
3.1. cHSP60 decreases eNOS expression, activity, mRNA stability, and promoter function in HCAECs
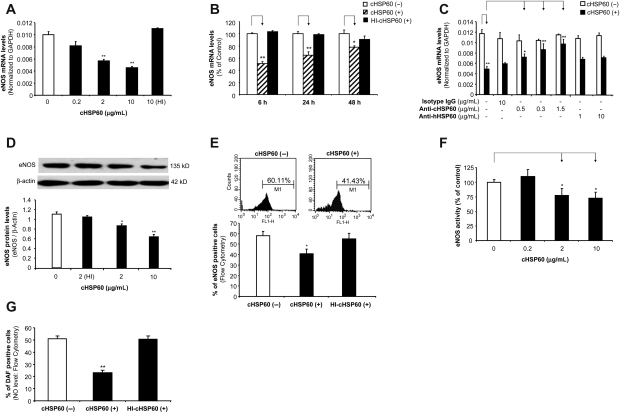
HCAECs were treated with cHSP60 in a concentration- and time-dependent manner. eNOS mRNA and protein levels were measured using real-time PCR and western blot as well as flow cytometry, respectively. When cells were treated with cHSP60 (2 or 10 µg/mL) for 24 h, eNOS mRNA levels were decreased by 34 and 42%, respectively, compared with controls (P < 0.01, n = 3, t-test, Figure 1A). When cells were cultured with cHSP60 (2 µg/mL) for 6, 24, or 48 h, all treatment groups showed a significant reduction of eNOS mRNA levels compared with controls (P < 0.05 or P < 0.01, n = 3, t-test, Figure 1B). HI-cHSP60 did not show any changes in all experimental groups. When co-cultured with antibodies against human HSP60 or cHSP60, only the anti-cHSP60 antibody effectively blocked cHSP60-induced eNOS downregulation in a concentration-dependent manner (P < 0.05 or P < 0.01, n = 3, t-test, Figure 1C), whereas anti-human HSP60 and isotype control IgG did not show any blocking effect.
Figure 1.
Effects of cHSP60 on eNOS mRNA and protein levels, enzyme activity, and cellular NO levels in HCAECs. Cells were treated with cHSP60 or HI-cHSP60 for 24 h. (A) Real-time PCR (eNOS mRNA in a concentration-dependent study). HCAECs were treated with cHSP60 (0, 0.2, 2, or 10 µg/mL) or HI-cHSP60 (10 µg/mL) for 24 h. (B) Real-time PCR (eNOS mRNA in a time course study). HCAECs were treated with cHSP60 (2 µg/mL) or HI-cHSP60 (10 µg/mL) for 6, 24, or 48 h. (C) Real-time PCR (eNOS mRNA in antibody blocking study). HCAECs were treated with cHSP60 (2 µg/mL) with or without anti-cHSP60 antibody (0.15, 0.3, or 1.5 µg/mL), anti-human HSP60 antibody (1 or 10 µg/mL) or isotype IgG (10 µg/mL) for 24 h. (D) Western blot analysis. Representative bands of eNOS and β-actin western blot staining are shown. Quantitative band density ratios were calculated and showed difference between cHSP60-treated and control groups. (E) Representative histograms of flow cytometry assay of eNOS staining are shown. Average percentage of eNOS positive cells was calculated and shown. (F) eNOS activity assay. (G) Cellular NO levels determined by DAF-FM DA staining and flow cytometry analysis. *P < 0.05, **P < 0.01, n = 3, t-test.
Western blotting and flow cytometry showed significant decreases in eNOS protein levels after cHSP60 (2 or 10 µg/mL) treatment for 24 h, compared with controls (P < 0.05 or P < 0.01, n = 3, t-test, Figure 1D and E). HI-cHSP60 did not show any significant effects. eNOS activities were also studied using an eNOS detection system. Similar to the real-time PCR and western blot data, cHSP60 (2 or 10 µg/mL) significantly decreased eNOS activity by 23 and 28%, respectively, compared with controls (P < 0.05, n = 3, t-test, Figure 1F). In addition, cellular NO levels were measured using the fluorescent dye DAF-FM DA (DAF). DAF staining is a unique method measuring NO production in living cells or solutions.17 cHSP60 at 2 µg/mL concentration decreased the NO-positive cell number by 55%, compared with controls (P < 0.01, t-test, Figure 1G). Thus, cHSP60 specifically reduces eNOS expression and its activities in HCAECs.
3.2. cHSP60 decreases eNOS mRNA stability and promoter activities in HCAECs
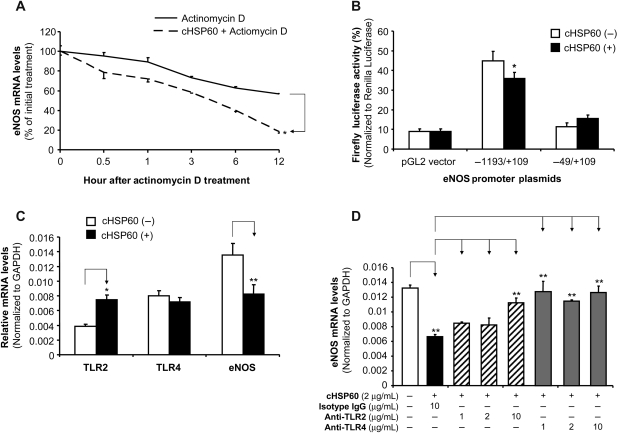
To study the possible mechanisms of cHSP60-induced eNOS downregulation in HCAECs, eNOS mRNA stability and eNOS promoter activities were determined in response to cHSP60 treatment. By using actinomycin D, a direct inhibitor of RNA polymerase II, we showed that the half-life of eNOS mRNA decreased from over 12 h in control cells to <6 h in cHSP60 (2 µg/mL)-treated cells (P < 0.05, n = 3, t-test, Figure 2A). By using eNOS promoter and reporter gene plasmid transfection experiments, we showed that functional eNOS promoter activities were significantly reduced in cHSP60-treated cells, compared with control cells (P < 0.05, n = 3, t-test, Figure 2B). This effect was specific because non-function (short) eNOS promoter or no promoter controls did not respond to cHSP60 treatment. Thus, cHSP60 decreases both eNOS mRNA stability and promoter activities in HCAECs.
Figure 2.
Effects of cHSP60 on eNOS mRNA stability and promoter activity as well as roles of TLRs in cHSP60 action in HCAECs. (A) eNOS mRNA stability. HCAECs were treated with 2.5 µg/mL actinomycin D (Act-D) in the presence or absence of 2 µg/mL cHSP60 for indicated time. Total RNA was harvested and analysed by real-time PCR using eNOS and GAPDH primers. Data are shown as the percentage of mRNA remaining in respect to cells before the addition of Act-D. (B) eNOS promoter activity. HCAECs were transiently co-transfected with an eNOS gene promoter construct and a pRL-SV40 plasmid as an internal control for 48 h. −1193/+109 bp eNOS promoter is functional, whereas −49/+109 bp eNOS promoter is not functional. After the transfection, the cells were treated with cHSP60 for another 24 h. Dual-luciferase assay was performed. (C) TLR-2 and TLR-4 expression. HCAECs were treated with cHSP60 (2 µg/mL) for 24 h. The mRNA levels of TLR-2, TLR-4, and eNOS were determined by real-time PCR. (D) Functional role of TLR-2 and TLR-4. HCAECs were treated with cHSP60 (2 µg/mL) in the presence or absence of anti-TLR-2 antibody (1, 2, and 10 µg/mL), or anti-TLR-4 antibody (1, 2, and 10 µg/mL) or isotype IgG (10 µg/mL) for 24 h. The eNOS mRNA levels were determined by real-time PCR. *P < 0.05, **P < 0.01, n = 3, t-test.
3.3. Expression and roles of TLR-2 and TLR-4 in cHSP60-induced eNOS downregulation in HCAECs
Since cHSP60 is able to interact with TLR-2 and TLR-4 to induce certain biologic responses such as inflammation and smooth muscle cell proliferation,18 we investigated whether TLR-2 and TLR-4 are involved in cHSP60-induced eNOS downregulation. We determined mRNA levels of TLR-2 and TLR-4 in the presence or absence of cHSP60 in HCACEs. Both TLR-2 and TLR-4 were expressed in HCAECs. In the absence of cHSP60 treatment, TLR-4 mRNA levels were much higher than TLR-2. While in the presence of cHSP60 treatment, TLR-2 mRNA levels increased to the level similar to that of TLR-4 mRNA, which had no change with cHSP60 treatment (Figure 2C). It is also confirmed that cHSP60 treatment significantly reduced eNOS mRNA levels in these experimental cells. Functional impact of TLRs was determined by using specific neutralizing antibodies against TLR-2 and TLR-4 in cHSP60-treated HCAECs. Three concentrations of antibodies (1, 2, and 10 µg/mL) were used in the experiments. Anti-TLR-2 antibody effectively blocked cHSP60-induced eNOS downregulation at 10 µg/mL, whereas anti-TLR-4 antibody had blocking effects at all three concentrations (P < 0.01, t-test, Figure 2D). Thus, TLR-4 may play a major role in cHSP60 functions in HCAECs.
3.4. cHSP60 increases superoxide anion production in HCAECs
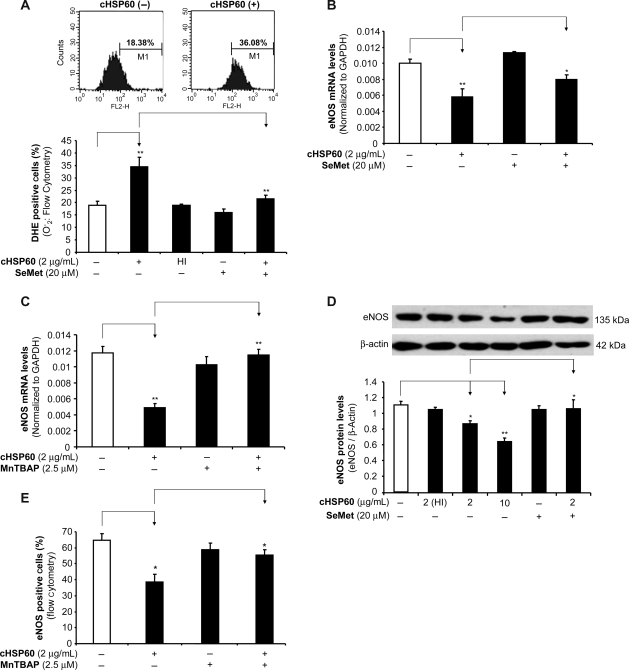
To study whether oxidative stress could play a role in cHSP60-induced eNOS downregulation, superoxide anion production in HCAECs was measured using a fluorescence dye DHE staining. When HCAECs were cultured with cHSP60 (2 µg/mL) for 24 h, the superoxide anion production was substantially increased by 81%, compared with controls (P < 0.01, n = 3, t-test, Figure 3A). Antioxidant SeMet (20 µM) effectively blocked cHSP60-induced superoxide anion overproduction. HI-cHSP60 at 10 µg/mL did not show any change in DHE staining. To confirm the functional link between superoxide anion and eNOS expression, both antioxidants SeMet and SOD mimetic MnTBAP were used in the cells with cHSP60 treatment. As expected, both SeMet and MnTBAP significantly blocked cHSP60-induced eNOS mRNA downregulation in HCAECs (P < 0.01, n = 3, t-test, Figure 3B and C). Western blot and flow cytometry also showed similar effects as real-time PCR data in SeMet- or MnTBAP-treated cells (P < 0.05 or P < 0.01, n = 3, t-test, Figure 3D and E).
Figure 3.
Role of superoxide anion in cHSP60-induced eNOS downregulation in HCAECs. Cells were treated with cHSP60 or HI-cHSP60 (2 µg/mL) in the presence or absence of antioxidant SeMet (20 µM) or MnTBAP (2.5 µM) for 24 h. (A) DHE staining and flow cytometry analysis for superoxide anion (O−2) production. (B) The eNOS mRNA levels were determined by real-time PCR for cHSP60 and SeMet treatment groups. (C) The eNOS mRNA levels were determined by real-time PCR for cHSP60 and MnTBAP groups. (D) The eNOS protein levels were determined by western blot for cHSP60 and SeMet treatment groups. (E) The eNOS protein levels were determined by flow cytometry for cHSP60 and MnTBAP treatment groups. *P < 0.05, **P < 0.01, n = 3, t-test.
3.5. Effects of cHSP60 on the expression and activities of ROS-related enzymes in HCAECs
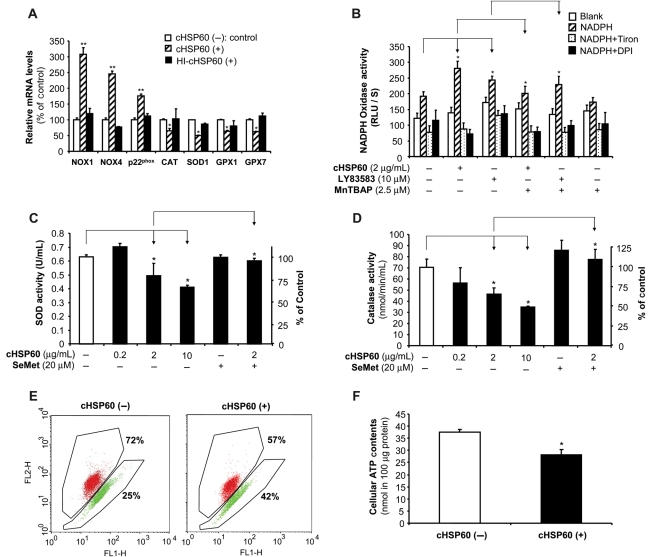
To study the potential sources of cHSP60-induced superoxide anion production, we determined several ROS-related enzymes in HCAECs. Cells treated with cHSP60 (2 µg/mL) for 24 h significantly increased the mRNA levels of NOX1 and NOX4 and p22phox and decreased the mRNA levels of CAT, SOD1, GPX1, and GPX7, compared with untreated cells or HI-cHSP60-treated cells (P < 0.05 or P < 0.01, n = 3, t-test, Figure 4A). NADPH oxidase activities were also significantly increased in the presence of cHSP60 or LY83583 (superoxide anion generator), and specificity of this assay was confirmed by using Tiron, intracellular scavenger of superoxide anion, and DPI, a flavoprotein inhibitor of NADPH oxidase as well as SOD mimetic MnTBAP (P < 0.05, n = 3, t-test, Figure 4B). The enzyme activities of CAT and SOD were studied with commercial assay kits. cHSP60 (2 or 10 µg/mL) treatment significantly reduced CAT activities by 35 and 51%, respectively, compared with controls (P < 0.05, n = 3, t-test, Figure 4C). Similarly, SOD activities were also reduced by 22 and 35%, respectively, compared with controls (P < 0.05, n = 3, t-test, Figure 4D). Co-culture with SeMet (20 µM) effectively reversed cHSP60-induced decrease in activities of both enzymes to the control levels (Figure 4C and D). Thus, cHSP60 induces the unbalance of ROS-related enzymes, which may be responsible, at least in part, for ROS increase in HCAECs.
Figure 4.
Effects of cHSP60 on ROS-related enzymes and mitochondrial membrane potential in HCAECs. Cells were treated with cHSP60 or HI-cHSP60 (2 µg/mL) or superoxide generator LY83583 (10 µM) in the presence or absence of antioxidant SeMet (20 µM) or MnTBAP (2.5 µM) for 24 h. (A) The mRNA levels of ROS-related enzymes (NOX1, NOX4, p22phox, CAT, SOD1, GPX1, and GPX7) were determined by real-time PCR. (B) NADPH oxidase activity assay. NAPDH, Tiron (intra cellular scavenger of superoxide anion) and DPI (a flavoprotein inhibitor of NADPH oxidase) were included in the assay. (C) SOD activity assay. (D) CAT activity assay. (E) JC-1 staining and flow cytometry analysis for mitochondrial membrane potential. (F) ATP contents were determined by ATPLite kit. *P < 0.05, **P < 0.01, n = 3, t-test.
3.6. cHSP60 decreases mitochondrial membrane potential and cellular ATP production
Mitochondria are known to be one of major sources of ROS generation from dysfunction of mitochondrial respiration chain.19 The mitochondrial membrane potential (Δψm) can serve as an indicator for the function of mitochondrial respiration chain. HCAECs were seeded on 6-well plates and cultured with or without cHSP60 (2 µg/mL) for 24 h. cHSP60 treatment substantially reduced mitochondrial membrane potential by 21% compared with controls (Figure 4E). Energy produced by mitochondrial respiration is used for ATP synthesis by a complex mechanism referred to as ‘oxidative phosphorylation’. Impaired mitochondrial respiration chain can lead to a decrease in ATP production. We determined ATP levels in HCAECs by using ATPLite kit. Indeed, cHSP60 (2 µg/mL) treatment significantly reduced ATP levels by 25% compared with controls (P < 0.05, n = 3, t-test, Figure 4F). Cell numbers in each culture condition were counted, and there was no difference between treatment and control groups. Thus, mitochondrial dysfunction may also contribute to the cHSP60-induced increase in ROS production.
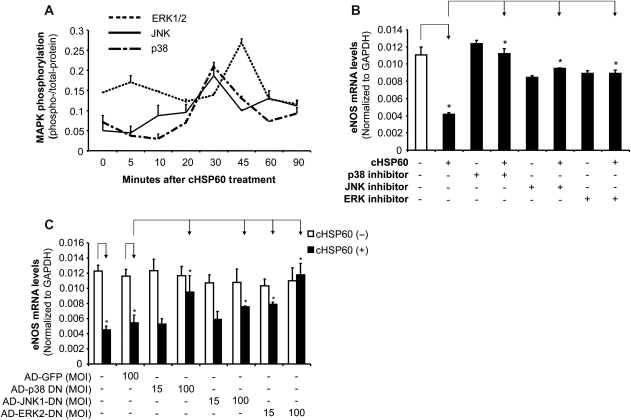
3.7. Roles of MAPKs in cHSP60-induced eNOS downregulation in HCAECs
Phosphorylation of MAPKs was investigated with a Bio-Plex luminex immunoassay. Increased phosphorylation levels of p38, JNK, and ERK2 was observed at 30–45 min after cHSP60 (2 µg/mL) treatment (Figure 5A). To confirm the functional role of the MAPK activation in the action of cHSP60, p38 inhibitor (SB239036, 1 µM), JNK inhibitor (SP600125, 40 µM), or ERK1/2 inhibitor (PD98059, 40 µM) were used to pre-treat HCAECs for 1 h before cells were cultured with cHSP60 (2 µg/mL) for 24 h. After culture, we determined the eNOS mRNA levels using real-time PCR. All three MAPK inhibitors effectively blocked cHSP60-induced eNOS mRNA decrease compared with cHSP60-treated groups (P < 0.05, n = 3, t-test, Figure 5B). In addition, we used adenovirus vectors (AD) containing dominant-negative mutant forms (DN) of p38, JNK1, and ERK2 to block cHSP60-induced eNOS downregulation. The transfection efficiency was 100% for 24 h transfection, demonstrated by signal from AD expressed green fluorescence protein (GFP). When cells were transfected with AD-p38-DN or AD-ERK2-DN, cHSP60-induced eNOS downregulation was completely blocked, whereas AD-JNK1-DN partially blocked cHSP60 effect (P < 0.05, t-test, Figure 5C).
Figure 5.
Roles of MAPKs in cHSP60-induced eNOS downregulation in HCAECs. (A) MAPK phosphorylation was determined by Bio-Plex luminex immunoassay. HCAECs were treated with cHSP60 (2 µg/mL) for different times (5, 10, 20, 30, 45, 60, and 90 min). (B) The eNOS mRNA levels were determined by real-time PCR for cHSP60 and MAPK chemical inhibitor treatment groups. HCAECs were treated with cHSP60 (2 µg/mL) in the presence or absence of inhibitors of p38, JNK, or ERK for 24 h. (C) The eNOS mRNA levels were determined by real-time PCR for cHSP60 and MAPK dominant-negative mutant (DN) adenovirus (AD) transfection groups. HCAECs were infected with AD-GFP (control), AD-p38-DN, AD-JNK-DN, or AD-ERK2-DN for 24 h and then treated with cHSP60 (2 µg/mL) for additional 24 h. *P < 0.05, n = 3, t-test.
3.8. cHSP60 induces endothelial dysfunction through eNOS downregulation and oxidative stress in porcine coronary arteries
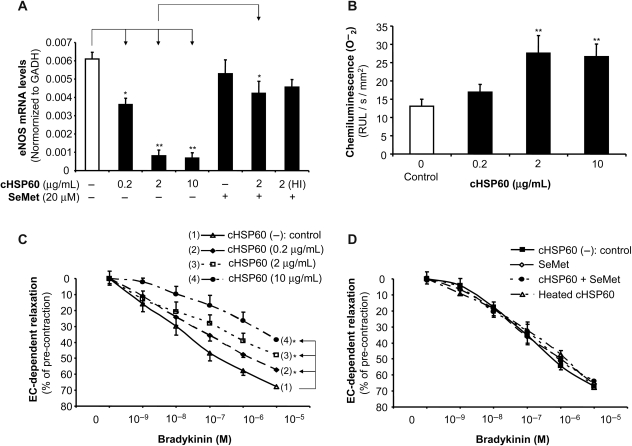
To further understand the role of cHSP60 in endothelial dysfunction at the level of vascular tissues, we used an established organ culture model of porcine coronary artery rings.14,15 Vessel rings were incubated with cHSP60 (0.2, 2, and 10 µg/mL), SeMet (20 µM), cHSP60 (2 µg/mL) plus SeMet (20 µM), or HI-cHSP60 (10 µg/mL) for 24 h. Real-time PCR was used to measure eNOS and GAPDH mRNA levels. The eNOS mRNA levels were reduced in a concentration-dependent fashion by 41, 87, or 89% for rings treated with 0.2, 2, or 10 µg/mL of cHSP60, respectively, compared with controls (P < 0.05 or P < 0.01, n = 4, t-test, Figure 6A). SeMet substantially blocked cHSP60-induced eNOS downregulation. HI-cHSP60 did not change in eNOS mRNA levels. In addition, superoxide anion production in porcine artery rings was measured using a lucigenin-enhanced chemiluminescence assay. When the rings were cultured with 2 or 10 µg/mL of cHSP60 for 24 h, the superoxide anion production was substantially increased by 111 or 103%, respectively, compared with controls (P < 0.01, n = 6, t-test, Figure 6B). Furthermore, the vasomotor function of porcine coronary artery rings was studied with the myograph tension system. Rings were cultured with different concentrations of cHSP60 for 24 h and subsequently subjected to contraction (U46619) and endothelium-dependent (bradykinin) and endothelium-independent (sodium nitroprusside) relaxation. Maximal contraction was not different between treatment and control groups. In response to bradykinin at 10−5 M, the endothelium-dependent relaxation was significantly reduced by 18, 39, and 52% for the rings treated with 0.2, 2, and 10 µg/mL of cHSP60, respectively, compared with controls (P < 0.05, n = 10, ANOVA, Figure 6C). Addition of SeMet completely blocked this effect (Figure 6D). HI-cHSP60 showed no effect on endothelium-dependent vasorelaxation. Thus, cHSP60 specifically induces endothelial dysfunction through eNOS downregulation and oxidative stress in porcine coronary arteries.
Figure 6.
Effects of cHSP60 on eNOS expression, superoxide anion production, and vasomotor functions in porcine coronary arteries. Porcine right coronary artery rings were cultured with cHSP60 (0.2, 2, or 10 µg/mL), with or without antioxidant SeMet (20 µM) for 24 h. (A) The eNOS mRNA levels were determined by real-time PCR. *P < 0.05, **P < 0.01, n = 4, t-test. (B) The superoxide anion (O−2) levels were determined by lucigenin-enhanced chemiluminescence assay. The data were normalized with area (mm2) of the ring and are expressed as relative light units (RLU/s/mm2). **P < 0.05, n = 6, t-test. (C) Vasomotor function was determined by myograph analysis for cHSP60 (0.2, 2, or 10 µg/mL) treatment group. After cHSP60 treatment, porcine right coronary artery rings were pre-contracted with thromboxane A2 analogue U46619 (3 × 10−8 M) and then subjected to endothelium-dependent relaxation by adding a series of concentrations of bradykinin (10−9–10−5 M). *P < 0.05, n = 10, ANOVA. (D) Vasomotor functions determined by myograph analysis for HI-cHSP60, SeMet, and SeMet plus cHSP60 treatment groups. The endothelium-dependent relaxation in response to a series of concentrations of bradykinin in each treatment group did not show any change compared with untreated controls.
4. Discussion
In this study, we present evidence to prove that cHSP60 is able to induce endothelial dysfunction in HCAECs and porcine coronary arteries. Specifically, cHSP60 significantly induces eNOS downregulation at both mRNA and protein levels through the inhibition of eNOS promoter activity and the decrease in eNOS mRNA stability in HCAECs. TLR-2 and TLR-4 and MAPKs are directly involved in the cHSP60 action. In addition, cHSP60 increases superoxide anion production through the unbalance of ROS-related enzymes and mitochondrial dysfunction in HCAECs. Furthermore, cHSP60-induced eNOS downregulation and oxidative stress were confirmed in the porcine coronary artery ring culture model. cHSP60 reduces endothelium-dependent vasorelaxation in porcine coronary arteries. These data may uncover the molecular mechanisms of C. pneumoniae pathogenesis in the vascular system.
eNOS synthesizes NO in vascular endothelial cells where it plays an important role in the control of vessel tension and platelet aggregation. However, under various pathological conditions, eNOS may become dysfunctional or its expression may be decreased.20 These abnormalities may not only impair endothelium-dependent vasorelaxation, but also accelerate atherosclerotic lesion formation.21 In the present study, we demonstrated direct effects of cHSP60 on cultured HCAECs and porcine coronary arteries. Using real-time PCR analysis, we showed that cHSP60 decreased eNOS mRNA levels in a time- and concentration-dependent manner. Western blot and flow cytometry analyses also confirmed that the treatment of cHSP60 decreased eNOS protein levels. cHSP60 induces eNOS downregulation through the inhibition of eNOS promoter activity and decrease in eNOS mRNA stability in HCAECs. Since recombinant cHSP60 used in this study was prepared from Escherichia coli, we include HI-cHSP60 in the critical experiments to exclude the possibility of the contamination of lipopolysacharide in the cHSP60 preparation. HI-cHSP60 did not show any effects on eNOS mRNA and protein levels. Furthermore, we also showed that cHSP60 significantly reduced eNOS activity as well as NO levels in cultured HCAECs. Along with the data we observed in porcine coronary arteries that cHSP60 significantly decreased endothelium-dependent vasorelaxation, it is clear that cHSP60 can directly affect the eNOS levels in vascular endothelial cells. Numerous reports have linked the C. pneumoniae infection and cHSP60 to cardiovascular diseases.2–5 However, to the best of our knowledge, this is the first report which shows a direct effect of cHSP60 on the eNOS system in endothelial cells. A previous study indicated that intracellular infection with C. pneumoniae reduced eNOS production in human umbilical core endothelial cells, and however, direct effects of cHSP60 on eNOS expression and molecular mechanisms are not studied.22
In the last several decades, ROS have been shown to be the key biomediators for vascular inflammation and atherogenesis.23 Human investigations support the oxidative stress hypothesis of atherogenesis.23,24 In the present study, we detected superoxide anion in human and porcine coronary endothelial cells. Data showed that cHSP60 significantly induced an increase in superoxide anion production, which could be one of the mechanisms for eNOS dysfunction. Superoxide anion is produced by a variety of sources including mitochondrial respiration chain, and NADH/NADPH oxidase.24 In addition, there are several cellular mechanisms that counterbalance the production of ROS, including enzymatic and non-enzymatic pathways.25 Among them, the best-characterized enzymatic pathways are CAT,26 SOD,27 and GPX.28 SOD facilitates the formation of H2O2 from superoxide anion and GPX coordinates the catalysis of H2O2 to water. In order to study the potential sources of increased superoxide anion in cHSP60-treated cells, we tested the mitochondrial membrane potential and ATP production in the cells. Our data showed a significant decrease in both above tests, indicating mitochondrial dysfunction and superoxide overproduction. In addition, we also studied the expression and activities of several ROS-related enzymes mentioned above. In response to the treatment of cHSP60, the mRNA levels of NADPH oxidase subunits NOX1, NOX4, and p22phox were increased, whereas the mRNA levels of CAT, SOD1, GPX1, and GPX7 were decreased. More importantly, NADHP oxidase activity was increased, whereas CAT and SOD activities were decreased in cHSP60-treated HCAECs. Thus, these data indicate that increased superoxide anion production in cHSP60-treated endothelial cells may be due to dysfunction of mitochondria and unbalance of ROS-related enzymes.
Since we demonstrated that cHSP60 induces oxidative stress, we further tested the blocking effects of antioxidants SeMet and SOD mimetic MnTBAP on cHSP60-treated cells. SeMet was chosen because it is a dietary antioxidant with unique mechanisms different from other types of antioxidants and it could be ready for clinical use. Indeed, SeMet effectively blocked cHSP60-induced eNOS downregulation and decrease in CAT and SOD activities. SeMet is the most prevalent form of dietary selenium.29 It is an effective antioxidant via enhancing activities of GPX.30 In addition, SeMet is able to directly interact with some oxidant molecules or oxidant generating ions.31,32 However, it is not clear whether SeMet supplement can prevent endothelial dysfunction and vascular disease in humans.
It is well known that the low level of GSH is a critical factor contributing oxidative stress, whereas antioxidant activity of SeMet can act at GSH levels. However, this mechanism is not investigated in the present study. It is not clear whether cHSP60 could reduce GSH levels or SeMet could increase GSH levels in endothelial cells. A recent study demonstrated that decreased endothelial GSH was partly responsible for the age-related loss of eNOS activity and endothelium-dependent vasorelaxation function in elderly rats, and (R)-α-lipoic acid (LA) can effectively increase cellular GSH levels and improve age-related endothelial dysfunction.33 LA is able to induce GSH synthesis.33
Recently, we have demonstrated that SeMet can effectively block lysophosphatidylcholine-induced oxidative stress and endothelial dysfunction in porcine coronary arteries.34 However, we cannot confirm whether SeMet acts on superoxide anion. Therefore, we used a second antioxidant MnTBAP, which has an SOD-like activity. Its blocking effects on cHSP60-induced eNOS downregulation can confirm the critical role of superoxide anion in endothelial dysfunction. Similar results have been reported by other researchers. Fukuoka et al.36 have reported that several natural products including Sesamol, a dietary free radical scavenger,35 can inhibit proliferation of rat vascular smooth muscle cells induced by HSP60 from C. pneumoniae. Thus, antioxidant therapy may provide an effective strategy to prevent vascular diseases mediated with these risk factors such as C. pneumoniae infection and others.
MAPKs relay many signalling pathways from the extracellular compartment to the cell nucleus through sequential kinase reactions that target transcription factor modification. They are important in regulating cell growth, migration, and differentiation in response to various extracellular stimuli.37 Three major subfamilies of structurally related MAPKs have been identified in mammalian cells, which are termed p44/42 MAPK (extracellular-signal-regulated kinase 1/2; ERKl/2), p38 MAPK, and Jun N-terminal kinases/stress-activated protein kinases (JNK/SAPKs). The pattern of MAPK activation in response to oxidative stress varies in dependence of the oxidant strength as well as cell types. Many MAPK pathways are still unclear. For instance, the MAPK pathways that couple endothelial activation from receptor levels to the expression of several key genes such as eNOS are largely unknown. In the present study, we demonstrated that p38, JNK, and ERK2 were activated in response to cHSP60 stimulation, and we also demonstrated that p38-, JNK-, or ERK1/2-specific inhibitors or dominant-negative mutant forms of these molecules effectively blocked cHSP60-induced eNOS downregulation in HCAECs.
In summary, the present study demonstrates that cHSP60 significantly decreases eNOS expression and NO production through oxidative stress and MAPKs activation. cHSP60 also significantly impaired endothelium-dependent vasorelaxation in cultured porcine coronary artery rings. cHSP60-induced mitochondrial dysfunction and unbalance of cellular redox enzymes may be the underlying mechanism of increased superoxide anion production. These data support the hypothesis that cHSP60 may directly contribute to the vascular disease formation during the C. pneumoniae infection.
Supplementary material
Funding
This work was partially supported by research grants from the National Institutes of Health (HL65916 and HL72716 to C.C.) and by the Baylor College of Medicine, Houston, TX, USA.
Supplementary Material
Acknowledgements
We would like to thank Grant N. Pierce (St Boniface General Hospital Research Centre, Manitoba, Canada) for his gift of recombinant cHSP60 and to thank Philip A. Marsden (University of Toronto, Toronto, Ontario, Canada) for his gift of eNOS promoter constructs.
Conflict of interest: none declared.
References
- 1.Krüll M, Maass M, Suttorp N, Rupp J. Chlamydophila pneumoniae. Mechanisms of target cell infection and activation. Thromb Haemost. 2005;94:319–326. doi: 10.1160/TH05-04-0261. [DOI] [PubMed] [Google Scholar]
- 2.Watson C, Alp NJ. Role of Chlamydia pneumoniae in atherosclerosis. Clin Sci (Lond) 2008;114:509–531. doi: 10.1042/CS20070298. [DOI] [PubMed] [Google Scholar]
- 3.Alamowitch S, Labreuche J, Touboul PJ, Eb F, Amarenco P for the GENIC Investigators. Chlamydia pneumoniae seropositivity in aetiological subtypes of brain infarction and carotid atherosclerosis: a case–control study. J Neurol Neurosurg Psychiatry. 2008;79:147–151. doi: 10.1136/jnnp.2007.126862. [DOI] [PubMed] [Google Scholar]
- 4.Cuffini C, Alberto-Guzmán L, Villegas N, Eduardo-Alonso C, Martínez-Riera L, Rodríguez-Fermepín M, et al. Isolation of Chlamydophila pneumoniae from atheromas of the carotid artery and their antibiotics susceptibility profile. Enferm Infecc Microbiol Clin. 2006;24:81–85. doi: 10.1157/13085013. [DOI] [PubMed] [Google Scholar]
- 5.Hauer AD, de Vos P, Peterse N, ten Cate H, van Berkel TJ, Stassen FR, et al. Delivery of Chlamydia pneumoniae to the vessel wall aggravates atherosclerosis in LDLr−/− mice. Cardiovasc Res. 2006;69:280–288. doi: 10.1016/j.cardiores.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Cannon CP, Braunwald E, McCabe CH, Grayston JT, Muhlestein B, Giugliano RP, et al. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med. 2005;352:1646–1654. doi: 10.1056/NEJMoa043528. [DOI] [PubMed] [Google Scholar]
- 7.Hirono S, Dibrov E, Hurtado C, Kostenuk A, Ducas R, Pierce GN. Chlamydia pneumoniae stimulates proliferation of vascular smooth muscle cells through induction of endogenous heat shock protein 60. Circ Res. 2003;93:710–716. doi: 10.1161/01.RES.0000095720.46043.F2. [DOI] [PubMed] [Google Scholar]
- 8.Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1547–1559. doi: 10.1161/01.atv.0000029720.59649.50. [DOI] [PubMed] [Google Scholar]
- 9.Wick G. Atherosclerosis—an autoimmune disease due to an immune reaction against heat-shock protein 60. Herz. 2000;25:87–90. doi: 10.1007/pl00001957. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Ming XF. Recent advances in understanding endothelial dysfunction in atherosclerosis. Clin Med Res. 2006;4:53–65. doi: 10.3121/cmr.4.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bredt DS. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic Res. 1999;31:577–596. doi: 10.1080/10715769900301161. [DOI] [PubMed] [Google Scholar]
- 12.Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998;105:32S–39S. doi: 10.1016/s0002-9343(98)00209-5. [DOI] [PubMed] [Google Scholar]
- 13.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 14.Chai H, Yang H, Yan S, Li M, Lin PH, Lumsden AB, et al. Effects of 5 HIV protease inhibitors on vasomotor function and superoxide anion production in porcine coronary arteries. J Acquir Immune Defic Syndr. 2005;40:12–19. doi: 10.1097/01.qai.0000172368.05327.7b. [DOI] [PubMed] [Google Scholar]
- 15.Chai H, Zhou W, Lin P, Lumsden A, Yao Q, Chen C. Ginsenosides block HIV protease inhibitor ritonavir-induced vascular dysfunction of porcine coronary arteries. Am J Physiol Heart Circ Physiol. 2005;288:H2965–H2971. doi: 10.1152/ajpheart.01271.2004. [DOI] [PubMed] [Google Scholar]
- 16.Chan Y, Fish JE, D'Abreo C, Lin S, Robb GB, Teichert AM, et al. The cell-specific expression of endothelial nitric-oxide synthase: a role for DNA methylation. J Biol Chem. 2004;279:35087–35100. doi: 10.1074/jbc.M405063200. [DOI] [PubMed] [Google Scholar]
- 17.Kojima H, Urano Y, Kikuchi K, Higuchi T, Hirata Y, Nagano T. Fluorescent indicators for imaging nitric oxide production. Angew Chem Int Ed Engl. 1999;38:3209–3212. doi: 10.1002/(sici)1521-3773(19991102)38:21<3209::aid-anie3209>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Da Costa CU, Wantia N, Kirschning CJ, Busch DH, Rodriguez N, Wagner H, et al. Heat shock protein 60 from Chlamydia pneumoniae elicits an unusual set of inflammatory responses via Toll-like receptor 2 and 4 in vivo. Eur J Immunol. 2004;34:2874–2884. doi: 10.1002/eji.200425101. [DOI] [PubMed] [Google Scholar]
- 19.Genova ML, Pich MM, Bernacchia A, Bianchi C, Biondi A, Bovina C, et al. The mitochondrial production of reactive oxygen species in relation to aging and pathology. Ann N Y Acad Sci. 2004;1011:86–100. doi: 10.1007/978-3-662-41088-2_10. [DOI] [PubMed] [Google Scholar]
- 20.Kawashima S. The two faces of endothelial nitric oxide synthase in the pathophysiology of atherosclerosis. Endothelium. 2004;11:99–107. doi: 10.1080/10623320490482637. [DOI] [PubMed] [Google Scholar]
- 21.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 22.Bouwman JJ, Visseren FL, Bevers LM, van der Vlist WE, Bouter KP, Diepersloot RJ. Azithromycin reduces Chlamydia pneumoniae-induced attenuation of eNOS and cGMP production by endothelial cells. Eur J Clin Invest. 2005;35:573–582. doi: 10.1111/j.1365-2362.2005.01541.x. [DOI] [PubMed] [Google Scholar]
- 23.Madamanchi NR, Hakim ZS, Runge MS. Oxidative stress in atherogenesis and arterial thrombosis: the disconnect between cellular studies and clinical outcomes. J Thromb Haemost. 2005;3:254–267. doi: 10.1111/j.1538-7836.2004.01085.x. [DOI] [PubMed] [Google Scholar]
- 24.Azumi H, Inoue N, Ohashi Y, Terashima M, Mori T, Fujita H, et al. Superoxide generation in directional coronary atherectomy specimens of patients with angina pectoris: important role of NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2002;22:1838–1844. doi: 10.1161/01.atv.0000037101.40667.62. [DOI] [PubMed] [Google Scholar]
- 25.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 26.Kirkman HN, Rolfo M, Ferraris AM, Gaetani GF. Mechanisms of protection of catalase by NADPH. Kinetics and stoichiometry. J Biol Chem. 1999;274:13908–13914. doi: 10.1074/jbc.274.20.13908. [DOI] [PubMed] [Google Scholar]
- 27.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Haan JB, Bladier C, Lotfi-Miri M, Taylor J, Hutchinson P, Crack PJ, et al. Fibroblasts derived from Gpx1 knockout mice display senescent-like features and are susceptible to H2O2-mediated cell death. Free Radic Biol Med. 2004;36:53–64. doi: 10.1016/j.freeradbiomed.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Pedrero Z, Madrid Y. Novel approaches for selenium speciation in foodstuffs and biological specimens: a review. Anal Chim Acta. 2009;634:135–152. doi: 10.1016/j.aca.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Alexander J. Selenium. Novartis Found Symp. 2007;282:143–149. [PubMed] [Google Scholar]
- 31.Ramoutar RR, Brumaghim JL. Effects of inorganic selenium compounds on oxidative DNA damage. J Inorg Biochem. 2007;101:1028–1035. doi: 10.1016/j.jinorgbio.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Battin EE, Perron NR, Brumaghim JL. The central role of metal coordination in selenium antioxidant activity. Inorg Chem. 2006;45:499–501. doi: 10.1021/ic051594f. [DOI] [PubMed] [Google Scholar]
- 33.Smith AR, Visioli F, Frei B, Hagen TM. Lipoic acid significantly restores, in rats, the age-related decline in vasomotion. Br J Pharmacol. 2008;153:1615–1622. doi: 10.1038/bjp.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safaya R, Chai H, Kougias P, Lin P, Lumsden A, Yao Q, et al. Effect of lysophosphatidylcholine on vasomotor functions of porcine coronary arteries. J Surg Res. 2005;126:182–188. doi: 10.1016/j.jss.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Joshi R, Kumar MS, Satyamoorthy K, Unnikrisnan MK, Mukherjee T. Free radical reactions and antioxidant activities of sesamol: pulse radiolytic and biochemical studies. J Agric Food Chem. 2005;53:2696–2703. doi: 10.1021/jf0489769. [DOI] [PubMed] [Google Scholar]
- 36.Fukuoka K, Sawabe A, Sugimoto T, Koga M, Okuda H, Kitayama T, et al. Inhibitory actions of several natural products on proliferation of rat vascular smooth muscle cells induced by HSP60 from Chlamydia pneumoniae J138. J Agric Food Chem. 2004;52:6326–6329. doi: 10.1021/jf0351164. [DOI] [PubMed] [Google Scholar]
- 37.Inagaki N, Ito M, Nakano T, Inagaki M. Spatiotemporal distribution of protein kinase and phosphatase activities. Trends Biochem Sci. 1994;19:448–452. doi: 10.1016/0968-0004(94)90128-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.